Peer-review started: February 15, 2017
First decision: March 7, 2017
Revised: March 14, 2017
Accepted: April 4, 2017
Article in press: April 5, 2017
Published online: August 2, 2017
Processing time: 159 Days and 2.6 Hours
The skin facilitates a number of key roles but its functioning can be impaired by disease. Atopic eczema is a chronic inflammatory disease where the skin barrier has become leaky, and inflammation occurs. It affects up to 20% of children and 3% of adults worldwide, manifesting as red itchy patches of skin with varying severity. This review aims to investigate the leaky skin barrier and immune mechanisms from the perspective of potential novel treatments. The complexity of atopic eczema as a disease is what makes it difficult to treat. Genome-wide association studies have highlighted possible genetic variations associated with atopic eczema, however in some cases, individuals develop the disease without these genetic risk factors. Loss of function mutations in the filaggrin gene are one of these associations and this is plausible due to its key role in barrier function. The Th2 immune response is the link with regards to the immune mechanisms as atopic inflammation often occurs through increased levels of interleukin (IL)-4 and IL-13. Eczematous inflammation also creates susceptibility to colonisation and damage by bacteria such as Staphylococcus aureus. Potential novel treatments are becoming ever more specific, offering the hope of fewer side effects and better disease control. The best new treatments highlighted in this review target the immune response with human beta defensin 2, phosphodiesterase-4 inhibitors and monoclonal antibodies all showing promise.
Core tip: Atopic eczema (atopic dermatitis) is an itchy inflammatory skin disease with complex aetiology, including impairment in barrier function and concomitant inflammation. Increased understanding of the molecular mechanisms in eczema pathogenesis has opened up opportunities for new therapeutic targets. This review summaries current understanding and highlights some novel treatments in development.
- Citation: Bell DC, Brown SJ. Atopic eczema treatment now and in the future: Targeting the skin barrier and key immune mechanisms in human skin. World J Dermatol 2017; 6(3): 42-51
- URL: https://www.wjgnet.com/2218-6190/full/v6/i3/42.htm
- DOI: https://dx.doi.org/10.5314/wjd.v6.i3.42
Atopic eczema (also called atopic dermatitis) is a skin disease that has shown a rise in prevalence in Africa, Eastern Asia and Western Europe[1]. It is a chronic inflammatory disease that manifests as red patches of itchy skin and in severe cases excoriated or infected lesions[2]. Inflammation is believed to occur when the skin barrier becomes leaky and an immune response is stimulated; vice versa, the inflammatory response can itself impair the skin barrier function[3].
There are two forms of eczema: Atopic, when an increased IgE response occurs, and non-atopic when this response is not observed. Eighty percent of cases are atopic[4] and the feature of atopy (raised IgE) is what relates the disease to other allergic responses such as food allergies, allergic rhinitis and asthma which can all show an IgE response. The so-called “atopic march” describes the progressive acquisition of atopic diseases in a step-wise manner throughout childhood[5]. Therefore children affected by one atopic disease will often show phenotypes of the others too[5]. Atopic eczema is predominantly a childhood disease that affects up to 20% of children[6] and this is one of the reasons why it is among the most common skin diseases worldwide[2]. In the 2010 WHO article into the global burden skin diseases, atopic eczema was ranked first by causing the most number of days that people were not at full health[7]. The effects of atopic eczema are not limited to skin. Children with more extreme atopic eczema suffer from reduced sleep and increased psychological problems[8]. Atopic eczema not only has a large effect on the health of the sufferer but it also has a substantial economic effect. In the United States, the direct cost for the treatment of atopic eczema may be as high as $3.8 billion per year[9], studies in the United Kingdom are not recent enough to compare as one of the most recent was in 1996[10,11]. This shows from an economic stance that research into a more cost effective treatment is of great importance.
The development of specific treatment modalities in atopic eczema is difficult due to the complexity of this disease. For example, there are a number of strong genetic risk factors associated with the disease and variations in these genes are often seen in atopic eczema; however in a subset of cases, a mutation is present but there is no disease[12]. Another feature involved with atopic eczema is environmental allergens such as dust or animal hair which can precipitate the disease or elicit a flare up. Therefore the disease is now believed to be caused by a combination of both genetic and environmental factors[13]; this complexity is why treatments are only partially effective and why there is currently no cure[14].
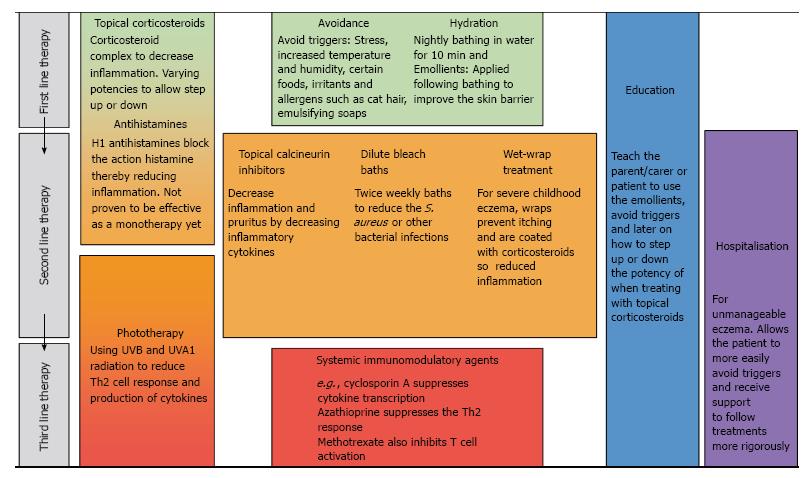
Atopic eczema arises due to interactions between a leaky skin barrier and the immune response that occurs both in the skin and the systemic circulation; therefore current treatments aim to reduce the inflammation and repair the skin barrier at sites of inflamed or dry skin. Due to the complexity and range of severity of the disease, there are a number of different treatments[15]. These have been summarised in Figure 1. The most commonly used treatment is the application of emollients; these act by increasing the lipid content in the stratum corneum (outermost layer of the skin) to repair the barrier, thereby improving hydration[15]. However, in all but the mildest cases emollients are insufficiently effective so a combination therapy is used with another agent targeting the inflammatory response. Topical corticosteroids act through the corticosteroid-receptor complex to downregulate synthesis of the proteins involved in inflammation[16]. Topical corticosteroids come in different forms from sprays to creams and ointments but more potent steroids must be used sparingly as they have been found to reduce dermal thickness[17]. Topical calcineurin inhibitors also target the immune mechanisms and act by binding to intracellular protein macrophilin-12, thereby decreasing the production of inflammatory cytokines interleukin (IL)-4 and IL-13; this treatment does not decrease dermal thickness[18,19].
Other treatments include wet-wraps[15,20], oral antihistamines[13] and phototherapy[21] where ultraviolet light is administered to the skin for its immunosuppressive effect. Atopic eczema sufferers frequently have to undergo two or more treatments (Figure 1), one for the disease itself and the other for co-existent bacterial or viral infection[22].
Staphylococcus aureus (S. aureus) is a bacterium that may be carried in the nose and flexural skin of some individuals and on the apparently healthy skin of atopic individuals. In individuals with atopic eczema it can cause infections within actively inflamed lesions and lead to increased skin barrier damage[22]. There is a more permissive environment for the growth of S. aureus because atopic skin shows a reduction in the expression of antimicrobial peptides[23]. Topical corticosteroids have been found to reduce the colonisation by S. aureus[24], but the most effective treatment involves combining these topical corticosteroids with an antimicrobial preparation[25]. However, this combination therapy only shows efficacy in the short-term as over a long period there is no significant benefit in comparison to corticosteroids alone. Therefore a more effective treatment is required to prevent S. aureus re-infection.
The stepwise approach to treating atopic eczema, shown in Figure 1, highlights the varying severity in this disease. It also illustrates the range of treatments in use, because of the variation in individual response to each therapy. The currently available treatments target atopic eczema in a rather non-specific way; it is hoped that novel treatments, such as monoclonal antibodies, will be able to target the specific problem(s) in each individual’s atopic eczema.
This review will focus on the skin barrier and the immune mechanisms of the skin and how irregular function of both lead to atopic eczema, as well as the novel and theoretical treatments designed for targeting each component of the disease.
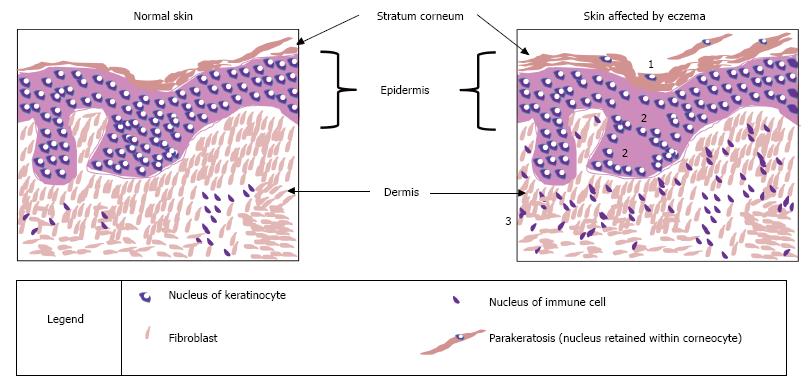
The skin is the largest organ in the body[26] and it plays a number of key roles in survival. A central function is to act as a physical barrier to prevent the entry of allergens and irritants while also vitally retaining water within the body[4]. The skin is composed of three main layers: epidermis, dermis and hypodermis[27]. The outermost layer of the epidermis is called the stratum corneum (Figure 2); this contributes to the control of trans-epidermal water loss (TEWL)[28], i.e., water lost through evaporation. The stratum corneum includes 18-20 layers constructed from dead cells containing keratin called corneocytes; this is surrounded by a matrix of lipids mainly consisting of ceramides and cholesterol[29]. The epidermis provides the physical skin barrier function through the matrix of lipids but also through corneodesmosomes and tight junctions with the stratum granulosum layer below[29].
An essential component of the barrier function of the skin is filaggrin (filament-aggregating protein), an intracellular protein[30] important for formation of the stratum corneum[31]. Filaggrin is formed from the dephosphorylation and proteolysis of profilaggrin when the keratinocytes in the stratum granulosum are undergoing differentiation to the corneocytes of the stratum corneum[32]. Filaggrin monomers bind to keratin molecules to strengthen the filament network and contribute to the changes in shape of keratinocytes as they mature into corneocytes. Filaggrin also plays a key role in control of TEWL. Filaggrin undergoes proteolysis to release hygroscopic amino acids at the surface of the stratum corneum, when the outer skin starts to become dehydrated. These amino acids contribute to natural moisturising factor (NMF) for the skin, maintaining hydration of the stratum corneum and controlling TEWL[33]. Another key role played by filaggrin and its degradation products is in the control of skin pH. The acidic pH of skin acts as an antimicrobial defensive mechanism to limit bacterial colonisation[34]. If the pH of the skin is increased, filaggrin proteolysis can contribute acidic amino acids to return it to the optimal slightly acidic pH[34].
Tight junctions hold the keratinocytes together, control the flow of fluids paracellularly and are key as they act as another barrier; the stratum corenum acts as the first physical barrier to allergens and irritants while tight junctions form the second[35]. This barrier is particularly important if the stratum corneum becomes diseased, since the tight junctions provide a second line of defence against allergens, to prevent their entry into the skin and the resultant immune response.
The difficulty in describing the causes of atopic eczema are that the mutations or genetic variants being proposed as the culprits of the skin barrier dysfunction only occur in a proportion of affected individuals. There have been a number of genetic studies aiming to highlight potential risk factors for atopic eczema; they have discovered links between certain mutations or genetic variants that are associated with increased risk for the disease[36-40]. The majority of candidate gene association studies point to null mutations in the filaggrin gene, FLG, and genes involved with the type 2 T helper lymphocytes (Th2 cell) function[41,42]. Loss of function mutations in FLG were first identified in 2006 and this remains the strongest genetic risk factor for atopic eczema identified to date[43].
The section above highlighted the importance of the protein filaggrin in a number of key roles involved with producing the skin barrier; therefore it is understandable that a loss of function mutation in FLG may increase the risk of atopic eczema. Other related diseases such as ichthyosis vulgaris, atopic asthma, allergic rhinitis and food allergies are also often associated with mutations in filaggrin[44]. Filaggrin is key in cross-linking keratins to flatten the shape of cells, in the stratum corneum; consequently null mutations will lead to malformed corneocytes and allergens may be able to enter through this leaky skin barrier and incite an inflammatory response[26]. In atopic eczema there is an increased rate of TEWL and again it is possible that filaggrin may play a role, as null mutations mean filaggrin cannot be degraded to form NMF, hence skin hydration is reduced[26]. Another often vital part of the disease is the colonisation by bacteria and this is more likely to occur in the alkaline conditions of the skin when filaggrin is absent or reduced in amount[45]. This is one of possibly several factors that allows binding of bacteria such as S. aureus, and it can contribute to the development of atopic eczema or worsen its severity[46]. The main mechanism by which S. aureus damages the skin barrier is through secretion of SspA/V8 protease. This protease acts to degrade the proteins in the corneodesmosomes in the stratum corneum but also proteins in the tight junctions of the stratum granulosum, thereby compromising both elements of the skin barrier and allowing entry of allergens and irritants[47,48].
In some cases of atopic eczema it has been shown that key proteins involved with tight junction function, claudin-1 and claudin-23, are reduced[35]. It was believed that mutations in the filaggrin gene may also affect tight junction functionality, however mouse models demonstrated that filaggrin insufficiency did not have a direct effect[49]. Other mouse models have demonstrated the importance of claudin-1 in maintaining normal levels of TEWL: When mice lack this protein, they die within a day[50]. On closer inspection, it was observed that claudin-deficient mice died of dehydration as a result of increased TEWL and this was due to non-functioning tight junctions[50]. Therefore, the reduction in claudin-1 seen in atopic eczema patients may contribute to their increased TEWL and dry skin. Tight junctions are vital for paracellular control of fluids, as well acting as a physical barrier, and what is often observed with atopic eczema is spongiosis, where oedema is occurring between the keratinocytes in the epidermis (Figure 2).
Another characteristic of atopic eczema which can be seen as scaliness (white flaky skin) when directly observing the skin, or by light microscopy of a histological section of diseased skin, is an increased thickness of the stratum corneum[51]. Normally epidermal cells undergo transformation from keratinocytes of the stratum basale, in the lower epidermis, to corneocytes of the stratum corneum, in the upper epidermis, and begin to shed off the skin; however in atopic eczema this cornification process is disrupted[52]. In atopic eczema the keratinocytes retain their nucleus and remain attached instead of shedding, contributing to the thickened stratum corneum. This feature can also be seen in Figure 2.
The balance of immune mechanisms in the skin is a closely regulated process, which involves a number of different immune and non-immune cells interacting to protect the body from pathogens[53]. However, this balance is susceptible to a number of diseases. Above, we have mostly described how skin barrier dysfunction leads to an increased immune response thereby causing atopic eczema; nevertheless the disease may also be caused by immune dysfunction leading to skin barrier damage. It has been demonstrated that IL-4 and IL-13, the two cytokines that are greatly increased in atopic eczema, are able to significantly decrease the expression of filaggrin[54]. When IL-4 and IL-13 were incubated with keratinocytes for 24 h they decrease the expression of filaggrin[54]. Hence these two interleukins can cause damage to the barrier via their effects on keratinocyte differentiation[54]. This study also highlighted that environmental allergens such as soap and detergents would cause the same damaging effects through increased levels of IL-4 and IL-13[54]. A different study demonstrated that histamine may also contribute to immune dysfunction causing a leaky skin barrier and atopic eczema[55]. This study again observed keratinocyte differentiation as a measure of barrier damage but also investigated the important barrier proteins keratin 1 and keratin 10, loricrin and filaggrin[55]. The study showed that expression of these proteins was reduced by as much as 80%-95% in the presence of histamine thereby affecting keratinocyte differentiation and the skin barrier[55]. The final part of this study demonstrated that histamine also caused down-regulation of the claudins involved in tight junction formation and therefore this may also contribute to the leaky skin barrier in atopic eczema[55].
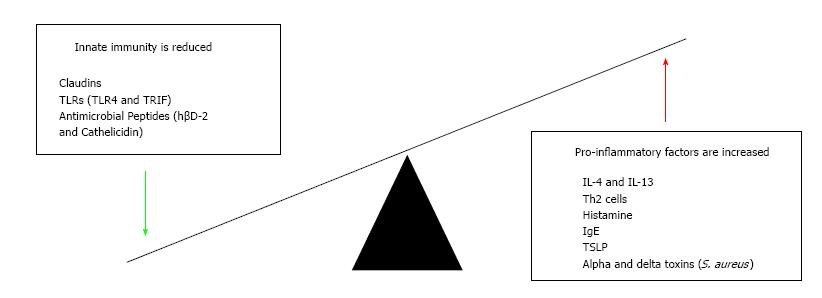
The adaptive and innate immune responses have both been highlighted as possibly playing a role in atopic eczema[56]. The candidate gene and genome-wide association studies mentioned earlier have illustrated that variation in genes involved with the adaptive response of the Th2 cells is associated with risk of atopic eczema. In a number of cases of atopic eczema there will be increased levels of the Th2 cell and its cytokines IL-4 and IL-13; these are important for instigating inflammation[56]. IL-4 is key for production of eosinophils and importantly IgE, which then acts through FcεRI receptors to cause mast cells degranulation, stimulating inflammation[57]. IL-13 has not been as extensively studied in skin, however its mechanism of inflammation appears to occur through interacting with the IL-4Rα receptor[58]. Another mechanism by which atopic eczema may occur is when someone begins to scratch, causing mechanical damage. The traumatised keratinocytes release thymic stromal lymphopoietin (TSLP), another cytokine. Studies have demonstrated that TSLP levels are increased in skin affected by atopic eczema compared to normal skin[59]. TSLP acts on dendritic cells which activate Th2 cells producing IL-4 and IL-13 resulting in a cycle of increased inflammation and atopic eczema[59,60].
The innate immune system may also play a role in causing an individual’s atopic eczema. One of the first lines of response to pathogens is by antimicrobial peptides which are secreted and activated once toll-like receptors (TLR) identify pathogens[61]. Defects in these TLRs have been implicated in potentially allowing the colonisation of bacteria and therefore instigating atopic eczema[62]. A study using a mouse model found that mice with defective TLR4 or TIR-domain-containing adapter-inducing interferon-β (TRIF) had increased levels of TEWL, resulting in atopic eczema[62]. The peptides themselves are found to be reduced in eczema-affected skin and therefore fail to prevent colonisation and damage by pathogens such as S. aureus or infection with herpes simplex resulting in eczema herpeticum[63]. Cathelicidin and human beta defensin 2 (hβD-2) have been shown to be reduced in atopic eczema, lowering the threshold for this damage to occur[64,65]. S. aureus releases alpha and delta toxins which activate the adaptive immune response resulting in increased Th2 cell activity[53,66] and driving further inflammation (Figure 3).
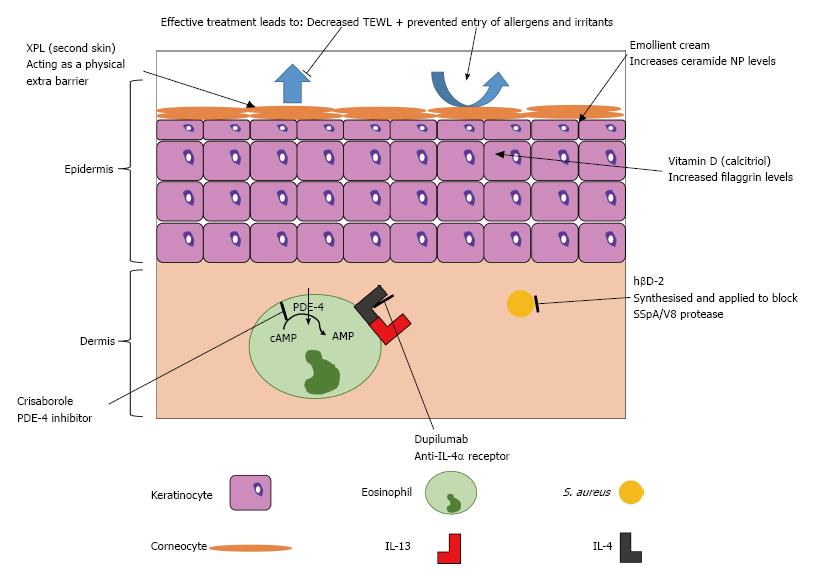
This knowledge of the molecular mechanisms in skin barrier function and immune response is creating opportunities for novel treatment approaches for atopic eczema, summarised in Figure 4 and Table 1.
| Repairing the damaged skin barrier | Reducing atopic inflammation |
| XPL (second skin) | Crisaborole |
| Emollients with increased ceramide NP levels | Dupilumab |
| Vitamin D (calcitriol) | hβD-2 |
One potential therapeutic avenue may involve using vitamin D to decrease the severity of atopic eczema; studies have shown that it is important in both barrier repair and modulation of the immune mechanism[67,68]. A study in mice demonstrated that those treated by phototherapy had greatly increased levels of filaggrin which also lead to decreased time for barrier repair to occur[69]. This was believed to be due to the action of vitamin D on keratinocytes to increase levels of calcitriol, thereby normalizing the faulty keratinocyte differentiation seen in atopic eczema, to improve barrier function[69,70]. Oral supplementation of vitamin D has not shown a therapeutic effect, so alternative methods of administration are required.
The knowledge of a central role for dry skin in atopic eczema has stimulated interest in the development of bespoke emollients as treatment for xerosis[71]. In one study a standard emollient (the control) was compared to an emollient cream containing 5% urea, a skin ceramide N-stearoyl phytosphingosine (NP) and lactate[71]. When skin that had previously been treated with the cream containing ceramide NP was changed to the control there was an increase in TEWL from 11.58 to 11.94 g/m2 per hour; this suggested that skin barrier function was improved more by using investigated cream than the control[71]. However this improvement between the creams may be due to the sodium lauryl sulfate in the control having an emulsifying effect and increasing barrier damage[71]. When hydration was also considered, the application of the ceramide NP cream showed greater hydration compared to the control, suggesting improvement of the stratum corneum barrier[71]. There is the possibility of damage if the ceramide NP cream is used with atopic eczema as it increases pH slightly and further work is needed to define the optimal emollient treatment for atopic eczema.
Another potential treatment aimed at the leaky skin barrier involves using a synthetic elastic “second skin”[72]. This skin is made of a cross-linked polymer (XPL) and has been used initially as anti-ageing solution where it can be applied to remove signs such as wrinkled skin; it has demonstrated dramatic results, especially around the eyes[72]. It has been subsequently proposed as a potential treatment for atopic eczema as the XPL will act as an extra skin barrier, for up to 24 h, preventing entry of allergens or irritants to affected areas of skin[72]. However this very interesting application remains speculative, as no research has yet been conducted.
It has recently been highlighted that increasing levels of the antimicrobial peptide hβD-2 can be used to reduce damage caused to the skin barrier by S. aureus[48]. In atopic eczema there are reduced levels of hβD-2 so its IL-1β defensive mechanism cannot prevent damage by the SSpA/V8 protease[48]. It was demonstrated that purified recombinant or synthesised hβD-2 could decrease skin barrier damage by 15% and 10% respectively[48]; this may be a useful avenue for future topical treatments.
Phosphodiesterase-4 (PDE-4) inhibitors can be taken orally to prevent cyclic-AMP degradation in cells involved in immune mechanisms of atopic eczema; however, this often leads to side effects such as nausea and diarrhoea[73]. An ointment based PDE-4 inhibitor has therefore been developed called crisaborole; it is one of the few non-steroidal based ointments developed recently[73]. It has just finished phase 3 clinical trials and has demonstrated improvements as high as 41% compared to a placebo moisturiser, in terms of atopic eczema severity scores[73]. This drug may soon be approved by the FDA for treatment of atopic eczema.
A novel treatment that presents the greatest opportunity to powerfully target atopic eczema inflammation involves using monoclonal antibodies which are currently being developed to treat several different atopic diseases[74]. Currently one of the only commercially available monoclonal antibody treatments for atopic disease is omalizumab which is licenced for the treatment of asthma[74]. It targets the IgE Cε3 domain which leads to decreased severity in asthma and has the potential to be used in atopic eczema[74].
However a more promising monoclonal antibody treatment is dupilumab, which is an anti-IL-4α receptor, so stops the action of IL-4, thereby preventing the inflammation by both IL-4 and IL-13[74]. It is currently showing promising results in phase 2 trials (up to 85% of patients showing a 50% reduction in eczema severity score)[75] and phase 3 trials (in which up to 38% of patients were clear or almost clear after 16 wk of treatment)[76]. Drawbacks of this treatment are that it involves an injection which is more invasive than the other treatments and that the long-term safety is currently unknown[74].
Atopic eczema is a complex and chronic inflammatory disease of the skin that affects a large proportion of people. The pathomechanisms include a leaky skin barrier and an immune response: Both are able to occur first thereby causing the other. The problems associated with atopic eczema extend far beyond skin disease, affecting a whole family and not just the individual or child affected, causing mental health problems as well as economic impact. Mutations in the gene encoding filaggrin (FLG) have been highlighted as an important part of the disease; filaggrin plays a number of roles in maintaining the skin barrier so this is plausible. However, not every case of atopic eczema has these null mutations in FLG and the same can be seen with the immune mechanisms of the disease. The Th2 cell response often occurs in atopic eczema and is central to causing the atopic inflammation. Bacterial infection contributes to atopic eczema pathogenesis and this is potentiated via reduced antimicrobial peptides or mutations in filaggrin leading to a reduction in the acidic pH of skin.
The multitude of causes is what has brought about the variety of treatments for atopic eczema. Different treatments are effective or ineffective in different individuals. The ideal treatment for atopic eczema would be able to target and repair the leaky skin barrier but also normalise the increased immune response of atopic skin. In milder atopic eczema, the best treatments often involve educating patients and children to avoid their own triggers and more education may improve overall treatment. Novel treatments have become more specific in targeting molecular mechanisms in atopic eczema, which, it is hoped, will make them more effective and with fewer side effects. However a considerable amount of research is still required to develop the most effective treatment to target both key mechanisms - the skin barrier dysfunction and immune response - to fully control this complex disease.
Sara J Brown holds a Wellcome Trust Senior Research Fellowship in Clinical Science (ref 106865/Z/15/Z).
Manuscript source: Unsolicited manuscript
Specialty type: Dermatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Atzori L, Cuevas-Covarrubias SA, Husein-ElAhmed H, Kaliyadan F, Palmirotta R S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Deckers IA, McLean S, Linssen S, Mommers M, van Schayck CP, Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS One. 2012;7:e39803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 432] [Cited by in RCA: 379] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 2. | Zaniboni MC, Samorano LP, Orfali RL, Aoki V. Skin barrier in atopic dermatitis: beyond filaggrin. An Bras Dermatol. 2016;91:472-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 842] [Cited by in RCA: 750] [Article Influence: 53.6] [Reference Citation Analysis (3)] |
| 4. | Leung DY. New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergol Int. 2013;62:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 219] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 5. | Cork MJ, Danby SG, Vasilopoulos Y, Hadgraft J, Lane ME, Moustafa M, Guy RH, Macgowan AL, Tazi-Ahnini R, Ward SJ. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009;129:1892-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 486] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 6. | Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, Williams H; ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2807] [Cited by in RCA: 2817] [Article Influence: 148.3] [Reference Citation Analysis (1)] |
| 7. | Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, Marks R, Naldi L, Weinstock MA, Wulf SK. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 696] [Cited by in RCA: 874] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 8. | Absolon CM, Cottrell D, Eldridge SM, Glover MT. Psychological disturbance in atopic eczema: the extent of the problem in school-aged children. Br J Dermatol. 1997;137:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Arkwright PD, Motala C, Subramanian H, Spergel J, Schneider LC, Wollenberg A; Atopic Dermatitis Working Group of the Allergic Skin Diseases Committee of the AAAAI. Management of difficult-to-treat atopic dermatitis. J Allergy Clin Immunol Pract. 2013;1:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 10. | Sach TH, McManus E, Mcmonagle C, Levell N. Economic evidence for the prevention and treatment of atopic eczema: a protocol for a systematic review. Syst Rev. 2016;5:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Herd RM, Tidman MJ, Prescott RJ, Hunter JA. The cost of atopic eczema. Br J Dermatol. 1996;135:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Rodríguez E, Baurecht H, Herberich E, Wagenpfeil S, Brown SJ, Cordell HJ, Irvine AD, Weidinger S. Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J Allergy Clin Immunol. 2009;123:1361-70.e7. [PubMed] |
| 13. | Apfelbacher CJ, van Zuuren EJ, Fedorowicz Z, Jupiter A, Matterne U, Weisshaar E. Oral H1 antihistamines as monotherapy for eczema. Cochrane Database Syst Rev. 2013;CD007770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66 Suppl 1:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 750] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 15. | Nowicki R, Trzeciak M, Wilkowska A, Sokołowska-Wojdyło M, Ługowska-Umer H, Barańska-Rybak W, Kaczmarski M, Kowalewski C, Kruszewski J, Maj J. Atopic dermatitis: current treatment guidelines. Statement of the experts of the Dermatological Section, Polish Society of Allergology, and the Allergology Section, Polish Society of Dermatology. Postepy Dermatol Alergol. 2015;32:239-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Lebwohl M, Friedlander SF. New strategies for optimizing the treatment of inflammatory dermatoses with topical corticosteroids in an era of corticosteroid-sparing regimens. J Am Acad Dermatol. 2005;53:S1-S2. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Aschoff R, Schmitt J, Knuschke P, Koch E, Bräutigam M, Meurer M. Evaluation of the atrophogenic potential of hydrocortisone 1% cream and pimecrolimus 1% cream in uninvolved forehead skin of patients with atopic dermatitis using optical coherence tomography. Exp Dermatol. 2011;20:832-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Gutfreund K, Bienias W, Szewczyk A, Kaszuba A. Topical calcineurin inhibitors in dermatology. Part I: Properties, method and effectiveness of drug use. Postepy Dermatol Alergol. 2013;30:165-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Martins JC, Martins C, Aoki V, Leonardi-Bee J, Gois AFT, Ishii HA, da Silva EMK. Topical tacrolimus for atopic dermatitis: a systematic review. Brit J Dermatol. 2014;170:E43-E44. |
| 20. | Devillers AC, Oranje AP. Wet-wrap treatment in children with atopic dermatitis: a practical guideline. Pediatr Dermatol. 2012;29:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Darné S, Leech SN, Taylor AE. Narrowband ultraviolet B phototherapy in children with moderate-to-severe eczema: a comparative cohort study. Br J Dermatol. 2014;170:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Ong PY, Leung DY. Bacterial and Viral Infections in Atopic Dermatitis: a Comprehensive Review. Clin Rev Allergy Immunol. 2016;51:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 23. | Park HY, Kim CR, Huh IS, Jung MY, Seo EY, Park JH, Lee DY, Yang JM. Staphylococcus aureus Colonization in Acute and Chronic Skin Lesions of Patients with Atopic Dermatitis. Ann Dermatol. 2013;25:410-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Gong JQ, Lin L, Lin T, Hao F, Zeng FQ, Bi ZG, Yi D, Zhao B. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double-blind multicentre randomized controlled trial. Br J Dermatol. 2006;155:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 25. | Friedman BC, Goldman RD. Anti-staphylococcal treatment in dermatitis. Can Fam Physician. 2011;57:669-671. [PubMed] |
| 26. | Matsui T, Amagai M. Dissecting the formation, structure and barrier function of the stratum corneum. Int Immunol. 2015;27:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 235] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 27. | Chen AI, Balter ML, Chen MI, Gross D, Alam SK, Maguire TJ, Yarmush ML. Multilayered tissue mimicking skin and vessel phantoms with tunable mechanical, optical, and acoustic properties. Med Phys. 2016;43:3117-3131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 28. | Engesland A, Škalko-Basnet N, Flaten GE. In vitro models to estimate drug penetration through the compromised stratum corneum barrier. Drug Dev Ind Pharm. 2016;42:1742-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Menon GK, Cleary GW, Lane ME. The structure and function of the stratum corneum. Int J Pharm. 2012;435:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 258] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 30. | Gruber R, Elias PM, Crumrine D, Lin TK, Brandner JM, Hachem JP, Presland RB, Fleckman P, Janecke AR, Sandilands A. Filaggrin genotype in ichthyosis vulgaris predicts abnormalities in epidermal structure and function. Am J Pathol. 2011;178:2252-2263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 31. | Kezic S, Kemperman PM, Koster ES, de Jongh CM, Thio HB, Campbell LE, Irvine AD, McLean WH, Puppels GJ, Caspers PJ. Loss-of-function mutations in the filaggrin gene lead to reduced level of natural moisturizing factor in the stratum corneum. J Invest Dermatol. 2008;128:2117-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 220] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 32. | Cabanillas B, Novak N. Atopic dermatitis and filaggrin. Curr Opin Immunol. 2016;42:1-8. [PubMed] [DOI] [Full Text] |
| 33. | Robinson M, Visscher M, Laruffa A, Wickett R. Natural moisturizing factors (NMF) in the stratum corneum (SC). I. Effects of lipid extraction and soaking. J Cosmet Sci. 2010;61:13-22. [PubMed] |
| 34. | Bandier J, Johansen JD, Petersen LJ, Carlsen BC. Skin pH, atopic dermatitis, and filaggrin mutations. Dermatitis. 2014;25:127-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, Berger AE, Zhang K, Vidyasagar S, Yoshida T. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127:773-786.e1-e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 575] [Cited by in RCA: 513] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 36. | Barnes KC. An update on the genetics of atopic dermatitis: scratching the surface in 2009. J Allergy Clin Immunol. 2010;125:16-29.e1-e11; quiz 30-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 37. | Esparza-Gordillo J, Weidinger S, Fölster-Holst R, Bauerfeind A, Ruschendorf F, Patone G, Rohde K, Marenholz I, Schulz F, Kerscher T. A common variant on chromosome 11q13 is associated with atopic dermatitis. Nat Genet. 2009;41:596-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 224] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 38. | Sun LD, Xiao FL, Li Y, Zhou WM, Tang HY, Tang XF, Zhang H, Schaarschmidt H, Zuo XB, Foelster-Holst R. Genome-wide association study identifies two new susceptibility loci for atopic dermatitis in the Chinese Han population. Nat Genet. 2011;43:690-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 39. | Paternoster L, Standl M, Chen CM, Ramasamy A, Bønnelykke K, Duijts L, Ferreira MA, Alves AC, Thyssen JP, Albrecht E, Baurecht H, Feenstra B, Sleiman PM, Hysi P, Warrington NM, Curjuric I, Myhre R, Curtin JA, Groen-Blokhuis MM, Kerkhof M, Sääf A, Franke A, Ellinghaus D, Fölster-Holst R, Dermitzakis E, Montgomery SB, Prokisch H, Heim K, Hartikainen AL, Pouta A, Pekkanen J, Blakemore AI, Buxton JL, Kaakinen M, Duffy DL, Madden PA, Heath AC, Montgomery GW, Thompson PJ, Matheson MC, Le Souëf P; Australian Asthma Genetics Consortium (AAGC), St Pourcain B, Smith GD, Henderson J, Kemp JP, Timpson NJ, Deloukas P, Ring SM, Wichmann HE, Müller-Nurasyid M, Novak N, Klopp N, Rodríguez E, McArdle W, Linneberg A, Menné T, Nohr EA, Hofman A, Uitterlinden AG, van Duijn CM, Rivadeneira F, de Jongste JC, van der Valk RJ, Wjst M, Jogi R, Geller F, Boyd HA, Murray JC, Kim C, Mentch F, March M, Mangino M, Spector TD, Bataille V, Pennell CE, Holt PG, Sly P, Tiesler CM, Thiering E, Illig T, Imboden M, Nystad W, Simpson A, Hottenga JJ, Postma D, Koppelman GH, Smit HA, Söderhäll C, Chawes B, Kreiner-Møller E, Bisgaard H, Melén E, Boomsma DI, Custovic A, Jacobsson B, Probst-Hensch NM, Palmer LJ, Glass D, Hakonarson H, Melbye M, Jarvis DL, Jaddoe VW, Gieger C; Genetics of Overweight Young Adults (GOYA) Consortium, Strachan DP, Martin NG, Jarvelin MR, Heinrich J, Evans DM, Weidinger S; EArly Genetics & Lifecourse Epidemiology (EAGLE) Consortium. Meta-analysis of genome-wide association studies identifies three new risk loci for atopic dermatitis. Nat Genet. 2011;44:187-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 261] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 40. | Cookson WO, Ubhi B, Lawrence R, Abecasis GR, Walley AJ, Cox HE, Coleman R, Leaves NI, Trembath RC, Moffatt MF. Genetic linkage of childhood atopic dermatitis to psoriasis susceptibility loci. Nat Genet. 2001;27:372-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 246] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 41. | Brown SJ, Relton CL, Liao H, Zhao Y, Sandilands A, Wilson IJ, Burn J, Reynolds NJ, McLean WH, Cordell HJ. Filaggrin null mutations and childhood atopic eczema: a population-based case-control study. J Allergy Clin Immunol. 2008;121:940-46.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 42. | Bin L, Leung DY. Genetic and epigenetic studies of atopic dermatitis. Allergy Asthma Clin Immunol. 2016;12:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 43. | Smith FJ, Irvine AD, Terron-Kwiatkowski A, Sandilands A, Campbell LE, Zhao Y, Liao H, Evans AT, Goudie DR, Lewis-Jones S. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006;38:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 687] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 44. | Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 814] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 45. | Lambers H, Piessens S, Bloem A, Pronk H, Finkel P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int J Cosmet Sci. 2006;28:359-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 490] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 46. | Lin YT, Wang CT, Chiang BL. Role of bacterial pathogens in atopic dermatitis. Clin Rev Allergy Immunol. 2007;33:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Tamber S, Cheung AL. SarZ promotes the expression of virulence factors and represses biofilm formation by modulating SarA and agr in Staphylococcus aureus. Infect Immun. 2009;77:419-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Wang B, McHugh BJ, Qureshi A, Campopiano DJ, Clarke DJ, Fitzgerald JR, Dorin JR, Weller R, Davidson DJ. IL-1β-Induced Protection of Keratinocytes against Staphylococcus aureus-Secreted Proteases Is Mediated by Human β-Defensin 2. J Invest Dermatol. 2017;137:95-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | Yokouchi M, Kubo A, Kawasaki H, Yoshida K, Ishii K, Furuse M, Amagai M. Epidermal tight junction barrier function is altered by skin inflammation, but not by filaggrin-deficient stratum corneum. J Dermatol Sci. 2015;77:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 50. | Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099-1111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1125] [Cited by in RCA: 1178] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 51. | Alsaad KO, Ghazarian D. My approach to superficial inflammatory dermatoses. J Clin Pathol. 2005;58:1233-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Yang M, Chang JM. Successful treatment of refractory chronic hand eczema with calcipotriol/betamethasone ointment: A report of three cases. Exp Ther Med. 2015;10:1943-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol. 2014;14:289-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 593] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 54. | Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, DeBenedetto A, Schneider L, Beck LA, Barnes KC, Leung DY. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2009;124:R7-R12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 355] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 55. | Gschwandtner M, Mildner M, Mlitz V, Gruber F, Eckhart L, Werfel T, Gutzmer R, Elias PM, Tschachler E. Histamine suppresses epidermal keratinocyte differentiation and impairs skin barrier function in a human skin model. Allergy. 2013;68:37-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 56. | Brandt EB, Sivaprasad U. Th2 Cytokines and Atopic Dermatitis. J Clin Cell Immunol. 2011;2:110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 446] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 57. | Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125:S73-S80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 977] [Cited by in RCA: 871] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 58. | Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111:677-690; quiz 691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 439] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 59. | Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1418] [Cited by in RCA: 1550] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 60. | Corrigan CJ, Jayaratnam A, Wang Y, Liu Y, de Waal Malefyt R, Meng Q, Kay AB, Phipps S, Lee TH, Ying S. Early production of thymic stromal lymphopoietin precedes infiltration of dendritic cells expressing its receptor in allergen-induced late phase cutaneous responses in atopic subjects. Allergy. 2009;64:1014-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 61. | Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2009;124:R13-R18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 62. | Brandt EB, Gibson AM, Bass S, Rydyznski C, Khurana Hershey GK. Exacerbation of allergen-induced eczema in TLR4- and TRIF-deficient mice. J Immunol. 2013;191:3519-3525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 63. | Howell MD, Wollenberg A, Gallo RL, Flaig M, Streib JE, Wong C, Pavicic T, Boguniewicz M, Leung DY. Cathelicidin deficiency predisposes to eczema herpeticum. J Allergy Clin Immunol. 2006;117:836-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 64. | Mallbris L, Carlén L, Wei T, Heilborn J, Nilsson MF, Granath F, Ståhle M. Injury downregulates the expression of the human cathelicidin protein hCAP18/LL-37 in atopic dermatitis. Exp Dermatol. 2010;19:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 65. | Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1464] [Cited by in RCA: 1397] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 66. | Breuer K, Wittmann M, Kempe K, Kapp A, Mai U, Dittrich-Breiholz O, Kracht M, Mrabet-Dahbi S, Werfel T. Alpha-toxin is produced by skin colonizing Staphylococcus aureus and induces a T helper type 1 response in atopic dermatitis. Clin Exp Allergy. 2005;35:1088-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 67. | Russell M. Assessing the relationship between vitamin D3 and stratum corneum hydration for the treatment of xerotic skin. Nutrients. 2012;4:1213-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Mutgi K, Koo J. Update on the role of systemic vitamin D in atopic dermatitis. Pediatr Dermatol. 2013;30:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 69. | Hong SP, Kim MJ, Jung MY, Jeon H, Goo J, Ahn SK, Lee SH, Elias PM, Choi EH. Biopositive effects of low-dose UVB on epidermis: coordinate upregulation of antimicrobial peptides and permeability barrier reinforcement. J Invest Dermatol. 2008;128:2880-2887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 70. | Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Dardenne O, Xie Z, Arnaud RS, Feingold K, Elias PM. Mice lacking 25OHD 1alpha-hydroxylase demonstrate decreased epidermal differentiation and barrier function. J Steroid Biochem Mol Biol. 2004;89-90:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 71. | Danby SG, Brown K, Higgs-Bayliss T, Chittock J, Albenali L, Cork MJ. The Effect of an Emollient Containing Urea, Ceramide NP, and Lactate on Skin Barrier Structure and Function in Older People with Dry Skin. Skin Pharmacol Physiol. 2016;29:135-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 72. | Yu B, Kang SY, Akthakul A, Ramadurai N, Pilkenton M, Patel A, Nashat A, Anderson DG, Sakamoto FH, Gilchrest BA. An elastic second skin. Nat Mater. 2016;15:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 73. | Jarnagin K, Chanda S, Coronado D, Ciaravino V, Zane LT, Guttman-Yassky E, Lebwohl MG. Crisaborole Topical Ointment, 2%: A Nonsteroidal, Topical, Anti-Inflammatory Phosphodiesterase 4 Inhibitor in Clinical Development for the Treatment of Atopic Dermatitis. J Drugs Dermatol. 2016;15:390-396. [PubMed] |
| 74. | Landolina N, Levi-Schaffer F. Monoclonal antibodies: the new magic bullets for allergy: IUPHAR Review 17. Br J Pharmacol. 2016;173:793-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 75. | Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, Rocklin R, Ming JE, Ren H, Kao R, Simpson E. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 1028] [Article Influence: 93.5] [Reference Citation Analysis (0)] |
| 76. | Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, Silverberg JI, Deleuran M, Kataoka Y, Lacour JP. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med. 2016;375:2335-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1407] [Article Influence: 156.3] [Reference Citation Analysis (0)] |