Published online Jul 27, 2015. doi: 10.5313/wja.v4.i2.39
Peer-review started: February 2, 2015
First decision: March 6, 2015
Revised: April 24, 2015
Accepted: May 7, 2015
Article in press: May 8, 2015
Published online: July 27, 2015
Processing time: 174 Days and 17 Hours
Airway complications after lung transplantation remain a significant cause of morbidity and mortality. Many of these occur at the anastomotic sites, which are susceptible due to poor collateral circulation. Of the possible complications, bronchial dehiscence is particularly formidable. These cases have been successfully treated bronchoscopically with metallic stents, which likely promote healing through granulation tissue formation. However, limited options exist in cases where the dehiscence fails to heal following stent placement. Here, we present the case report of a 65-year-old male who developed bronchial dehiscence status post bilateral lung transplantation for idiopathic pulmonary fibrosis that failed to heal with simple stent placement. Eventually, the patient underwent amniotic membrane grafting with stenting as a novel therapy for non-healing bronchial dehiscence, for which we describe the anesthetic management. His anesthetic plan included inhalational induction with sevoflurane, propofol infusion for total intravenous anesthesia, rocuronium for muscle relaxation, and closed-circuit assisted ventilation. His existing tracheostomy was used as the airway for oxygenation and induction. In summary, our anesthetic plan for the lung transplant patient was effective; future amniotic membrane grafting for bronchial dehiscence through bronchoscopy may follow a similar technique. Ultimately, the choice of anesthesia in this patient population requires judicious consideration of the requirements of the procedure as well as the pathophysiology of the transplanted lung.
Core tip: Bronchial dehiscence is a significant airway complication following lung transplantation and most commonly occurs at anastomotic sites due to poor collateral perfusion. This complication is often difficult to treat, especially when widespread. Severe disease has been treated with the temporary placement of metallic stents within the airway to promote healing, but limited options exist when stenting fails. This case report presents the anesthetic considerations for a lung transplant patient undergoing bronchoscopic placement of an amniotic membrane graft as a novel solution for non-healing bronchial dehiscence after multiple failed attempts with metallic stent placement.
- Citation: Feng TR, Gildea TR, Doyle DJ. Anesthesia for bronchoscopic amniotic membrane grafting to treat non-healing bronchial dehiscence. World J Anesthesiol 2015; 4(2): 39-43
- URL: https://www.wjgnet.com/2218-6182/full/v4/i2/39.htm
- DOI: https://dx.doi.org/10.5313/wja.v4.i2.39
Since the first human lung transplantation, improvements in patient selection, surgical technique, and immunosuppression have led to increased overall survival[1-3]. Nevertheless, airway complications remain an important cause of morbidity and mortality[4,5]. The incidence of complications at most centers ranges from 7%-18% with a 2%-4% mortality rate[1]. These complications arise partly because bronchial arterial circulation is not reestablished during transplantation and requires approximately 2 wk for rearterialization[4-6]. Thus, initial bronchial perfusion depends on retrograde collaterals from the pulmonary artery, making the anastomotic sites particularly susceptible to ischemia[1,4,5,7].
Of the anastomotic complications, dehiscence is particularly difficult to treat, especially when widespread and clinically significant[2]. Partial dehiscence is often managed conservatively with surveillance and aggressive antibiotic therapy. More severe cases have been treated with temporary placement of metallic stents, which promote healing through excessive granulation tissue formation[1]. Other methods have also been used, including endoscopic application of cyanoacrylate glue[8] and surgical repair with homograft aorta[9].
Here, we present the anesthetic management of a lung transplant recipient with non-healing bronchial dehiscence treated with the novel application of amniotic tissue grafting via bronchoscopy. As interventional bronchoscopic procedures have become more sophisticated and capable of treating more severe disease, anesthesia for bronchoscopy has evolved alongside them. In providing anesthesia for such patients, thorough preoperative evaluation with ample consideration of transplanted lung physiology and requirements within the bronchoscopy suite setting is imperative.
A 65-year-old male underwent bilateral lung transplantation for end-stage lung disease secondary to idiopathic pulmonary fibrosis. Though he tolerated the procedure well, his immediate post-operative course was complicated by cardiac insufficiency, pulmonary hypertension, acute kidney injury, hypotension, and coagulopathy. He soon underwent percutaneous tracheotomy due to debilitation and extended ventilation requirement.
Three weeks later, he developed acute hypoxic decompensation with sepsis, pneumomediastinum and pneumothorax. Bronchoscopy revealed partial dehiscence of the right anastomosis with a large fistula into the mediastinum. A non-covered metallic stent was placed in the right main stem bronchus and was subsequently replaced and repositioned several times. However, the dehiscence continued to worsen and extend. Pseudomonas aeruginosa was also isolated from the bronchial wash and treated with antibiotics. The decision was made to place an amniotic membrane graft via bronchoscopy.
Pre-anesthetic assessment demonstrated a patient status post tracheostomy and ASA class IV, weighing 78 kg. His blood pressure was 119/57 mmHg, pulse was 90 beats/min, temperature was 35.5 °C, and arterial oxygen saturation was 99% on 4 L via tracheostomy collar. He had bilateral chest tubes in place and bilateral rhonchi on auscultation. His hemoglobin and hematocrit were 8.7 g/dL and 26.3%, respectively. Serum electrolytes were within normal limits. Chest X-ray showed no pneumothoraces.
His tracheostomy was used as the airway for oxygenation and for pure sevoflurane inhalational induction at 5 L/min over 2-3 min. We then switched to total intravenous anesthesia (TIVA) using a propofol infusion, starting at 125 mcg/kg per minute and rate adjusted to a Bispectral Index of less than 50 throughout the case. Following induction, the patient’s blood pressure dropped to 82/51 mmHg, which was treated with propofol titration to 100 mcg/kg per minute and 200 mcg of phenylephrine. A 50 mg dose of rocuronium was given for paralysis.
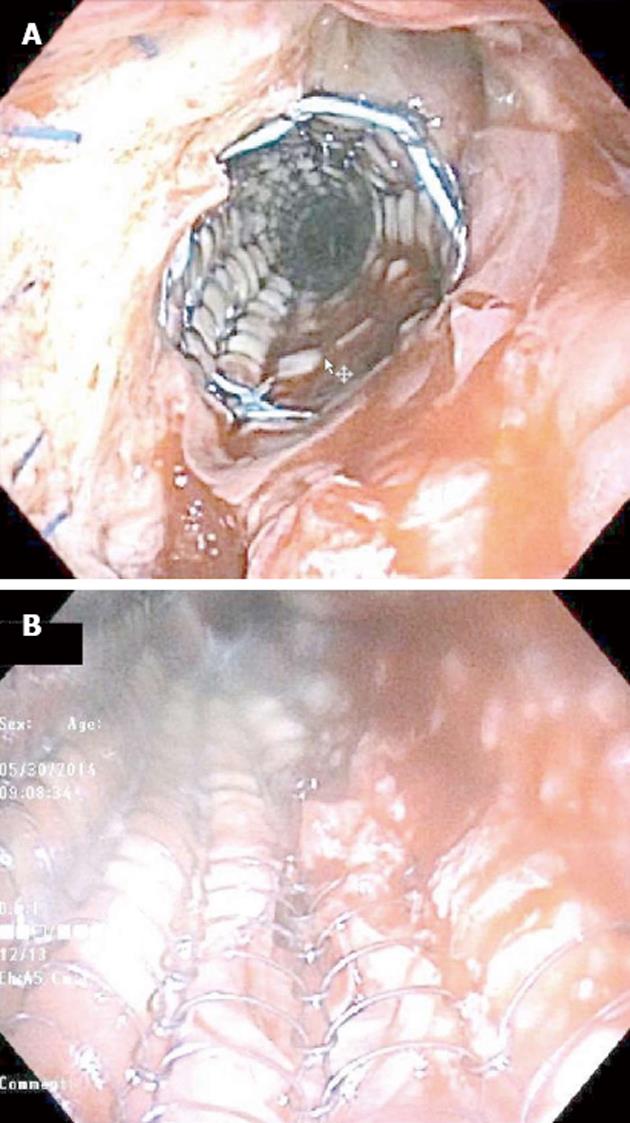
After the patient was anesthetized and stabilized, rigid bronchoscopy was performed. A closed circuit was attempted due to the large fistula into the mediastinum; wet gauze was packed into the mouth to facilitate ventilation and limit circuit leaks. The stent was peeled out of granulation tissue with forceps and then removed with flexible instruments. A 2 cm by 4 cm EpiFix amniotic tissue graft was draped over a balloon and deployed over the fistula and the defect in the posterior wall and the mediastinal fistula of the right main bronchus as planned. An uncovered Ultraflex stent was passed through the rigid bronchoscope into the right main stem bronchus and was deployed over the amniotic tissue. However, the amniotic tissue was dislodged and required repositioning; its final location was under the stent and partially covering the fistula, which was the best positioning possible (Figure 1).
Throughout the case, a high oxygen flow rate of 15 L/min was maintained due to expected circuit leaks and suctioning from bronchoscopy. An assisted ventilation setting was used with a respiratory rate of 12-15 respirations/min. Two more 10 mg doses of rocuronium were given during the case. Due to a down-trending blood pressure, the patient was started on a phenylephrine infusion at 30 mcg/min; he was later titrated to 40 mcg/min.
Prior to emergence, the propofol infusion rate was decreased to 75 mcg/kg per minute and 5 mg of neostigmine with 0.6 mg of glycopyrrolate were given for reversal of rocuronium. Ondansetron 4 mg was given for nausea prophylaxis. Once the patient was confirmed to be breathing spontaneously at 14 respirations/min, he was switched to blow-by oxygen through mask on 6 L/min. He was transported to the post-anesthesia care unit (PACU) without any post-operative complications. His blood pressure in the PACU was 103/42 mmHg with a pulse of 70 beats/min, temperature of 35.1 °C, and oxygen saturation of 100% on room air.
Weeks later, the patient passed away due to complications related to liver failure and sepsis; bronchoscopy cultures prior to death were negative.
Management of airway complications varies depending on clinical symptoms and severity, and can range from medical management to interventional bronchoscopy to open surgical repair[1]. Among the various methods to treat airway complications, stent placement is becoming increasing popular and is effective for several types of complications[1,7]. The different types of stents and their associated advantages and disadvantages have been described at length in the literature[1,6,7,10-13]. In bronchial dehiscence, bronchoscopically deployed uncovered metallic stents have been useful in treatment, as they promote excessive granulation tissue formation that provides a platform for healing[6]. They also have the added benefit of preventing stenosis through a constant outward radial force, which is important at the anastomosis due to its tendency to become stenotic upon healing[2,6].
Amniotic membrane grafts are processed from human placenta and comprise the innermost layer of the amniotic cavity. Due to its anti-inflammatory, stem cell proliferating, and epithelialization-promoting effects, these grafts are particularly useful in healing and have been used in ophthalmology and for reduction of post-laminectomy epidural adhesions[14-17]. Furthermore, Kheirkhah et al[18] demonstrated its antibacterial effects in treating acute Pseudomonas keratitis. Though its mechanism of action is poorly defined, its structure and properties likely lend to epithelia cell migration and attachment[15]. Thus, in this case report, amniotic tissue was bronchoscopically placed as a potential substrate to treat the bronchial dehiscence and Pseudomonas infection.
As bronchoscopic procedures gradually became more sophisticated, anesthesia for bronchoscopy evolved alongside them. The increasing complexity and duration of bronchoscopic cases inevitably require deep sedation or general anesthesia[19,20]. Rigid bronchoscopy is most often performed under general anesthesia with neuromuscular blockade[19]. TIVA is preferred with a continuous variable rate infusion of propofol, as this is thought to minimize undesirable cardiorespiratory effects compared to bolus doses[19,21]. The benefits of propofol are its rapid onset (< 2 min) and rapid offset (< 15 min)[19,22,23]. It has also been shown to have the lowest complication rate and improved patient neuropsychometric recovery[22], as well as improved tolerance, total amnesia, and decreased cough[19,21]. Drug choice for neuromuscular blockade depends on the required duration of action; typically rocuronium or vecuronium is used[19,23].
The choice of inhalational induction vs intravenous induction depends on both clinical circumstance and patient preference. Our patient’s existing tracheostomy lent itself well to inhalational induction with sevoflurane since this merely involved connecting the tracheostomy tube to the patient breathing circuit. The switch to TIVA is typically made once the rigid bronchoscope is placed to prevent room contamination, as the anesthetic circuit is not closed[19,24]. Gauze packing in the nose and mouth can help with circuit leakage[19]. A study by Thwaites et al[25] comparing sevoflurane to propofol use on induction demonstrated a significantly slower onset with sevoflurane, but a lower incidence of apnea and shorter time to establish spontaneous ventilation[25]. Other advantages of sevoflurane included smoother transition to maintenance, less associated hypotension, and earlier emergence[25]. However, patient preference appeared to lean towards propofol[25].
Several ventilation strategies can be employed during bronchoscopic procedures, including spontaneous ventilation, high-frequency jet ventilation, and closed-circuit positive pressure ventilation[19]. Spontaneous ventilation is typically ideal[19,24]; thus, muscle relaxants are only recommended for coughing, movement, or dangerous airway manipulation[24]. Unfortunately, spontaneous ventilation is often not feasible due to the deeper sedation required for cough and sympathetic drive suppression during bronchoscopy[19]. Jet ventilation is a poor choice due to the added risk of further dissecting into the mediastinum through the fistula. The high airflow would also make amniotic tissue graft positioning difficult due to the graft’s paper-like consistency and propensity to become displaced. Furthermore, an attempt to maintain a closed circuit is recommended in patients with airway fistulas due to the possibility of further exacerbation of existing pneumomediastinum and pneumothorax.
In lung transplant recipients, thorough consideration of transplanted lung physiology is also prudent. In the immediate post-operative month, total lung capacity and FEV1 tend to decrease; significant improvement in respiratory function and gas exchange of the transplanted lungs is only gradually seen with time[26]. Furthermore, transplanted lungs are highly susceptible to pulmonary edema from fluid overload as lymphatic drainage is interrupted during harvesting[26]. In single lung transplants, ventilation and perfusion in the native and transplanted lungs may also be unequally distributed due to a difference in compliance[26]. In single lung transplants for emphysema, ventilator flow is mostly directed toward the more compliant native lung, whereas the opposite is true for single lung recipients with pulmonary fibrosis[26]. Bilateral transplantation does not appear to require ventilator precautions other than avoiding barotrauma due to an overall decrease in compliance[26]. Barotrauma and aggressive tracheobronchial stimulation can be avoided with gentle intubation and moderate-to-deep anesthesia[26]. Fiberoptic bronchoscopic intubation may also be helpful in avoiding complications at the site of bronchial anastomosis[26].
In conclusion, our anesthetic plan for the lung transplant patient was effective for the procedure; future amniotic membrane grafting for bronchial dehiscence through bronchoscopy may follow a similar technique. Ultimately, the choice of anesthesia in this patient population requires judicious consideration of the requirements of the procedure as well as the physiology of the transplanted lung.
A 65-year-old male with a history of bilateral lung transplantation complicated by bronchial dehiscence that failed treatment with metallic stent placement, who underwent bronchoscopic amniotic membrane grafting as a novel therapy for non-healing bronchial dehiscence.
He initially presented with acute hypoxic decompensation with sepsis, pneumomediastinum and pneumothorax, and bronchial dehiscence of the right anastomosis with a large fistula into the mediastinum was confirmed via bronchoscopy. Prior to his procedure, he was hemodynamically stable and presented with a tracheostomy, bilateral chest tubes, and bilateral rhonchi on physical exam.
Hemoglobin 8.7 g/dL; hematocrit 26.3%; serum electrolytes within normal limits.
Chest X-ray showed no pneumothoraces pre-procedurally.
The patient underwent bronchoscopic placement of an amniotic membrane graft underlying a stent as a potential substrate to treat the non-healing bronchial dehiscence and superimposed Pseudomonas infection.
The reported cases of amniotic membrane grafting as a treatment have been mainly in the domain of ophthalmology and for the reduction of post-laminectomy epidural adhesions; use of amniotic membrane grafting for the treatment of bronchial dehiscence has not been reported in the literature and the anesthetics for this procedure has not been discussed.
This case report outlines an anesthetic plan that was successful for the grafting procedure and can be used as a guideline in the future when bronchoscopically treating non-healing bronchial dehiscence with amniotic membranes. In this patient population, it is particularly important to carefully consider the comorbidities of the patient, the requirements of the procedure, and the physiology of the transplanted lung.
This is an interesting case report on the anesthetic management of bronchoscopic amniotic membrane grafting. The procedure is new for this kind of application and thus the paper is original and has merit. The report will be a useful reading for all anesthesiologist involved in lung surgery. The manuscript is well written and easily readable.
P- Reviewer: Luchetti M, Tanabe S S- Editor: Ji FF L- Editor: A E- Editor: Yan JL
| 1. | Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc. 2009;6:79-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 186] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | Ferraroli GM, Ravini M, Torre M, Valvassori L, Belloni PA. Successful treatment of bronchial dehiscence with endobronchial stent in lung transplantation. Diagn Ther Endosc. 2000;6:183-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Chhajed PN, Malouf MA, Tamm M, Spratt P, Glanville AR. Interventional bronchoscopy for the management of airway complications following lung transplantation. Chest. 2001;120:1894-1899. [PubMed] |
| 4. | Fernández-Bussy S, Majid A, Caviedes I, Akindipe O, Baz M, Jantz M. Treatment of airway complications following lung transplantation. Arch Bronconeumol. 2011;47:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Kshettry VR, Kroshus TJ, Hertz MI, Hunter DW, Shumway SJ, Bolman RM. Early and late airway complications after lung transplantation: incidence and management. Ann Thorac Surg. 1997;63:1576-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 138] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Mughal MM, Gildea TR, Murthy S, Pettersson G, DeCamp M, Mehta AC. Short-term deployment of self-expanding metallic stents facilitates healing of bronchial dehiscence. Am J Respir Crit Care Med. 2005;172:768-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Herrera JM, McNeil KD, Higgins RS, Coulden RA, Flower CD, Nashef SA, Wallwork J. Airway complications after lung transplantation: treatment and long-term outcome. Ann Thorac Surg. 2001;71:989-993; discussion 993-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Maloney JD, Weigel TL, Love RB. Endoscopic repair of bronchial dehiscence after lung transplantation. Ann Thorac Surg. 2001;72:2109-2111. [PubMed] |
| 9. | McGiffin D, Wille K, Young K, Leon K. Salvaging the dehisced lung transplant bronchial anastomosis with homograft aorta. Interact Cardiovasc Thorac Surg. 2011;13:666-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Kapoor BS, May B, Panu N, Kowalik K, Hunter DW. Endobronchial stent placement for the management of airway complications after lung transplantation. J Vasc Interv Radiol. 2007;18:629-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Gildea TR, Murthy SC, Sahoo D, Mason DP, Mehta AC. Performance of a self-expanding silicone stent in palliation of benign airway conditions. Chest. 2006;130:1419-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Saad CP, Ghamande SA, Minai OA, Murthy S, Pettersson G, DeCamp M, Mehta AC. The role of self-expandable metallic stents for the treatment of airway complications after lung transplantation. Transplantation. 2003;75:1532-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Chhajed PN, Malouf MA, Tamm M, Glanville AR. Ultraflex stents for the management of airway complications in lung transplant recipients. Respirology. 2003;8:59-64. [PubMed] |
| 14. | Tao H, Fan H. Implantation of amniotic membrane to reduce postlaminectomy epidural adhesions. Eur Spine J. 2009;18:1202-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Rahman I, Said DG, Maharajan VS, Dua HS. Amniotic membrane in ophthalmology: indications and limitations. Eye (Lond). 2009;23:1954-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Arora R, Mehta D, Jain V. Amniotic membrane transplantation in acute chemical burns. Eye (Lond). 2005;19:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Adly OA, Moghazy AM, Abbas AH, Ellabban AM, Ali OS, Mohamed BA. Assessment of amniotic and polyurethane membrane dressings in the treatment of burns. Burns. 2010;36:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Kheirkhah A, Tabatabaei A, Zavareh MK, Khodabandeh A, Mohammadpour M, Raju VK. A controlled study of amniotic membrane transplantation for acute Pseudomonas keratitis. Can J Ophthalmol. 2012;47:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | José RJ, Shaefi S, Navani N. Anesthesia for bronchoscopy. Curr Opin Anaesthesiol. 2014;27:453-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Abdelmalak BB, Gildea TR, Doyle DJ. Anesthesia for bronchoscopy. Curr Pharm Des. 2012;18:6314-6324. [PubMed] |
| 21. | José RJ, Shaefi S, Navani N. Sedation for flexible bronchoscopy: current and emerging evidence. Eur Respir Rev. 2013;22:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Morris JM, Kwon PH, Zanders BT. Monitoring, Sedation, and Anesthesia for Flexible Fiberoptic Bronchoscopy. In: Haranath SP, ed. Global Perspectives on Bronchoscopy. InTech, 2012. [Accessed 2014; Sept 5] Available from: http://www.intechopen.com/books/global-perspectives-on-bronchoscopy/monitoring-sedation-and-anesthesia-for-flexible-bronchoscopy.. |
| 23. | Gillbe C, Hillier J. Anaesthesia for bronchoscopy, tracheal and airway surgery. Anaesth Intensive Care Med. 2005;6:422-425. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Barato EE, Bernal A, Carvajal FB, Giraldo C, Echeverri F, Martínez DA, Peralta CA, Salazar DF, Salcedo EE, Sandoval ME. Anesthesia Considerations for Interventional Pulmonology Procedures. Rev Colomb Anestesiol. 2011;39:316-328. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Thwaites A, Edmends S, Smith I. Inhalation induction with sevoflurane: a double-blind comparison with propofol. Br J Anaesth. 1997;78:356-361. [PubMed] |
| 26. | Feltracco P, Falasco G, Barbieri S, Milevoj M, Serra E, Ori C. Anesthetic considerations for nontransplant procedures in lung transplant patients. J Clin Anesth. 2011;23:508-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |