Published online Sep 30, 2022. doi: 10.5313/wja.v11.i1.1
Peer-review started: May 6, 2022
First decision: August 1, 2022
Revised: August 14, 2022
Accepted: September 13, 2022
Article in press: September 13, 2022
Published online: September 30, 2022
Processing time: 146 Days and 3 Hours
Heart failure is generally regarded as a progressive and irreversible medical condition. The EVAHEART is an implantable left ventricular assist system.
We report the anesthesia management of a 56-year-old male patient with dilated cardiomyopathy undergoing an EVAHEART implantation. Transesophageal echocardiography is crucial to ensure the correct positioning of the device and the proper aortic valve outflow. Because the continuous blood flow device functions best under low systemic and pulmonary vascular resistance, milrinone is the preferred drug. Our patient was accompanied by pulmonary hypertension, so during the operation, nitric oxide was used to reduce pulmonary artery pressure.
The cardiac output achieved by the patient with the assistance of EVAHEART can reach 4 L/min, which of course depends on the front load, rear load, and pump speed.
Core Tip: The EVAHEART is an implantable left ventricular assist system. We report the anesthesia management of a 56-year-old male patient with dilated cardiomyopathy undergoing an EVAHEART implantation. The anesthesia and perioperative management of EVAHEART implantation differ from those for traditional auxiliary devices. The first is the physiological characteristics of the non-pulsating continuous blood flow, for which the device has the best pump function under relatively low average arterial pressure and systemic vascular resistance. Second, transesophageal echocardiogram (TEE) is used to verify the correct position of the EVAHEART and to verify that the native heart has only partial auxiliary functions. Blood flow through the left ventricular outflow tract is sufficient to open the aortic valve. Third, perioperative TEE is used to guide the volume supplement, the adjustment of vasoactive drugs, and the adjustment of pump speed. Due to the continuous shortage of heart donors, EVAHEART implantation is the last hope for patients with end-stage heart failure. In the future, an increasing number of people will receive this kind of surgery, so we hope that this report can help them.
- Citation: Wu SG, He W. Anesthesia management of a patient undergoing implantation of a left ventricular assist system: A case report. World J Anesthesiol 2022; 11(1): 1-7
- URL: https://www.wjgnet.com/2218-6182/full/v11/i1/1.htm
- DOI: https://dx.doi.org/10.5313/wja.v11.i1.1
The global incidence of heart failure is approximately 26 million. As the population of China ages, the number of patients with heart failure increases[1]. The final treatment for end-stage heart failure is heart transplant. However, the continuous shortage of donor hearts necessitates the search for other surgical options. The ventricular assist device is the last resort to save critically ill patients, and the scenario in which cardiovascular anesthesiologists will face patients who will be implanted with ventricular assist devices will become more common.
EVAHEART (Yongrenxin Medical Equipment Co., Ltd., Chongqing, China) is an implantable left ventricular assist system (LVAD). Its main function is to build up the patient's auxiliary flow channel and assist in their blood circulation. Blood flows into the pump from the left ventricle through an inflow vessel. The centrifugal force generated by the rotation of an impeller in the pump pushes blood into the outflow vessel, which then enters the ascending aorta, thus assisting in the patient’s blood circulation.
EVAHEART has pulsatile blood flow and excellent blood compatibility, which makes it close to the real heart. Its unique continuous flow and non-stagnation may minimize the chance of thrombosis. In addition, because of its adjustable output, the pump can be used as an auxiliary device, whereby it can facilitate to the greatest extent the function of the heart[2,3].
However, little is known about the unconventional anesthetic management of patients undergoing implantation of the implantable LVAD. In our case, transesophageal echocardiogram (TEE) monitoring was vital throughout the perioperative period, and various pulmonary arterial pressure lowering agents were required. Hence, this article serves to provide additional information on this subject matter.
Chest tightness and shortness of breath after repeated activity for 6 years.
A 56-year-old man was suffering from severe dilated cardiomyopathy. The patient had been hospitalized three times in the past 6 years. Recently, he was readmitted due to chest tightness and shortness of breath and sought surgical treatment. Transthoracic ultrasound revealed that the entire heart was enlarged, with the left and right ventricular wall functions diffusely weakened. Moreover, the left and right heart functions were incomplete, with a widened tricuspid ring and high regurgitation of the tricuspid valve. Pulmonary hypertension was moderate, the inferior vena cava was widened, and the systemic reflux was obstructed. The ejection fraction was only 14.6%.
The patient reported no other medical history such as hypertension and diabetes.
The patient denied any personal and family history of illness.
The patient’s height was 175 cm and his weight was 70 kg. He was clear and energetic, and had cyanosis of lips, no swelling of superficial cervical lymph nodes, no swelling of jugular veins, and clear breath sounds in both lungs. His cardiac rhythm was uniform, and no obvious murmurs was heard in the apical area. The abdomen was flat and soft, without tenderness and rebound pain, and the liver and spleen were not reached under the ribs. Murphy's sign was negative. There was no percussion pain in bilateral kidney area and no tenderness in bilateral ureter running area. There was no edema in both lower limbs. Neurological tests were negative. Allen's test was positive.
The patient was accompanied by renal insufficiency (creatinine 126 mmol/L) and hepatic dysfunction (alanine aminotransferase 205 U/L and aspartate aminotransferase 152 U/L). Low cardiac output, pulmonary edema, and periodic inotropic drug dependence were also present.
Transthoracic ultrasound revealed that the entire heart was enlarged, with the left and right ventricular wall functions diffusely weakened. Moreover, the left and right heart functions were incomplete, with a widened tricuspid ring and high regurgitation of the tricuspid valve. Pulmonary hypertension was moderate, the inferior vena cava was widened, and the systemic reflux was obstructed. The ejection fraction was only 14.6%.
Dilated cardiomyopathy (congestive cardiomyopathy); Enlargement of the whole heart; Left ventricular dysfunction.
Due to the patient's height, weight, and health condition, it was difficult for him to wait for a suitable heart donor, and LVAD implantation became the first choice for treatment. Therefore, the patient chose to implant the EVAHEART LVAD via median thoracotomy at our hospital, where cardiopulmonary bypass (CPB), aortic intubation, superior and inferior vena cava intubation, and external connection are routinely performed.
The patient was classified as having grade IV cardiac function according to the New York Heart Association and was treated according to the plan approved by our hospital. We obtained the patient's written informed consent to disclose his case.
While the patient was awake, a monitor was used to track the invasive blood pressure through the left radial artery catheter (note that the intensive care unit had already completed the arterial and internal jugular vein punctures). After the operation, the patient inhaled oxygen (3 L/min) via a mask, and dexmedetomidine was pumped continuously (1 μg/kg for more than 15 min, then 0. 5 μg/kg/h continuously). Sixty drops/min of 500 mL compound electrolyte was given intravenously. For induction in a semi-recumbent position, the patient was given 3 mg midazolam, 10 mg etomidate, 50 mg rocuronium, and 30 μg sufentanil intravenously. Propofol, cisatracurium, and sufentanil were given at small doses for maintenance. After anesthesia induction, a 7.5 F internal jugular vein sheath was implanted through the right internal jugular vein to ensure that blood products could be given quickly. The monitoring of pulmonary artery pressure and mixed venous blood oxygen saturation was performed by implanting a floating catheter through the sheath. An ultrasound device inserted into the esophagus was used to evaluate cardiac function at any time during the operation and help the operator locate the EVAHEART. During the operation, autotransfusion was used, and any lost blood was purified and returned to the patient for blood protection. To reduce blood loss, a prothrombin complex (600 IU in total) and fibrinogen (1 g in total) were given after the CPB. At the same time, the intake of crystalloid fluids was minimized.
A comprehensive TEE was performed before the CPB, although the patient had already undergone TTE examination before the operation. The TEE was used to evaluate his left and right ventricular functions and to check whether a thrombus was in the heart cavity, especially in the apex of the left ventricle, which is the insertion point of the EVAHEART into the blood port. The aortic valve function was evaluated because moderate to severe dysfunction will lead to blood regurgitation to the left ventricle when the device is activated. Atrial septal defects and patent foramen ovale were checked to prevent left ventricular depression from causing right-to-left shunting when the device was activated, thus reducing systemic oxygenation. The function of the mitral valve was evaluated for stenosis or regurgitation. If the mitral valve is narrow or closed incompletely, it is unfavorable to the implantation of the device, as blood from the left atrium enters the left ventricle and is then sucked into the blood vessel. Finally, the ascending aorta was evaluated for calcification, plaque, or dilatation, as those may affect aortic intubation.
After sternotomy, it was found that the entire heart was grossly enlarged, and the contraction of both the left and right heart was diffusely weakened, especially the left heart. The mitral valve leaflets were slender and moved freely. The quality of the leaflets was good, the annulus was enlarged, the anastomosis was poor, and severe regurgitation occurred. The quality of the tricuspid valve leaflets was good, and the annulus was enlarged, resulting in severe regurgitation. Intraoperative esophageal ultrasound also confirmed the above lesions. A mitral valvuloplasty was performed, followed by a tricuspid valvuloplasty. Finally, a set of the EVAHEART LVAD was implanted.
Using the proper length of the blood vessel as a marker, the ascending aorta was cut longitudinally at the proper anastomosis position, and the artificial blood vessel was sutured to the ascending aorta. The appropriate position of the apex (located between the anterior descending branch and the second diagonal branch, 2 cm away from the anterior descending branch) was selected, and the anastomosis position of the blood vessel was marked. The thrombus and obstructed trabeculae in the apical ventricle were cleared. The preset thread was tightly attached to the blood vessel wall, sewn into the skirt, and knotted, and the blood vessel was implanted and fixed. An appropriate surgical incision was made at the outlet of the pump cable, and a special tunnel knife was used to make a subcutaneous tunnel. The head and handle of the tunnel knife were removed, and the pump cable was passed through the upper right abdomen. The blood pump was placed into heparin-containing saline, the start button of the blood pump drive pressed, the test run, and the power supply checked. The blood inlet tube was connected to the blood pump and tightened with a surgical wrench. The blood outlet tube was connected with the blood pump, which was not tightened and fully exhausted. With the head down, the aortic clamp was released, and the circulation opened and rebounded after defibrillation. The vascular blocking forceps were loosened slightly to facilitate the tightening of the surgical wrench after the side hole of the connecting port was fully exhausted. The blood pump was started at 1000 rpm, and after confirming that it had been safely started, the anesthesiologist held down the carotid artery, released the blocking forceps of the blood vessel, and released it after pressing the carotid artery for 30 s. Mechanical ventilation was resumed, and nitric oxide (NO) inhalation was started. With the circulation in parallel, the exhaust effect was checked via esophageal ultrasound, and any changes in left ventricular volume were noted. The flow of the CPB was gradually reduced, and the speed of the blood pump was increased. At the same time, TEE was used to verify that the pump was located at the top of the left ventricle and that the inlet was axially aligned with the mitral valve opening. It is best to use the standard four-cavity and two-cavity central plane in the middle esophagus. The position of the device can be adjusted during surgery, but this patient's position was fine. The flow was gradually reduced after the circulation was stabilized. The contractility of the right ventricular myocardium was low, and it was stopped by the flow reduction test. The patient’s blood pressure greatly dropped, but it did not improve after increasing vasoactive drug administration and volume adjustment. Esophageal ultrasound indicated that the right heart was noticeably full, the pulmonary artery was visibly widened, the left ventricular volume was clearly deficient, and the left ventricle was shaking. Pulmonary hypertension and right heart failure were considered. Then, extracorporeal membrane oxygenation (ECMO) was connected via right atrial and pulmonary artery intubation. After ECMO transfer, the patient’s hemodynamics gradually improved.
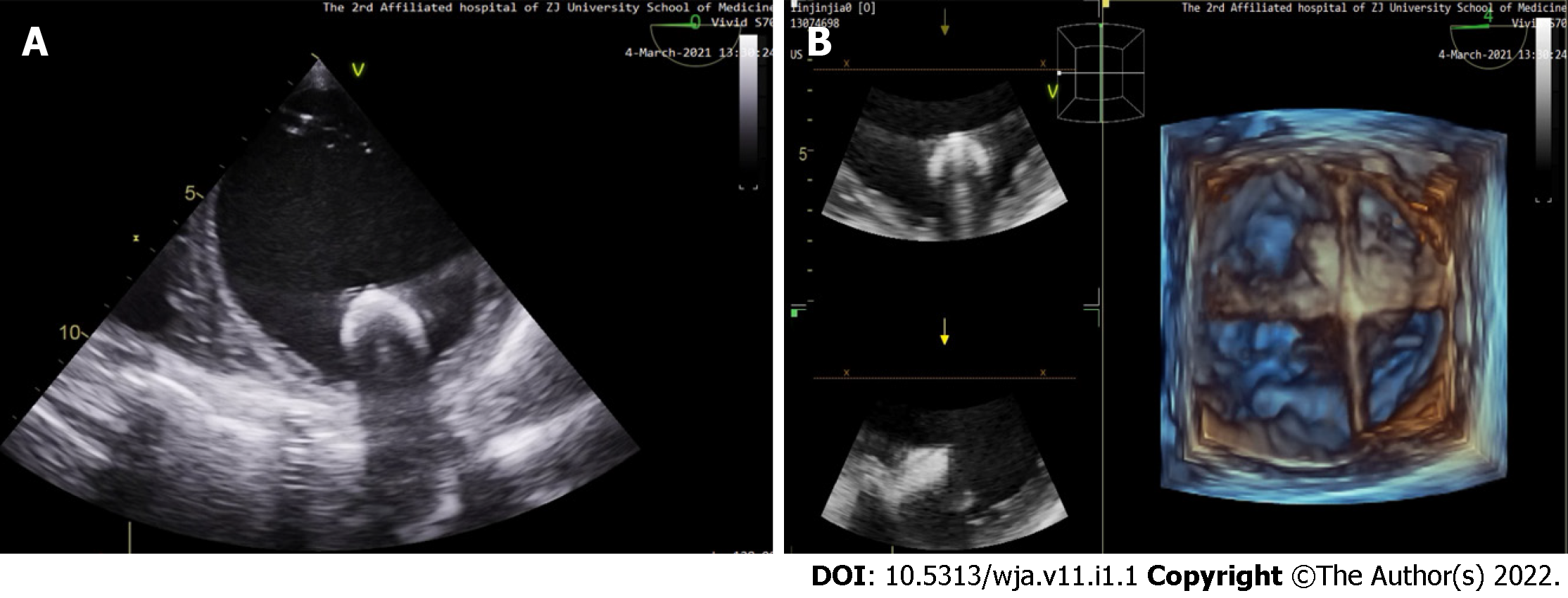
The satisfactory positioning of the device was confirmed using esophagus ultrasound (Figure 1). Adequate ventilation, stable circulation, reversal of heparin with protamine, and sufficient reversal with activated clotting time were all confirmed. The preparation of quick infusion devices and infusion warmers is necessary because massive bleeding may occur, especially when preparing to stop CPB. During the entire CPB process, the temperature should not be lower than 34 ℃.
Because the EVAHEART can pump enough flow from the left ventricle, the main physiological problem is the maintenance of the left ventricular inflow. Almost all patients have pulmonary hypertension, so we should reduce the pulmonary artery pressure as much as possible. Hence, the use of pulmonary vasodilators (such as inhaled NO and injected phentolamine into the pulmonary artery) is necessary. Under the guidance of the pulmonary artery pressure, the right ventricular afterload should be reduced. At least 10 L/min of fresh gas flow is required during NO inhalation to avoid nitrogen dioxide accumulation. If necessary, positive inotropic drugs (such as milrinone) should be used to support right heart function. Because LVADs work best at lower systemic and pulmonary vascular resistance, it is recommended to prioritize drugs such as milrinone, dobutamine, or isoprenaline[4]. Conversely, some patients need to increase systemic vascular resistance, so in those cases, it is recommended to use vasopressin, renin, or norepinephrine.
The rotational speed of EVAHEART is adjustable; it can be adjusted according to left ventricular volume and cardiac function to maintain stable circulation. To avoid aortic root stasis and the subsequent reduction of coronary blood flow, the pump speed can be adjusted to allow the aortic valve to open. It can be verified by TEE during the operation and can be observed in the arterial pressure waveform. If the pump speed is too fast, the formation of the arterial pressure wave decreases, and the absence of a pulse means the aortic valve is closed. The existence of the pulsatile waveform indicates that blood is flowing out through the aortic valve. Aortic valve opening depends on left heart function, volume, and pump speed. TEE can guide volume supplement, pump speed adjustment, and heart function improvement.
The patient was transferred to the intensive care unit under propofol maintenance. Based on the need, vasopressors and pulmonary vasodilators were used to maintain hemodynamic stability. While in the intensive care unit, esophageal ultrasound was continuously monitored. Rehydration, adjustment of vasoactive drugs, and adjustment of pump speed were guided according to the esophageal ultrasound, while NO was continuously inhaled. The stability of the patient's hemodynamics was maintained. Postoperatively, the ECMO was removed on the fourth day, the endotracheal tube was pulled out on the tenth day, and the patient could walk on the fourteenth day. Compared with the preoperative values, the hemodynamic parameters of the patient were improved. The liver and kidney functions were improved, indicating that the perfusion was good and that organ function could be maintained. Anticoagulant therapy was used to keep the international standardized ratio between 1.5 and 2.0. At the most recent follow-up, the patient was fully self-sufficient.
The anesthesia management of patients with LVAD implantation differs from other cardiac anesthesia in several important aspects. For example, the physiology is unique in that the blood flow in the aorta is non-pulsating and continuous. After LVAD implantation, the power of the pump and the inherent power of the heart work together to fully support organ perfusion. Since the pump provides continuous pressure and flow during contraction and relaxation, organ perfusion will further increase. Pump speed and mean arterial pressure are the main variables that determine the blood flow through the LVAD. Therefore, the goal of anesthesia is to maintain a relatively low mean arterial pressure (60-75 mmHg) and a corresponding low systemic vascular resistance to optimize blood flow and organ perfusion.
Anesthesiologists have difficulty in observing the heart directly and comprehensively due to factors such as surgical vision and operation. TEE can achieve this goal. TEE examination is very important before, during, and after LVAD implantation. It can determine the correct position of the pump in the ventricle, which is located at the top, with the inlet axially aligned with the mitral valve opening. In addition, via continuous TEE monitoring, the pump flow rate is gradually reduced, and when the pump speed decreases, the pulse pressure will be gradually increased, thus verifying the opening point of the aortic valve. However, aortic valve opening depends not only on pump speed but also on left ventricular volume and contraction force, which can be monitored by TEE.
After LVAD implantation, with the operation of the pump, the left atrium and left ventricle pressure can be reduced. However, some residual volume in the left atrium and left ventricle must be retained. The main goal of the LVAD is not to completely replace left ventricular function but rather to play an auxiliary role. The auxiliary function of the left heart can normalize the cardiac index by improving the intrinsic ventricular function. By reducing the left ventricular volume and end diastolic pressure, the myocardium of patients with chronic heart failure can be partially restored. The blood flow through the left ventricular outflow tract and aortic valve after implantation of the axial flow device must be maintained, not only to maximize the potential of improving intrinsic ventricular function but also to minimize the risk of thrombosis on the aortic valve.
After LVAD implantation, left ventricular function will be greatly improved. Some patients are often accompanied by an increase in pulmonary artery pressure. The right heart function is our key concern. The right ventricular function and left ventricular volume need to be monitored continuously by TEE during the perioperative period. TEE is especially used to detect right heart failure. If the left ventricular volume decreases severely when low volume or high pump speed is set, right heart failure will occasionally occur. EVAHEART can produce high flow with an increase in rotational speed, which leads to the left ventricle collapsing and the left ventricular septum moving, potentially leading to right ventricular dysfunction[3].
During the perioperative period of LVAD implantation, we have to address a very important problem, which is how the blood in the right heart can smoothly reach the left heart through the pulmonary circulation. Therefore, the function of the right heart and the reduction of pulmonary vascular resistance are very important. If the right heart function is poor or the pulmonary vascular resistance is high, the right ventricle will have difficulty in providing the preload to maintain the cardiac output. These two goals can be achieved by using drugs that strengthen the right heart function and help reduce pulmonary vascular resistance, such as milrinone, dobutamine, or isoprenaline, or drugs that directly dilate the pulmonary artery, such as NO and prostaglandin[1,5]. Some surgeons may consider using epidural anesthesia combined with general anesthesia to complete the LVAD implantation, which can not only reduce systemic vascular resistance but can also be used for postoperative analgesia. However, due to possible coagulation dysfunction and postoperative anticoagulation, we do not recommend using this technique.
Because EVAHEART cannot completely resist systemic vascular resistance, low systemic vascular resistance can improve pump function. However, if the patient shows very low systemic vascular resistance, it may be an adverse effect of using a large number of right heart support drugs, or it may be a related syndrome caused by severe heart failure. At this time, a small dose of vasopressin can be considered. Vasopressin is known to have systemic effects, but its effect on pulmonary vessels is relatively small[5].
In summary, the anesthesia management of patients with EVAHEART implantation differs from the management of other cardiac anesthesia. The first is the physiological characteristics of the non-pulsating continuous blood flow, for which the device has the best pump function under relatively low average arterial pressure and systemic vascular resistance. Second, TEE is used to verify the correct position of the EVAHEART and to verify that the native heart has only partial auxiliary functions. Blood flow through the left ventricular outflow tract is sufficient to open the aortic valve. Third, perioperative TEE is used to guide the volume supplement, the adjustment of vasoactive drugs, and the adjustment of pump speed. Due to the continuous shortage of heart donors, EVAHEART implantation is the last hope for patients with end-stage heart failure.
In the future, an increasing number of people will receive this kind of surgery, so we hope that this report can help them.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sharma D, India; Wake AD, Ethiopia S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Hao G, Wang X, Chen Z, Zhang L, Zhang Y, Wei B, Zheng C, Kang Y, Jiang L, Zhu Z, Zhang J, Wang Z, Gao R; China Hypertension Survey Investigators. Prevalence of heart failure and left ventricular dysfunction in China: the China Hypertension Survey, 2012-2015. Eur J Heart Fail. 2019;21:1329-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 2. | Bartoli CR, Kang J, Motomura T. Decreased RPM reduces von Willebrand factor degradation with the EVAHEART LVAS: implications for device-specific LVAD management. J Card Surg. 2020;35:1477-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Newman K, Montes R, Campos J, Marquez-Maya N, Vu V, Zebrowski E. Reducing regional flow stasis and improving intraventricular hemodynamics with a tipless inflow cannula design: An in vitro flow visualization study using the EVAHEART LVAD. Artif Organs. 2019;43:834-848. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Ma W, Suhitharan T, Shah SS, Kothandan H. Anesthesia for left ventricular assisted device insertion in a patient with multiple organ failure. Saudi J Anaesth. 2021;15:210-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Sugawara Y, Mizuno Y, Oku S, Goto T. Effects of vasopressin during a pulmonary hypertensive crisis induced by acute hypoxia in a rat model of pulmonary hypertension. Br J Anaesth. 2019;122:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |