Published online Dec 10, 2012. doi: 10.5317/wjog.v1.i4.46
Revised: September 3, 2012
Accepted: October 23, 2012
Published online: December 10, 2012
Developing countries suffer the highest burden of cervical cancers but have the lowest resources. Effective cervical cytology screening programme, along with a network of diagnostic and therapeutic colposcopy centres, like developed countries, is almost impossible to be reproduced in developing countries. Visual inspection methods [e.g., Visual inspection with Lugol’s iodine (VILI) and Visual Inspection with Acetic Acid (VIA)] which are cheaper, require less expertise and have the advantage of possible treatment in one setting have been shown to be effective alternatives. The sensitivity to detect CIN2+, by VIA and VILI, have been shown to be 80% and 91% respectively, with a specificity rate of 92% and 85% respectively. Screening by human papillomavirus (HPV) testing has high sensitivity (96.4%) but low specificity (94.1%) to detect CIN2+, when compared to Pap Smear (sensitivity, 55.4% and specificity, 96.8%). A single lifetime HPV testing in a large unscreened population has been shown to significantly reduce cervical cancer incidence and mortality when compared to cervical cytology, VIA or no screening. HPV testing of self-collected vaginal specimens also helps to overcome religious and socio-cultural barriers towards pelvic examination amongst women in developing countries. Current HPV testing methods are expensive, skill/infrastructure demanding and takes time to produce results. A cheaper HPV test, called careHPV™, which is able to provide results within 2.5 h and requires minimal skill/infrastructure to operate, was designed for use in developing countries. One stop screen and treat facilities using VIA or rapid HPV testing, and cryotherapy, can overcome non-compliance to follow-up which is a major issue in developing countries. Cure rates of 81.4% for CIN1, 71.4% for CIN2 and 68.0% for CIN3 at 6 mo after treatment have been reported. Incorporating telemedicine with cervicography of VIA or VILI or even telecolposcopy, has great potential in cervical cancer screening, especially in countries with vast geographical areas.
- Citation: Rao SJ. Trends in cervical cancer screening in developing countries. World J Obstet Gynecol 2012; 1(4): 46-54
- URL: https://www.wjgnet.com/2218-6220/full/v1/i4/46.htm
- DOI: https://dx.doi.org/10.5317/wjog.v1.i4.46
World Bank classifies 145 countries in the world with Gross National Income per capita ranging from less than USD $1005 to USD $12 275 as developing countries[1]. In 1989, a cut-off of USD $6000 has been set as an explicit benchmark to divide between lower-middle and upper-middle income countries[1]. About 80% or 4.8 billion out of the world’s 6 billion people live in the less developed regions[2]. To make the situation worse, the burden of poverty is further compounded by high burden of diseases in the less affluent countries. Amongst others, global figures show that more than 85% of cervical cancer cases occur in the developing world and in these countries it remains as the most common cancer among women as opposed to second or third placing elsewhere[3].
Cervical cancer in the less developed regions of the world has an estimated incidence of 453 000 cases per year and death rate of 242 000 cases per year as opposed to the more developed regions of the world whereby the incident and mortality rates are almost 6-7 times lesser[4]. Age Standardise Incidence Rate for cervical cancer in developing countries range from 15 to 55 per 100 000 people compared to less than 10 per 100 000 people in the developed countries[5]. 88% of mortality caused by cervical cancer in 2008 occurred in the developing countries[4]. Many of these countries have limited facilities for cervical cancer screening and treatment such as surgery, chemotherapy or radiotherapy. Resorting to traditional and alternative treatments or even just “waiting to die” are not uncommon in these places. Early detection and treatment will inevitably reduce the burden of the disease in these resource limited nations. Effective national Papanicalou Smear screening has been shown to reduce cervical cancer incidence by 80% only when it has at least 70% coverage which is unrealistic in many developing countries[6-8]. Stage per stage, morbidity and mortality of cervical cancer is significantly reduced the earlier the treatment is instituted and thus the importance of early and effective screening. The aim of this review is to provide a comprehensive and in-depth overview of the trends in the methods of screening for cervical cancers in developing countries.
The basis of cervical cytology methods of screening for cervical cancer was described by Dr. George Papanicolaou[9,10] since the early 19th century and is commonly known nowadays as Pap Smear or less commonly, Papanicolaou Smear. Even though the effectiveness of cervical cytology has never been analysed in randomised controlled trials, sufficient evidence from observational studies has led to its widespread adoption as the main cervical cancer screening strategy across the world[11-14]. From its initial description of using a rubber suction bulb with curved glass pipette onto the posterior vaginal fornix and subsequently ether-alcohol cellular fixation on the microscope slide, the current technique has evolved into using Ayre’s spatula or cervical brush directly onto the cervix and cellular fixation on the microscope slide using alcohol/ether-alcohol or newer cellular stabilization agents (e.g., CytoFix®)[15]. It is interesting to note here that a Cochrane Review found no difference in the diagnostic outcome of both the Ayre’s spatula and spatula’s with extended tips (e.g., Aylesbury) even though the former collected less endocervical cells[16].
