Peer-review started: October 6, 2021
First decision: January 12, 2022
Revised: January 24, 2022
Accepted: February 27, 2022
Article in press: February 27, 2022
Published online: April 25, 2022
Processing time: 195 Days and 4.5 Hours
Although it is well established that coronavirus disease 2019 (COVID-19) is associated with inflammation and a prothrombotic state leading to stroke and venous thromboembolism (VTE), the nuances of this association are yet to be uncovered[1]. Many studies link elevations in inflammatory markers to cases of thromboembolism. Most reports of thromboembolism associated with COVID-19 occur in the venous circulation during or just after the initial hospitalization due to COVID-19[2]. It is unclear how long the hypercoagulable effect of COVID-19 lasts.
We present a unique case of a 65-year-old-female who presented to her primary care doctor with a sore throat, cough, fatigue, congestion, diarrhea, headache, and anosmia. She tested positive for severe acute respiratory syndrome coronavirus 2 and received a bamlanivimab infusion 9 days later. After recovering from the acute illness, she received the Pfizer-BioNTech COVID-19 vaccine. Months later, she presented to the Emergency Department (ED) complaining of right sided shoulder pain and motor weakness in her left hand while trying to type on a keyboard. On presentation to the ED, her calculated Padua prediction score for risk of VTE was two and inflammatory markers were not elevated. She was found to have a brachiocephalic artery occlusion as well as an ischemic stroke which was treated with heparin.
This case suggests hypercoagulability due to COVID-19 may extend further than current literature suggests, to at least six months.
Core Tip: Acute severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is associated with hypercoagulability. However, in this unique case, a patient suffered two separate thromboembolisms, one in the brachiocephalic artery and an ischemic stroke. With only two risk factors at baseline and no corresponding elevation in inflammatory markers, we hypothesize that the clots grew slowly over time which would not cause acutely elevated inflammatory markers. Although her SARS-CoV-2 infection occurred six months prior to her Emergency Department presentation, we hypothesize the hypercoagulable state caused by coronavirus disease 2019 was contributory. Additionally, it is unlikely the vaccination alone caused her thrombosis, yet it is still possible it had a contributory effect.
- Citation: Kilby KJ, Anderson-Quiñones C, Pierce KR, Gabrah K, Seth A, Brunson A. Late ischemic stroke and brachiocephalic thrombus in a 65-year-old patient six months after COVID-19 infection: A case report. World J Hematol 2022; 9(2): 13-19
- URL: https://www.wjgnet.com/2218-6204/full/v9/i2/13.htm
- DOI: https://dx.doi.org/10.5315/wjh.v9.i2.13
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is associated with inflammation and a prothrombotic state, with increases in fibrin, fibrin degradation products, fibrinogen, and D-dimer[1,3]. It is common for hospitalized SARS-CoV-2 positive patients to receive higher than usual prophylactic doses of anticoagulants due to the established association between coronavirus disease 2019 (COVID-19) and thromboembolism[4]. However, when patients are not admitted to the hospital, they typically do not receive anticoagulation therapy unless there is another indication. Rivaroxaban can be used for venous thromboembolism (VTE) prophylaxis for acutely ill medical patients. For this indication, it is administered orally as a 10 mg dose once daily for 31 to 39 days (including hospitalization and post-discharge)[5,6]. Some providers consider using rivaroxaban in this way for high-risk COVID-19 patients who are discharged from the hospital. The International Society on Thrombosis and Haemostasis confirms it is “reasonable to consider extended-duration thromboprophylaxis with low molecular weight heparin or a direct oral anticoagulant for at least two weeks and up to six weeks post-hospital discharge in selected COVID-19 patients who are at low risk for bleeding and with key VTE risk factors such as advanced age, stay in the Intensive Care Unit, cancer, a prior history of VTE, thrombophilia, severe immobility, an elevated D-dimer, and an IMPROVE VTE score of four or more[7].” We present a case of a 65-year-old-female who was not hospitalized for her SARS-CoV-2 infection and six months (197 days) later presented to the hospital with a thrombus in the brachiocephalic artery and an acute ischemic stroke in the right anterior cerebral artery (ACA)/middle cerebral artery (MCA) watershed region with no apparent inciting factors.
A 65-year-old-female presented to the Emergency Department (ED) complaining of right sided shoulder pain and motor weakness in her left hand while trying to type on a keyboard. In the ED, she experienced worsening left upper extremity weakness which improved after one hour.
The patient’s symptoms began after waking up and had a sudden onset.
The patient has a past medical history of depression, anxiety, asthma, gastroesophageal reflux disease, and iron deficiency anemia. She had no prior history of right arm, shoulder, or clavicular injury on the right upper extremity. She tested positive for SARS-CoV-2 six months (197 days) prior to hospital presentation. After testing positive, she presented to her primary care doctor with a three-day history of fever (38.6 °C), sore throat, cough, fatigue, congestion, diarrhea, headache, and anosmia. Nine days following COVID-19 symptom onset, she received a bamlanivimab infusion through the left antecubital vein in an outpatient setting due to unrelenting symptoms. She became afebrile two days after the infusion and presenting symptoms remitted. She did not require hospitalization during her acute infection with COVID-19. After recovering from the acute illness, she received her first and second dose, 103 and 124 days later, respectively, of the Pfizer-BioNTech COVID-19 vaccine (0.3 mL) in the left deltoid. Both vaccinations caused soreness in the left arm, but no other adverse effects were reported after vaccination. The second vaccination occurred two and a half months (73 days) prior to her presentation to the ED. There was no period of upper limb immobilization.
The patient reported having a normal colonoscopy about six years prior and a mammogram three years prior. No pap smear or pelvic exam in the last few years was reported. The patient is unaware of any family or personal history of blood clots.
Physical exam was significant for localized motor weakness and hyperreflexia in the left arm, and slight decreased sensation to light touch in her left foot. The National Institutes of Health Stroke Scale score was one with right arm drift. The calculated HAS-BLED score was zero indicating no contraindication to anticoagulation.
Inflammatory markers were not elevated on admission or throughout her hospital stay. During the hospital admission, inflammatory markers were as follows: Erythrocyte Sedimentation Rate (ESR) 2 mm/hr, Troponin < 0.04 ng/mL, C-reactive protein (CRP) < 3 mg/L, Ferritin 4 ng/mL, Lactate Dehydrogenase 161 U/L, D-Dimer < 0.27 ug/mL. Pro B-Type Natriuretic Peptide (ProBNP) and Procalcitonin were not performed during this hospital admission. The SARS-CoV-2 ribonucleic acid rapid test collected on admission was negative. A lipid panel revealed elevated cholesterol (235 mg/dL) and low-density lipoprotein (129 mg/dL).
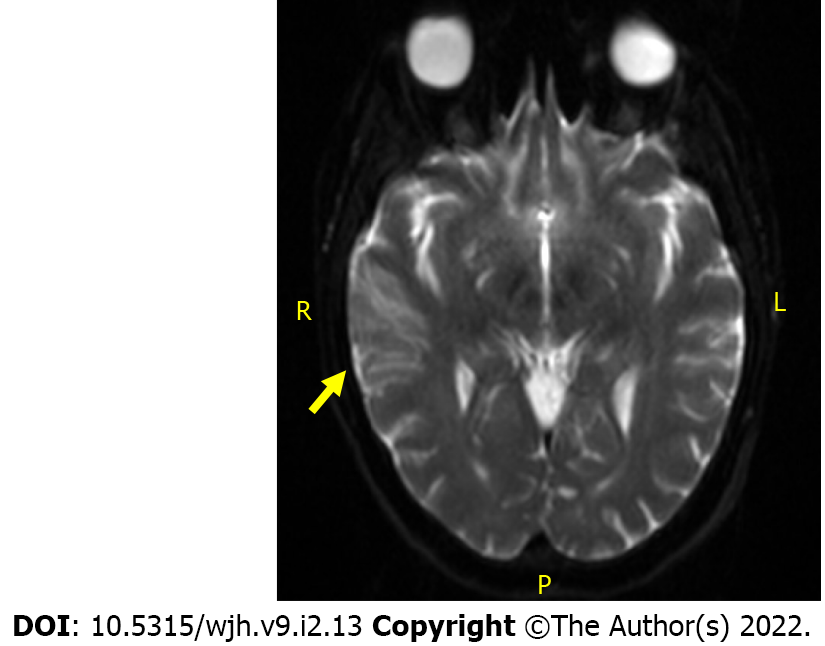
Brain magnetic resonance imaging showed subacute multifocal embolic appearing infarcts in the MCA watershed extending from anterior to posterior with involvement of the right hand knob and motor strip distribution (Figure 1).
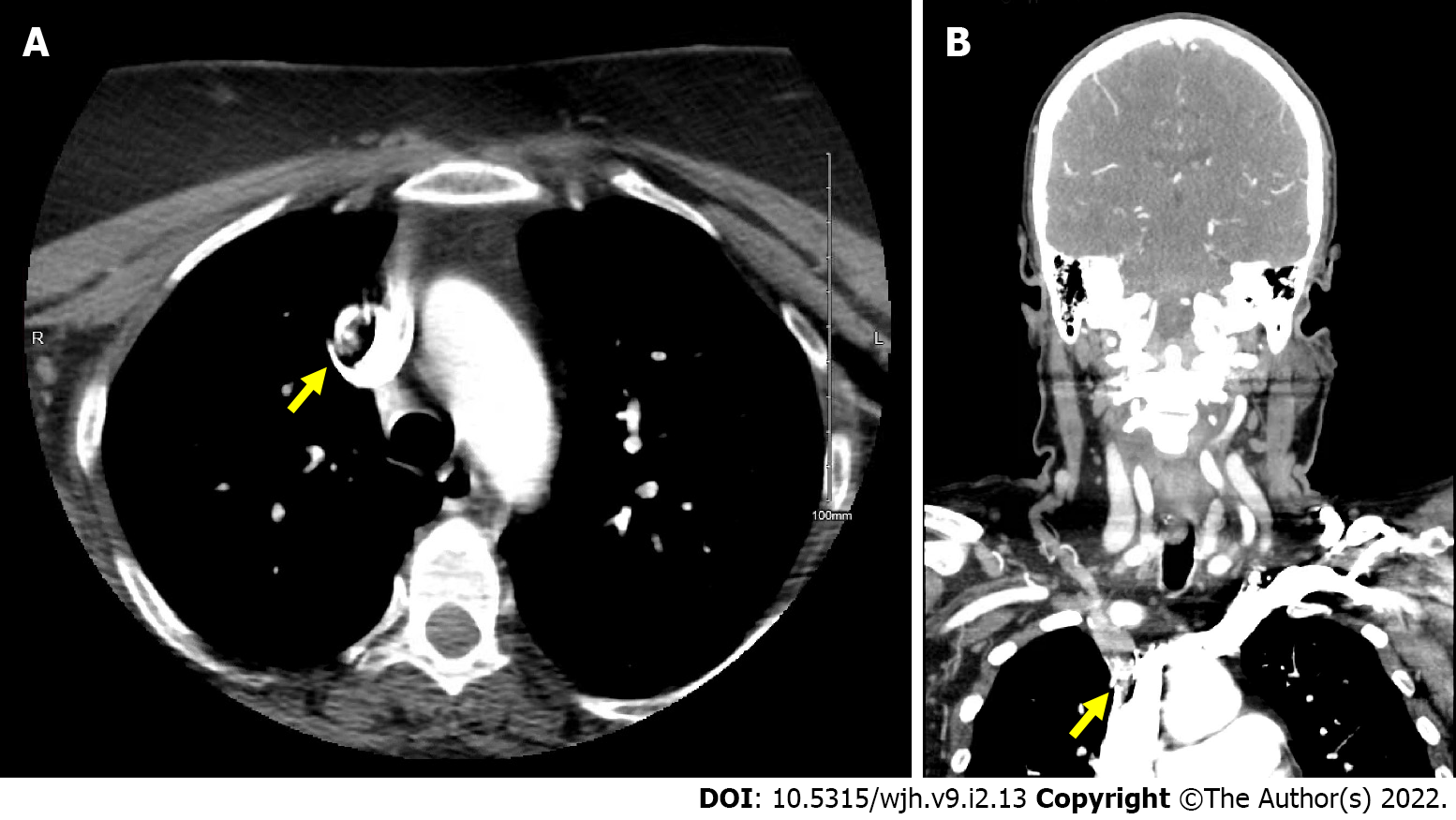
The Head and Neck Computed Tomography (CT) Angiogram found a non-occlusive filling defect in the right proximal brachiocephalic artery (Figure 2).
To determine the cause of the stroke, a transthoracic echocardiogram (TTE) and a transesophageal echocardiogram were performed. Testing showed normal heart function with an ejection fraction of 60 to 65% and no patent foramen ovale.
The final diagnosis of the presented case is a thrombus in the brachiocephalic artery and an acute ischemic stroke in the right ACA/MCA watershed region.
The patient was started on a heparin drip at 12 units/kg/h which was adjusted as necessary to maintain an activated partial thromboplastin time between 85 and 107 s. Oral aspirin 325 mg was administered and then transitioned to 81 mg daily. No tissue plasminogen activator (TPA) was given, as she was outside of the TPA administration window. Heparin was discontinued and apixaban 5 mg twice daily was started after a repeat Head and Neck CT Angiogram was normal.
To rule out malignancy related hypercoagulability, a colonoscopy and mammogram were recommended outpatient after discharge.
At discharge, new medications for the patient included aspirin 81 mg daily, apixaban 5 mg twice daily and atorvastatin 40 mg daily.
COVID-19 is associated with an increased risk of thromboembolism. It has been related to venous thrombosis more frequently than arterial thrombosis[8]. Many recent studies have concluded that thromboembolisms related to a SARS-CoV-2 infection are commonly accompanied by elevated inflammatory markers. A study by Guan and colleagues found disease severity was associated with elevated CRP (≥ 10 mg/L). Eighty-one and a half percent (110/135) of severe cases vs 56.4% (371/658) of non-severe cases presented with elevated CRP (P < 0.001)[9]. Similarly, three studies from China found CRP was elevated in patients with COVID-19 and linked to severity of disease[10-12]. Elevated ferritin levels have also been associated with severity[10,13]. In a prospective study, D-dimer, fibrinogen, and fibrin degradation product (FDP) levels were higher in patients with COVID-19, compared to healthy controls (P < 0.001 for all three comparisons). Higher values of D-dimer and FDP were seen in patients with more severe disease than in those with milder manifestations (P < 0.05 for both comparisons)[1]. Several studies have found D-dimer elevated in patients with COVID-19 and associated with worse outcomes[10,12-15]. In the unique case presented here, the patient's thromboembolisms did not follow the typical presentation because there was no corresponding elevation in inflammatory markers and the timing of the thromboembolism was well outside the reported time frame of the post COVID-19 infection period. One possible explanation for this, is that the clots grew slowly over time which would not cause acutely elevated inflammatory markers.
It is also important to consider the patient's risk factors for a thromboembolism. History of a VTE, malignancy, age greater than 50, venous stasis, vascular injury, medications such as estrogen, and a hypercoagulable state can all increase a patient's risk for thromboembolism. At baseline, our patient was independently performing activities of daily living. Her established risk factors include her age (65 years) and her BMI of 30.45 which is categorized as obese. Her calculated Padua score at the time of COVID-19 onset was two (obesity and acute illness). On presentation to the ED six months later, her calculated Padua prediction score for risk of VTE was two (obesity and acute ischemic stroke). During admission, it increased to five due to reduced mobility. Because her Padua score was less than four there was no indication to initiate VTE prophylaxis until she was admitted to the hospital. She suffered two separate embolisms with only two risk factors at baseline, leading us to hypothesize that her recent SARS-CoV-2 infection caused a hypercoagulable state which led to her hospital admission.
We cannot however rule out the potential impact of the bamlanivimab infusion or Pfizer-BioNTech COVID-19 vaccination on her coagulable state. Administration of the Johnson & Johnson/Janssen vaccine was temporarily paused due to several reports of thrombosis. It has since been resumed as these events were determined to be very rare, especially above age 50. The combined incidence of thrombosis from at least one dose of the Pfizer or Moderna vaccines in women less than 50 years old was one case per 222951 vaccinated. The median time-to-event was three days[16]. Incidence in older women is not well defined. Both 0.3 mL doses of the Pfizer-BioNTech COVID-19 vaccine were administered to the left deltoid and after each vaccination, the left arm became sore. Given this information, it is unlikely the vaccination alone caused her thrombosis, yet it is still possible it had a contributory effect. Additionally, she received a bamlanivimab infusion. Bamlanivimab is not reported to cause or be associated with increased risk of thrombosis but there is not a lot of data on the long-term effects yet. Therefore, we do not believe this infusion played significantly into this patient's clinical course aside from decreasing her likelihood of being hospitalized at the time of her SARS-CoV-2 infection. Many proposed mechanisms for the association of SARS-CoV-2 infection and hypercoagulability stem from endothelial injury that leads to an inflammatory response. It is unclear how long the hypercoagulability associated with COVID-19 lasts. A case report described three critically ill SARS-CoV-2 positive patients who each suffered multiple cerebral infarctions. These patients ranged from 65 to 70 years old; similar to the patient presented in our case. Their thrombotic event occurred 10, 18, and 33 days from disease onset[17]. This case report suggests COVID-19 associated hypercoagulability may last beyond that timeframe. The case we have presented here suggests hypercoagulability may extend even further beyond this period as the thromboses occurred six months after a SARS-CoV-2 infection.
The sequelae of COVID-19 are numerous and are associated with significant complications, one of which is thromboembolism. Without a more complete understanding of this disease process, determining appropriate prognostic indicators and therapeutics has yet to be fully elucidated. In this case report, we discussed a patient who presented with a serious and unexpected complication possibly related to a SARS-CoV-2 infection months prior. Larger case series and cohort studies are needed to determine if the presentation described in this case report has been observed in other patient populations. These additional studies will allow for better understanding of the risks associated with prolonged hypercoagulability in the setting of COVID-19.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Aktas G, Turkey; Ong H, Malaysia; Roganovic J; Croatia S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Han H, Yang L, Liu R, Liu F, Wu KL, Li J, Liu XH, Zhu CL. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 788] [Article Influence: 157.6] [Reference Citation Analysis (0)] |
| 2. | Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management. Thromb Res. 2020;194:101-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 497] [Cited by in RCA: 448] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 3. | Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, Brown TS, Der Nigoghossian C, Zidar DA, Haythe J, Brodie D, Beckman JA, Kirtane AJ, Stone GW, Krumholz HM, Parikh SA. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J Am Coll Cardiol. 2020;75:2352-2371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1381] [Cited by in RCA: 1365] [Article Influence: 273.0] [Reference Citation Analysis (0)] |
| 4. | Parks AL, Auerbach AD, Schnipper JL, Anstey JE, Sterken DG, Hecht TEH, Fang MC; Hospital Medicine Reengineering Network (HOMERuN). COVID-19 coagulopathy and thrombosis: Analysis of hospital protocols in response to the rapidly evolving pandemic. Thromb Res. 2020;196:355-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Cohen AT, Spiro TE, Büller HR, Haskell L, Hu D, Hull R, Mebazaa A, Merli G, Schellong S, Spyropoulos AC, Tapson V; MAGELLAN Investigators. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 456] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 6. | Xarelto (rivaroxaban). XARELTO® has been prescribed more than 80 million times in the US alone. Available from: https://www.xarelto-us.com/. |
| 7. | Spyropoulos AC, Levy JH, Ageno W, Connors JM, Hunt BJ, Iba T, Levi M, Samama CM, Thachil J, Giannis D, Douketis JD; Subcommittee on Perioperative, Critical Care Thrombosis, Haemostasis of the Scientific, Standardization Committee of the International Society on Thrombosis and Haemostasis. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1859-1865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 492] [Cited by in RCA: 549] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 8. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18856] [Article Influence: 3771.2] [Reference Citation Analysis (7)] |
| 9. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4960] [Cited by in RCA: 5514] [Article Influence: 1102.8] [Reference Citation Analysis (1)] |
| 10. | Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, Ng OT, Marimuthu K, Ang LW, Mak TM, Lau SK, Anderson DE, Chan KS, Tan TY, Ng TY, Cui L, Said Z, Kurupatham L, Chen MI, Chan M, Vasoo S, Wang LF, Tan BH, Lin RTP, Lee VJM, Leo YS, Lye DC; Singapore 2019 Novel Coronavirus Outbreak Research Team. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA. 2020;323:1488-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1346] [Cited by in RCA: 1364] [Article Influence: 272.8] [Reference Citation Analysis (0)] |
| 11. | Deng Y, Liu W, Liu K, Fang YY, Shang J, Zhou L, Wang K, Leng F, Wei S, Chen L, Liu HG. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J (Engl). 2020;133:1261-1267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 445] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 12. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18183] [Article Influence: 3636.6] [Reference Citation Analysis (0)] |
| 13. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30084] [Article Influence: 6016.8] [Reference Citation Analysis (3)] |
| 14. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14754] [Article Influence: 2950.8] [Reference Citation Analysis (0)] |
| 15. | Lippi G, Favaloro EJ. D-dimer is Associated with Severity of Coronavirus Disease 2019: A Pooled Analysis. Thromb Haemost. 2020;120:876-878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 394] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 16. | Sessa M, Kragholm K, Hviid A, Andersen M. Thromboembolic events in younger women exposed to Pfizer-BioNTech or Moderna COVID-19 vaccines. Expert Opin Drug Saf. 2021;20:1451-1453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, Xie J, Yan X, Zhou X, Liu Z, Wang J, Du B, Qin Y, Gao P, Qin X, Xu Y, Zhang W, Li T, Zhang F, Zhao Y, Li Y. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020;382:e38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1532] [Cited by in RCA: 1606] [Article Influence: 321.2] [Reference Citation Analysis (0)] |