INTRODUCTION
Intrahepatic cholestasis of pregnancy (ICP) is a metabolic disease occurring in the second and third trimester. Experiments show that bile acids have cytotoxic effects, leading to apoptosis and necrosis in vivo. Exposure to high blood bile acids in pregnancy can cause damage to the fetal heart, liver, lungs and other organs[1]. In addition, bile acids can increase the expression of oxytocin receptors in the uterine muscle fibers increasing sensitivity to oxytocin[2]. These factors can lead to vasospasm and hypoxia at the surface of the placenta and increased vascular permeability[3]. Thus ICP is an important risk factor for perinatal morbidity and mortality[4]. The etiology and pathogenesis of ICP is not clear. It may be related to the mutation of genes such as MDR3, BSEP, ATP8B1[5-7], imbalance of estrogen and progesterone[8], or thyroid hormones[9]. These changes may cause metabolism disorders. When this happens in the liver, the Na+-K+-ATP activity and the biliary bile salt transportation function may decrease, inducing intrahepatic cholestasis. Over the past decade, the relationship of placental immunological changes has been found to be increasingly related to the development of ICP[10]. The embryo, as an alloantigen with paternal ingredients, is essentially an allogeneic allograft to the uterus. The maternal immune system identifies the fetus as an allograft, gradually establishing a balance of tolerance between the maternal and fetal components of the pregnancy.
Once this balance is disrupted, the maternal-fetal interface’s unique dynamics in the local immune microenvironment cannot be maintained, resulting in direct placental trophoblast and decidual cell damage, while a number of inflammatory cytokines are released into the peripheral blood, resulting in changes of the immune microenvironment in liver tissue, inducing cholestasis.
Maternal-fetal immune regulation is a core issue of reproductive immunology. How the maternal-fetal immune interaction influences ICP has plagued scientists. Maternal-fetal interface immune regulation during pregnancy in ICP has been a hot research topic in recent years. A systemic review on the correlation between the etiology and pathology of the maternal fetal interface immunity will help to clarify the immunological mechanisms of ICP and promote and improve the diagnosis, treatment, and understanding of ICP.
RESEARCH ADVANCES ON IMMUNE TOLERANCE BETWEEN THE MATERNAL-FETAL INTERFACE
The maternal-fetal interface is a direct maternal and fetal tissue contact surface, which consists of a large number of immune cells, decidual cells and fetal trophoblast cells. Nevertheless, the number of immune cells, surface antigens, and the effect on receptors in the maternal-fetal interface are very different compared to other organs. Such immune conditions in the maternal-fetal interface protect the placenta as an immune privileged organ. This kind of immune tolerance occurs in the interaction between decidual cells and trophoblast cells.
In general, the immune tolerance embodied in the maternal-fetal interface consists of cellular and humoral immune tolerance. Maternal decidual immune cells include NK cells and T lymphocytes. Studies demonstrate that more than 80% of the maternal-fetal interface lymphocytes are NK cells and the decidual NK cells are mainly CD56+, which secrete cytokines and growth factors to induce local immunosuppression and help embryo development[11]. It is reported that the type of T lymphocytes in pregnancy also shows a decreased CD4+/CD8+ ratio, as well as a helper T cell (Th)1-Th2 shift phenomenon[12]. Th1 secrete interleukin (IL)-2, IL-12, interferon (IFN)-γ and tumor necrosis factor (TNF)-β/α mediated cytotoxic response; Th2 secrete IL-4, IL-5, IL-6 and IL-10, inducing a humoral immune response, and stimulation of B lymphocytes produces antibodies[13]. Studies have shown a shift in the balance of cytokine profiles away from Th1 type reaction to Th2 type reaction in ICP. It disrupts the immune tolerance balance between mother and fetus[10,14]. Th3 secrete transforming growth factor β1 (TGF-β1) which has a strong immunosuppressive effect on cytotoxic T cells, natural killer cells, T cells and natural killer T cells[15]. It is well established that the fetal trophoblast does not express classical human lymphocyte antigen (HLA)-A and HLA-B which can be identified by maternal decidual T lymphocytes, but has a high expression of non-classical HLA-G and HLA-E, and HLA-I like proteins[16,17]. Thus, damage to the fetus from NK cells and T lymphocytes of the mother is prevented.
Kovats et al[18] found that maternal expression of leukemia inhibitory factor (LIF) begins early in pregnancy. LIF can selectively induce HLA-G expression in the maternal-fetal interface on the outer membrane of the chorionic trophoblast cells[18]. CD8+ and CD4+ T cells interact with HLA-G in trophoblast cells to activate the Fas/Fasl signaling pathway, inducing CD8+ T cell apoptosis, and inhibiting the proliferation of CD4+ T-positive cells. This may be the reason why T cells in the decidua are far less numerous than in the outer periphery circulation[19]. Bainbridge proved that the leading strand of HLA-G can strongly induce the expression of HLA-E in the outer chorionic trophoblasts[20]. And the decidual NK cells expression of CD94/NKG2A receptors is 5 fold that of the periphery circulation[21]. Carretero found that the CD94/NKG2A receptor and HLA-E binding by SHP-1 molecules recruits the intracellular delivery of immune inhibitory signals. At the same time, it is found that the HLA-G is the ligand for the expression of the KIR 2DL4 and ILT2 receptors in NK cells in the decidual membrane. Their interaction could inhibit the activity of NK cells[19]. Thus, trophoblast cells are immune to injury by the expression of non-MHC-I receptors to inhibit antigens from NK cells. In addition, HLA-G and HLA-E could inhibit the killing effect of macrophages and T cells on the decidual membrane[22].
Humoral immune tolerance at the maternal fetal interface is effected through blocking antibody (BA) IgG and its subclasses. BA are produced idiotypically of anti-anti-HLA antibodies which are reactive with the fetal half of the self HLS antigens[23]. It can bind the epitope surface antigen of the trophoblast to avoid the maternal killer T cell recognition, allowing the fetus to escape the maternal immune response[24]. Studies show that the HLA-G antigen can stimulate maternal BA production. Furthermore, placental BA concentration is 3 fold that of the maternal symmetric antibody concentration. Th2 factor IL-6 can regulate the production of blocking antibodies through glycosylation[25].
IMMUNE TOLERANCE DISORDER AND THE PATHOGENESIS OF ICP
In recent years, the study of immune pathogenesis in ICP has become a popular research area. More and more research shows that the dynamic imbalance of the maternal fetal interface is closely related to immune tolerance in ICP[26]. Patients with ICP usually demonstrate changes in humoral and cellular immune tolerance associated with antigens, receptors and cytokines, and these changes are not only reflected in the maternal fetal interface but also in maternal peripheral blood.
Studies have shown that the level of IgG in the serum of pregnant women with ICP is significantly decreased, but IgM, IgA and C3, C4 show no significant changes. This suggests that decreased IgG blocking antibody leads to a weakened immune protection effect and failure of maternal fetal immune tolerance.
Cellular immune microenvironment changes in ICP
Studies have shown that TNF-α concentration is higher in peripheral blood of women with ICP than in pregnant women without ICP. It is also confirmed that a decreased TGF-β1 in ICP placental tissue can promote secretion of TNFα and IL-1. The expression of TNF-α and IL-1 are increased in placental tissue in patients with ICP, which promote the secretion of TGF-1[27]. Recent studies have found that TNF-α increase significantly in serum of ICP patients. The increase of TNF-α is positively related to the severity of ICP, which demonstrates that TNF-α may be involved in the occurrence of ICP[28]. Prior to these findings, it was found that an increasing IFN-γ expression in placental tissue from ICP patients as well as TNF-α was related to the occurrence of ICP[29,30]. Increases of IFN-γ and IL-4 may play an important role in the pathogenesis of ICP[31]. These results suggest that ICP generally shows an increased Th1 type cytokine phenomenon, leading to a increase of the Th1-Th2 ratio demonstrating pathological changes of cell immune imbalance[32].
Immune cellular surface antigen changes in ICP
Changes of Th2-Th1 cells in the maternal fetal interface are likely to represent the cellular immune reaction activity of the maternal-fetal interface. The changes of the cellular immune surface antigen of the maternal fetal interface in ICP have received much research attention. A decrease in CD8+ cells and an increase in CD4+ cells in ICP patients lead to an increase of CD4/CD8 ratio. Meanwhile, it is also found that NK cells decrease significantly[14,33]. In recent studies, it is found that leukocyte CD3 antigen, CD4/CD8 ratio, and Th1 were all elevated in the ICP group compared to the control group, while CD8 and Th2 were lower in the ICP group than in the control group, Women with ICP have abnormal expression of T cells and helper T cells in peripheral blood[26,34].
The results from these studies demonstrate that the surface antigen on Th2 cells and NK cells have enhanced cellular immune function, and promote the Th1/Th2 type cytokine balance via Th1. The lack of such balance may be a cause of the liver cell damage noted to be present in ICP.
Trophoblast cell surface antigen and decidual cell receptor changes in ICP
Immune tolerance in pregnancy begins as immune recognition of decidual cells and trophoblast cells. High expression of specific inhibitory ligands on trophoblast cells and specific inhibitory receptors of NK cells in decidua are important factors of immune tolerance. Changes in the expression of trophoblast cell surface antigens and decidual cell receptors inevitably lead to immune dysfunction, which may cause ICP. Peng et al[21] found that decreased expression of HLA-G and HLA-E protein in trophoblast cells are related to ICP. It may be one of the important mechanisms involved in pregnancy immune tolerance imbalance disorders[21]. Dexamethasone increases expression of HLA-G and HLA-E, which may be the critical pharmacological mechanism in the treatment of ICP. Other studies found increased Th1 and decreased HLA-2G in placental tissues from ICP patients, which demonstrate that changes of surface antigens on trophoblast cells may be another cause of ICP[29]. In a different study, downward expression of VEGF receptors was found in syncytiotrophoblast, cytotrophoblast, placental vascular endothelial cells and villi in ICP placentas but it is not clear which is the cause and which is the effect[35].
IMMUNE ETIOLOGY STUDIES ON ICP
Genetic factors influence immune balance of ICP
Genetic factors in the pathogenesis of ICP have been studied for several decades. Many studies have shown that ICP may be the result of multiple factors including a well recognized genetic predispositions[36-39]. That some liver cell secretory transport genes act as genetic factors in the pathogenesis of ICP has been confirmed, but the relationship between immunologically related genes and ICP is not clear. Some scholars believe that the HLA-II antigen promotes the production of blocking antibody. The compatibility is high when the mother and fetus have similar HLA-II antigen expression. The higher the compatibility of the maternal fetal HLA-II antigen, the less the immune response. The strength of the maternal immune system response to paternal antigens of the fetus correlates to the susceptibility of the onset of ICP[40]. Nowak pointed out that women with activation of killer cell immunoglobulin-like receptor (KIR) gene and the KIR inhibitory receptor gene ratio between 0.33-0.83 were prone to have spontaneous abortion while women with a ratio between 0.86-1.25 tend to have a NK cell protective effect, suggesting that the KIR genotypes most likely associate with pathologic pregnancies, such as spontaneous abortion[30,41]. Guimond found that women with missing T cells may have a normal pregnancy but those missing NK cells exhibit pathological changes of pregnancy. He believes these results demonstrate that NK cell immune activity not only has potential immune protective effects, but also is the main cellular immune recognition mediated by the maternal fetal interface[31,42]. These prompted explanation of immune genetic factors in the pathogenesis of ICP, including the ligand NK cell surface antigen, receptor, and their interaction in trophoblast cells. Unfortunately the genetic mechanism for the pathogenesis of ICP in immune gene is still not clear.
Pathogenesis factors disrupt immune tolerance during pregnancy
Without a doubt, research results have clarified our understanding of the changes in immune cell activation and other immune factors in ICP. But factors disrupting the balance of immune tolerance in the maternal fetal interface is not clear. The study of immune tolerance disorders has received little attention. Excluding genetic susceptibility factors, it is not difficult to understand why some factors which cause strong immune responses likely to be associated with ICP. In addition, maternal factors such as nerve - endocrine interactions also affect the decidual immune microenvironment. Social and psychological stress may cause an increase of proinflammatory cytokines. Stress during pregnancy causes changes of IL-6 and IL-10 levels[43,44]. Even though IL-6 and IL-10 level are associated with ICP[45], further studies are necessary to clarify whether prenatal stress may be part of the pathogenesis of ICP.
TO INVESTIGATE THE IMMUNE IMBALANCE AS A MECHANISM OF ICP
Is immune dysfunction the cause of ICP or the result? If the immune imbalance is the result of ICP, then what is the pathogenesis? Previous studies of immunologic changes in ICP seem unable to determine immune dysfunction and ICP causality. It is difficult to carry out research on the causality of ICP because changes in immune regulation cannot be monitored before the onset of the maternal-fetal interface to clarify the immune changes with the onset of ICP. However, we still have some evidence to support that immune regulation disorders can cause the occurrence of ICP. Peng found that dexamethasone can treat ICP and up-regulate HLA-G and HLA-E, which suggests that the expression of surface antigen on trophoblast in ICP can be effectively influenced. This supports the notion that immune imbalance is more likely the initiating factor of ICP[46].
In addition, the up-regulation of Th1 type cytokines such as IL-2, IL-12, IFN-γ, and TNF-α/β and their release into the maternal circulation can generate liver injury and induce ICP. Interaction of TNF-α and IL-2 can promote NK cell change into cytotoxic LAK cells, resulting in damage to liver cells. TNF can also activate neutrophils, promote their aggregation in the liver and prompt degranulation, releasing proteases and oxygen free radicals, causing liver cell damage[27,47]. In addition, TNF-α can promote the uptake, synthesis, and secretion of bile acids in liver cells resulting in cholestasis[45,48]. Furthermore, TNF-α increases the synthesis and secretion of placental estrogen, which is associated with ICP[49].
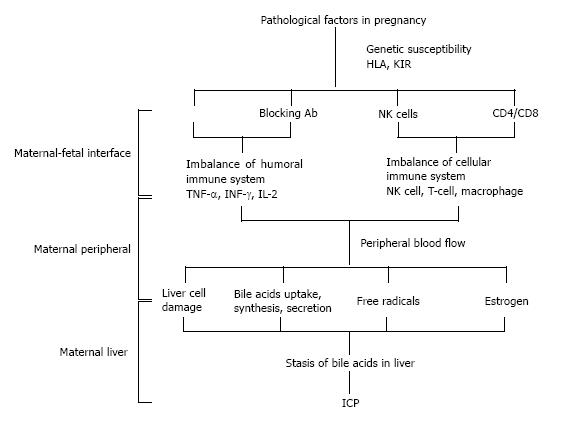
In conclusion, immune pathogenesis leading to ICP might including the following: In the genetically susceptible population, pathogenic factors promote changes in gene expression of trophoblast leukocyte antigen and receptors on decidual NK cells, which lead to an unbalance of blocking antibody and cytokine pathways. Then the cellular immune system activates and secretes Th1 cytokines into the maternal circulation causing damage to the liver cells, resulting in ICP (Figure 1).
Figure 1 Pathogenesis of intrahepatic cholestasis of pregnancy caused by immune intolerance.
ICP: Intrahepatic cholestasis of pregnancy; KIR: Killer cell immunoglobulin-like receptor; IFN: Interferon; IL: Interleukin; TNF: Tumor necrosis factor; HLA: Human lymphocyte antigen.
Thus, these pathological changes contribute to an increased fetal morbidity due to maternal changes, mediated (at least in part) by disruption of the maternal-fetal immune balance. Because of the etiological factor and the pathogenesis of ICP, there is currently no effective clinical standard to prevent and cure ICP. Any approach that modulates the immune tolerance of the maternal-fetal interface toward the natural state could provide insight in the understanding of ICP, which could lead to a targeted treatment.
P- Reviewer: Mohammed Usta IHAB S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