Published online Feb 10, 2015. doi: 10.5317/wjog.v4.i1.16
Peer-review started: January 17, 2014
First decision: February 13, 2014
Revised: October 29, 2014
Accepted: October 31, 2014
Article in press: November 3, 2014
Published online: February 10, 2015
Processing time: 391 Days and 18.9 Hours
AIM: To examine the association of hepatoma-derived growth factor (HDGF) expression with the prognosis of patients with cervical cancer of the uterus (CC).
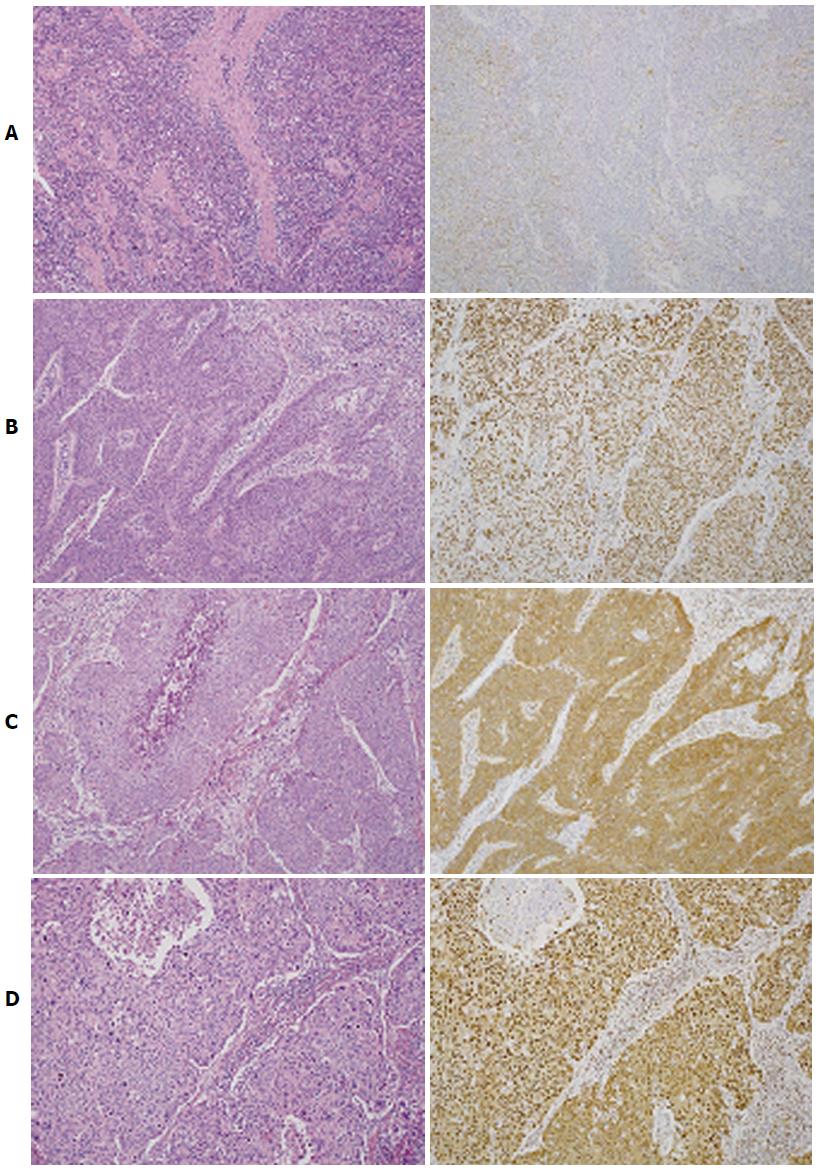
METHODS: HDGF is a unique nuclear growth factor, and it may play an important role in the development and progression of carcinoma. HDGF expression in 88 CC patients aged 23 to 76 years (median, 54 years) was analyzed by immunohistochemistry. A rabbit polyclonal antibody against the C-terminal amino acids (aa 231-240) of the human HDGF sequence was used as primary antibody at a dilution of 1:5000. This specific anti-HDGF antibody was purified using C-terminal peptide-conjugated Sepharose columns. Staining of endothelial cells in the noncancerous areas of each specimen was used as an internal positive control. Samples with more than 80% of tumor cells showing positive immunoreactivity in both the nucleus and cytoplasm were regarded as HDGF index level 2, more than 80% positive immunoreactivity in either the nucleus or cytoplasm as level 1, and less than 80% in both the nucleus and cytoplasm as level 0. The chi-square test and Fisher’s exact probability test were used to examine the relationship between HDGF expression and clinicopathologic parameters, and statistical significance was examined by the log-rank test. Multivariate analysis of factors related to survival was performed using Cox’s proportional hazards regression model. Statistical significance was set at P < 0.05.
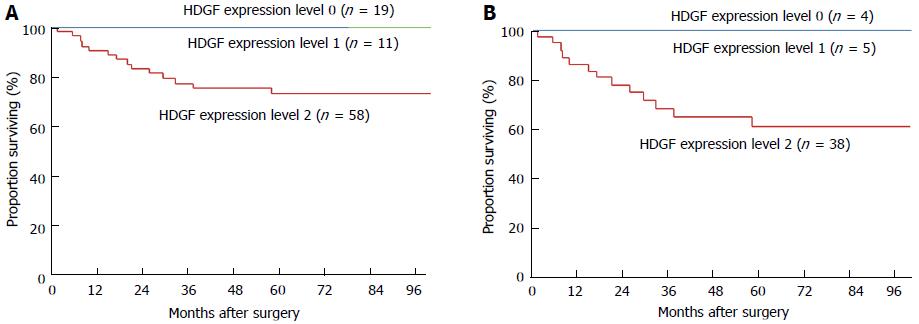
RESULTS: The five-year overall survival rate was 82.9%. Fourteen patients died due to tumors, nine of whom had tumor recurrence at 2-21 mo (median, 10 mo) after surgery. Tumor recurrence in five patients was determined at the time of the patients’ deaths. Nineteen cases were regarded as HDGF index level 0, 11 as level 1, and 58 as level 2. Patients with level 2 expression showed higher rates of histological classification of keratinized squamous cell carcinoma and adenosquamous carcinoma (44.8% of level 2 patients and 13.3% in levels 0 and 1), deep invasion (pT2-4 in 65.5% of level 2 patients, and 30.0% in levels 0 and 1), the presence of lymphatic invasion (50.0% in level 2, and 20.0% in levels 0 and 1), and the presence of lymph node metastasis (37.9% in level 2, and 6.7% in levels 0 and 1). Patients with an HDGF index of level 2 CC showed poorer 5-year overall survival rates than those with level 0 or 1 CC (74.0% and 100%, respectively, P = 0.0036). Univariate analysis revealed that histological classification (P = 0.04), depth of tumor invasion (P = 0.0001), vascular invasion (P = 0.004), and lymph node metastasis (P = 0.0001) were significant factors affecting overall survival in addition to HDGF expression. Multivariate analysis revealed HDGF expression level and lymph node metastasis as independent prognostic factors for overall survival (P = 0.0148 and P = 0.0197, respectively). The prognostic significance of HDGF was further analyzed in pT1 and pT2-4 patient groups, respectively. Among patients with pT1 CC, one the 39 analyzed patients died during the study, and no difference was observed among patients with HDGF index level 0, 1, or 2 CC. However, prognostic significance of the HDGF index was observed in the pT2-4 patient group, in which the mortality rates of patients with HDGF index level 2 CC and those with level 0 or 1 CC significantly differed (P = 0.0463).
CONCLUSION: The HDGF expression level is of prognostic significance in CC.
Core tip: Hepatoma-derived growth factor (HDGF) is a unique nuclear growth factor, playing an important role in the development and progression of carcinomas. Prognostic importance of HDGF expression has been reported in several cancers. In the present study, HDGF expression in cervical cancer was examined by immunohistochemistry, showing increased HDGF expression as a marker of deep invasion, lymphatic invasion, and lymph node metastasis. In addition, HDGF expression was an independent prognostic factor for overall survival.
- Citation: Song M, Tomoeda M, Jin YF, Kubo C, Yoshizawa H, Kitamura M, Nagata S, Ohta Y, Kamiura S, Nakamura H, Tomita Y. Hepatoma-derived growth factor expression as a prognostic marker in cervical cancer. World J Obstet Gynecol 2015; 4(1): 16-23
- URL: https://www.wjgnet.com/2218-6220/full/v4/i1/16.htm
- DOI: https://dx.doi.org/10.5317/wjog.v4.i1.16
Cervical cancer of the uterus (CC) is one of the most common carcinomas in females globally[1,2]. Human papilloma virus infection through sexual transmission is causative of CC carcinogenesis[3,4], and the rate of CC development in females of reproductive age is increasing worldwide[5,6]. More than 25% of patients with CC are under 40 years of age, and its occurrence in nulliparous women is increasing[7]. Due to the rise of mass screening, many cases of CC are detected at an early stage of the disease, and the number of curable patients is increasing[8]. Nevertheless, it is desirable to protect the fertility of nulliparous patients and patients of child-bearing age during treatment[5,6]. Thus, fertility-sparing surgeries, such as vaginal radical trachelectomy or abdominal radical trachelectomy, have been introduced[9,10].
Appropriate candidates for fertility-sparing surgery are patients with tumors classified by the International Federation of Gynecology and Obstetrics (FIGO) as stage IA1 with lymphovascular space involvement, IA2, and IB1 with tumors less than 2 cm in size[11]. In IB1 patients with tumors larger than 2 cm in size, higher risks of extrauterine spread and recurrence have been statistically demonstrated[12]. In cases in which lymph node metastasis is diagnosed or highly suspected, the surgery should be radicalized, or chemoradiotherapy should be undertaken instead[13].
Hepatoma-derived growth factor (HDGF) is a heparin-binding protein purified from the conditioned medium of the hepatocellular carcinoma (HCC) cell line HuH-7, which can proliferate autonomously in a serum-free chemically defined medium[14,15]. HDGF is an acidic 26 kDa protein consisting of 230 amino acids with no hydrophobic signal sequence in its N-terminus, and it has high affinity for the glycosaminoglycans heparin and heparan sulfate[16,17]. Exogenously supplied HDGF stimulates the proliferation of fibroblasts, endothelial cells, vascular smooth muscle cells, pulmonary epithelial cells and hepatocytes, as well as HCC, lung cancer and colon cancer cells, through the stimulation of ERK phosphorylation[18-20]. HDGF translocates to the nucleus, and this nuclear targeting stimulates cell proliferation[18].
Following these observations, we hypothesized that HDGF expression in human malignant tumors might influence metastasis and patient prognosis. Indeed, several reports have shown the correlation between increased HDGF expression and poor prognosis in cancers[21-24]. However, there have been no reports evaluating the correlation of HDGF expression with CC clinicopathologic features or prognosis.
In the present study, which included 88 patients with CC who were undergoing surgery, the expression levels of HDGF and the relationship between HDGF expression and clinicopathological features and prognosis were analyzed.
Tumor tissue was collected from 88 patients with CC who underwent surgical resection between January 1995 and March 2002 at the Gynecology Department, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka, Japan. Patient ages ranged from 23 to 76 years (median, 54 years). Informed consent for the use of the specimens was obtained from all patients. The surgical procedures performed included cervical conization alone in two patients, total hysterectomy alone in five, total hysterectomy plus salpingo-oophorectomy in one, total hysterectomy plus salpingo-oophorectomy plus pelvic lymph node dissection in one, modified radical hysterectomy alone in three, modified radical hysterectomy plus salpingo-oophorectomy in two, modified radical hysterectomy plus pelvic lymph node dissection in one, modified radical hysterectomy plus salpingo-oophorectomy plus pelvic lymph node dissection in one, radical hysterectomy in six, radical hysterectomy plus salpingo-oophorectomy in 52, and radical hysterectomy plus salpingo-oophorectomy plus pelvic lymph node dissection in 14. Samples obtained from uterine lesions and dissected lymph nodes were fixed in 10% formalin and routinely processed for paraffin embedding. Histologic sections cut at a thickness of 4 μm were stained with hematoxylin and eosin, and immunoperoxidase procedures were performed [Avidin-Biotin Complex (ABC) method]. Histologic sections were reviewed by one of the authors (Tomita Y) to define the extent and mode of cancer invasion in the uterus as well as lymph node metastasis. Tumor stages were classified according to the FIGO and pTNM classification[25].
After surgery, all samples were examined by laboratory procedures, such as routine peripheral blood cell counts, and all patients underwent chest roentgenogram, computed tomography of the abdomen, colposcopic examination, and smear cytology at 6-12-mo intervals. Neoadjuvant chemotherapy was performed in nine patients. Adjuvant therapy was performed in 57 patients, radiotherapy alone in 26 patients, chemotherapy alone in 21, and combined chemo- and radiotherapy in nine. The chemotherapeutic protocols were as follows; fluorouracil (5-FU) or its derivative alone in six patients, cisplatinum (CDDP) or its derivative alone in 23, 5-FU and CDDP in one, and irinotecan in one. The follow-up period for survivors ranged from 12-145 (median, 88.2) mo. This study protocol was approved by the institutional review board of Osaka Medical Center for Cancer and Cardiovascular Diseases.
Immunohistochemical studies were completed using the avidin-biotin-peroxidase complex method. Antigen retrieval was performed by heating the sections in 10 mmol/L citrate buffer (pH 6.0) for 5 min. A rabbit polyclonal antibody against the C-terminal amino acids (aa 231-240) of the human HDGF sequence was used as the primary antibody at a dilution of 1:5000. This specific anti-HDGF antibody was purified using C-terminal peptide-conjugated Sepharose columns[16,17]. Non-immunized rabbit IgG (Vector Labs; Burlingame, CA) was used as a substitute for the primary antibody to verify the possibility of false-positive responses due to the non-specific binding of IgG or the secondary antibody. Counterstaining was performed with hematoxylin.
All immunohistochemically stained sections were blindly examined without any prior knowledge of the clinicopathologic parameters or patient outcome. Staining of endothelial cells in the noncancerous areas of each specimen was used as an internal positive control. Consistent HDGF expression in endothelial cells has been reported[19,20]. The HDGF expression pattern was independently evaluated in both the nucleus and cytoplasm; cells showing staining intensity similar to or stronger than that in the nucleus or cytoplasm of endothelial cells were regarded as nucleus positive or cytoplasm positive, respectively. Samples with more than 80% of tumor cells showing positive immunoreactivity in both the nucleus and cytoplasm were regarded as HDGF index level 2, more than 80% positive immunoreactivity in either the nucleus or cytoplasm as level 1, and less than 80% in both the nucleus and cytoplasm as level 0.
SAS software (Statistical Analysis System Institute, Cary, NC) was used for all statistical analyses. The chi-square test and Fisher’s exact probability test were used to examine the relationship between HDGF expression and clinicopathologic parameters of prognosis. The cumulative survival rate was calculated by the Kaplan-Meier method, and statistical significance was examined by the log-rank test[26]. Multivariate analysis of factors related to survival was performed using Cox’s proportional hazards regression model[27]. Statistical significance was set at P < 0.05.
Histologically, 27 tumors were keratinizing squamous cell carcinoma, 60 were non-keratinizing squamous cell carcinoma, and one was adenosquamous carcinoma. Tumor cells invaded less than 5 mm (pT1a) in 14 patients, more than 5 mm but confined to the cervix (pT1b) in 27, beyond the uterus without parametrial invasion (pT2a) in 15, to the parametrium but not to the pelvic wall or lower third of the vagina (pT2b) in 28, to the pelvic wall or lower third of the vagina (pT3) in three, and to the mucosa of the bladder or rectum (pT4) in one. Sixty-four patients were node negative (pN0), and 24 had regional lymph node metastasis (pN1).
The five-year overall survival rate was 82.9%. Fourteen patients died due to the tumors, nine of whom had tumor recurrence at 2-21 mo (median, 10 mo) after surgery. Tumor recurrence in five patients was determined at the time of the patients’ deaths.
The HDGF staining pattern in CC varied; 65 cases (73.9%) showed strong staining in the nuclei of more than 80% of tumor cells and were thus regarded as nucleus positive, while 62 cases (70.5%) with strong cytoplasmic staining in more than 80% of tumor cells were cytoplasm positive. Fifty-eight cases (65.9%) determined as both nucleus and cytoplasm positive were regarded as HDGF index level 2. Eleven cases with HDGF expression either in the nucleus or the cytoplasm were classified as HDGF index level 1 and 19 others as HDGF index level 0 (Figure 1).
The correlations between HDGF tumor expression and clinicopathological factors are shown in Table 1. In comparison to CC with HDGF index level 0 or 1, level 2 CC showed higher rates in the following categories: histological classification of keratinized squamous cell carcinoma and adenosquamous carcinoma (44.8% of level 2 patients and 13.3% in levels 0 and 1), deep invasion (pT2-4 in 65.5% of level 2 patients and 30.0% in levels 0 and 1), the presence of lymphatic invasion (50.0% in level 2 and 20.0% in levels 0 and 1), and the presence of lymph node metastasis (37.9% in level 2 and 6.7% in levels 0 and 1).
| Factor | Category | No. ofpatients | HDGF expression | P value | ||
| Level 0 | Level 1 | Level 2 | ||||
| n = 88 | n = 19 | n = 11 | n = 58 | |||
| Age, yr | 51.6 ± 13.8 | 46.7 ± 15.5 | 55.6 ± 12.1 | 52.4 ± 13.2 | 0.17 | |
| Histological differentiation | 1: Keratinized SQ | 29 | 2 (7) | 2 (7) | 25 (86) | 0.037 |
| 2: Nonkeratinized SQ | 58 | 17 (29) | 9 (16) | 32 (55) | ||
| 3: Adenosquamous carcinoma | 1 | 0 (0) | 0 (0) | 1 (100) | ||
| Lymphatic permeation | 1: Absent | 53 | 18 (34) | 6 (11) | 29 (55) | 0.0006 |
| 2: Present | 35 | 1 (3) | 5 (14) | 29 (83) | ||
| Vascular permeation | 1: Absent | 65 | 18 (28) | 6 (9) | 41 (63) | 0.019 |
| 2: Present | 23 | 1 (4) | 5 (22) | 17 (74) | ||
| Depth of invasion (pT) | 1: T1 | 41 | 15 (37) | 6 (15) | 20 (49) | 0.028 |
| 2: T2 | 43 | 4 (9) | 4 (9) | 35 (81) | ||
| 3: T3 | 3 | 0 (0) | 1 (33) | 2 (67) | ||
| 4: T4 | 1 | 0 (0) | 0 (0) | 1 (100) | ||
| Lymph node metastasis (pN) | 1: Absent | 64 | 12 (19) | 9 (14) | 36 (56) | 0.0004 |
| 2: Present | 24 | 0 (0) | 2 (8) | 22 (92) | ||
| Stage | 1: I | 43 | 15 (35) | 7 (16) | 21 (49) | 0.014 |
| 2: II | 36 | 4 (11) | 4 (11) | 28 (88) | ||
| 3: III | 5 | 0 (0) | 0 (0) | 5 (100) | ||
| 4: IV | 4 | 0 (0) | 0 (0) | 4 (100) | ||
| Types of surgery | 1: Conization | 2 | 2 (100) | 0 (0) | 0 (0) | 0.0001 |
| 2: Total hysterectomy | 7 | 7 (100) | 0 (0) | 0 (0) | ||
| 3: Modified radical hysterectomy | 7 | 4 (57) | 0 (0) | 3 (43) | ||
| 4: Radical hysterectomy | 72 | 6 (8) | 11 (15) | 55 (76) | ||
| Salpingo-oophorectomy | 1: Not performed | 17 | 12 (71) | 2 (12) | 3 (18) | 0.0001 |
| 2: Performed | 71 | 7 (10) | 9 (13) | 55 (77) | ||
| Pelvic lymph node dissection | 1: Not performed | 71 | 14 (20) | 9 (13) | 48 (68) | 0.7 |
| 2: Performed | 17 | 5 (29) | 2 (12) | 10 (59) | ||
| Adjuvant/neoadjuvantchemotherapy | 1: Not performed | 53 | 16 (30) | 5 (9) | 32 (60) | 0.03 |
| 2: Performed | 35 | 3 (9) | 6 (17) | 26 (74) | ||
| Adjuvant radiotherapy | 1: Not performed | 52 | 16 (31) | 9 (17) | 27 (52) | 0.003 |
| 2: Performed | 36 | 3 (8) | 2 (6) | 31 (86) | ||
Patients with HDGF index level 2 CC showed poorer 5-year overall survival rates than those with level 0 or 1 CC (74.0% vs 100%, P = 0.0036; Table 2, Figure 2A). In addition to HDGF expression, univariate analyses revealed that histological classification, depth of tumor invasion, vascular invasion, and lymph node metastasis were significant factors affecting overall survival (Table 2).
| Factor | Category | No. of patientsn = 88 | No. of deathsn = 14 | 5-yroverall survival rate | P value |
| Age (yr) | 1: ≤ 50 | 37 | 4 | 89% | 0.27 |
| 2: > 50 | 51 | 10 | 79% | ||
| Histological differentiation | 1: Keratinized SQ | 29 | 5 | 81% | 0.04a |
| 2: Nonkeratinized SQ | 58 | 8 | 85% | ||
| 3: Adenosquamous carcinoma | 1 | 1 | 0% | ||
| Lymphatic permeation | 1: Absent | 53 | 6 | 88% | 0.15 |
| 2: Present | 35 | 8 | 75% | ||
| Vascular permeation | 1: Absent | 65 | 6 | 90% | 0.005 |
| 2: Present | 23 | 8 | 63% | ||
| Depth of invasion (pT) | 1: T1 | 41 | 1 | 97% | 0.001b |
| 2: T2 | 43 | 10 | 75% | 0.0001d | |
| 3: T3 | 3 | 2 | 0% | ||
| 4: T4 | 1 | 1 | 0% | ||
| Lymph node metastasis (pN) | 1: Absent | 64 | 2 | 97% | 0.0001 |
| 2: Present | 24 | 12 | 49% | ||
| Stage | 1: I | 43 | 2 | 95% | 0.0001d |
| 2: II | 36 | 4 | 88% | ||
| 3: III | 5 | 4 | 20% | ||
| 4: IV | 4 | 4 | 0% | ||
| Hepatoma-derived growth factor expression | 1: Level 0 | 19 | 0 | 100% | 0.0036a |
| 2: Level 1 | 11 | 0 | 100% | ||
| 3: Level 2 | 58 | 14 | 74% |
Multivariate analysis of factors significant in the univariate analyses revealed that HDGF index and lymph node metastasis were independent prognostic factors for overall survival (Table 3).
| Factor | Category | χ2value | P value |
| Histological differentiation | 1: Keratinized and nonkeratinized squamous cell carcinoma | 0.51 | 0.474 |
| 0: Adenosquamous carcinoma | |||
| Vascular permeation | 1: Absent | 0.16 | 0.686 |
| 0: Present | |||
| Depth of invasion (pT)-A | 1: T1 | 1.19 | 0.275 |
| 0: T2-4 | |||
| Depth of invasion (pT)-B | 1: T1 and 2 | 3.06 | 0.0801 |
| 0: T3 and 4 | |||
| Lymph node metastasis (pN) | 1: Absent | 5.43 | 0.0197 |
| 0: Present | |||
| Hepatoma-derived growth factor expression | 1: Level 0 and 1 | 5.94 | 0.0148 |
| 0: Level 2 |
The prognostic significance of HDGF was further analyzed in pT1 and pT2-4 patient groups, respectively. Among patients with pT1 CC, one of the 39 analyzed patients died during the study, and no difference was observed among the groups. However, prognostic significance of the HDGF index was observed in the pT2-4 patient group, in which a significant difference in mortality was present between patients with HDGF index level 2 CC and those with level 0 or 1 CC (Figure 2B).
To identify optimal cutoff for the HDGF index in CC, statistical significance was examined at multiple cutoff levels, including < 50%, < 80%, < 90%, and < 100%. The prognostic significance was the greatest when the cutoff level was set at 80%. This categorization was therefore employed.
In the present study, CCs with HDGF index level 2 showed higher frequencies of deep tumor invasion (pT2-4), the presence of lymph node metastasis, and lymphatic invasion compared to level 1 and 2 cases, which are indicators of tumor progression in CC. In addition, patients with HDGF index level 2 CC showed a higher mortality rate compared with those with level 0 or 1. Previous studies have demonstrated that HDGF has a range of biological functions, including DNA synthesis, proliferation, growth stimulating activity, and vascular development[17-20]. The present study clearly indicates that HDGF is also involved in CC progression.
Fourteen of the 56 patients with HDGF level 2 expression died, whereas no deaths were observed among the 30 patients with HDGF level 0/1 expression. Although the distribution of patients was biased, the present results clearly indicate that HDGF expression is a useful marker to detect patients with CC who have a favorable prognosis. Indeed, multivariate analyses revealed the HDGF expression level to be an independent prognostic factor for CC. Analysis of the HDGF index together with other independent prognostic factors such as lymphatic invasion, vascular invasion, and lymph node metastasis might be a useful tool in predicting prognosis and determining appropriate therapeutic modalities for patients with CC.
Although patients with HDGF index level 0 or 1 can expect a favorable outcome after surgery, the occurrence of lymph node metastasis varied between the two groups. As patients with HDGF index level 0 CC showed no lymph node metastasis, they may suitable for fertility-sparing surgery. In contrast, patients with HDGF index level 1 or 2 exhibited a higher risk of lymph node metastasis; thus, standard surgeries such as radical hysterectomy are more preferable when the FIGO stage is higher than IA.
In conclusion, HDGF expression, as determined by immunohistochemistry, could be used as a new prognostic factor for CC. Although further study is still needed to elucidate the precise role of HDGF in CC malignancy, our results imply that HDGF may be a useful tool for determining the appropriate treatment of CC.
Cervical cancer of the uterus (CC) is one of the most common carcinomas in females, and its occurrence in nulliparous women is increasing. Fertility-sparing surgeries have been introduced to these patients, therefore assessment of histological grading of tumor is important. Expression of Hepatoma-derived growth factor (HDGF) in human malignant tumors might influence metastasis and patient prognosis, and reports have shown the correlation between increased HDGF expression and poor prognosis in several cancers, however, there have been no reports evaluating the correlation of HDGF expression and clinicopathologic features and prognosis of CC.
HDGF expression as determined by immunohistochemistry could be used as a new prognostic factor for CC. Although further study is still needed to elucidate the precise role of HDGF in CC malignancy, the present report might imply that HDGF may be a useful tool in determining appropriate treatment of CC.
Fertility-sparing surgery, such as vaginal radical trachelectomy or abdominal radical trachelectomy, are introduced to patients with CC with the International Federation of Gynecology and Obstetrics (FIGO) stage IA1 with lymphovascular space involvement, IA2, and IB1 with tumors less than 2 cm in size. In cases where lymph node metastasis is diagnosed or highly suspected, the surgery should be radicalized, or chemoradiotherapy should be undertaken instead. The present study showed the correlation between HDGF expression and lymph node metastasis, then HDGF immunohistochemistry might be involved in the decisionmaking of CC treatment.
Patients with HDGF index level 0 CC showed no lymph node metastasis, they are suitable for fertility-sparing surgery. In contrast, patients with HDGF index level 1 or 2 have higher risk of lymph node metastasis; thus standard surgeries such as radical hysterectomy is more preferable when the FIGO stage is higher than IA.
HDGF: a heparin-binding protein purified from the conditioned medium of the hepatocellular carcinoma (HCC) cell line HuH-7, which can proliferate autonomously in a serum-free chemically defined medium. HDGF is an acidic 26 kDa protein consisting of 230 amino acids with no hydrophobic signal sequence in its N-terminus, and it has high affinity for the glycosaminoglycans heparin and heparan sulfate. Exogenously supplied HDGF stimulates the proliferation of fibroblasts, endothelial cells, vascular smooth muscle cells, pulmonary epithelial cells and hepatocytes, as well as HCC, lung cancer and colon cancer cells, through the stimulation of extracellular signal-regulated kinase phosphorylation. HDGF translocates to the nucleus, and this nuclear targeting stimulates cell proliferation.
The author gives information about the expression levels of HDGF in 88 patients with cervical cancer undergoing surgery, and the relationship between HDGF expression and clinicopathological features and prognosis. The research group has great experiences in this area.
P- Reviewer: Kim SY, Rangel-Corona R S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Curado MP, Edwards B, Shin HR, Ferlay J, Heanue M, Boyle P, Storm H. Cancer Incidence in Five Continents, Volume IX. Lyon: IARC Scientific Publication 2009; . |
| 2. | Park S, Bae J, Nam BH, Yoo KY. Aetiology of cancer in Asia. Asian Pac J Cancer Prev. 2008;9:371-380. [PubMed] |
| 3. | Steben M, Duarte-Franco E. Human papillomavirus infection: epidemiology and pathophysiology. Gynecol Oncol. 2007;107:S2-S5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 174] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1829] [Cited by in RCA: 1883] [Article Influence: 104.6] [Reference Citation Analysis (0)] |
| 5. | Oehler MK, Wain GV, Brand A. Gynaecological malignancies in pregnancy: a review. Aust N Z J Obstet Gynaecol. 2003;43:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Creasman WT. Cancer and pregnancy. Ann N Y Acad Sci. 2001;943:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Orr JW. Cervical cancer. Surg Oncol Clin N Am. 1998;7:299-316. [PubMed] |
| 8. | Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2010;60:99-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 287] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 9. | Plante M, Gregoire J, Renaud MC, Roy M. The vaginal radical trachelectomy: an update of a series of 125 cases and 106 pregnancies. Gynecol Oncol. 2011;121:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 10. | Olawaiye A, Del Carmen M, Tambouret R, Goodman A, Fuller A, Duska LR. Abdominal radical trachelectomy: Success and pitfalls in a general gynecologic oncology practice. Gynecol Oncol. 2009;112:506-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Abu-Rustum NR, Sonoda Y. Fertility-sparing surgery in early-stage cervical cancer: indications and applications. J Natl Compr Canc Netw. 2010;8:1435-1438. [PubMed] |
| 12. | Abu-Rustum NR, Neubauer N, Sonoda Y, Park KJ, Gemignani M, Alektiar KM, Tew W, Leitao MM, Chi DS, Barakat RR. Surgical and pathologic outcomes of fertility-sparing radical abdominal trachelectomy for FIGO stage IB1 cervical cancer. Gynecol Oncol. 2008;111:261-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Dornhöfer N, Höckel M. New developments in the surgical therapy of cervical carcinoma. Ann N Y Acad Sci. 2008;1138:233-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Nakamura H, Kambe H, Egawa T, Kimura Y, Ito H, Hayashi E, Yamamoto H, Sato J, Kishimoto S. Partial purification and characterization of human hepatoma-derived growth factor. Clin Chim Acta. 1989;183:273-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Nakamura H, Izumoto Y, Kambe H, Kuroda T, Mori T, Kawamura K, Yamamoto H, Kishimoto T. Molecular cloning of complementary DNA for a novel human hepatoma-derived growth factor. Its homology with high mobility group-1 protein. J Biol Chem. 1994;269:25143-25149. [PubMed] |
| 16. | Izumoto Y, Kuroda T, Harada H, Kishimoto T, Nakamura H. Hepatoma-derived growth factor belongs to a gene family in mice showing significant homology in the amino terminus. Biochem Biophys Res Commun. 1997;238:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Enomoto H, Yoshida K, Kishima Y, Kinoshita T, Yamamoto M, Everett AD, Miyajima A, Nakamura H. Hepatoma-derived growth factor is highly expressed in developing liver and promotes fetal hepatocyte proliferation. Hepatology. 2002;36:1519-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Kishima Y, Yamamoto H, Izumoto Y, Yoshida K, Enomoto H, Yamamoto M, Kuroda T, Ito H, Yoshizaki K, Nakamura H. Hepatoma-derived growth factor stimulates cell growth after translocation to the nucleus by nuclear localization signals. J Biol Chem. 2002;277:10315-10322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Everett AD, Narron JV, Stoops T, Nakamura H, Tucker A. Hepatoma-derived growth factor is a pulmonary endothelial cell-expressed angiogenic factor. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1194-L1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Everett AD, Lobe DR, Matsumura ME, Nakamura H, McNamara CA. Hepatoma-derived growth factor stimulates smooth muscle cell growth and is expressed in vascular development. J Clin Invest. 2000;105:567-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Yoshida K, Tomita Y, Okuda Y, Yamamoto S, Enomoto H, Uyama H, Ito H, Hoshida Y, Aozasa K, Nagano H. Hepatoma-derived growth factor is a novel prognostic factor for hepatocellular carcinoma. Ann Surg Oncol. 2006;13:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Yamamoto S, Tomita Y, Hoshida Y, Takiguchi S, Fujiwara Y, Yasuda T, Doki Y, Yoshida K, Aozasa K, Nakamura H. Expression of hepatoma-derived growth factor is correlated with lymph node metastasis and prognosis of gastric carcinoma. Clin Cancer Res. 2006;12:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Uyama H, Tomita Y, Nakamura H, Nakamori S, Zhang B, Hoshida Y, Enomoto H, Okuda Y, Sakon M, Aozasa K. Hepatoma-derived growth factor is a novel prognostic factor for patients with pancreatic cancer. Clin Cancer Res. 2006;12:6043-6048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Yamamoto S, Tomita Y, Hoshida Y, Morii E, Yasuda T, Doki Y, Aozasa K, Uyama H, Nakamura H, Monden M. Expression level of hepatoma-derived growth factor correlates with tumor recurrence of esophageal carcinoma. Ann Surg Oncol. 2007;14:2141-2149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol Obstet. 2009;105:107-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 516] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 26. | Kaplan E, Meier P. Non-parametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457-81. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32610] [Cited by in RCA: 31239] [Article Influence: 466.3] [Reference Citation Analysis (0)] |
| 27. | Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187-220. |