Peer-review started: August 10, 2016
First decision: September 12, 2016
Revised: September 25, 2016
Accepted: November 16, 2016
Article in press: November 16, 2016
Published online: February 6, 2017
Processing time: 163 Days and 3.8 Hours
The role of plasmapheresis in liver failure and hepatic encephalopathy is undefined and its use as a strategy to salvage patients with severe allograft dysfunction after liver transplantation remains investigational. We present a case of early allograft dysfunction following deceased donor liver transplantation (DDLT) where plasmapheresis was effective as a bridge to recovery and possibly avoiding a retransplantation. A 16 years old boy, known to have decompensated Wilson’s disease underwent DDLT at our Public Sector Hospital. He received a healthy liver from a brain-dead donor, whose liver was considered too large for the boy. The graft was reduced in situ to a left lobe graft. Surgery was uneventful and the recipient was well for the initial 96 h. On Doppler and further computed tomography scan, a partial portal vein thrombus was noted. He was reexplored and a Fogarty endothombecteomy was performed. Following the second surgery, he developed severe allograft dysfunction with a peak bilirubin of 40 mg/dL. He underwent imaging to rule out technical causes for the dysfunction, followed by a liver biopsy, which revealed acute cellular rejection. Multiple cycles of plasmapheresis were initiated. Over the next two weeks, the graft demonstrated a gradual recovery. He was discharged on the 30th postoperative day, with a serum bilirubin of 5.5 mg/dL. He remains well on follow-up, with the liver function tests improving further. Our report demonstrates the beneficial effect of plasmapheresis, which appears to be an effective treatment option for early allograft dysfunction following liver transplantation and may obviate the need for retransplantation.
Core tip: We demonstrate the beneficial effects of plasmapheresis, which appears to be an effective treatment option for early allograft dysfunction following liver transplantation and may obviate the need for retransplantation.
- Citation: Rammohan A, Sachan D, Logidasan S, Sathyanesan J, Palaniappan R, Rela M. Role of plasmapheresis in early allograft dysfunction following deceased donor liver transplantation. World J Hematol 2017; 6(1): 24-27
- URL: https://www.wjgnet.com/2218-6204/full/v6/i1/24.htm
- DOI: https://dx.doi.org/10.5315/wjh.v6.i1.24
With expanding indications and increasing demand for liver transplantation (LT) donor organ shortage is a major limitation. Early allograft dysfunction (EAD) is not an uncommon entity, especially in transplantation with organs from marginal donors[1]. The incidence of EAD varies between 1.4%-23%, with a median range of 5%-6%[1-3]. This wide range of incidences is attributable to the myriad of definitions which exist for EAD although most definitions are a combination of elevated bilirubin, international normalized ratio (INR), transaminases, and hepatic encephalopathy.
EAD leads to increased morbidity and may result in mortality and liver support therapies need to be instituted[2-5]. In severe forms, retransplantation may be the only treatment modality. If the duration of early graft dysfunction passes uneventfully, the patient often recovers spontaneously[1-6]. Measures like liver support devices lessen the hepatic metabolic burden and may help in the recovery of graft function[2-4]. Plasmapheresis has been used in acute liver failure, but its role in supporting dysfunctional liver allografts remains unclear[7,8].
In this brief report, we present a case of allograft dysfunction following deceased donor liver transplantation (DDLT) where the graft was salvaged using multiple cycles of plasmapheresis.
A 16-year-old boy weighing 33 kg, with decompensated Wilson’s disease underwent DDLT at our Public Sector Hospital. He received a healthy liver from a 34-year-old brain-dead donor. The donor had an initial Sodium value of 194 meq/L, which was controlled and brought down to 164 meq/L at the time of organ retrieval. The donor had one episode of significant hypotension. As the donor liver was considered too large for the boy, it was reduced in situ into a left lobe graft.
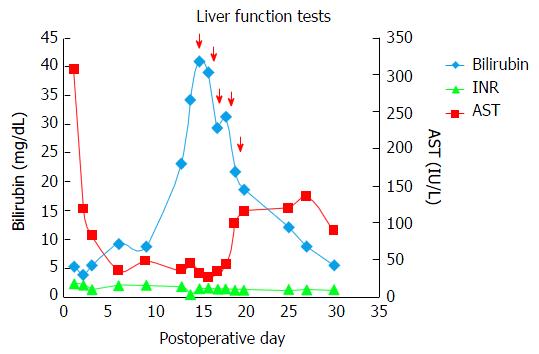
The recipient operation was uneventful with a total blood loss of 1000 mL. The end lactate was 4.2 mmol/L from a peak of 10.2 mmol/L in the anhepatic phase. The total cold ischemia time was 210 min. The graft had an accessory artery from the left gastric artery, taken as a cuff from the celiac axis and anastomosed to the common hepatic artery of the recipient. The surgery was uneventful and the recipient was well initially; being extubated on the 1st postoperative day (POD). Immunosuppression was initiated with steroids (Methyl-Prednisolone 0.25 mg/kg per day) and calcineurin inhibitors (Tacrolimus 0.03 mg/kg per day) from POD 1. On the 5th POD, his drain output increased from 600 mL in 24 h to 1700 mL and his serum bilirubin which had dropped to 3.8 mg/dL, went upto 9.3 mg/dL. Doppler showed poor flow in the portal vein. On further imaging, he was noted to have a partial portal vein thrombus. He underwent emergency re-exploration when a Fogarty endothrombectomy was done and the graft was revascularised with an iliac vein interposition graft for the portal venous anastomosis. During the second surgery, the graft was noted to be very stiff. Following this, he developed severe allograft dysfunction (rising serum bilirubin > 10 mg/dL over 3 consecutive days in the absence of biliary complications). Over the next 5 d his bilirubin increased up to 23.5 mg/dL, while his transaminases remained normal. He underwent repeat imaging which ruled out technical causes for the dysfunction including a patent portal vein. Liver biopsy was performed which was suggestive of moderate acute cellular rejection, there was no evidence of antibody mediated rejection. He received pulsed steroid therapy (Methyl-Prednisolone 20 mg/kg per day on consecutive three days). Despite the steroid pulse, the graft dysfunction did not abate and the hyperbilirubinemia persisted on an upward trend, peaking at 40.8 mg/dL on the 15th POD. In an effort to salvage the graft, plasmapheresis was initiated on the 15th POD.
Plasmapheresis was done on 5 consecutive days using continuous flow centrifugal technology based Spectra Optia Apheresis system (Terumo BCT, Denver, CO, United States). Acid citrate dextrose-A anticoagulation and dual vascular access were used. Patient's total blood volume (BV) was calculated as per Nadler's formula and Plasma volume (PV) was calculated according to the formula PV = BV × (1 - Hematocrit)[8]. 1.0 PV was processed in each session with 100% replacement using 5% albumin solution and blood group specific fresh frozen plasma. The inlet: Anticoagulant ratio was kept 1:12 to 1:15 and blood flow rate kept between 45-50 mL/min. Baseline calcium was monitored before each procedure and 20 mL 10% calcium gluconate was given prophylactically during the procedure to prevent citrate toxicity. Continuous monitoring of pulse and blood pressure was carried out during the procedure to prevent any adverse events related to the procedure. No serious adverse effects were observed during the procedure. Complete blood count, INR, liver function tests, renal function tests, arterial ammonia, arterial blood gas analysis, were performed every 12 hourly irrespective of the timings of the plasmapheresis.
His bilirubin showed a steady fall and by the 5th cycle of plasmapheresis it had dropped to 15 mg/dL (Figure 1). In the interim, he had an episode of fever with chills, and grew K. pneumonia in his blood culture. This was successfully treated with appropriate antibiotics (Piperacillin-Tazobactum). No obvious source for the infection could be discerned. He was discharged on the 30th POD being asymptomatic, tolerating oral diet well, with stable vital signs and with a serum bilirubin of 5.5 mg/dL. He remains well on follow-up, with the latest liver function tests showing a total bilirubin of 1 mg/dL, 4 mo after transplantation.
Although the pathophysiological basis for early allograft dysfunction has not been wholly elucidated; it appears to be a critical interplay between donor factors, recipient characteristics, and intra-operative events[1,2,4,5].
Despite a few studies including one by Park et al[4] having shown plasmapheresis to be beneficial in severe graft dysfunction; the role of plasmapheresis remains undefined in graft dysfunction[1-4]. The mechanisms by which plasmapheresis is beneficial hasn’t been completely elucidated, but it does remove the plasma containing free and protein-bound toxic substrates and infuse fresh plasma, as well as clotting factors and albumin, thus functioning as a liver support; creating a milieu conducive to liver regeneration[1-6]. Plasmapheresis is an important adjunct in the treatment of hepatic encephalopathy as it improves blood-clotting, hyperbilirubinemia, and hyperammonemia; acting as a bridge to LT[7,8].
In a series from Japan, all 46 patients with liver failure following LT improved with plasmapheresis[5]. In another recent study by Choe et al[3] consisting of 143 patients with EAD of whom 107 underwent Plasmapheresis. There was a significant improvement in the 1-mo and 1-year survival of this subgroup of patients as compared to those who did not undergo plasmapheresis. A report from Johns Hopkin also suggested that plasmapheresis may aid in the recovery of primary allograft nonfunction following liver transplantation[9,10].
As demonstrated in our patient, a single plasmapheresis session cannot be expected to provide a definite beneficial effect in patients with a failing liver graft[2,3]. Repeated sessions appear necessary to achieve cumulative effects. The timing and interval of plasmapheresis must be adjusted on a case-by-case basis, by daily determination of patient’s general condition and liver graft function[2-4]. In a dysfunctional liver, the liver enzymes often fluctuate, depending on the condition of the liver graft, and hence cannot be used to assess the effectiveness of plasmapheresis. Prothrombin time is readily affected by the plasma infusion and is also not a predictable marker of the effectiveness of plasmapheresis[3,11]. Serum bilirubin appears to be the most reliable parameter to base decision regarding the initiation, continuation and termination of plasmapheresis[2,4,11].
In countries, where retransplantation may not be a feasible option due to the lack of availability of donor grafts and/or the huge financial burden involved, plasmapheresis appears to be a readily available artificial liver support system with the added advantage of being economical, simple and easy to use.
Apart from benefiting the patient, effective management of early allograft dysfunction helps a unit improve its overall efficiency and sets a benchmark for excellence in care by showing an enhancement in healthcare delivery in general.
In conclusion, our report demonstrates the beneficial effect of plasmapheresis, which appears to be an effective treatment option for early allograft dysfunction following DDLT and may obviate the need for retransplantation.
Early liver allograft dysfunction.
Arterial complications, venous complications, biliary complications, rejection, infection.
Early liver allograft dysfunction.
Early liver allograft dysfunction.
Early liver allograft dysfunction.
Plasmapheresis.
Plasmapheresis - total plasma exchange.
Useful not to disregard a simple but very effective procedure such as plasmapheresis in treating early allograft dysfunction.
The paper is good, although it doesn’t seem to get different conclusion from the larger case series already published.
Manuscript source: Unsolicited manuscript
Specialty type: Hematology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Dellacasa CM, Fukuda S, Qin JM, Schroeder T, Surov S S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Camci C, Akdogan M, Gurakar A, Gilcher R, Rose J, Monlux R, Alamain S, Wright H, Sebastian A, Nour B. The impact of total plasma exchange on early allograft dysfunction. Transplant Proc. 2004;36:2567-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 2. | Deschênes M, Belle SH, Krom RA, Zetterman RK, Lake JR. Early allograft dysfunction after liver transplantation: a definition and predictors of outcome. National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Transplantation. 1998;66:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 180] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Choe W, Kwon SW, Kim SS, Hwang S, Song GW, Lee SG. Effects of therapeutic plasma exchange on early allograft dysfunction after liver transplantation. J Clin Apher. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 4. | Park CS, Hwang S, Park HW, Park YH, Lee HJ, Namgoong JM, Yoon SY, Jung SW, Park GC, Jung DH. Role of plasmapheresis as liver support for early graft dysfunction following adult living donor liver transplantation. Transplant Proc. 2012;44:749-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Yamamoto R, Nagasawa Y, Marubashi S, Furumatsu Y, Iwatani H, Iio K, Matsui I, Dono K, Imai E, Monden M. Early plasma exchange for progressive liver failure in recipients of adult-to-adult living-related liver transplants. Blood Purif. 2009;28:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (2)] |
| 6. | Lee JY, Kim SB, Chang JW, Park SK, Kwon SW, Song KW, Hwang S, Lee SG. Comparison of the molecular adsorbent recirculating system and plasmapheresis for patients with graft dysfunction after liver transplantation. Transplant Proc. 2010;42:2625-2630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Akdogan M, Camci C, Gurakar A, Gilcher R, Alamian S, Wright H, Nour B, Sebastian A. The effect of total plasma exchange on fulminant hepatic failure. J Clin Apher. 2006;21:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Hwang S, Ha TY, Ahn CS, Kim KH, Lee SG. Reappraisal of plasmapheresis as a supportive measure in a patient with hepatic failure after major hepatectomy. Case Rep Gastroenterol. 2007;1:162-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51:224-232. [PubMed] |
| 10. | Mandal AK, King KE, Humphreys SL, Maley WR, Burdick JF, Klein AS. Plasmapheresis: an effective therapy for primary allograft nonfunction after liver transplantation. Transplantation. 2000;70:216-220. [PubMed] |
| 11. | Bektas M, Idilman R, Soykan I, Soydan E, Arat M, Cinar K, Coban S, Tuzun A, Bozkaya H, Ormeci N. Adjuvant therapeutic plasma exchange in liver failure: assessments of clinical and laboratory parameters. J Clin Gastroenterol. 2008;42:517-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |