Published online Jul 28, 2023. doi: 10.5313/wja.v12.i1.1
Peer-review started: April 29, 2023
First decision: June 1, 2023
Revised: June 14, 2023
Accepted: July 3, 2023
Article in press: July 3, 2023
Published online: July 28, 2023
Processing time: 88 Days and 11.7 Hours
Malignant hyperthermia (MH) is a hypermetabolic disorder of skeletal muscles triggered by exposure to volatile anesthetics and depolarizing muscular relaxants. It manifests with clinical presentations such as tachycardia, muscle rigidity, hyperpyrexia, and rhabdomyolysis in genetically predisposed individuals with ryanodine receptor or calcium voltage-gated channel subunit alpha1 S mutations. Local anesthetics, such as lidocaine, are generally considered safe; however, complications can arise, albeit rarely. Lidocaine administration has been reported to induce hypermetabolic reactions resembling MH in susceptible individuals. The exact mechanism by which lidocaine might trigger MH is not fully under
We present the case of MH in a 43-year-old male patient with an unknown genetic predisposition following a lidocaine injection during a dental procedure. This case serves as a reminder that while the occurrence of lidocaine-induced MH is rare, lidocaine can still trigger this life-threatening condition. Therefore, caution should be exercised when administering lidocaine to individuals who may be susceptible to MH. It is important to note that prompt intervention played a crucial role in managing the patient’s symptoms. Upon recognizing the early signs of MH, agg
This case highlighted the significance of vigilant monitoring and swift action in mitigating the detrimental effects of lidocaine-induced MH. Caution should be exercised when administering lidocaine to individuals who may be predisposed to MH. It is very important to be aware and vigilant of the signs and symptoms of MH as early recognition and treatment intervention are important to prevent serious complications to decrease mortality.
Core Tip: Malignant hyperthermia is preventable in the clinical setting with the use of anesthetics like succinylcholine or inhaled versions like halothane or sevoflurane. Clinical findings like hypercarbia in the operating room or hyperthermia, tachycardia, muscle rigidity, and rhabdomyolysis are the sequela that follows. These symptoms rarely occur with the usage of local anesthetics like lidocaine. Given their wide application in the clinical setting, it is paramount for clinicians to be aware of the likelihood of malignant hyperthermia being caused by local anesthetics and to manage the symptoms as early as possible.
- Citation: Obi MF, Ubhi M, Namireddy V, Noel C, Sharma M, Campos FN, Garg Y. Malignant hyperthermia as a rare complication of local lidocaine injection: A case report. World J Anesthesiol 2023; 12(1): 1-7
- URL: https://www.wjgnet.com/2218-6182/full/v12/i1/1.htm
- DOI: https://dx.doi.org/10.5313/wja.v12.i1.1
Malignant hyperthermia (MH) is an inherited pharmacogenetic condition that affects skeletal muscles, resulting in hypermetabolism. It is an autosomal-dominant genetically heterogeneous ion channelopathy that is triggered by anesthesia. Due to advancements in identifying and treating MH, its incidence has decreased since it was first recognized in 1960. Currently, MH affects 1 in 50000 to 1 in 250000 adults and 1 in 15000 children[1]. People with mutations in ryanodine receptor (RYR1) and calcium voltage-gated channel subunit alpha1 S (CACNA1S) genes (which are responsible for regulating intracellular calcium balance) are susceptible to MH. In these individuals, calcium homeostasis is dis
Certain medical conditions, including central core disease, multiminicore disease, King-Denborough syndrome, and MH-like syndrome with STAC3 mutations, can increase the susceptibility to MH. These conditions are associated with genetic mutations in the RYR1 gene, which is also implicated in most cases of MH. The RYR1 gene encodes the ryanodine receptor responsible for regulating calcium release in muscle cells. The mutations in these conditions disrupt the normal function of the ryanodine receptor, leading to abnormal calcium ion channels and muscle dysfunction. As a result, individuals with these conditions may be more prone to experiencing MH when exposed to triggers that cause hypermetabolic reactions in skeletal muscles. In the case of MH-like syndrome with STAC3 mutations, the STAC3 gene is involved in the excitation-contraction coupling process, which controls calcium release in muscle cells. Mutations in the STAC3 gene interfere with its normal function and disrupt the release of calcium ions, increasing the risk of MH-like syndrome[2].