Apart from Pap Smear which is also referred to as the conventional method, liquid based cytology is another method of cervical cytology employing similar method of collecting cervical cells with a cervical brush which is then washed into a liquid fixative solution (e.g., ThinPrep®, SurePath™) and finally vortexed, filtered and monolayer plated in the cytology laboratory[17,18]. Additionally, these liquid based specimens can be utilised further for human papillomavirus (HPV), gonorrhoea and chlamydia testing[19,20]. Generally, despite some conflicting large scale research outcomes, the common opinion is that the liquid based cytology is better than conventional smears in the sense of specimen adequacy, detection of glandular abnormalities, possibility of additional tests and overcoming blood/other contaminants in the smear[21,22]. However, liquid based cytology solutions are patented and additional laboratory facilities are needed to process these samples. Altogether, they add a significant cost to cervical cytology screening programme and thus, liquid based cytology is neither a suitable alternative nor is there any established programme employing this technique among developing countries.
Traditionally, performing cervical cytology screening involves three visits (when abnormality is detected), namely initial cervical smear, colposcopic diagnosis usually with biopsy and finally definitive treatment depending on the biopsy result[23]. At each step, there will also be communication of the test results to the patient. Thus, despite being available in developing countries, cervical cytology screening tend to be an expensive exercise and frequently impractical as it involves a relatively sophisticated infrastructure and system, skilled personnel (colposcopists, cytotechnicians, cytopathologists etc.), functional referral and communication system, transportation and loss of wages issue for multiple attendance and consequently non compliance by the patients[24]. A cost-effectiveness analysis of different modalities of cervical screening in developing countries has shown that the most cost-effective strategies were the ones that required least visits, which have shortest linkage to treatment and relied less on laboratory facilities[25]. These criteria are not completely fulfilled by cervical cytology screening. Moreover, cervical cytology by Pap Smear technique showed low sensitivity, even at the lowest cut off of atypical squamous cells of undetermined significance for CIN2+ (57%; 95%CI: 38%-76%) but the specificity was rather high (93%; 95%CI: 89%-97%)[26]. Visual inspection methods described below which are widely advocated for cervical cancer screening in developing countries, have higher sensitivity rates but lower specificity compared to Pap Smear[27].
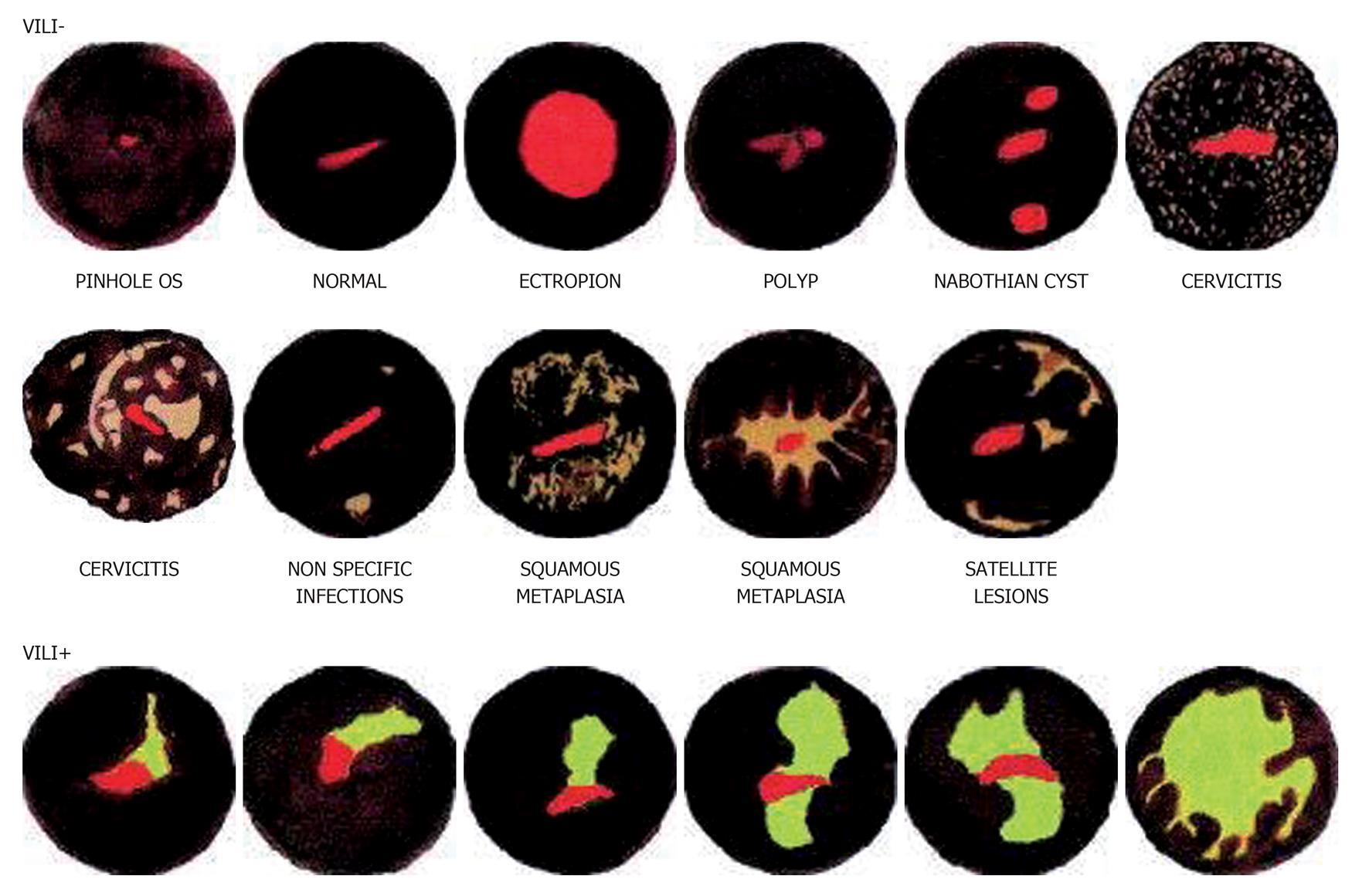
Interestingly, naked eye inspection of the cervix stained with Lugol’s iodine, known as the Schiller’s test, is historically (since 1930s) the first described method of cervical screening[28,29]. Visual inspection with Lugol’s iodine (VILI) is similar to Schiller’s test whereby Lugol’s iodine solution is applied onto the cervix and visualized with the naked eye to identify mustard-yellow iodine non-uptake areas on the cervix[30]. The abnormal cervical epithelium which contains little or no glycogen will not stain black with Lugol’s iodine but remains colourless, pale or stained mustard-yellow[31]. A meta-analysis of 11 cross sectional studies involving over 58 000 women in Africa and India showed VILI to have a sensitivity of 91% (95%CI: 88%-94%) and specificity of 85% (95%CI: 81%-88%) for the detection of CIN2 or worse[26]. Despite these impressive numbers, only few colposcopists employ this technique as Lugol’s iodine staining of the cervix is considered to be less refined, tends to obscure finer details and is less acceptable to the woman if it stains her clothes compared to acetic acid. However there is no published data to support this perception. A pilot study amongst field health workers (non-specialists) trained to perform cervicoscopy however reports that it is easier to detect the colour patterns produced by iodine staining rather than acetic acid[30] (Figure 1).
Another technique of visual inspection with the naked eye is Visual Inspection with Acetic Acid (VIA); also known as Acetic Acid Test (AAT) or Vinegar Acid Test. VIA is often interchangeably used with the term cervicoscopy even though in the strictest sense, cervicoscopy simply means visualizing the cervix with the naked eye after applying a staining solution and should be applicable to VILI as well[32,33]. A task force by the International Academy of Cytology has recommended the usage of the term Direct Visual Inspection (DVI) to mean inspecting the cervix with naked eye after application of 3%-5% acetic acid[34]. DVI with a special chemiluminescent light and using magnification is called speculoscopy[35]. A large trial involving more than 1100 patients in South Africa showed no added benefit in using this technique and the usage of the special light increases its cost[36]. DVI should not be confused with Unaided Visual Inspection (UVI) which is visual inspection of the cervix without using any staining solution, also known as “downstaging”, which is now a discarded stand alone technique due to its unacceptable inaccuracies in detecting cervical disease[37]. The term VIA will be used in this paper in keeping with its more popular usage.
In VIA, acetic acid of 3%-5% concentration, depending on the centre, is applied onto the cervix and the cervix is visualized to identify the acetowhite areas which are concentrated in the abnormal cervical epithelium[38,39]. Abnormal epithelium usually have higher content of precipitated nuclear protein due to hyperchromasia which prevents the reflection of the underlying pink stroma with rich blood vessels and thus appears white[39]. A meta-analysis involving 26 studies where VIA was utilised for primary screening showed that VIA has a sensitivity of 80% (range: 79%-82%), specificity of 92% (range: 91%-92%) and positive predictive value of 10% (range: 9%-10%) for the detection of CIN2 and worse[40]. Despite having less sensitivity than VILI (but higher than cytology), VIA has rapidly gained popularity in many developing countries. An attractive feature of cervical screening by VIA is the short duration of time needed to train non-physician field health workers, ranging from 7 to 21 d which is very useful in resource limited developing countries especially if it has vast geographical areas with large population[30,41]. However, a large trial involving 34 087 women undergoing VIA in India by non-physician field health workers reported issues of declining rates of cervical abnormality detection, implying progressive drop in skills and the need for yearly re-training[42,43].
Frequently, cervical cancer screening facilities in developing countries face the issue of concurrent sexually transmitted diseases amongst the women being screened. A study amongst 2754 women in South Africa has also shown that the efficacy of VIA is not affected by co-existing Neisseria gonorrhoeae, Trichomonas vaginalis or Chlamydia trachomatis infection of the genital tract but the presence of HIV infection significantly decreased its specificity[44]. Due to the wider availability of affordable anti-retroviral therapy, more women with HIV are living longer and cervical cancer screening strategies need to be tailored accordingly[45-47]. Despite its limitations amongst patients with HIV, VIA still seem to perform better than cytology in this group. In a recent cross-sectional study involving 303 HIV-positive women, Sahasrabuddhe et al[48] have showed that at CIN2+ disease threshold, the sensitivity, specificity and positive and negative predictive value estimates of VIA were 80.0%, 82.6%, 47.6% and 95.4%, respectively, compared to 60.5%, 59.6%, 22.4% and 88.7% for the atypical squamous cells of undetermined significance or severe (ASCUS+) cut-off on cytology, 60.5%, 64.6%, 24.8% and 89.4% for the low- grade squamous intraepithelial cells or severe (LSIL+) cut-off on cytology and 20.9%, 96.0%, 50.0% and 86.3% for high-grade squamous intraepithelial lesion or severe (HSIL+) cut-off on cytology.
Various other problems are present in visual inspection of the cervix strategies. Squamo-columnar junction where pre-cancerous lesions commonly occur, tend to migrate inward into the endocervical canal with increasing age. Contrary to cytology where shed cells from deep inside the endocervical canal may be picked up; direct visualization can be increasingly difficult in older women in order to obtain adequate view of the transformation zone. Training non-physician health workers to use the endocervical forceps can become more technical even though it is not impossible. Real world performance may differ from the promising figures of controlled research settings. Variations in defining acetowhite or Lugol’s non-uptake area will alter efficacy rates amongst centres, issues of training and re-training standards need to be addressed and quality control strategies need to be incorporated (Figure 2).
Low level magnification (2-4.5 ×) using hand held devices (e.g., Gynoscope, AVIscope or just ordinary magnifying lense) in conjunction with VIA, also known as VIA with magnification or VIAM, has been trialled in India, South Africa, Mexico, Peru, Costa Rica and a few other developing countries with the hope of increasing the efficacy of VIA[45,49-53]. However, these trials have shown that low level magnification did not give any added benefit to VIA. A meta-analysis of 3 studies involving over 18 000 patients has shown that VIA has a pooled sensitivity and specificity of 60.3% (95%CI: 53.6%-66.7%) and 86.8% (95%CI: 86.3%-87.3%) respectively, in detecting high-grade squamous intraepithelial lesions (HSIL)[49]. VIAM on the other hand, showed a sensitivity of 64.2% (95%CI: 57.6%-70.4%) and specificity of 86.8% (95%CI: 86.2%-87.3%)[49]. There are no reported trials of magnification techniques using VILI.
Taking digital or 35-mm photographs of the cervix after the application of acetic acid which is referred to as cervicography producing cervicographs/cervicograms/cervigrams has also been trialled in developing countries. It was first introduced by Adolph Stafl in 1982[54]. There are no published studies assessing the usage of cervicography after the application of Lugol’s iodine even though technically it utilises the same principles. It has been evaluated both as a primary screening tool and as an addition to other screening methods. A large trial involving 8640 women in Costa Rica showed that cervicography has an overall sensitivity of 49.3% and specificity of 95.0% in detecting high-grade squamous epithelial lesions or cancer, as opposed to 77.2% sensitivity with 94.2% specificity for cytology in the same study[55]. Cervicography was particularly not recommended for postmenopausal women in this study as it only had a sensitivity of 26.9% for women 50 years of age and older[55].
Since the establishment of persistent high risk HPV infection as a precursor for cervical cancer, various techniques of detecting them either for triaging, co-testing, test of cure or primary screening have been developed. Currently, the following tests which detects presence/absence of or identifies specific types of high risk HPV (DNA or E6/E7 viral messenger RNA) in cervical specimen, have been approved by the United States Food and Drug Administration; Hybrid Capture® 2 (HC2; detects DNA presence/absence of 13 HPV types), Cervista™HPV HR test (detects DNA presence/absence of 14 HPV types), Cervista™ HPV 16/18 (specifically detects DNA presence/absence of HPV 16/18), cobas HPV test (identifies specific HPV16 and HPV18 DNA and a pooled result of DNA presence/absence of 12 other HPV types) and Aptima® mRNA test (identifies messenger RNA of 14 HPV types)[56]. The Canadian Cervical Cancer Screening Trial involving 10154 women has shown that the sensitivity of HPV testing for detecting CIN2+ was 94.6% (95%CI: 84.2%-100.0%) and the specificity was 94.1% (95%CI: 93.4%-94.8%) compared to 55.4% (95%CI: 33.6%-77.2%) sensitivity and 96.8% (95%CI: 96.3%-97.3%) specificity for Pap Smear[57]. Despite its high sensitivity being offset by reduced specificity, HPV testing has been repeatedly trialled as a primary screening tool both in developing and developed countries especially since an Italian study showed an overall reduction of cervical cancer incidence through HPV screening[58]. A study among 2900 women in South Africa has shown that the specificity of HPV testing to detect CIN2+ can be increased to 90% but at the expense of reducing sensitivity to 79% by increasing the positivity threshold (expressed as relative light units per positive control specimen, RLU/PC) from > 1 RLU/PC to > 8 RLU/PC[59].
A large trial involving 131 746 women was performed in India, to compare HPV testing using HC2 as a single lifetime screening strategy vs cervical cytology, VIA or standard care which is the control group (i.e., no screening at all which is standard for that population group and geographical area)[43]. The HPV testing group has shown a significantly reduced incidence of cervical cancer and cervical cancer mortality rate compared to cervical cytology and VIA[43]. Cumulative data over 8 years revealed that compared to the control group, hazard ratio for the incidence of cervical cancer was 1.05 (95%CI: 0.77%-1.43%) for HPV testing, 1.34 (95%CI: 0.99%-1.82%) for cervical cytology and 1.30 (95%CI: 0.95%-1.78%) for VIA[43]. Hazard ratio for death due to cervical cancer was 0.52 (95%CI: 0.33%-0.83%) for HPV testing, 0.89 (95%CI: 0.62-1.27) for cervical cytology and 0.86 (95%CI: 0.60%-1.25%) for VIA[43]. The authors also emphasized that the drawback to widespread usage of HPV testing in developing countries is high cost (USD $20-$30 per test), requiring at least 24-48 h to provide the results and need for sophisticated laboratory infrastructure[43].
A cheap HPV test called careHPV™ (Qiagen, Gaithersburg, USA), which costs around USD $5 per test and able to provide results (detecting 14 high risk HPV types) within 2.5 h, has been developed specifically for usage in low-resource public-health settings to screen women 30 years of age and older[60,61]. It is yet to be marketed commercially. A cross sectional study involving 2388 women in China tested with careHPV™ showed that it had significantly better sensitivity at 90.0% (95%CI: 83.0%-97.0%) and specificity at 84.2% (95%CI: 82.7%-85.7%) for detecting CIN2+ from cervical specimens compared to VIA which showed sensitivity of 41. 4% (95%CI: 29.9%-53.0%) and specificity of 94.5% (95%CI: 93.6%-95.4%)[61]. careHPV™ performed comparably to HC2 and cervical specimens yielded better results compared to self-collected vaginal specimens; all of which were also tested concurrently[61]. A cost-effectiveness analysis from this study results also showed that for a 70% participation rate, once a lifetime screening at the age of 35 years would reduce cancer mortality by 8% (for VIA) to 12% (for careHPV™) over the long term, with a cost-effectiveness ratio of USD $557 (for VIA) to USD $959 (for careHPV™) per life year saved compared to no intervention; referenced to a 2008 GDP per capita in Shanxi Province of USD $2975[62]. Lower cost, easy and shorter learning curve to learn to use the technique, good cost-effectiveness and rapid results make careHPV™ an attractive option for developing countries[63].
HPV testing also paves the way for analysing self-collected specimen from the vagina by the women using various methods such as tampon, swab, cytobrush, vaginal lavage or custom made device[64]. No good evidence is available to compare between self-sampling methods. Cultural, religious and even socio-economic barriers among women (which are particularly prevalent in developing countries) may hinder participation in cervical cancer screening programmes which traditionally requires speculum aided collection of cervical specimen. This was studied in a randomised controlled trial comparing conventional cervical cytology and cytobrush for self-sampling among 25061 Mexican women[65]. This study revealed higher acceptability for self-sampling among women at 98% compared to 89% among women who underwent conventional cytology[65]. It also showed that HPV analysis of self-sampled cervical specimen displayed higher sensitivity, lower specificity and lower positive predictive value compared to cervical cytology for detecting CIN2 or worse[65]. Analysis of 2530 self-collected vaginal specimen using careHPV™ in China showed a sensitivity of 81.4% (95%CI: 72.3%-90.5%) and specificity of 82.4% (95%CI: 80.8%-83.9%) for the detection of CIN2 or worse[61].
Detecting cervical pre-cancerous lesions by VIA or presence of high risk HPV by rapid testing methods will enable the provision of immediate treatment or further referral if frank cancer or larger lesions are detected[66]. This is an attractive modality in developing countries where “one stop centres” are useful to overcome issues of communication, recall system failure or unavailability and non-compliance or non-feasibility of multiple visits by the women[67]. The preferred treatment method in this strategy is cryotherapy as it has been shown to have lower and milder complication rates, requires less skill than electrical excision, can be performed by trained non-physician health worker and is cheaper than laser ablation[68-70]. A large trial involving 6555 women in South Africa showed that the prevalence of CIN2+ after 6 and 12 mo, was significantly lower in the VIA and cryotherapy arm, 2.23% (95%CI: 1.57%-2.89%) at 6 mo and 2.91% (95%CI: 2.12%-3.69%) at 12 mo compared to the control group (delayed evaluation), 3.55% (95%CI: 2.71%-4.39%) at 6 mo and 5.41% (95%CI: 4.32%-6.50%) at 12 mo[71]. A study in India where 1026 women underwent VIA and cryotherapy, cure rates of 81.4% for CIN 1, 71.4% for CIN 2 and 68.0 for CIN 3 were reported at 6 mo follow-up[69]. With the availability of rapid HPV testing such as careHPV™ or utilising VIA, along with cryotherapy, “see and treat (or refer)” is a promising avenue for developing countries.
With the aid of modern digital technology, tele-VILI, tele-VIA, tele-cervicography or even tele-colposcopy, either as still digital images or even real-time teleconference images is a reality of the modern era. Utilisation of digital cameras linked to the internet via laptop or even multimedia messaging system (MMS) via mobile phones, especially modern smartphones, will enable non-physician health workers in remote areas to capture cervical images and transmit them to experts in centralised secondary or tertiary centres for further opinion. This method is adopted in a centre in Zambia and named eC3 (Electronic Cervical Cancer Control)[72]. Limitations of this approach are loss of stereoscopic view and depth perception as the images are 2 dimensional, potential distortions of the images (due to factors such as technique, lighting and camera battery which in turn can cause wrong diagnosis and decisions) and cost of infrastructure, maintenance and repair that can be forbidding in certain countries[72].
Large numbers of the world’s population live in developing countries where cervical cancer is at endemic proportions and causes most mortality. The successes of developed countries to inhibit cervical cancer rates to very low levels by high intensity cervical cancer screening programmes could not be emulated in low and middle income countries. This is mainly due to economic factors leading to limited health resources, lack of technical expertise and competing interests from more pressing health issues (such as maternal and infant health, infectious diseases like tuberculosis, malaria and HIV/AIDS). Socio-political barriers such as low literacy/education rates, poor access to health facilities, religious taboos, poverty, war and civil unrest, also contribute to this problem. Large proportions of the developing countries’ population who are unscreened or under-screened and usually being the ones with higher risk factors for cervical cancer is a huge public health challenge.
Primary prevention of cervical cancer with HPV vaccine is still beyond reach for many poorer countries. Efforts from the GAVI Alliance (formerly the “Global Alliance for Vaccines and Immunisation”) and other similar organisations to provide subsidised and even free HPV vaccines in poorer countries will inevitably suppress cervical cancer rates in these countries. In the continuum, the importance of secondary prevention by utilising the most cost-effective cervical cancer screening strategy could not be over emphasized. There is no one technique which will meet the needs of all developing countries and each health authority would need to work in collaboration with the local medical fraternity to determine the best option. Single lifetime HPV testing, careHPV™, VIA, one stop screen and treat centres, self-collecting of vaginal specimen and tele-cervicography seem to offer promising avenues in cervical cancer screening in the developing countries.
Peer reviewers: Chunxia Cao, PhD, Department of Medicine, University of Florida, 2033 Mowry Rd., CGRC, Room 365, Gainesville, FL32610, United States; Christos R Iavazzo, MD, MSc, PhD, Second Department of Obstetrics and Gynecology, University of Athens, Aretaieio Hospital, Athens, Greece
S- Editor Jiang L L- Editor A E- Editor Zheng XM
| 1. | World Bank. 2012 World Bank Classification According To GNI. Available from: http://data.worldbank.org/country. |
| 2. | Population, Education and Development. Department of Economic and Social Affairs, Population Division. New York: United Nations 2003; . |
| 3. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25546] [Article Influence: 1824.7] [Reference Citation Analysis (7)] |
| 4. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Cancer Incidence and Mortality Worldwide: IARC. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer; 2010. Available from: http://globocan.iarc.fr. |
| 5. | Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, Boyle P. Cancer Incidence in Five Continents. Vol. IX. Lyon: IARC Scientific Publications 2007; . |
| 6. | Lăără E, Day NE, Hakama M. Trends in mortality from cervical cancer in the Nordic countries: association with organised screening programmes. Lancet. 1987;1:1247-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 408] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 7. | Quinn M, Babb P, Jones J, Allen E. Effect of screening on incidence of and mortality from cancer of cervix in England: evaluation based on routinely collected statistics. BMJ. 1999;318:904-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 338] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 8. | Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Womens Health (Larchmt). 2012;21:1031-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 237] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 9. | Papanicolaou GN, Traut HF. The diagnostic value of vaginal smears in carcinoma of the uterus. 1941. Arch Pathol Lab Med. 1997;121:211-224. [PubMed] |
| 10. | Traut HF, Papanicolaou GN. Cancer of the Uterus: The Vaginal Smear in Its Diagnosis. Cal West Med. 1943;59:121-122. [PubMed] |
| 11. | Aklimunnessa K, Mori M, Khan MM, Sakauchi F, Kubo T, Fujino Y, Suzuki S, Tokudome S, Tamakoshi A, Motohashi Y. Effectiveness of cervical cancer screening over cervical cancer mortality among Japanese women. Jpn J Clin Oncol. 2006;36:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Taylor R, Morrell S, Mamoon H, Wain G, Ross J. Decline in cervical cancer incidence and mortality in New South Wales in relation to control activities (Australia). Cancer Causes Control. 2006;17:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Screening for squamous cervical cancer: duration of low risk after negative results of cervical cytology and its implication for screening policies. IARC Working Group on evaluation of cervical cancer screening programmes. Br Med J (Clin Res Ed). 1986;293:659-664. [PubMed] |
| 14. | Clarke EA, Anderson TW. Does screening by "Pap" smears help prevent cervical cancer A case-control study. Lancet. 1979;2:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 154] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | PAPANICOLAOU GN. The cell smear method of diagnosing cancer. Am J Public Health Nations Health. 1948;38:202-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Martin-Hirsch P, Jarvis G, Kitchener H, Lilford R. Collection devices for obtaining cervical cytology samples. Cochrane Database Syst Rev. 2000;CD001036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Hologic Inc. ThinPrep(R) Package Insert. Available from: http://www.thinprep.com/. |
| 18. | BD SurePath(TM). SurePath(TM) Package Insert. Available from: http://www.bd.com/tripath/physicians/surepath.asp. |
| 19. | Hawthorne CM, Farber PJ, Bibbo M. Chlamydia/gonorrhea combo and HR HPV DNA testing in liquid-based pap. Diagn Cytopathol. 2005;33:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Griffith WF, Stuart GS, Gluck KL, Heartwell SF. Vaginal speculum lubrication and its effects on cervical cytology and microbiology. Contraception. 2005;72:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Akamatsu S, Kodama S, Himeji Y, Ikuta N, Shimagaki N. A comparison of liquid-based cytology with conventional cytology in cervical cancer screening. Acta Cytol. 2012;56:370-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Cox JT. Liquid-based cytology: evaluation of effectiveness, cost-effectiveness, and application to present practice. J Natl Compr Canc Netw. 2004;2:597-611. [PubMed] |
| 23. | Singla S, Mathur S, Kriplani A, Agarwal N, Garg P, Bhatla N. Single visit approach for management of cervical intraepithelial neoplasia by visual inspection & amp; loop electrosurgical excision procedure. Indian J Med Res. 2012;135:614-620. [PubMed] |
| 24. | Kim JJ, Salomon JA, Weinstein MC, Goldie SJ. Packaging health services when resources are limited: the example of a cervical cancer screening visit. PLoS Med. 2006;3:e434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahé C, Wright TC. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med. 2005;353:2158-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 438] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 26. | Arbyn M, Sankaranarayanan R, Muwonge R, Keita N, Dolo A, Mbalawa CG, Nouhou H, Sakande B, Wesley R, Somanathan T. Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. Int J Cancer. 2008;123:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 196] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 27. | Saxena U, Sauvaget C, Sankaranarayanan R. Evidence-based screening, early diagnosis and treatment strategy of cervical cancer for national policy in low- resource countries: example of India. Asian Pac J Cancer Prev. 2012;13:1699-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Schiller W. Early diagnosis of carcinoma of the cervix. Surg Gynecol Obstet. 1933;66:210–220. |
| 29. | Schiller W. Leucoplakia , leucokeratosis , and carcinoma of the cervix. Am J Obstet Gynecol. 1938;35:17–38. |
| 30. | Sankaranarayanan R, Wesley R, Thara S, Dhakad N, Chandralekha B, Sebastian P, Chithrathara K, Parkin DM, Nair MK. Test characteristics of visual inspection with 4% acetic acid (VIA) and Lugol's iodine (VILI) in cervical cancer screening in Kerala, India. Int J Cancer. 2003;106:404-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Sarian LO, Derchain SF, Naud P, Roteli-Martins C, Longatto-Filho A, Tatti S, Branca M, Erzen M, Serpa-Hammes L, Matos J. Evaluation of visual inspection with acetic acid (VIA), Lugol's iodine (VILI), cervical cytology and HPV testing as cervical screening tools in Latin America. This report refers to partial results from the LAMS (Latin AMerican Screening) study. J Med Screen. 2005;12:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Sankaranarayanan R, Wesley R, Somanathan T, Dhakad N, Shyamalakumary B, Amma NS, Parkin DM, Nair MK. Visual inspection of the uterine cervix after the application of acetic acid in the detection of cervical carcinoma and its precursors. Cancer. 1998;83:2150-2156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Gaffikin L, Lauterbach M, Blumenthal PD. Performance of visual inspection with acetic acid for cervical cancer screening: a qualitative summary of evidence to date. Obstet Gynecol Surv. 2003;58:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Wright TC, Menton M, Myrtle JF, Chow C, Singer A. Visualization techniques (colposcopy, direct visual inspection, and spectroscopic and other visual methods). Summary of task force 7. Acta Cytol. 2002;46:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Lonky SA. Speculoscopy. Am J Obstet Gynecol. 1999;180:772-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 36. | Cronjé HS, van Rensburg E, Cooreman BF, Niemand I, Beyer E. Speculoscopy vs. the acetic acid test for cervical neoplasia. Int J Gynaecol Obstet. 2000;69:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Basu P, Sankaranarayanan R, Mandal R, Roy C, Das P, Choudhury D, Datta K, Karamakar S, Tsu V, Chakrabarti RN. Evaluation of downstaging in the detection of cervical neoplasia in Kolkata, India. Int J Cancer. 2002;100:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Sankaranarayanan R, Nessa A, Esmy PO, Dangou JM. Visual inspection methods for cervical cancer prevention. Best Pract Res Clin Obstet Gynaecol. 2012;26:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 39. | Sankaranarayanan R, Wesley RS. A Practical Manual on Visual Screening for Cervical Neoplasia. IARC Technical Publication No. 41. Lyon: IARC Press 2003; Available from: http://screening.iarc.fr/doc/viavilimanual.pdf. |
| 40. | Sauvaget C, Fayette JM, Muwonge R, Wesley R, Sankaranarayanan R. Accuracy of visual inspection with acetic acid for cervical cancer screening. Int J Gynaecol Obstet. 2011;113:14-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Sankaranarayanan R, Rajkumar R, Theresa R, Esmy PO, Mahe C, Bagyalakshmi KR, Thara S, Frappart L, Lucas E, Muwonge R. Initial results from a randomized trial of cervical visual screening in rural south India. Int J Cancer. 2004;109:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Sankaranarayanan R, Nene BM, Dinshaw KA, Mahe C, Jayant K, Shastri SS, Malvi SG, Chinoy R, Kelkar R, Budukh AM. A cluster randomized controlled trial of visual, cytology and human papillomavirus screening for cancer of the cervix in rural India. Int J Cancer. 2005;116:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 43. | Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, Hingmire S, Malvi SG, Thorat R, Kothari A. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 852] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 44. | Denny L, Kuhn L, Pollack A, Wright TC. Direct visual inspection for cervical cancer screening: an analysis of factors influencing test performance. Cancer. 2002;94:1699-1707. [PubMed] |
| 45. | Bailey H, Thorne C, Semenenko I, Malyuta R, Tereschenko R, Adeyanova I, Kulakovskaya E, Ostrovskaya L, Kvasha L, Cortina-Borja M. Cervical screening within HIV care: findings from an HIV-positive cohort in Ukraine. PLoS One. 2012;7:e34706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Adler DH. The impact of HAART on HPV-related cervical disease. Curr HIV Res. 2010;8:493-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Chalermchockcharoenkit A, Chayachinda C, Thamkhantho M, Komoltri C. Prevalence and cumulative incidence of abnormal cervical cytology among HIV-infected Thai women: a 5.5-year retrospective cohort study. BMC Infect Dis. 2011;11:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Sahasrabuddhe VV, Bhosale RA, Kavatkar AN, Nagwanshi CA, Joshi SN, Jenkins CA, Shepherd BE, Kelkar RS, Sahay S, Risbud AR. Comparison of visual inspection with acetic acid and cervical cytology to detect high-grade cervical neoplasia among HIV-infected women in India. Int J Cancer. 2012;130:234-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Sankaranarayanan R, Shastri SS, Basu P, Mahé C, Mandal R, Amin G, Roy C, Muwonge R, Goswami S, Das P. The role of low-level magnification in visual inspection with acetic acid for the early detection of cervical neoplasia. Cancer Detect Prev. 2004;28:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Denny L, Kuhn L, Pollack A, Wainwright H, Wright TC. Evaluation of alternative methods of cervical cancer screening for resource-poor settings. Cancer. 2000;89:826-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 51. | Pérez-Cruz E, Winkler JL, Velasco-Mondragón E, Salmerón-Castro J, García F, Davis-Tsu V, Escandón-Romero C, Hernández-Avila M. [Screening and follow-up for cervical cancer prevention in rural Mexico using visual inspection]. Salud Publica Mex. 2005;47:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Winkler JL, Lewis K, Del Aguila R, Gonzales M, Delgado JM, Tsu VD, Sellors JW. Is magnification necessary to confirm visual inspection of cervical abnormalities A randomized trial in Peru. Rev Panam Salud Publica. 2008;23:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Rodríguez AC, Morera LA, Bratti C, Herrero R, Cox JT, Morales J, Alfaro M, Hutchinson M, Castle PE, Hildesheim A. Performance of direct visual inspection of the cervix with acetic acid and magnification in a previously screened population. J Low Genit Tract Dis. 2004;8:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 54. | Stafl A. Cervicography: a new method for cervical cancer detection. Am J Obstet Gynecol. 1981;139:815-825. [PubMed] |
| 55. | Schneider DL, Herrero R, Bratti C, Greenberg MD, Hildesheim A, Sherman ME, Morales J, Hutchinson ML, Sedlacek TV, Lorincz A. Cervicography screening for cervical cancer among 8460 women in a high-risk population. Am J Obstet Gynecol. 1999;180:290-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Wright TC, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis. 2007;11:201-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 57. | Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, Ratnam S, Coutlée F, Franco EL. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 737] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 58. | Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, Ghiringhello B, Girlando S, Gillio-Tos A, De Marco L. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 692] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 59. | Kuhn L, Denny L, Pollack A, Lorincz A, Richart RM, Wright TC. Human papillomavirus DNA testing for cervical cancer screening in low-resource settings. J Natl Cancer Inst. 2000;92:818-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 142] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 60. | Kuhn L; QIAGEN. Gaithersburg, USA. The careHPVTM Test QIAGENcares. Lyon: IARC Press 2009; Available from: http://www.qiagen.com/about/whoweare/qiagencares/the-carehpv-test.pdf. |
| 61. | Qiao YL, Sellors JW, Eder PS, Bao YP, Lim JM, Zhao FH, Weigl B, Zhang WH, Peck RB, Li L. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncol. 2008;9:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 345] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 62. | Shi JF, Canfell K, Lew JB, Zhao FH, Legood R, Ning Y, Simonella L, Ma L, Kang YJ, Zhang YZ. Evaluation of primary HPV-DNA testing in relation to visual inspection methods for cervical cancer screening in rural China: an epidemiologic and cost-effectiveness modelling study. BMC Cancer. 2011;11:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 63. | Gage JC, Ajenifuja KO, Wentzensen N, Adepiti AC, Stoler M, Eder PS, Bell L, Shrestha N, Eklund C, Reilly M. Effectiveness of a simple rapid human papillomavirus DNA test in rural Nigeria. Int J Cancer. 2012;131:2903-2909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 64. | Gök M, Heideman DA, van Kemenade FJ, Berkhof J, Rozendaal L, Spruyt JW, Voorhorst F, Beliën JA, Babovic M, Snijders PJ. HPV testing on self collected cervicovaginal lavage specimens as screening method for women who do not attend cervical screening: cohort study. BMJ. 2010;340:c1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 65. | Lazcano-Ponce E, Lorincz AT, Cruz-Valdez A, Salmerón J, Uribe P, Velasco-Mondragón E, Nevarez PH, Acosta RD, Hernández-Avila M. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet. 2011;378:1868-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 66. | Sahasrabuddhe VV, Parham GP, Mwanahamuntu MH, Vermund SH. Cervical cancer prevention in low- and middle-income countries: feasible, affordable, essential. Cancer Prev Res (Phila). 2012;5:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 67. | Sahasrabuddhe VV, Parham GP, Mwanahamuntu MH, Vermund SH. Cervical cancer prevention in low- and middle-income countries: feasible, affordable, essential. Cancer Prev Res (Phila). 2012;5:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 68. | Mwanahamuntu MH, Sahasrabuddhe VV, Pfaendler KS, Mudenda V, Hicks ML, Vermund SH, Stringer JS, Parham GP. Implementation of 'see-and-treat' cervical cancer prevention services linked to HIV care in Zambia. AIDS. 2009;23:N1-N5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 69. | Sankaranarayanan R, Rajkumar R, Esmy PO, Fayette JM, Shanthakumary S, Frappart L, Thara S, Cherian J. Effectiveness, safety and acceptability of 'see and treat' with cryotherapy by nurses in a cervical screening study in India. Br J Cancer. 2007;96:738-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 70. | Denny L, Kuhn L, De Souza M, Pollack AE, Dupree W, Wright TC. Screen-and-treat approaches for cervical cancer prevention in low-resource settings: a randomized controlled trial. JAMA. 2005;294:2173-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 246] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 71. | Denny L, Kuhn L, Hu CC, Tsai WY, Wright TC. Human papillomavirus-based cervical cancer prevention: long-term results of a randomized screening trial. J Natl Cancer Inst. 2010;102:1557-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 72. | Parham GP, Mwanahamuntu MH, Pfaendler KS, Sahasrabuddhe VV, Myung D, Mkumba G, Kapambwe S, Mwanza B, Chibwesha C, Hicks ML. eC3--a modern telecommunications matrix for cervical cancer prevention in Zambia. J Low Genit Tract Dis. 2010;14:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 73. | Quinley KE, Gormley RH, Ratcliffe SJ, Shih T, Szep Z, Steiner A, Ramogola-Masire D, Kovarik CL. Use of mobile telemedicine for cervical cancer screening. J Telemed Telecare. 2011;17:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |