Published online May 12, 2015. doi: 10.5318/wjo.v5.i2.73
Peer-review started: November 29, 2014
First decision: December 12, 2014
Revised: January 12, 2015
Accepted: March 4, 2015
Article in press: March 5, 2015
Published online: May 12, 2015
Processing time: 171 Days and 6.7 Hours
Aggressive posterior retinopathy of prematurity (ROP), previously referred to as “Rush disease”, is a rapidly progressive form of ROP. This form of ROP typically presents in very low birth weight babies of early gestational age. Historically, anatomical and functional outcomes have been poor with standard treatment. This review is designed to discuss current knowledge and treatment regarding this aggressive form of ROP. Recommendations regarding management of these difficult cases are detailed.
Core tip: Neonates with aggressive posterior retinopathy of prematurity often have unfavorable visual outcomes due to the aggressive and destructive nature of the disease. Treatment options, including laser and anti-vascular endothelial growth factor therapy can change the course of the disease, but both with potential side effects. Case studies and recommendations regarding the management of these complicated cases are reviewed.
- Citation: Pulido CM, Quiram PA. Current understanding and management of aggressive posterior retinopathy of prematurity. World J Ophthalmol 2015; 5(2): 73-79
- URL: https://www.wjgnet.com/2218-6239/full/v5/i2/73.htm
- DOI: https://dx.doi.org/10.5318/wjo.v5.i2.73
Retinopathy of prematurity (ROP) occurs in premature infants of early gestational age and low birth weight. While screening and treatment options have advanced, it remains a major cause of childhood blindness in middle and high income countries[1]. Aggressive posterior ROP (APROP) is a rapidly progressing form of the disease characterized by “plus” disease and a more posterior location. The advent of anti-vascular endothelial growth factor (VEGF) therapy for the treatment of retinal neovascularization has provided a new treatment approach for ROP[2,3]. The purpose of this article is to review the current knowledge regarding ROP and discuss treatment guidelines regarding APROP.
In normal retinal development, vasculogenesis begins around 17 wk postmenstrual age (PMA)[4]. Vessels originate at the optic nerve and grow peripherally towards the ora serrata. Normal development can continue until about 39-40 wk, near the time of birth[4].
Abnormal angiogenesis related to ROP can be divided into two phases of oxygenation[4]. Phase I begins at the time of premature birth when increased levels of oxygen relative to the in utero environment cause downregulation of VEGF. A decrease in VEGF terminates vessel formation at the vascular-avascular junction. In Phase II, large areas of avascular retina trigger the release of hypoxia-induced factors, which leads to greater VEGF production. In turn, elevated VEGF drives the abnormal angiogenesis characteristic of ROP. Elevated VEGF levels in eyes with active ROP have been well documented. For example, in infants with Stage 4 ROP, VEGF is present in the vitreous at significantly higher levels compared to non-ROP controls[5]. Infants with active neovascularization demonstrate the highest levels of VEGF, further confirming the causative impact of VEGF in ROP pathogenesis.
In addition to the role in retinal development and ROP pathogenesis, VEGF is an important growth factor in normal development of many organ systems, including central nervous system pathways, lungs, and solid organs[6,7]. The long term effect of VEGF suppression following anti-VEGF therapy in the eye or systemic circulation is unknown.
ROP is characterized by zones and stages. Zone 1 is a circular area extending from the optic disc with a radius twice the distance from the center of the disc to the center of the macula. Zone 2 forms a ring around Zone 1 extending to the nasal ora serrata. Zone 3 is the remaining retinal area on the temporal ora.
Stage 1 ROP is defined as a flat demarcation line between the vascular and avascular regions of the retina. Progression to Stage 2 is indicated by the development of an elevated ridge at the avascular/vascular junction. Stage 3 is signified by abnormal neovascularization at the ridge. Stage 4 has two designations. Stage 4A is a partial retinal detachment not involving the macula and Stage 4B is a partial retinal detachment including the macula. Stage 5 is total retinal detachment. Vascular activity is denoted by the presence of “plus disease” which indicates increased blood flow to the point of causing vascular dilation and tortuosity. Other indicators of plus disease include engorgement of the iris vessels, vitreous haze, and pupillary rigidity.
APROP (formerly known as Rush disease) is defined as Zone 1 or posterior Zone II ROP with Stage 3 and the presence of plus disease. The neovascularization often appears flat and anterior to the ridge tissue. In APROP, eyes can rapidly progress from Stage 1 to Stage 3 with a high risk for progressing to retinal detachment.
Indicators for the potential development of ROP are low birth weight and early gestational age. In the Early Treatment of Retinopathy of Prematurity Study (ETROP), which enrolled infants born from 2000-2002, the incidence of ROP amongst infants weighing < 1251 g was 68%[8]. This finding was very similar to the earlier Cryotherapy for Retinopathy of Prematurity study (CRYO-ROP), which enrolled patients from 1986-1987, suggesting a fairly steady incidence of ROP despite advances in neonatal care and better outcomes for premature infants[8]. The ETROP study did show an increased percentage of infants with Zone 1 ROP over the CRYO-ROP study, possibly due to the greater survival of extremely premature infants. The ETROP study also indicated a racial disparity, with Caucasian infants more likely to develop severe ROP than African-American infants[8]. Worldwide, developing nations are reporting more cases of ROP cases as they acquire better neonatal care. Other developing countries report ROP at higher average birth weights, suggesting the need to tailor screening protocols based on the population[1].
After ROP develops, many eyes spontaneously regress without treatment. It is common for the areas of ROP to involute with down grading of the stage followed by continued growth of normal retinal vessels into the periphery. A study of 82 infants with subthreshold disease showed a predictable course of involution[9]. All 82 infants reached complete involution with the majority reaching complete involution between 39-75 wk PMA. On average, the higher the stage of ROP, the longer it took for involution to be completed[9].
Unfortunately APROP usually leads to less favorable outcomes. One study from Australia found that in a cohort of 304 infants with ROP, 2.5% had developed APROP[10]. Rates of retinal detachment for infants exhibiting APROP treated with laser vary, but appear to remain high. A study of 22 eyes treated by laser found an 18.2% detachment rate[11]. A larger study of 109 eyes with APROP treated by laser showed a 17.4% detachment rate[12]. Risk factors for progressing to detachment despite confluent laser photocoagulation were gestational age of less than 29.5 wk, hemorrhages, need for repeat treatment, and new onset fibrovascular traction after treatment. The BEAT-ROP study showed a lower detachment rate, with only a 2.9% detachment rate for APROP treated with intravitreal bevacizumab and 2.7% for laser[2]. However, BEAT-ROP focused on outcomes within 54 wk post-menstrual age, and data indicates that bevacizumab treatment may delay the timeline of recurrence[3].
The standards set by the cCRYO-ROP trial recommended treatment at Stage 3 ROP with at least 5 contiguous or 8 total clock hour sectors in Zone 1 or 2 with plus disease[13-25]. The ETROP study built upon these results by setting an earlier treatment threshold for laser photocoagulation[26-41]. The study showed treatment benefit for any stage in Zone 1 with plus disease, Stage 3 Zone 1 with or without plus disease, and Stage 2 or 3 with plus disease in Zone 2 (type 1 ROP). For type 2 ROP (Zone 1, Stage 1 or 2 without plus and Zone 2, Stage 3 without plus) close observation is recommended.
The BEAT-ROP trial tested the efficacy of intravitreal bevacizumab (IVB) injection versus laser ablation in a randomized trial[2]. Recurrence of ROP within 54 wk PMA for laser in Zone 1 disease was significantly higher than with IVB (42% vs 6%). However for Zone 2 disease the difference between the two therapies was not significant. The trial also showed that while laser permanently ablated the retina, IVB allowed for continued vascularization in the peripheral retina.
A chief critique of Bevacizumab Eliminates the Angiogenic Threat of Retinopathy of Prematurity (BEAT-ROP) was the trial’s end point of 54 wk. The mean age at which infants with Zone 1 ROP were treated was 34.5 ± 1.4 wk for IVB and 33.7 ± 1.6 wk for laser. The mean interval between recurrence and treatment was 19.2 ± 8.6 wk for IVB and 6.4 ± 6.7 wk for laser in infants with Zone 1 ROP. Given the ranges encompassed by 1 or 2 standard deviations from the means, many recurrences may have fallen outside of the 54 wk endpoint[3]. This suggests that for Zone 1 ROP, where IVB showed a statistically significant better outcome, the BEAT-ROP trial may not have given a full assessment of bevacizumab’s ability to prevent recurrence. Furthermore this study was not powered for safety.
Several case reports and case series have indicated the need for a longer duration of monitoring after bevacizumab treatment[42-44]. In one series, 17 eyes in 9 patients developed recurrence after IVB at a mean age of 34.1 wk PMA[43]. The mean age of recurrence was 49.3 wks and the mean age of retinal detachment was 58.4 wk PMA. This series also indicated an altered pattern of recurrence after IVB. Recurrence after laser often presents anterior to the vascular-avascular junction. After IVB, recurrence was noted more posterior to the initial site of extraretinal fibrovascular proliferation. Anterior recurrence was seen in 47% of the eyes. Posterior recurrence alone appeared in 12% of eyes, and 41% showed in both areas[43]. Whereas regression following laser is predictable, treatment with IVB appears to result in short term regression with less predictable long term reactivation.
In addition to the late recurrence following IVB, there are concerns about the systemic effects of administering IVB injections in infants. While not statistically significant, out of the seven infants who died before the BEAT-ROP endpoint, five were in the IVB treatment arm. One study of 11 patients identified bevacizumab in the systemic circulation after IV injection[45]. There was a statistically significant negative correlation between the serum VEGF titers and the serum bevacizumab titers. Given the role of VEGF in various developmental processes, systemic bevacizumab may pose a risk to preterm infants.
There has been great interest in the use of telemedicine in screening for ROP. With the number of pre-term infants rising globally and a limited pool of ROP screeners, telemedicine presents a method to satisfy the high demand for screening. The Photographic Screening for Retinopathy of Prematurity (PHOTO-ROP) study investigated the use of telemedicine in conjunction with conventional bedside indirect ophthalmoscopy (BIO)[46-48]. After imaging both fundi using the RetCam-120, traditional BIO was performed. The reading center or bedside clinician then determined which eyes demonstrated clinically significant ROP (CSROP), or ROP severe enough to warrant on-site examination, or ETROP type 1, ROP severe enough to warrant treatment. Using BIO as the reference standard, digital imaging provided sensitive and specific detection of CSROP and ETROP type 1, suggesting it is an effective tool to use in conjunction with traditional screening. Using the reading center data as the reference standard, imaging showed high specificity and positive predictive values, but weaker sensitivity, negative predictive value, and accuracy, suggesting the limitations for using digital imaging as the primary screening modality[47].
The Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP) structured their trial to better assess the ability for digital imaging to be used as the primary screening tool[49-54]. Their study used RetCam II imaging without simultaneous bedside indirect ophthalmoscopy. Infants were imaged with the same frequency as recommended for BIO. If treatment-warranted ROP (TW-ROP) was identified, follow-up took place using BIO. Digital imaging showed a 100% sensitivity, 99.8% specificity, 93.8% positive predictive value, and 100% negative predictive value[43]. The success of the SUNDROP trial suggests that as imaging technology improves, so does the validity of using a telemedicine approach for ROP screening.
ROP is associated with the long term development of myopia, and more severe ROP is associated with worse visual outcomes[13,55]. Given this baseline tendency towards myopia, it has been difficult to definitively prove a connection between laser treatment and refractive error. Both the CRYO-ROP and ETROP trials found high rates of myopia in patients receiving ablation, but credited the tendency to greater severity of ROP[13,26]. One retrospective study showed that of 43 infants treated by laser, 73% scored 6/12 (20/40) or better on the Snellen acuity chart[56]. However, there was a strong correlation between the refractive error of each eye and the number of laser burns applied. Of the infants with APROP, all of whom received treatment, 40% developed myopia[10]. The authors cautioned that the correlation between refractive error and laser burns includes multiple confounding factors like the need for more laser burns stemming from more severe ROP. In the APROP subset they concede that laser often yields poor functional vision despite improved structural outcomes.
The landmark BEAT-ROP trial yielded favorable results, but questions over the full efficacy and safety of the drug remain[2-3]. The BEAT-ROP trial enabled a comparison of refractive outcomes between laser treatment and bevacizumab[57]. There was a significantly lower percentage of infants treated with IVB who developed high and very high myopia. The BEAT-ROP group also found a strong correlation between refractive error and laser burns. Given the study’s design of comparing infants with similar severity ROP but different treatment methodology, these results indicate laser ablation plays a role in the development of myopia. Myopia of prematurity, regardless of ROP status, stems from abnormal anterior segment development. The BEAT-ROP group hypothesizes that the greater preservation of the peripheral retina and extension of retinal vessels past the neovascular ridge in IVB treated eyes allows for the continued production of local growth factors necessary for normal anterior segment development, leading to better refractive outcomes[57].
While IVB seems to allow for better visual outcomes, it can result in abnormal vascularization of the retina. One study examined outcomes in infants with APROP or posterior Zone II with plus disease that regressed after one IVB injection[58]. Fluorescein angiography (FA) revealed incomplete vascularization of the peripheral retina in 11/20 (55%) of eyes. Of these, 9 showed fluorescein dye leakage at the vascular-avascular junction. In comparison, laser therapy completely prevents vascularization past the ridge. Treatment with IVB provides an opportunity for continued vascularization in the periphery, but the development of abnormal peripheral retina is also a potential outcome.
Prior to the 1940s premature birth was often fatal, resulting in no recognition of ROP. With advancement in neonatal survival, ROP emerged as a diagnosis with the baby boomer generation. One study examining 47 patients aged 45 or older that were diagnosed at birth with ROP, but received no treatment. In this study, 88.4% had posterior segment pathology resulting from ROP[59]. Retinal folds were seen most frequently, with retinal detachments, retinal pigmentation, lattice-like degeneration, and retinal tears. Early onset cataract was noted with 74.5% having undergone cataract surgery. Within this group, 51.2% exhibited BCVA of 20/200 or worse[59].
The CRYO-ROP trial began in the 1980s and ushered in the next wave of ROP infants, the ablation generation. The most recent publication reports the visual acuity and anatomical outcomes at 15 years[14]. Of particular interest was the development of retinal folds and detachments in eyes which had no evidence of unfavorable outcomes at 10 years. During this 5 year period, identification of progressive retinal disease occurred in 4.5% (6) of treated eyes and in 7.7% (7) of control eyes. Data from both generations highlights the importance of maintaining close follow-up with ROP patients well past infancy.
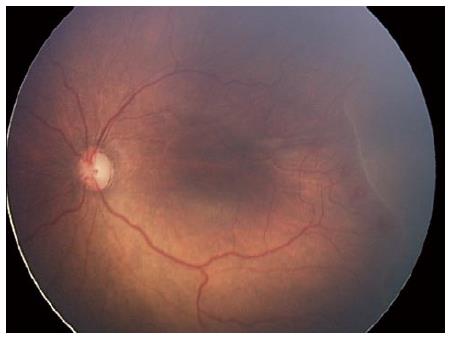
Report of a case: A male infant was born at 24 wk gestation with a birth weight of 420 g. At 32 wk, anterior segment examination showed a prominent tunica vasculosa lentis in both eyes and dilated fundus examination showed Stage 2, Zone 1 disease with preplus (Figure 1). One week later, the ROP had significantly worsened with presence of plus disease and flat Stage 3 with extensive hemorrhages at the junction of avascular and vascular retina.
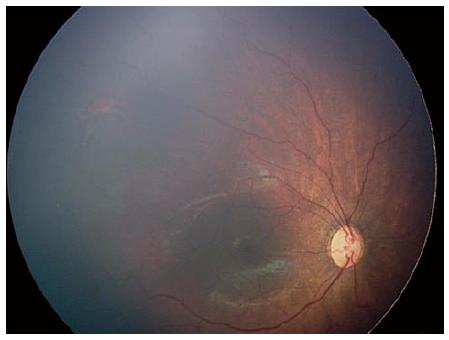
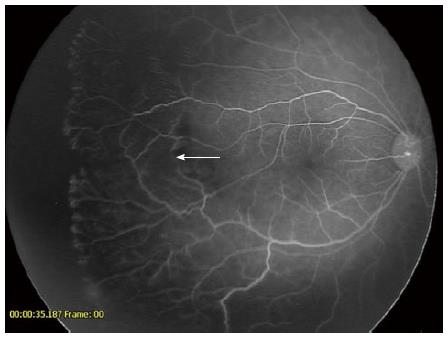
Informed consent for intravitreal bevacizumab injection was obtained from the patient’s parents. Intravitreal bevacizumab was injected without complication. One week following treatment, regression of Stage 3 and reduction of plus disease occurred. The active ridge completely regressed and normal vasculogenesis continued into Zone 2. At approximately 55 wk, the patient underwent an exam under anesthesia with Retcam photos and fluorescein angiography. Examination showed apparently normal vascularization to mid Zone 2 (Figure 2). Fluorescein angiogram showed evidence of the previous ridge (arrow). At the junction of vascular and avascular retina, areas of neovascularization were present with extensive areas of avascular retina in the periphery (Figure 3). Concern regarding late reactivation of ROP following IVB injection prompted laser photocoagulation to areas of avascular retina.
The data from the BEAT-ROP study, shows improved outcomes for Zone 1 APROP treated with IVB compared to laser, but no difference for posterior Zone 2 disease. Considering the importance of VEGF in the developing neonate[5,6] and the unknown long term systemic effects of IVB, the use of IVB is generally reserved for Zone 1 APROP. Reactivation and late retinal detachment following IVB is a serious concern with multiple reports citing retinal detachments beyond 60 wk PMA[43,44]. In order to closely monitor these neonates, we recommend weekly examinations following IVB until the child is discharged from the NICU. Following discharge, the infant is examined every 2 wk until 55-60 wk and then undergoes an exam under anesthesia, fluorescein angiogram and Retcam photos. If incomplete vascularization or neovascularization is noted, laser photocoagulation is performed. The infants are followed until 70 wk or until noted to have complete vascularization at time of EUA and FA. In our series of over 30 infants, no retinal detachments have occurred following this protocol.
APROP can present with uncontrolled neovascularization in Zone 1 that can rapidly progress to retinal detachment. Treatment with laser ablation alone can result in less than favorable outcomes. Use of anti-VEGF agents has shown promising results for the treatment of APROP, but because of unknown systemic and long-term effects on neonatal development, judicious use is recommended. In addition, long term follow up after IVB is necessary to monitor for the development of late recurrence.
P- Reviewer: Chaudhry IA, Inan UU, Mahendradas P, Sharif N S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK
| 1. | Gilbert C, Fielder A, Gordillo L, Quinn G, Semiglia R, Visintin P, Zin A; International NO-ROP Group. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics. 2005;115:e518-e525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 464] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 2. | Mintz-Hittner HA, Kennedy KA, Chuang AZ; BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1132] [Cited by in RCA: 1021] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 3. | Moshfeghi DM, Berrocal AM. Retinopathy of prematurity in the time of bevacizumab: incorporating the BEAT-ROP results into clinical practice. Ophthalmology. 2011;118:1227-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Smith LE, Hard AL, Hellström A. The biology of retinopathy of prematurity: how knowledge of pathogenesis guides treatment. Clin Perinatol. 2013;40:201-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Sonmez K, Drenser KA, Capone A, Trese MT. Vitreous levels of stromal cell-derived factor 1 and vascular endothelial growth factor in patients with retinopathy of prematurity. Ophthalmology. 2008;115:1065-1070.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Cleaver O, Melton DA. Endothelial signaling during development. Nat Med. 2003;9:661-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 363] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 7. | Ruhrberg C, Bautch VL. Neurovascular development and links to disease. Cell Mol Life Sci. 2013;70:1675-1684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Good WV, Hardy RJ, Dobson V, Palmer EA, Phelps DL, Quintos M, Tung B; Early Treatment for Retinopathy of Prematurity Cooperative Group. The incidence and course of retinopathy of prematurity: findings from the early treatment for retinopathy of prematurity study. Pediatrics. 2005;116:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 340] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 9. | Ni YQ, Huang X, Xue K, Yu J, Ruan L, Shan HD, Xu GZ. Natural involution of acute retinopathy of prematurity not requiring treatment: factors associated with the time course of involution. Invest Ophthalmol Vis Sci. 2014;55:3165-3170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Gunn DJ, Cartwright DW, Gole GA. Prevalence and outcomes of laser treatment of aggressive posterior retinopathy of prematurity. Clin Experiment Ophthalmol. 2014;42:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Drenser KA, Trese MT, Capone A. Aggressive posterior retinopathy of prematurity. Retina. 2010;30:S37-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Sanghi G, Dogra MR, Katoch D, Gupta A. Aggressive posterior retinopathy of prematurity: risk factors for retinal detachment despite confluent laser photocoagulation. Am J Ophthalmol. 2013;155:159-164.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Dobson V, Quinn GE, Summers CG, Hardy RJ, Tung B; Cryotherapy for Retinopathy of Prematurity Cooperative Group. Visual acuity at 10 years in Cryotherapy for Retinopathy of Prematurity (CRYO-ROP) study eyes: effect of retinal residua of retinopathy of prematurity. Arch Ophthalmol. 2006;124:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Palmer EA, Hardy RJ, Dobson V, Phelps DL, Quinn GE, Summers CG, Krom CP, Tung B; Cryotherapy for Retinopathy of Prematurity Cooperative Group. 15-year outcomes following threshold retinopathy of prematurity: final results from the multicenter trial of cryotherapy for retinopathy of prematurity. Arch Ophthalmol. 2005;123:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Msall ME, Phelps DL, Hardy RJ, Dobson V, Quinn GE, Summers CG, Tremont MR; Cryotherapy for Retinopathy of Prematurity Cooperative Group. Educational and social competencies at 8 years in children with threshold retinopathy of prematurity in the CRYO-ROP multicenter study. Pediatrics. 2004;113:790-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Hardy RJ, Palmer EA, Dobson V, Summers CG, Phelps DL, Quinn GE, Good WV, Tung B; Cryotherapy for Retinopathy of Prematurity Cooperative Group. Risk analysis of prethreshold retinopathy of prematurity. Arch Ophthalmol. 2003;121:1697-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: natural history ROP: ocular outcome at 5(1/2) years in premature infants with birth weights less than 1251 g. Arch Ophthalmol. 2002;120:595-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Cryotherapy for Retinopathy of Prematurity Cooperative Group. Contrast sensitivity at age 10 years in children who had threshold retinopathy of prematurity. Arch Ophthalmol. 2001;119:1129-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Cryotherapy for Retinopathy of Prematurity Cooperative Group. Effect of retinal ablative therapy for threshold retinopathy of prematurity: results of Goldmann perimetry at the age of 10 years. Arch Ophthalmol. 2001;119:1120-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter Trial of Cryotherapy for Retinopathy of Prematurity: ophthalmological outcomes at 10 years. Arch Ophthalmol. 2001;119:1110-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 225] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 21. | Quinn GE, Dobson V, Siatkowski R, Hardy RJ, Kivlin J, Palmer EA, Phelps DL, Repka MX, Summers CG, Tung B. Does cryotherapy affect refractive error? Results from treated versus control eyes in the cryotherapy for retinopathy of prematurity trial. Ophthalmology. 2001;108:343-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity. Snellen visual acuity and structural outcome at 5 1/2 years after randomization. Arch Ophthalmol. 1996;114:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 144] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Multicenter trial of cryotherapy for retinopathy of prematurity. 3 1/2-year outcome--structure and function. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 1993;111:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 90] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Multicenter trial of cryotherapy for retinopathy of prematurity. One-year outcome--structure and function. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 1990;108:1408-1416. [PubMed] |
| 25. | Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 1988;106:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 604] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 26. | Quinn GE, Dobson V, Davitt BV, Wallace DK, Hardy RJ, Tung B, Lai D, Good WV; Early Treatment for Retinopathy of Prematurity Cooperative Group. Progression of myopia and high myopia in the Early Treatment for Retinopathy of Prematurity study: findings at 4 to 6 years of age. J AAPOS. 2013;17:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Davitt BV, Christiansen SP, Hardy RJ, Tung B, Good WV; Early Treatment for Retinopathy of Prematurity Cooperative Group. Incidence of cataract development by 6 months’ corrected age in the Early Treatment for Retinopathy of Prematurity study. J AAPOS. 2013;17:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | VanderVeen DK, Bremer DL, Fellows RR, Hardy RJ, Neely DE, Palmer EA, Rogers DL, Tung B, Good WV; Early Treatment for Retinopathy of Prematurity Cooperative Group. Prevalence and course of strabismus through age 6 years in participants of the Early Treatment for Retinopathy of Prematurity randomized trial. J AAPOS. 2011;15:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Davitt BV, Quinn GE, Wallace DK, Dobson V, Hardy RJ, Tung B, Lai D, Good WV; Early Treatment for Retinopathy of Prematurity Cooperative Group. Astigmatism progression in the early treatment for retinopathy of prematurity study to 6 years of age. Ophthalmology. 2011;118:2326-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Dobson V, Quinn GE, Summers CG, Hardy RJ, Tung B, Good WV; Early Treatment for Retinopathy of Prematurity Cooperative Group. Grating visual acuity results in the early treatment for retinopathy of prematurity study. Arch Ophthalmol. 2011;129:840-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Quinn GE, Dobson V, Hardy RJ, Tung B, Palmer EA, Good WV; Early Treatment for Retinopathy of Prematurity Cooperative Group. Visual field extent at 6 years of age in children who had high-risk prethreshold retinopathy of prematurity. Arch Ophthalmol. 2011;129:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Christiansen SP, Dobson V, Quinn GE, Good WV, Tung B, Hardy RJ, Baker JD, Hoffman RO, Reynolds JD, Rychwalski PJ, Shapiro MJ; Early Treatment for Retinopathy of Prematurity Cooperative Group. Progression of type 2 to type 1 retinopathy of prematurity in the Early Treatment for Retinopathy of Prematurity Study. Arch Ophthalmol. 2010;128:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Good WV, Hardy RJ, Dobson V, Palmer EA, Phelps DL, Tung B, Redford M; Early Treatment for Retinopathy of Prematurity Cooperative Group. Final visual acuity results in the early treatment for retinopathy of prematurity study. Arch Ophthalmol. 2010;128:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 34. | Davitt BV, Dobson V, Quinn GE, Hardy RJ, Tung B, Good WV; Early Treatment for Retinopathy of Prematurity Cooperative Group. Astigmatism in the Early Treatment for Retinopathy Of Prematurity Study: findings to 3 years of age. Ophthalmology. 2009;116:332-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Quinn GE, Dobson V, Davitt BV, Hardy RJ, Tung B, Pedroza C, Good WV; Early Treatment for Retinopathy of Prematurity Cooperative Group. Progression of myopia and high myopia in the early treatment for retinopathy of prematurity study: findings to 3 years of age. Ophthalmology. 2008;115:1058-1064.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Good WV; Early Treatment for Retinopathy of Prematurity Cooperative Group. The Early Treatment for Retinopathy Of Prematurity Study: structural findings at age 2 years. Br J Ophthalmol. 2006;90:1378-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | VanderVeen DK, Coats DK, Dobson V, Fredrick D, Gordon RA, Hardy RJ, Neely DE, Palmer EA, Steidl SM, Tung B, Good WV; Early Treatment for Retinopathy of Prematurity Cooperative Group. Prevalence and course of strabismus in the first year of life for infants with prethreshold retinopathy of prematurity: findings from the Early Treatment for Retinopathy of Prematurity study. Arch Ophthalmol. 2006;124:766-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Jones JG, MacKinnon B, Good WV, Hardy RJ, Dobson V, Palmer EA, Phelps DL, Quintos M, Tung B; Early Treatment for Retinopathy of Prematurity Cooperative Group. The early treatment for ROP (ETROP) randomized trial: study results and nursing care adaptations. Insight. 2005;30:7-13. [PubMed] |
| 39. | Davitt BV, Dobson V, Good WV, Hardy RJ, Quinn GE, Siatkowski RM, Summers CG, Tung B; Early Treatment for Retinopathy of Prematurity Cooperative Group. Prevalence of myopia at 9 months in infants with high-risk prethreshold retinopathy of prematurity. Ophthalmology. 2005;112:1564-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Good WV; Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:233-248; discussion 248-250. [PubMed] |
| 41. | Hardy RJ, Good WV, Dobson V, Palmer EA, Phelps DL, Quintos M, Tung B; Early Treatment for Retinopathy of Prematurity Cooperative Group. Multicenter trial of early treatment for retinopathy of prematurity: study design. Control Clin Trials. 2004;25:311-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Ittiara S, Blair MP, Shapiro MJ, Lichtenstein SJ. Exudative retinopathy and detachment: a late reactivation of retinopathy of prematurity after intravitreal bevacizumab. J AAPOS. 2013;17:323-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Hu J, Blair MP, Shapiro MJ, Lichtenstein SJ, Galasso JM, Kapur R. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol. 2012;130:1000-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 44. | Patel RD, Blair MP, Shapiro MJ, Lichtenstein SJ. Significant treatment failure with intravitreous bevacizumab for retinopathy of prematurity. Arch Ophthalmol. 2012;130:801-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Sato T, Wada K, Arahori H, Kuno N, Imoto K, Iwahashi-Shima C, Kusaka S. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012;153:327-333.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 243] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 46. | Vinekar A, Trese MT, Capone A; Photographic Screening for Retinopathy of Prematurity (Photo-ROP) Cooperative Group. Evolution of retinal detachment in posterior retinopathy of prematurity: impact on treatment approach. Am J Ophthalmol. 2008;145:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Photographic Screening for Retinopathy of Prematurity (Photo-ROP) Cooperative Group. The photographic screening for retinopathy of prematurity study (photo-ROP). Primary outcomes. Retina. 2008;28:S47-S54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Balasubramanian M, Capone A, Hartnett ME, Pignatto S, Trese MT; Photographic Screening for Retinopathy of Prematurity (Photo-ROP) Cooperative Group. The Photographic Screening for Retinopathy of Prematurity Study (Photo-ROP): study design and baseline characteristics of enrolled patients. Retina. 2006;26:S4-S10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Fijalkowski N, Zheng LL, Henderson MT, Wang SK, Wallenstein MB, Leng T, Moshfeghi DM. Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP): five years of screening with telemedicine. Ophthalmic Surg Lasers Imaging Retina. 2014;45:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 50. | Fijalkowski N, Zheng LL, Henderson MT, Wallenstein MB, Leng T, Moshfeghi DM. Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP): four-years of screening with telemedicine. Curr Eye Res. 2013;38:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 51. | Silva RA, Murakami Y, Lad EM, Moshfeghi DM. Stanford University network for diagnosis of retinopathy of prematurity (SUNDROP): 36-month experience with telemedicine screening. Ophthalmic Surg Lasers Imaging. 2011;42:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Murakami Y, Silva RA, Jain A, Lad EM, Gandhi J, Moshfeghi DM. Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP): 24-month experience with telemedicine screening. Acta Ophthalmol. 2010;88:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Silva RA, Murakami Y, Jain A, Gandhi J, Lad EM, Moshfeghi DM. Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP): 18-month experience with telemedicine screening. Graefes Arch Clin Exp Ophthalmol. 2009;247:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Murakami Y, Jain A, Silva RA, Lad EM, Gandhi J, Moshfeghi DM. Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP): 12-month experience with telemedicine screening. Br J Ophthalmol. 2008;92:1456-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Nissenkorn I, Yassur Y, Mashkowski D, Sherf I, Ben-Sira I. Myopia in premature babies with and without retinopathy of prematurity. Br J Ophthalmol. 1983;67:170-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 100] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 56. | Gunn DJ, Cartwright DW, Yuen SA, Gole GA. Treatment of retinopathy of prematurity in extremely premature infants over an 18-year period. Clin Experiment Ophthalmol. 2013;41:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 57. | Geloneck MM, Chuang AZ, Clark WL, Hunt MG, Norman AA, Packwood EA, Tawansy KA, Mintz-Hittner HA; BEAT-ROP Cooperative Group. Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmol. 2014;132:1327-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 239] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 58. | Tahija SG, Hersetyati R, Lam GC, Kusaka S, McMenamin PG. Fluorescein angiographic observations of peripheral retinal vessel growth in infants after intravitreal injection of bevacizumab as sole therapy for zone I and posterior zone II retinopathy of prematurity. Br J Ophthalmol. 2014;98:507-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 59. | Smith BT, Tasman WS. Retinopathy of prematurity: late complications in the baby boomer generation (1946-1964). Trans Am Ophthalmol Soc. 2005;103:225-234; discussion 234-236. [PubMed] |