During an MH crisis, oxygen consumption increases and ATP decreases, resulting in anaerobic metabolism, muscle breakdown, lactic acidosis, and an increase in body temperature and heart rate. Symptoms include rhabdomyolysis, cyanosis, muscular contracture and rigidity, arrhythmia, coagulopathy, and hyperthermia. Left untreated, MH has an 80% mortality rate. However, with effective treatment and supportive measures, the mortality rate decreases to 5%. Treatment includes stopping the use of halogenated agents, supplying 100% oxygen through hyperventilation, and administering dantrolene sodium. Dantrolene sodium is a muscle relaxant that prevents calcium release from the endoplasmic reticulum by interacting with RYR1[1,3]. Diagnosis is based on clinical presentation, and clinicians must act swiftly to decrease mortality in susceptible individuals. Susceptibility confirmation is based on the halothane-caffeine contracture test, which is conducted 3 mo after the crisis[1]. There is some degree of controversy over whether lidocaine, a local anesthetic, causes MH. Studies indicate that the incidence is low. However, this case confirms that while the likelihood of lidocaine causing MH is minimal, it can still trigger it.
A 43-year-old man reported tooth pain that had started 2 d earlier, with progressive worsening.
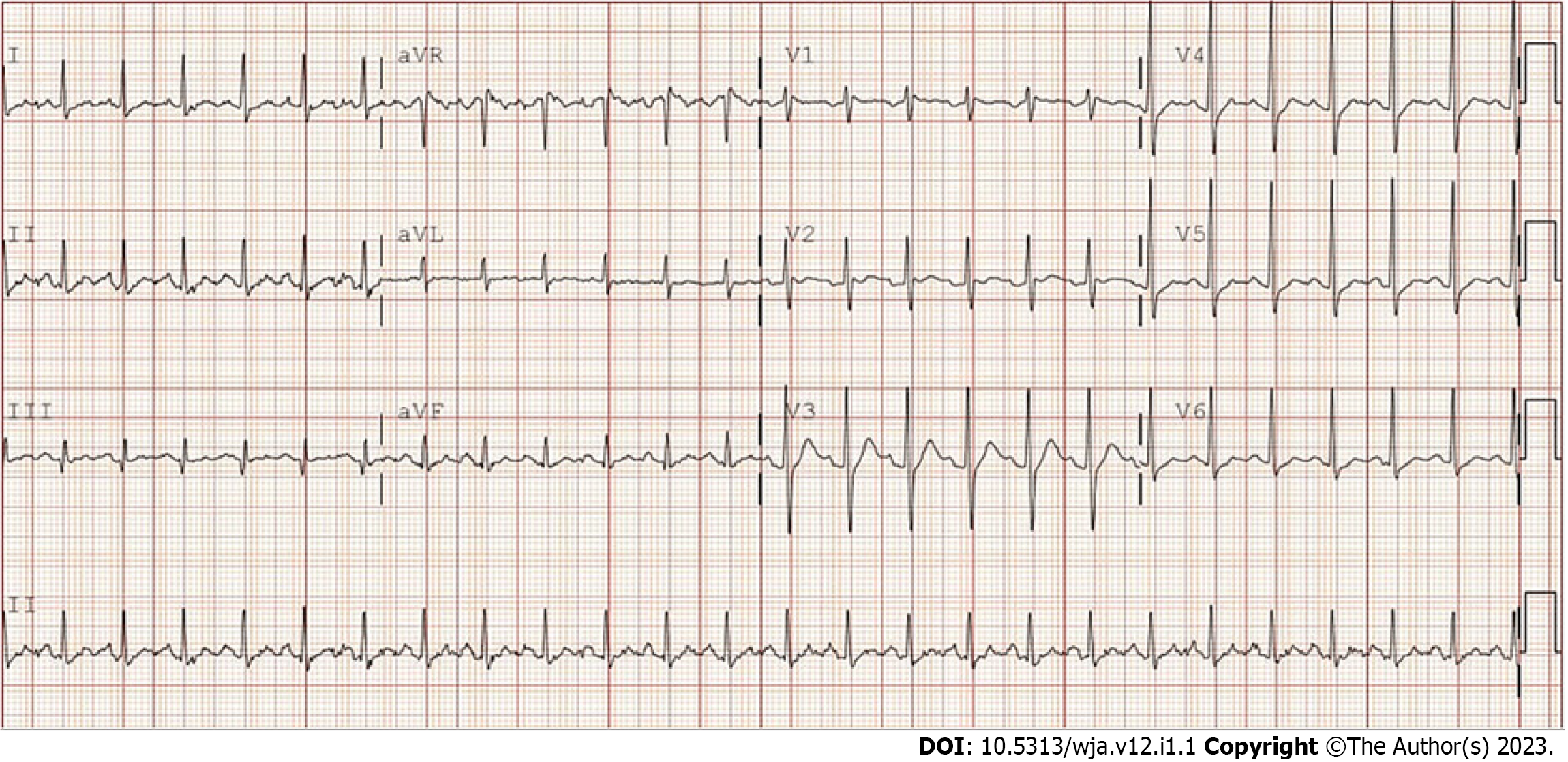
The patient arrived at the emergency department (ED) at 1:52 pm with a complaint of tooth pain. During the initial encounter, his vital signs were found to be stable. In the ED, he acknowledged that he was scheduled for a tooth extraction at approximately 2:30 pm that same afternoon. He reported that his tooth pain had started 2 d earlier and had become progressively worse, reaching six on the ten-point visual analog scale. The patient denied having any fever, chills, or shortness of breath. His current medication was amoxicillin (500 mg) and nonsteroidal anti-inflammatory drugs (400 mg). The dental team was consulted in the ED at 2:40 pm. Following an application of 3% lidocaine (with 2 mL epinephrine), the patient’s 10th tooth was extracted. His vitals at the time were stable. The patient was in no distress and was therefore discharged at 2:50 pm. Ten minutes after discharge, the patient returned to the ED with complaints of chills, trembling, shortness of breath, lower extremity rigidity, and tachypnea. He also reported feeling faint, but mentation was intact. The patient acknowledged that his symptoms started while he was on his way home. Electrocardiogram was obtained as indicated (Figure 1).
No significant past medical history.
No significant family history was reported.
This patient was observed to have visible chills and facial pallor. Vital signs on presentation showed a heart rate of 170 beats/min, blood pressure of 107/63 mmHg, respiratory rate of 44/min, and body temperature of 41.3 °C. He was hemodynamically unstable. Muscle rigidity was noted. The patient was alert and oriented to person, place, and time. Chest symmetry with respiration, no crackles or wheezing, and normal vesicular breath sounds were observed. Normal S1 and S2 and no pathological murmurs were heard.
The laboratory results were significant for pre-renal azotaemia, myoglobinuria, and elevated creatine phosphokinase (Table 1).
| Parameter | Result (normal range) |
| Chemistry | |
| Urine toxicology | Negative |
| High-sensitive troponin | 6.6 (3.0-58.9) ng/mL |
| Myoglobin | 197 (25-72) ng/mL |
| Creatine phosphokinase | 499 (10-120) µg/L |
| Calcium | 7.5 (8.6-10.3) mg/dL |
| Sodium | 144 (136-145) mEq/L |
| Potassium | 3.7 (3.5-5.2) mEq/L |
| Chloride | 114 (96-106) mmol/L |
| CO2 | 21 (23-29) mEq/L |
| Blood urea nitrogen | 15 (7-20) mg/dL |
| GFR | > 60 (> 60) mL/min/1.73 m2 |
| Creatine | 1.63 (0.7-1.3) mg/dL |
| Basic coagulation | |
| INR | 1.17 (0.88-1.13) |
| PTT | 32.7 (27.0-37.0) s |
| Prothrombin time | 13.7 (10.2–13.3) |
| Venous blood gas | |
| pH venous | 7.37 (7.31-7.41) |
| PCO2 venous | 36 (41-51) mmHg1 |
| HCO3 venous | 20.8 (21-28) mmol/L |
| PO2 venous | 78 (30-40) mmHg |
Chest X-ray indicated no acute cardiopulmonary disease, no pleural effusion, and no pneumothorax. Echocardiogram was significant for an ejection fraction of 55%-60%. No wall motion abnormalities and no valvulopathy were observed.
Based on the rapid onset of symptoms including increased breathing rate (tachypnea), elevated heart rate (tachycardia), muscle stiffness (rigidity), and high body temperature (hyperpyrexia) occurring within 10 min after the patient’s last normal state, along with laboratory findings of myoglobin in the urine, elevated creatine phosphokinase levels, acute kidney injury, and mild increase in carbon dioxide levels (hypercapnia), a diagnosis of MH was made. The patient was experiencing an acute state of increased metabolism. The clinical presentation strongly supported the diagnosis, although it was classified as a mild case since the symptoms appeared within a short time frame and prompt actions were taken to address and stop this life-threatening condition. Considering the swift response, the administration of dantrolene was delayed. Given the underlying pathophysiology of MH, the use of lorazepam before dantrolene could also be justified.
The patient received an immediate dose of lorazepam (2 mg) and intravenous acetaminophen (1 g) along with intravenous fluids. As a result, the patient’s respiratory rate improved from 44/min to 28-29/min. Subsequently, the patient was transferred to the intensive care unit for more thorough observation. Throughout the presentation, the patient remained conscious and aware of his surroundings. The administration of dantrolene was postponed due to the patient’s clinical improvement following the administration of lorazepam and intravenous fluids.
The patient was referred for genetic testing and counseling.
MH is a rare, potentially life-threatening autosomal dominant hypermetabolic crisis caused by defective cellular membrane dysfunction that occurs in people with a genetic predisposition. MH is caused by genetic mutations in skeletal muscle RYR1 or CACNA1S receptors; these receptors regulate intracellular calcium in muscle cells[3]. Potent volatile anesthetic agents such as halothane and desflurane, and the depolarizing muscle relaxant succinylcholine can cause MH in susceptible individuals having this skeletal muscle-cell genetic mutation.
The incidence of reported MH reactions ranged from 1 per 10000 to 1 per 250000 anesthetic administrations[3]. The use of these anesthetic agents in susceptible individuals triggers a massive release of calcium within the muscle cells, causing the MH symptoms. The influx of calcium into the cell causes sustained muscular contractions and breakdown (rhabdomyolysis), leading to clinical manifestations. They include hyperthermia, muscle rigidity, tachycardia, difficulty breathing, metabolic acidosis, an increase in carbon dioxide production, an increase in oxygen consumption, and hyperkalemia. These symptoms are all linked to hypermetabolic crisis[3].
While there is some controversy surrounding the use of amide-type local anesthetics (such as lidocaine) due to their potential to cause MH, research has shown that lidocaine is widely used, with a low incidence of MH. However, there have been literature reviews and studies conducted to evaluate the risk of MH in patients undergoing dental procedures. These studies have given rise to some debate about the safe administration of amide-type local anesthetics for individuals who are susceptible to MH. Despite the conclusion drawn by one report suggesting that there may not be a significant risk factor in the administration of such drugs to MH-susceptible individuals, our case supports the idea that caution should be exercised when administering lidocaine anesthetic, as it can indeed cause MH[4].
According to Minasian et al[4], members of the Malignant Hyperthermia Association of the United States conducted a survey to investigate the occurrence of MH-like reactions in individuals [all of whom were MH susceptible (MHS)] following amide local anesthesia. The survey involved 307 MHS respondents. Only one respondent reported symptoms indicative of MH after receiving amide local anesthetics. The report also highlighted that a significant proportion of the respondents (18%) had faced challenges in obtaining routine dental care due to their MHS status, with some having to undergo dental procedures without local anesthesia or refusing treatment altogether. As a result, the members of Malignant Hyperthermia Association of the United States concluded that amide local anesthetics could be safely administered to MHS patients without major risks, and a diagnosis of MHS could negatively impact the quality of dental care provided to MHS patients. Nonetheless, while administering amide local anesthesia, caution must still be exercised toward individuals with a history of susceptibility to MH. Ignoring potential risks due to the relatively low incidence of reported symptoms could lead to poor management and increased mortality.
As an amide type of local anesthetic, lidocaine can increase calcium release from the sarcoplasmic reticulum (SR) in muscle cells indirectly through its effect on cyclic adenosine monophosphate (cAMP) levels. It can trigger the cAMP/protein kinase A (PKA) signaling pathway[5]. cAMP is a signaling molecule that activates PKA, which can then phosphorylate and activate RYR1 in the SR. The RYR1 is the main calcium release channel in skeletal muscle cells, and its activation leads to the release of calcium from the SR into the cytosol of the muscle cell[6]. An increase in the release of calcium into the SR represents the pathophysiology behind the cause of MH. The hypermetabolic state seen in MH is due to abnormally elevated levels of calcium inside the muscle cell, which then causes rapid and sustained contraction of the muscle fibers, leading to their breakdown and rigidity.
Further research has shown that lidocaine can increase intracellular calcium by enhancing the permeability of the SR membrane, which is mediated by its direct effect on the SR Ca2+-dependent ATPase enzyme[7]. Although the effect of lidocaine is not normally a concern in healthy individuals, caution should be exercised in those individuals with genetic mutations or MH susceptibility since its use can intensify calcium release, which may in turn increase the risk of developing an MH reaction. Some case studies indicated that at a low concentration lidocaine can inhibit the release of calcium from the SR, which is the storage site of calcium ions in muscle cells. This can lead to a reduction in muscle contraction[8]. However, at high concentrations lidocaine can have the opposite effect leading to an increase in the efflux or release of calcium ions from the SR. This modulation of calcium handling by lidocaine has implications for individuals who are susceptible to MH. In individuals predisposed to MH, the ability of lidocaine to increase calcium efflux at high concentrations can potentially trigger an MH episode.
It is important to note that reports of lidocaine-induced MH are relatively rare. There have been a few documented cases where lidocaine has been associated with MH. For example, there was a case report of an MH episode occurring in a patient with underlying muscular dystrophy following extradural anesthesia with lidocaine. In another case of MH, the patient was symptomatic after intravenous lidocaine was administered for the treatment of ventricular arrhythmia[8]. Since there are few reported cases of lidocaine causing MH, further studies on the pathophysiology of local lidocaine inducing MH are needed.
Lorazepam increases the activity of the inhibitory neurotransmitter gamma-aminobutyric acid, thereby causing skeletal muscle relaxation. In this case report, lorazepam was given with intravenous fluids, which improved the patient’s symptoms. As a result, dantrolene administration was altered. The mechanism behind the patient’s improvement was due to an increase in gamma-aminobutyric acid, which can inhibit calcium influx inside the cell, leading to hyperpolarization and relaxation of muscle cells[9]. Based on the hypermetabolic state observed in MH, the tachyarrhythmias associated with MH were likely a result of elevated calcium levels within the cardiac myocytes, which disrupts the normal electrical activity by affecting the pacemaker cells and conduction system. Additionally, the acidotic state in MH can trigger premature excitability of the pacemaker cells and conduction system, resulting in tachyarrhythmia.
MH is a life-threatening hypermetabolic condition that occurs in people who are genetically predisposed to it. This genetic mutation is often seen in RYR1 or CACNA1S receptors, leading to an increase in intracellular calcium. This causes sustained muscle contractions and rigidity, leading to muscle breakdown and metabolic acidosis. Our case reported a patient with unknown MHS, who experienced MH symptoms after a lidocaine injection during a dental procedure, indicating that lidocaine can cause MH. Although the prevalence of lidocaine-induced MH is low, it is essential to understand that certain individuals with genetic susceptibility can still be vulnerable to MH from lidocaine use. It can affect the SR either directly through an increase in its membrane permeability (causing calcium influx) or indirectly through the cAMP/PKA signaling pathway. As such, caution should always be exercised prior to administration of lidocaine as it is the most used local anesthesia.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Amornyotin S, Thailand; Lee L, South Korea S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Zhao S
| 1. | Acosta IS, de Cos GV, Fernández MT. Malignant Hyperthermia Syndrome: A Clinical Case Report. EJIFCC. 2021;32:286-291. [PubMed] |
| 2. | Litman RS, Griggs SM, Dowling JJ, Riazi S. Malignant Hyperthermia Susceptibility and Related Diseases. Anesthesiology. 2018;128:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Rosenberg H, Pollock N, Schiemann A, Bulger T, Stowell K. Malignant hyperthermia: a review. Orphanet J Rare Dis. 2015;10:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 331] [Article Influence: 33.1] [Reference Citation Analysis (1)] |
| 4. | Minasian A, Yagiela JA. The use of amide local anesthetics in patients susceptible to malignant hyperthermia. Oral Surg Oral Med Oral Pathol. 1988;66:405-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Liu Y, Zhang J, Zan J, Zhang F, Liu G, Wu A. Lidocaine improves cerebral ischemia-reperfusion injury in rats through cAMP/PKA signaling pathway. Exp Ther Med. 2020;20:495-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Reiken S, Lacampagne A, Zhou H, Kherani A, Lehnart SE, Ward C, Huang F, Gaburjakova M, Gaburjakova J, Rosemblit N, Warren MS, He KL, Yi GH, Wang J, Burkhoff D, Vassort G, Marks AR. PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: defective regulation in heart failure. J Cell Biol. 2003;160:919-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Sánchez GA, Casadoumecq AC, Alonso GL, Takara D. Inhibitory effect of lidocaine on the sarcoplasmic reticulum Ca2+-dependent atpase from temporalis muscle. Acta Odontol Latinoam. 2010;23:92-98. [PubMed] |
| 8. | Tatsukawa H, Okuda J, Kondoh M, Inoue M, Terashima S, Katoh S, Ida K. Malignant hyperthermia caused by intravenous lidocaine for ventricular arrhythmia. Intern Med. 1992;31:1069-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Heidelberger R, Matthews G. Inhibition of calcium influx and calcium current by gamma-aminobutyric acid in single synaptic terminals. Proc Natl Acad Sci U S A. 1991;88:7135-7139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 1.5] [Reference Citation Analysis (0)] |