Peer-review started: January 12, 2017
First decision: February 16, 2017
Revised: April 26, 2017
Accepted: May 21, 2017
Article in press: May 22, 2017
Published online: August 6, 2017
Processing time: 210 Days and 23.7 Hours
Sickle cell disease (SCD) is the first molecular disease in the literature. Although the structural alteration and dysfunction of the sickle hemoglobin (HbS) are well understood, the many factors modifying the clinical signs and symptoms of the disease are under investigation. Besides having an abnormal electrophoretic mobility and solubility, HbS is unstable. The autooxidation rate of the abnormal HbS has been reported to be almost two times of the normal. There are two more components of the oxidative damage in SCD: Free radical induced oxidative damage during vaso-occlusion induced ischemia-reperfusion injury and decreased antioxidant capacity in the erythrocyte and in the circulation. We will discuss the effects of oxidative alterations in the erythrocyte and in the plasma of SCD patients in this review.
Core tip: Oxidative alterations in the plasma and erythrocyte of sickle cell disease may indicate disease progression and phenotype. Detected oxidative modifications may be used as disease markers. Novel drugs targetting oxidative damage of plasma and cellular components may be important as promising therapeutic options.
- Citation: Oztas Y, Yalcinkaya A. Oxidative alterations in sickle cell disease: Possible involvement in disease pathogenesis. World J Hematol 2017; 6(3): 55-61
- URL: https://www.wjgnet.com/2218-6204/full/v6/i3/55.htm
- DOI: https://dx.doi.org/10.5315/wjh.v6.i3.55
Sickle cell disease (SCD) is an autosomal recessive disease which was first reported by an American physician, James Herrick in 1904[1]. It was noted for being the first molecular disease after demonstration of the point mutation in beta globin gene in 1949[2]. Acidic glutamate residue at the sixth position was exchanged with a hydrophobic valine in the beta subunit of sickle hemoglobin (HbS). Solubility of abnormal HbS decreases in deoxygenation, dehydration and acidosis resulting with formation of long and solid polymers in the erythrocyte where it interacts with the cytoskeleton forcing the cell to get an almost sickled shape. Although the erythrocyte has a high capacity to move through the narrowest capillaries, the sickle erythrocyte loses its elasticity and tends to slow down and accumulate resulting with vaso-occlusion.
SCD is characterized by anemia, vaso-occlusion and chronic inflammation. Ischemia, and necrosis develop after vaso-occlusion concomitantly resulting with organ dysfunction[3]. Acute vaso-occlusive crisis is the most common clinical presentation that results with hospitalization. Pulmonary hypertension, leg ulcers, priapism and stroke may develop as a complication of vaso-occlusive crisis. On the other hand frequent transfusions result with iron deposition in the tissues and organs of the patients with resultant organ dysfunction[4]. Iron induces generation of free radicals that produce oxidative stress and damages cell membrane, organelles and DNA[5].
Although the structural alteration and dysfunction of the HbS are well understood, the many factors modifying the phenotype of the patients and clinical presentation are under investigation. Understanding the spectrum of biochemical alterations produced by this genetic disease, novel therapeutic approaches can be developed to increase the life quality of the patients.
The erythrocytes have always been subjected to oxidative stress because they already transport oxygen in the circulation[6]. While there is a continuous flow of oxygen in the erythrocyte, it additionally contains iron (Fe2+) bound to heme in the cytoplasm surrounded by a membrane containing unsaturated fatty acids[7]. However, under normal conditions Fe2+ is isolated in the pocket of heme group and the antioxidant enzymes work to prevent or limit the damage of the oxidant stress[8].
When deoxyhemoglobin binds oxygen, an electron from Fe2+ of hemoglobin is transferred to oxygen forming oxyhemoglobin also called superoxyhemoglobin[9]. Normally this is reversible, however occasionally O2 leaves hemoglobin in the form of superoxide and l ferric hemoglobin named methemoglobin (MetHb) is formed. Normal erythrocytes have some amount of MetHb and superoxide formation. As a result hydroxyl radical is formed by dysmutation catalyzed by H2O2 and Fe2+. Therefore there is always some amount of oxidative stress in the erythrocyte[10].
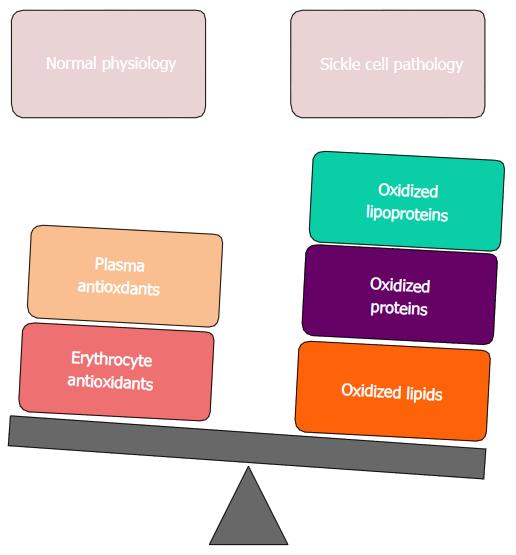
However, there is an excessive increase of oxidative stress in the sickle erythrocyte and plasma medium that the balance between antioxidants and oxidants is altered towards an increased production of oxidized lipids, proteins and lipoproteins (Figure 1).
A point mutation in beta globin gene results with an unstable HbS protein that has an abnormal electrophoretic mobility and solubility[11,12]. Therefore, MetHb formation and decomposition and heme release have tremendously increased[13]. It was first shown by Hebbel et al[9] that autooxidation of HbS was increased compared to normal hemoglobin, HbA. The autooxidation rate of HbS has been reported to be almost two times (1.7-1.9) of the normal with an increased formation of superoxide[9-14].
Excessive amount of lipid peroxidation has been observed in sickle erythrocytes[15] where the membrane damage due to peroxidation was demonstrated by increased permeability to potassium ion[16], altered membrane asymmetry[17], decreased erythrocyte deformability[18], dehydration[19,20] and hemolysis[21].
Iron and copper are particular elements that trigger Hb oxidation[22-24]. There are contradictory findings about the concentration of Fe2+ and Cu2+ in the sickle erythrocyte. Increased[24-26], similar or decreased amounts were reported in the sickle erythrocyte compared to normal[27,28]. Furthermore, there is an iron deposit on the membrane of the sickle erythrocyte that is different from normal. Heme bound iron[11] and unbound Fe2+ ion[29] were shown on the membrane. This is a factor that further increases the oxidative stress on the membrane. In addition, Hb auto-oxidation and radical formation thereby increased as mentioned above.
There are two more components of the oxidative damage in SCD: Free radical induced oxidative damage during vaso-occlusion induced ischemia-reperfusion injury and decreased antioxidant capacity in the erythrocyte and in the circulation[30]. Increased oxidative stress in the sickle erythrocyte disrupts the reducing power and defense mechanisms of the cell, thus facilitates further damage by other oxidative agents. Free heme in the sickle erythrocyte inhibits some enzymes that protect the cell from oxidation; there is a decreased activity of hexose mono phosphate pathway as well as decreased glutathione[30]. Although this metabolic deterioration has not been understood in the sickle erythrocytes, it should have a strong implication on the disease pathogenesis.
Membrane proteins of sickle erythrocytes were reported to have oxidative alterations[20-31]. Irregularities in the membranous distribution of band 3 and glycophorin, show that the membrane structure of the sickle erythrocyte is disrupted[20]. It has been observed that, accumulation of aggregates of hemichrome at the cytoplasmic region of Band 3 results in the merging of Band 3 molecules which in turn increases sickle cell fusion to endothelium and recognition by macrophages through increased immunoglobin G and complement activation at Band 3 merging sites[32]. Spectrin, which is a membrane skeleton protein, cannot properly bind to the sickle membrane as a result of the anomalies in the membrane proteins of the sickle cell. There is direct evidence that membrane proteins such as ankyrin, spectrin, Band 3 and Band 4 may have oxidative damage[31].
It has been shown that, membrane lipids of sickle cells also suffer oxidative damage[15]. Excessive lipid peroxidation accompanied by loss of membrane fluidity in biological membranes result in decreased membrane potential and increased permeability of H+ and other ions, followed with cell rupture and loss of cell contents and organelles.
Chronic intravascular hemolysis of SCD results with excessive production of heme that depletes endothelial nitric oxide[33]. Additionally vaso-occlusive crisis end up with ischemia-reperfusion injury where enzymes like xanthine oxidase, NADPH oxidase, nitric oxide synthase were activated inducing excessive production of free radicals[34,35]. Asymmetric dimethyl arginine, a nitric oxide inhibitor was found to be increased in SCD[36]. All these factors contribute to endothelial dysfunction and further aggravate oxidative stress resulting with a depletion of plasma antioxidants in SCD[37].
Plasma protein oxidation is monitored by measurement of protein carbonyl levels[38]. Increased protein carbonyl levels were reported in various diseases and regarded as a factor that might contribute to the disease pathology[39-41]. Carbonyl-modified plasma proteins were demonstrated to trigger endothelial dysfunction[42] which is regarded to be important in the pathogenesis of SCD. We reported increased protein oxidation by carbonyl modification in SCD patients’ plasma where carbonyl levels were correlated to plasma iron and hemolysate zinc levels[43]. Sulfhydrl groups measured in the plasma are mostly from proteins[44]. In fact protein sulfhydryl groups are important antioxidants that can break the oxidation chain. Albumin is the major plasma protein and was been shown to be a strong antioxidant[12]. We found decreased sulfhydryl content in the plasma of SCD patients[43]. All these posttranslational modifications occurred as a result of oxidative stress and needs further investigation to understand their effect on protein function and turnover.
Albumin is the major plasma protein that has antioxidant capacity due to its sulfhydryl groups[45]. Therefore it is a major target for oxidative injury. It was previously reported that free 34cysteine residue of albumin was the target for oxidizing agents[46,47]. A study using proteomics approach reported oxidative posttranslational modification of plasma albumin in the form of malondialdehyde adducts in SCD patients with pulmonary hypertension[48]. Our group showed that electrophoretic mobility of albumin from SCD patients was different than that of albumin from healthy controls[49]. The inflammatory and oxidative medium in SCD possibly targets albumin and induces structural modification. Methemalbumin formation was also reported in SCD patients[50]. This may be an antioxidant defense mechanism where plasma albumin binds oxidized heme and may by this way alleviate toxic effects of free heme on other low abundance proteins.
Malonyldialdehyde is a non-enzymatic oxidative by product of lipid peroxidation. Its main sources are oxidation of polyunsaturated fatty acids and cyclic endoperoxides released during eicosanoid synthesis[51]. Peroxidation of membrane lipids results in loss of membrane architecture that is essential for the deformability of the erythrocyte in passing through capillaries[52]. An erythrocyte with such membrane defects has a shorter life span and becomes a target for the reticuloendothelial system.
We previously reported MDA levels in the plasma and in the erythrocyte of SCD patients were higher than healthy controls[53]. Interestingly these patients had significantly lower blood cholesterol levels and there was a negative correlation between MDA and cholesterol in these patients.
Oxysterols are cholesterol oxidation products having metabolic roles as well[54]. 7-ketocholesterol is an oxysterol that is mostly formed due to increased oxidative stress[55]. There are two studies investigating cholesterol oxidation in the sickle erythrocytes. One study found sickle erythrocyte membranes contained higher 7-ketocholesterol levels than normal erythrocyte membranes[56]. In the other study, increased 7-ketocholesterol in sickle erythrocyte membrane was suggested to alter membrane dynamics and packaging capacity, therefore contributing to membrane pathology in SCD[57]. We found increased 7-ketocholesterol levels in SCD patients who also had hypocholesterolemia[58]. We suggested this cholesterol oxidation product, 7-ketocholesterol may modulate cholesterol biosynthesis at cytoplasmic or nuclear level.
Low-density lipoprotein (LDL) oxidation is a complex procedure in which both the proteins and lipids of the LDL are oxidized, resulting in extensive damage to its structure[59,60]. Macrophages, through increased proteoglycan binding, recognize and scavenge this cytotoxic remnant of native LDL forming foam cells[61,62]. The oxidation of LDL particles draws attention primarily because of their effect on atherosclerosis and coronary syndromes[63]. However, LDL leakage across endothelium and its subsequent oxidation by radicals can result in macrophage activation in all vascular structures. Furthermore, it is known that without oxidation, LDL particles do not result in the accumulation of cholesterol esters in blood vessels[64,65]; we can infer that if LDL is being oxidized, the result will be damage in vascular structure.
For example, oxidation of apolipoprotein B-100 component of LDL resulted in conformational change and increased endothelial uptake of LDL[66]. Being reported previously in patients with thalassemia[67], increased oxidation of LDL in patients with SCD patients might result with its increased clearance from plasma. This may be an explanation for decreased LDL as well as cholesterol levels in patients with SCA[68]. Possibly chronic hemolysis and increased erythropoietic activity are more important in the consumption of plasma pool of cholesterol and the development of hypocholesterolemia in patients with SCD[69]. However, the possible link between LDL oxidation and hypocholesterolemia should be investigated in further studies.
High-density lipoprotein (HDL) is known as the apolipoprotein that carries cholesterol back into the liver[70]; although HDL function is not as simple as this sentence suggests, its primary ability to accept cholesterol from LDL and macrophage foam cells is why HDL is considered protective against atherosclerosis[71,72]. Oxidized HDL on the other hand, loses its ability to remove cholesterol[73]. Contrary studies exist, it has been shown that specific forms of oxidized HDL (tyrosylated HDL) may in fact increase cholesterol uptake and decrease atherosclerotic plaque formation[74]. However, the specific nature of these oxidations and the lack of data about the in vivo formation of oxidized HDL raise questions on the reliability of this data for in vivo consideration.
Another important role of HDL is its anti-inflammatory function[75]. Oxidized HDL loses this function almost entirely and may even act pro-inflammatory during the acute phase response[75,76]. Furthermore, HDL levels are also decreased by ongoing inflammation[77,78]. This data suggests that the ongoing inflammatory state, increased acute phase reactants, and the constant oxidative stress that SCD patients undergo can result in a vicious cycle that is a major contributor to HDL dysfunction in SCD[79].
HDLs have additional functions; lipopolysaccharide binding, endothelial cell movement and function modulation, platelet-activating factor inhibition, anticoagulant activity inhibition, anti-oxidant enzyme effects, prostacyclin binding, stimulation of NO release; these are either direct effects through their plasma lipid transport role or effects through enzymes that travel alongside the apolipoprotein[78,80,81]. Paroxonase is one of these enzymes and was shown to have a decreased activity in SCD and researchers suggested that pediatric patients with SCD who had chronic oxidative stress might have a higher incidence of vaso-occlusive crisis[82]. However, SCD patients who had hydroxyurea had normal paroxonase activity. HDL has important antioxidant capacity and HDL mimetic peptides keep a potential to be a therapeutic agent in vascular inflammation[83]. 4F, an HDL mimetic, was shown to be beneficial against endothelial dysfunction in a mouse model of SCD[84].
SCD is regarded as a high oxidative stress situation, because of HbS. It is not unexpected that iron of heme can trigger many oxidative events that may damage erythrocyte and plasma macromolecules. Besides iron, vaso-occlusion induced ischemia-reperfusion injury and chronic inflammation also trigger oxidative damage at the cellular and at the circulation. There are many oxidative markers being studied in SCD. The clinical correlations of molecular alteration of proteins and lipids are important and they may modify disease presentation. New options of therapy in SCD will possibly involve antioxidants-either being synthetic or being biomimetic as adjuvant.
Manuscript source: Invited manuscript
Specialty type: Hematology
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Acuna-Castroviejo D, Al-Haggar M, Li Z S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Savitt TL. The second reported case of sickle cell anemia. Charlottesville, Virginia, 1911. Va Med Q. 1997;124:84-92. [PubMed] |
| 2. | Pauling L, Itano HA. Sickle cell anemia a molecular disease. Science. 1949;110:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 1229] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 3. | Powars DR. Sickle cell anemia and major organ failure. Hemoglobin. 1990;14:573-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Porter J, Garbowski M. Consequences and management of iron overload in sickle cell disease. Hematology Am Soc Hematol Educ Program. 2013;2013:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 5. | Bresgen N, Eckl PM. Oxidative stress and the homeodynamics of iron metabolism. Biomolecules. 2015;5:808-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 6. | Sivilotti ML. Oxidant stress and haemolysis of the human erythrocyte. Toxicol Rev. 2004;23:169-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Jakobik V, Burus I, Decsi T. Fatty acid composition of erythrocyte membrane lipids in healthy subjects from birth to young adulthood. Eur J Pediatr. 2009;168:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Pandey KB, Rizvi SI. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxid Med Cell Longev. 2010;3:2-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 264] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 9. | Hebbel RP, Morgan WT, Eaton JW, Hedlund BE. Accelerated autoxidation and heme loss due to instability of sickle hemoglobin. Proc Natl Acad Sci USA. 1988;85:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 228] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Misra HP, Fridovich I. The generation of superoxide radical during the autoxidation of hemoglobin. J Biol Chem. 1972;247:6960-6962. [PubMed] |
| 11. | Asakura T, Minakata K, Adachi K, Russell MO, Schwartz E. Denatured hemoglobin in sickle erythrocytes. J Clin Invest. 1977;59:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 135] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Liu SC, Zhai S, Palek J. Detection of hemin release during hemoglobin S denaturation. Blood. 1988;71:1755-1758. [PubMed] |
| 13. | Harrington JP, Keaton L. Unfolding and release of heme from human hemoglobins A, S and F. Int J Biochem. 1993;25:661-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Watkins JA, Claster S, Caughey WS. Enhanced Production of Oxy Radicals and Peroxide by Hemoglobin-S and Hemoglobin-F. Federation Proceedings. 1986;45: 1640-1640. |
| 15. | Rice-Evans C, Omorphos SC, Baysal E. Sickle cell membranes and oxidative damage. Biochem J. 1986;237:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Ney PA, Christopher MM, Hebbel RP. Synergistic effects of oxidation and deformation on erythrocyte monovalent cation leak. Blood. 1990;75:1192-1198. [PubMed] |
| 17. | Kuypers FA, Lewis RA, Hua M, Schott MA, Discher D, Ernst JD, Lubin BH. Detection of altered membrane phospholipid asymmetry in subpopulations of human red blood cells using fluorescently labeled annexin V. Blood. 1996;87:1179-1187. [PubMed] |
| 18. | Nash GB, Johnson CS, Meiselman HJ. Mechanical properties of oxygenated red blood cells in sickle cell (HbSS) disease. Blood. 1984;63:73-82. [PubMed] |
| 19. | Embury SH. The clinical pathophysiology of sickle cell disease. Annu Rev Med. 1986;37:361-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Liu SC, Yi SJ, Mehta JR, Nichols PE, Ballas SK, Yacono PW, Golan DE, Palek J. Red cell membrane remodeling in sickle cell anemia. Sequestration of membrane lipids and proteins in Heinz bodies. J Clin Invest. 1996;97:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Hebbel RP. Beyond hemoglobin polymerization: the red blood cell membrane and sickle disease pathophysiology. Blood. 1991;77:214-237. [PubMed] |
| 22. | Rifkind JM. Copper and the autoxidation of hemoglobin. Biochemistry. 1974;13:2475-2481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Winterbourn CC, Carrell RW. Oxidation of human haemoglobin by copper. Mechanism and suggested role of the thiol group of residue beta-93. Biochem J. 1977;165:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Winterbourn CC, McGrath BM, Carrell RW. Reactions involving superoxide and normal and unstable haemoglobins. Biochem J. 1976;155:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 200] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Repka T, Shalev O, Reddy R, Yuan J, Abrahamov A, Rachmilewitz EA, Low PS, Hebbel RP. Nonrandom association of free iron with membranes of sickle and beta-thalassemic erythrocytes. Blood. 1993;82:3204-3210. [PubMed] |
| 26. | Schaeffer K, Lofton JA, Powell SC, Osborne HH, Foster LH. Occurrence of copper in sickling erythrocytes. Proc Soc Exp Biol Med. 1968;128:734-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Prasad AS, Ortega J, Brewer GJ, Oberleas D, Schoomaker EB. Trace elements in sickle cell disease. JAMA. 1976;235:2396-2398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Darbari D, Loyevsky M, Gordeuk V, Kark JA, Castro O, Rana S, Apprey V, Kurantsin-Mills J. Fluorescence measurements of the labile iron pool of sickle erythrocytes. Blood. 2003;102:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Kuross SA, Hebbel RP. Nonheme iron in sickle erythrocyte membranes: association with phospholipids and potential role in lipid peroxidation. Blood. 1988;72:1278-1285. [PubMed] |
| 30. | Morris CR, Suh JH, Hagar W, Larkin S, Bland DA, Steinberg MH, Vichinsky EP, Shigenaga M, Ames B, Kuypers FA. Erythrocyte glutamine depletion, altered redox environment, and pulmonary hypertension in sickle cell disease. Blood. 2008;111:402-410. [PubMed] |
| 31. | Platt OS, Falcone JF. Membrane protein interactions in sickle red blood cells: evidence of abnormal protein 3 function. Blood. 1995;86:1992-1998. [PubMed] |
| 32. | Bosman GJ. Erythrocyte aging in sickle cell disease. Cell Mol Biol (Noisy-le-grand). 2004;50:81-86. [PubMed] |
| 33. | Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | George A, Pushkaran S, Konstantinidis DG, Koochaki S, Malik P, Mohandas N, Zheng Y, Joiner CH, Kalfa TA. Erythrocyte NADPH oxidase activity modulated by Rac GTPases, PKC, and plasma cytokines contributes to oxidative stress in sickle cell disease. Blood. 2013;121:2099-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 35. | Kaul DK, Hebbel RP. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. J Clin Invest. 2000;106:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 308] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 36. | Schnog JB, Teerlink T, van der Dijs FP, Duits AJ, Muskiet FA; CURAMA Study Group. Plasma levels of asymmetric dimethylarginine (ADMA), an endogenous nitric oxide synthase inhibitor, are elevated in sickle cell disease. Ann Hematol. 2005;84:282-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Ren H, Ghebremeskel K, Okpala I, Lee A, Ibegbulam O, Crawford M. Patients with sickle cell disease have reduced blood antioxidant protection. Int J Vitam Nutr Res. 2008;78:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1601] [Cited by in RCA: 1635] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 39. | Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1648] [Cited by in RCA: 1611] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 40. | Inagi R, Miyata T. Oxidative protein damage with carbohydrates and lipids in uremia: ‘Carbonyl stress’. Blood Purif. 1999;17:95-98. [PubMed] |
| 41. | de Arriba SG, Krügel U, Regenthal R, Vissiennon Z, Verdaguer E, Lewerenz A, García-Jordá E, Pallas M, Camins A, Münch G. Carbonyl stress and NMDA receptor activation contribute to methylglyoxal neurotoxicity. Free Radic Biol Med. 2006;40:779-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Banfi C, Brioschi M, Barcella S, Veglia F, Biglioli P, Tremoli E, Agostoni P. Oxidized proteins in plasma of patients with heart failure: role in endothelial damage. Eur J Heart Fail. 2008;10:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Oztas Y, Durukan I, Unal S, Ozgunes N. Plasma protein oxidation is correlated positively with plasma iron levels and negatively with hemolysate zinc levels in sickle-cell anemia patients. Int J Lab Hematol. 2012;34:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Wayner DD, Burton GW, Ingold KU, Barclay LR, Locke SJ. The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochim Biophys Acta. 1987;924:408-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 586] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 45. | Bruschi M, Candiano G, Santucci L, Ghiggeri GM. Oxidized albumin. The long way of a protein of uncertain function. Biochim Biophys Acta. 2013;1830:5473-5479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Turell L, Carballal S, Botti H, Radi R, Alvarez B. Oxidation of the albumin thiol to sulfenic acid and its implications in the intravascular compartment. Braz J Med Biol Res. 2009;42:305-311. [PubMed] |
| 47. | Candiano G, Petretto A, Bruschi M, Santucci L, Dimuccio V, Prunotto M, Gusmano R, Urbani A, Ghiggeri GM. The oxido-redox potential of albumin methodological approach and relevance to human diseases. J Proteomics. 2009;73:188-195. [PubMed] |
| 48. | Odhiambo A, Perlman DH, Huang H, Costello CE, Farber HW, Steinberg MH, McComb ME, Klings ES. Identification of oxidative post-translational modification of serum albumin in patients with idiopathic pulmonary arterial hypertension and pulmonary hypertension of sickle cell anemia. Rapid Commun Mass Spectrom. 2007;21:2195-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Ozgunes N, Oztas Y, Unal S, Yaman H. Structural modification of plasma albumin in sickle cell anemia. Acta Haematol. 2015;133:67-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 50. | Hanson MS, Piknova B, Keszler A, Diers AR, Wang X, Gladwin MT, Hillery CA, Hogg N. Methaemalbumin formation in sickle cell disease: effect on oxidative protein modification and HO-1 induction. Br J Haematol. 2011;154:502-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Yagi K. Lipid peroxides and human diseases. Chem Phys Lipids. 1987;45:337-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 868] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 52. | Manwani D, Frenette PS. Vaso-occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Hematology Am Soc Hematol Educ Program. 2013;2013:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | Oztas YE, Sabuncuoglu S, Unal S, Ozgunes H, Ozgunes N. Hypocholesterolemia is associated negatively with hemolysate lipid peroxidation in sickle cell anemia patients. Clin Exp Med. 2011;11:195-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Kandutsch AA, Chen HW. Inhibition of sterol synthesis in cultured mouse cells by 7alpha-hydroxycholesterol, 7beta-hydroxycholesterol, and 7-ketocholesterol. J Biol Chem. 1973;248:8408-8417. [PubMed] |
| 55. | Lyons MA, Brown AJ. 7-Ketocholesterol. Int J Biochem Cell Biol. 1999;31:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Kucuk O, Lis LJ, Dey T, Mata R, Westerman MP, Yachnin S, Szostek R, Tracy D, Kauffman JW, Gage DA. The effects of cholesterol oxidation products in sickle and normal red blood cell membranes. Biochim Biophys Acta. 1992;1103:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Szostek R, Kucuk O, Lis LJ, Tracy D, Mata R, Dey T, Kauffman JW, Yachnin S, Westerman MP. Effect of inserted oxysterols on phospholipid packing in normal and sickle red blood cell membranes. Biochem Biophys Res Commun. 1991;180:730-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | Yalcinkaya A, Lay İ, Samadi A, Unal S, Akbiyik F, Oztas Y. Increased plasma 7-ketocholesterol levels may explain the hypocholesterolemia of sickle cell disease patients. Acta Medica. 2017;48: 1-4. |
| 59. | Gieseg SP, Pearson J, Firth CA. Protein hydroperoxides are a major product of low density lipoprotein oxidation during copper, peroxyl radical and macrophage-mediated oxidation. Free Radic Res. 2003;37:983-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995;91:2488-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 1098] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 61. | Hessler JR, Morel DW, Lewis LJ, Chisolm GM. Lipoprotein oxidation and lipoprotein-induced cytotoxicity. Arteriosclerosis. 1983;3:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 368] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 62. | Borén J, Olin K, Lee I, Chait A, Wight TN, Innerarity TL. Identification of the principal proteoglycan-binding site in LDL. A single-point mutation in apo-B100 severely affects proteoglycan interaction without affecting LDL receptor binding. J Clin Invest. 1998;101:2658-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 208] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 63. | Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7301] [Cited by in RCA: 7415] [Article Influence: 353.1] [Reference Citation Analysis (1)] |
| 64. | Parthasarathy S, Raghavamenon A, Garelnabi MO, Santanam N. Oxidized low-density lipoprotein. Methods Mol Biol. 2010;610:403-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 207] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 65. | Heery JM, Kozak M, Stafforini DM, Jones DA, Zimmerman GA, McIntyre TM, Prescott SM. Oxidatively modified LDL contains phospholipids with platelet-activating factor-like activity and stimulates the growth of smooth muscle cells. J Clin Invest. 1995;96:2322-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 238] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 66. | Lankin VZ, Tikhaze AK, Kapel’ko VI, Shepel’kova GS, Shumaev KB, Panasenko OM, Konovalova GG, Belenkov YN. Mechanisms of oxidative modification of low density lipoproteins under conditions of oxidative and carbonyl stress. Biochemistry (Mosc). 2007;72:1081-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Livrea MA, Tesoriere L, Maggio A, D’Arpa D, Pintaudi AM, Pedone E. Oxidative modification of low-density lipoprotein and atherogenetic risk in beta-thalassemia. Blood. 1998;92:3936-3942. [PubMed] |
| 68. | Rahimi Z, Merat A, Haghshenass M, Madani H, Rezaei M, Nagel RL. Plasma lipids in Iranians with sickle cell disease: hypocholesterolemia in sickle cell anemia and increase of HDL-cholesterol in sickle cell trait. Clin Chim Acta. 2006;365:217-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 69. | Shalev H, Kapelushnik J, Moser A, Knobler H, Tamary H. Hypocholesterolemia in chronic anemias with increased erythropoietic activity. Am J Hematol. 2007;82:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 70. | Glomset JA. The plasma lecithins: cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155-167. [PubMed] |
| 71. | Heinecke JW. Mechanisms of oxidative damage of low density lipoprotein in human atherosclerosis. Curr Opin Lipidol. 1997;8:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 197] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 72. | Rader DJ, Puré E. Lipoproteins, macrophage function, and atherosclerosis: beyond the foam cell? Cell Metab. 2005;1:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 73. | Nagano Y, Arai H, Kita T. High density lipoprotein loses its effect to stimulate efflux of cholesterol from foam cells after oxidative modification. Proc Natl Acad Sci USA. 1991;88:6457-6461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 180] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 74. | Bergt C, Oram JF, Heinecke JW. Oxidized HDL: the paradox-idation of lipoproteins. Arterioscler Thromb Vasc Biol. 2003;23:1488-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, La Du BN, Fogelman AM, Navab M. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96:2758-2767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 634] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 76. | Zhang M, Gao X, Wu J, Liu D, Cai H, Fu L, Mei C. Oxidized high-density lipoprotein enhances inflammatory activity in rat mesangial cells. Diabetes Metab Res Rev. 2010;26:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 77. | Tietge UJ, Maugeais C, Cain W, Grass D, Glick JM, de Beer FC, Rader DJ. Overexpression of secretory phospholipase A(2) causes rapid catabolism and altered tissue uptake of high density lipoprotein cholesteryl ester and apolipoprotein A-I. J Biol Chem. 2000;275:10077-10084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 139] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 78. | Han CY, Tang C, Guevara ME, Wei H, Wietecha T, Shao B, Subramanian S, Omer M, Wang S, O’Brien KD. Serum amyloid A impairs the antiinflammatory properties of HDL. J Clin Invest. 2016;126:266-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 79. | Soupene E, Borja MS, Borda M, Larkin SK, Kuypers FA. Featured Article: Alterations of lecithin cholesterol acyltransferase activity and apolipoprotein A-I functionality in human sickle blood. Exp Biol Med (Maywood). 2016;241:1933-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 1001] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 81. | Barter P. Effects of inflammation on high-density lipoproteins. Arterioscler Thromb Vasc Biol. 2002;22:1062-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 82. | El-Ghamrawy MK, Hanna WM, Abdel-Salam A, El-Sonbaty MM, Youness ER, Adel A. Oxidant-antioxidant status in Egyptian children with sickle cell anemia: a single center based study. J Pediatr (Rio J). 2014;90:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 83. | White CR, Datta G, Mochon P, Zhang Z, Kelly O, Curcio C, Parks D, Palgunachari M, Handattu S, Gupta H. Vasculoprotective Effects of Apolipoprotein Mimetic Peptides: An Evolving Paradigm In Hdl Therapy (Vascular Disease Prevention, In Press.). Vasc Dis Prev. 2009;6:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 84. | Ou J, Ou Z, Jones DW, Holzhauer S, Hatoum OA, Ackerman AW, Weihrauch DW, Gutterman DD, Guice K, Oldham KT. L-4F, an apolipoprotein A-1 mimetic, dramatically improves vasodilation in hypercholesterolemia and sickle cell disease. Circulation. 2003;107:2337-2341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |