Published online Jul 18, 2014. doi: 10.5312/wjo.v5.i3.319
Revised: January 21, 2014
Accepted: April 3, 2014
Published online: July 18, 2014
Osteoarthritis (OA) is one of the most common degenerative joint diseases in aging population. Obesity is an important risk factor for initiation and progression of OA. It is accepted that excess body weight may lead to cartilage degeneration by increasing the mechanical forces across weight-bearing joints. However, emerging data suggest that additional metabolic factors released mainly by white adipose tissue may also be responsible for the high prevalence of OA among obese people. Adipocyte-derived molecules ‘‘adipokines’’ have prompt much interest in OA pathophysiological research over the past decade since they play an important role in cartilage and bone homeostasis. Therefore, the aim of this review is to summarize the current knowledge on the role of adipokines including leptin, adiponectin, visfatin and resistin in OA and their potential to be used as biomarkers for earlier diagnosis, classifying disease severity, monitoring disease progression, and testing pharmacological interventions for OA. In OA patients, leptin, visfatin and resistin showed increased production whereas adiponectin showed decreased production. Leptin and adiponectin are far more studied than visfatin and resistin. Importantly, altered adipokine levels also contribute to a wide range of diseases. Further experiments are still crucial for understanding the relationship between adipokines and OA.
Core tip: Osteoarthritis (OA) is one of the most common degenerative joint diseases in aging population. Obesity is an important risk factor for initiation and progression of OA. Adipokines have prompt much interest in OA pathophysiological research over the past decade since they play an important role in cartilage and bone homeostasis. Therefore, the aim of this review is to summarize the current knowledge on the role of adipokines including leptin, adiponectin, visfatin and resistin in OA and their potential to be used as biomarkers for earlier diagnosis, classifying disease severity, monitoring disease progression, and testing pharmacological interventions for OA.
- Citation: Poonpet T, Honsawek S. Adipokines: Biomarkers for osteoarthritis? World J Orthop 2014; 5(3): 319-327
- URL: https://www.wjgnet.com/2218-5836/full/v5/i3/319.htm
- DOI: https://dx.doi.org/10.5312/wjo.v5.i3.319
The coexistence of obesity and osteoarthritis (OA) has increased remarkably nowadays. OA is the most common degenerative joint disease which affects more than 37% of people whose age are over 60 years[1]. Due to aging of the population, the prevalence of OA continues to increase in the near future[2]. Osteoarthritis is characterized by articular cartilage degradation, subchondral bone sclerosis, osteophyte formation, and synovial inflammation. The etiology of OA is largely complicated because it includes both genetic and non-genetic factors[3]. Obesity is considered as a worldwide health problem with low-grade inflammatory status. It has long been recognized as an important risk factor for initiation and progression of OA. Since obesity is a modifiable risk factor, it has received much interest in OA clinical study.
It is primarily accepted that excess body weight may leads to cartilage degeneration by increasing the mechanical forces across weight-bearing joints. However, several studies have revealed the association between obesity and OA in non-weight-bearing joints such as those in fingers and wrists. For example, a study reported a two-fold increase in hand OA risk in obese individuals[4]. Moreover, emerging data suggest that additional metabolic factors released mainly by white adipose tissue (WAT) may also be responsible for the high prevalence of OA among obese people[5].
In general, radiography is used to confirm the diagnosis of OA because it can reveal clinical changes at the joint margin, such as the bony outgrowth and joint space narrowing. However, these radiographic evidences are seen only after substantial cartilage loss has already taken place. To avoid severe joint pain or dysfunction, as well as total joint replacement surgery, early detection, especially in the preradiographic stage of the disease are required. Biomarkers offer a potential alternative mean for earlier diagnosis of nonsymptomatic OA. Nowadays, bone and cartilage biomarkers responsible for cartilage degradation are still frequently used in classifying disease severity, monitoring disease progression, and testing pharmacological interventions. Nevertheless, adipocyte-derived molecules ‘‘adipokines’’ have prompted much interest in OA pathophysiological research over the past decade due to the fact that they play an important role in cartilage and bone homeostasis. Moreover, the association of adipokines with obesity, together with its pro- or anti-inflammatory properties suggests that adipokines might be another crucial mediator that links inflammation with obesity and OA. Therefore, the aim of this review is to include the current knowledge of the role of adipokines including leptin, adiponectin, visfatin and resistin in OA and their potential to be used as biomarkers for OA.
The production of most adipokines is increased with obesity, except for adiponectin. Adipokine levels are gender dependent, which normally higher in women than in men even after adjusted for body mass index (BMI). This might contribute to higher prevalence of OA in females. Adipokines are produced in knee OA joints by infrapatellar fat pads (IPFPs), synovium, chondrocytes, osteoblasts, as well as osteoclasts[6,7]. It was suggested that systemic (plasma) and local (synovial fluid) adipokine levels would be related with cartilage degeneration and synovial inflammation[8]. The information regarding adipokine levels are summarized in Table 1.
| Adipokines | Association with BMI | Plasma levels between genders | Plasma levels between groups | Levels in OA patients |
| Leptin | positive | women > men | OA > control | SF > plasma |
| Adiponectin | negative | women > men | control > OA | plasma > SF |
| Visfatin | positive | unclear | OA > control | SF > plasma |
| Resistin | unclear | women > men | OA > control | plasma > SF |
The leptin concentration in plasma was positively correlated with BMI, in both healthy controls and OA patients. Obese individuals generally display higher levels of circulating leptin than their non-obese counterparts[9,10]. Premenopausal women show about 3 times higher plasma leptin concentration than men[11]. It has been reported that higher leptin concentration in plasma was associated with higher odds ratio of having knee OA, after age, ethnicity and BMI adjustments[12]. Interestingly, synovial leptin levels were 3 to 11 times higher than those in matched plasma sample[6]. Therefore, local leptin may play more distinct roles in bone metabolism regulation than systemic leptin.
Adiponectin circulates in high concentrations (0.01% of total plasma protein) in the blood exceeding those in the paired synovial fluid[7]. Plasma adiponectin levels are negatively correlated with BMI, lower in obese people and increase with weight loss[13,14]. Women have significantly higher plasma adiponectin levels than men[15]. Unlike other adipokines, plasma adiponectin levels were reported to be lower in OA patients than in healthy individuals[16]. In OA patients, adiponectin levels in plasma were almost 100 times higher than in synovial fluid, and these levels showed an inverse correlation[17]. However, Distel et al[18] have shown the increased adiponectin levels in the IPFPs of knee OA. It has been reported that the amount of HMW relative to total adiponectin in OA synovial fluid was lower than in OA plasma, whereas that of the hexamer was similar and that of the trimer was higher in OA synovial fluid than in OA plasma[19].
Visfatin levels are increased in obese individuals compared with lean people[20], which can be reduced by weight loss[21]. Although very recent study reported no significant differences in plasma visfatin levels between genders, it seems to be higher in female than in male[22]. OA patients have higher circulating and local visfatin concentrations compared with controls, with levels in OA synovial fluid are greater than paired OA plasma[23]. It has been shown that OA cartilage and synovium release higher amounts of visfatin than control samples[24]. Moreover, the visfatin expression in OA IPFPs is also higher than in the matched subcutaneous adipose tissue[25].
Plasma resistin levels were significantly higher than matched synovial levels and increased in obese individuals without direct association with BMI[26]. Resistin levels in females showed significantly higher than in males. It can be detected in inflamed synovium joints, such as rheumatoid arthritis (RA) and OA[6,27]. It was demonstrated that resistin levels in both plasma and synovial fluid were elevated after traumatic joint injuries[28]. In radiographic hand OA patients, plasma resistin levels were higher than in non-radiographic hand OA and controls[29]. Interestingly, leptin deficient (ob/ob and db/db) mouse models showed elevated levels of circulating resistin, suggesting that resistin levels are slightly dependent upon leptin levels[30].
Adipokines exert both catabolic and anabolic roles in cartilage, chondrocytes, osteoblasts and osteoclasts as summarized in Table 2.
| Adipokines | Proteases | Cytokines | Inflammation | Cartilage | Bone |
| Leptin | ↑MMP-1 ↑MMP-3 ↑MMP-9 ↑MMP-13 ↑Cysteine proteases ↑ADAMTS-4 ↑ADAMTS-5 | ↑IL-1β↑IL-6 ↑IL-8 ↓FGF ↑TNF-α↑IGF-1 ↑TGF-β↑GRO ↑MCP-1 | ↑NOS2 ↑iNOS ↑PGE2 ↑COX-2 | ↓↑Chondrocyte proliferation ↑Proteoglycan synthesis ↑Collagen synthesis | ↑Osteoblast proliferation ↑Ossification ↑ALP ↑OC |
| Adiponectin | ↑MMP-1 ↑MMP-3 ↑MMP-9 ↑↓MMP-13 ↑TIMP-1 ↑TIMP-2 | ↑IL-6 ↑IL-8 ↑MCP-1 ↑VCAM-1 | ↑NOS2 ↑PGE2 ↑VEGF | ↑Chondrocyte proliferation ↑Proteoglycan synthesis ↑Collagen synthesis ↑Matrix mineralization | ↑Osteoblast proliferation ↑Osteoclast differentiation ↑RANKL ↓OPG |
| Visfatin | ↑MMP-3, ↑MMP-13, ↑ADAMTS-4, ↑ADAMTS-5 | ↑IL-1β↑IL-6 ↑TNF-α | ↑NO ↑PGE2 | ↓Chondrocyte phenotype ↓Proteoglycan synthesis ↓Collagen synthesis | ↑Osteoblast proliferation ↓Osteoclast differentiation |
| Resistin | ↑MMP-1 ↑MMP-13 ↑ADAMTS-4 | ↑IL-6 ↑TNF-α | ↑PGE2 | ↓Proteoglycan synthesis ↓Collagen synthesis | ↑Osteoblast proliferation ↑Osteoclast differentiation |
In vivo injection of leptin into the rat knee joints shows catabolic effects in OA cartilage by increasing the production of metalloproteinases (MMPs) enzymes such as MMP-1, -3, -9 and -13, as well as cysteine proteases at both gene and protein levels[31,32]. In parallel, human OA cartilage treated with small interfering RNA (siRNA) targeted for leptin showed decreased MMP-13 expression[33]. Moreover, Bao et al[34] have demonstrated that the gene expression of two important aggrecanases, a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-4 and -5, were considerably increased after treatment with leptin, whereas it decreases the anabolic factors such as basic fibroblast growth factors (FGF) production in mouse articular cartilage. These evidences suggest a prominent catabolic effect of leptin on cartilage metabolism in OA joints.
In cultured chondrocytes, OA chondrocytes produce higher leptin concentrations than normal chondrocytes. Leptin can stimulate chondrocytes to secrete higher levels of key mediators in cartilage degradation such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-6, IL-8, growth-related oncogene (GRO) and monocyte chemo-attractant protein-1 (MCP-1)[32,35-37]. It has been shown that leptin had proinflammatory and catabolic effects on chondrocyte proliferation. Leptin reduced proliferation of OA chondrocytes after the 48-hour treatment and reduced chondrocyte proliferation in both control and OA after the 7-day treatment[38].
However, anabolic activities of leptin in cartilage metabolism have also been reported, suggesting that catabolic effects of leptin may trigger compensatory anabolic responses. Dumond et al[9] have showed that the production of insulin-like growth factor-1 (IGF-1) and transforming growth factor-β (TGF-β) can be induced by intra-articular injection of leptin. In addition, Figenschau et al[39] demonstrated increased chondrocyte proliferation and enhanced proteoglycans and collagen synthesis after leptin incubation. Recent studies revealed that leptin can also promote proliferation, differentiation, type X collagen production and cytoskeletal remodeling in chondrocytes[40-42]. The ob/ob mice showed reduced type X collagen synthesis in growth plates[43].
Leptin increases the proliferation and differentiation of osteoblasts by inhibiting adipogenic differentiation of bone marrow cells. It has been found that leptin acts as a regulator for bone growth by inducing collagen synthesis, osteoblast proliferation and differentiation, bone mineralization, as well as endochondral ossification[44-46]. The increased synthesis of leptin in OA subchondral osteoblasts is associated with the osteoblast dysfunction by increasing levels of alkaline phosphatase (ALP), osteocalcin (OC), collagen type I, and TGF-β[47]. The results of immunohistological studies showed that osteophytes expressed high levels of leptin[5].
Nitric oxide (NO) is a proinflammatory mediator which promotes apoptosis, chondrocyte phenotype loss, as well as MMPs activation. The combination of leptin and interferon-γ can activate the production of type 2 nitric oxide synthase (NOS2) in cultured chondrocytes[48]. Leptin, alone or in synergy with IL-1β, has also been reported to enhance the production of inducible nitric oxide synthase (iNOS), prostaglandin E2 (PGE2) and cyclooxygenasse (COX)-2 in human OA cartilage and chondrocytes[49,50]. Surprisingly, the incidence of knee OA between leptin deficient (ob/ob) obese mice and leptin receptor deficient (db/db) obese mice was not different when compared with wild-type mice[51], suggesting that obesity alone was unable to induce knee OA and therefore leptin has a significant role in OA pathophysiology.
Adiponectin seems to have both catabolic and anabolic effects on pathological changes of several tissues/cells involved in the initiation and progression of OA. Adiponectin and adiponectin receptors have been identified in human chondrocytes[6]. Adiponectin exert a proinflammatory function by stimulating NOS2, MCP-1, MMP-1, -3, -9 and-13, IL-6, IL-8, PGE2, and vascular endothelial growth factor (VEGF) production from chondrocytes and cartilage[36,52,53]. Adiponectin can induce vascular cell adhesion molecule 1 (VCAM-1) expression in murine and human chondrocytes, suggesting its role to perpetuate cartilage degradation by modulating molecules responsible for leukocyte infiltration at inflamed joints[54]. In addition, adiponectin levels in OA synovial fluid was correlated with aggrecan degradation[55].
Adiponectin enhances proliferation and mineralization of human osteoblasts[56]. The stimulation of osteoblasts with adiponectin increased the production of the inflammatory mediators IL-6, IL-8, and MCP-1. In grade 1 (non-ossified) osteophytes, adiponectin were detectable in connective tissue fibroblasts. In grade 2–5 (ossified osteophytes) a lower extent of adiponectin was expressed by osteoblasts, suggesting its involvement in early osteophyte formation[57]. By contrast, adiponectin stimulates receptor activator of nuclear factor kappa-B ligand (RANKL) and inhibits the production of osteoprotegerin (OPG) in osteoblasts, which in turn indirectly activates osteoclasts[58].
Interestingly, several studies have shown a protective effect of adiponectin in knee OA. Chen et al[17] demonstrated down-regulated IL-1β induced MMP-13 production and up-regulated tissue inhibitor of metalloproteinases (TIMP)-1 and -2 production in primary chondrocytes at both mRNA and protein levels. Moreover, adiponection can stimulate release of antiinflammatory molecules such as IL-10 and IL-1 receptor antagonist[59,60], suggesting the protective role against cartilage damage[17]. In addition, adiponectin has been shown to increase murine chondrocyte proliferation, aggrecan synthesis, matrix mineralization, and upregulated type II and type X collagen expression[61].
Visfatin affects the expression of chondrocyte-specific genes involved in extracellular matrix (ECM) formation. For example, it was observed that visfatin plus IGF-1 reduces the production of proteoglycans and collagen type II[62]. Similarly, visfatin-treated mouse articular chondrocytes showed increased MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5 expression[24], suggesting a deleterious role of visfatin in articular cartilage. A recent study had shown that visfatin counteracted anabolic IGF-1 signaling, and therefore reduced IGF-1-mediated proteoglycan synthesis in human chondrocytes[62].
Moreover, elevated level of visfatin can reduce the expression of factors essential for the maintenance of the chondrocyte phenotype such as sex determining region Y)-box 9 (SOX-9) and type II collagen[63]. On the other hand, visfatin has also showed some anabolic properties. It was demonstrated that the inhibition of visfatin by pharmacological or siRNA techniques decreased the production of human chondrocyte specific matrix genes such as collagen type2 alpha1 (COL2A1) and aggrecan (ACAN)[64]. Moreover, visfatin has been shown to induce the production of IL-1β, TNF-α, and IL-6 in lymphocytes[65].
It has been shown that visfatin is related to inflammation at the cartilage level by increasing MMP activity and NO production, as well as proteoglycan release in OA cartilage matrix[66]. To note, visfatin plus IL-1β stimulation is able to induce the synthesis of PGE2, a relevant catabolic factor, in murine and human OA chondrocytes. The knockdown of visfatin expression by using a siRNA confirms this effect[24].
Visfatin could influence differentiation of mesenchymal stem cells to adipocytes or osteoblasts in vitro[67]. Visfatin is expressed in osteoblasts and osteoclasts in ossified osteophytes[57]. Apart from the effect of visfatin on osteoblast proliferation and collagen type I synthesis[68], it has been mentioned that visfatin also participates in osteoclast formation by inhibiting osteoclastogenesis[65], suggesting its role in osteophyte formation.
Although the study regarding the role of resistin in OA is sparse, some studies showed its direct effect on cartilage matrix and cytokine production. In the weeks immediately after joint injury, both plasma and synovial fluid levels of resistin were elevated. Resistin increased expression of MMP-1,-13, and ADAMTS-4 in human articular chondrocytes. In addition, resistin can stimulate inflammatory cytokines, such as IL-6 and TNF-α, as well as PGE2 synthesis. Furthermore, resistin stimulates proteoglycan degradation, as well as inhibited the production of proteoglycan and type II collagen in mouse and human cartilage explants[69]. It is produced in osteoblasts and osteoclasts in ossified osteophytes. Recombinant mouse resistin stimulates osteoblast proliferation and osteoclast differentiation, indicating a role in osteophyte formation[70].
In a 5-year cohort study, plasma leptin levels seemed to be positively associated with the occurrence of radiographic knee OA. Moreover, it showed a positive association with knee OA progression in subjects who have radiographic knee OA at baseline. However, the association disappeared after adjustment for BMI[71]. In addition, leptin expression has been reported to be associated with the radiographic severity of OA, suggesting a potential role of leptin as a possible biomarker for quantitative detection of OA[72]. In advanced grade OA cartilage, leptin and its long isoform receptor (Ob-Rb) levels in synovial fluid were significantly increased compared to healthy or adjacent mildly affected cartilage[38]. In addition, elevated plasma leptin levels have been detected in the end-stage knee OA patients compared with controls, independent of BMI, age and gender. On the contrary, no association was found between plasma leptin levels and cartilage damage or synovial inflammation parameters in OA patients[8]. In addition, Iwamoto’s group did not find any association between plasma leptin levels and knee OA with grade 4 Kellgren-Lawrence (KL) scores, and Berry et al[71] found no association between baseline plasma leptin levels and 2-year alterations of cartilage volume and defects in knee OA patients.
Plasma adiponectin levels were significantly increased in end-stage knee OA patients compared with healthy controls independent of age, gender and BMI[8]. Compared to less severely affected subjects, Koskinen et al[73] found increased plasma adiponectin levels in patients with the radiologically most severe OA, grade 4-5 Ahlback scores, compared with patients who have less severe disease. Likewise, a significant association between plasma adiponectin levels and the Lequesne index was found[74]. Filková et al[15] also found that plasma adiponectin levels were higher in erosive OA patients than in nonerosive OA patients. The study of Gandhi et al[75] showed an elevation in the adiponectin expression in IPFP from end-stage knee OA compared with that from early stage OA.
However, some clinical data support the protective roles of adiponectin as a molecule against cartilage damage in OA. Honsawek and Chayanupatkul showed an inverse correlation between plasma adiponectin and radiographic knee OA severity. They found increased adiponectin levels in grade 2 KL-scores knee OA patients compared with controls, but decreased levels in grade 4 KL-scores knee OA patients[76]. In addition, it has been reported that patients with high adiponectin levels had a decreased risk for hand OA progression[4]. However, another study showed no association between plasma adiponectin levels and radiographic hand OA severity[77]. In addition, Berry et al[71] did not find any association between baseline plasma adiponectin levels, cartilage volume changes and defects in knee OA subjects in a 2-year study. Interestingly, leptin/adiponectin ratio in synovial fluid was proposed to be a predictor of pain in knee OA patients. A lower leptin/adiponectin ratio correlated with lower knee OA pain when measured by the McGill Pain Questionnaire-Short Form (MPQ-SF) pain scale[78].
Levels of visfatin in plasma and synovial fluid appeared to be associated with lipid metabolism, inflammation and clinical disease activity. Plasma visfatin concentrations showed a positive correlation with C-reactive protein (CRP), an inflammatory marker, indicating that it may be related to lipid metabolism and inflammatory process[79,80]. Visfatin levels in synovial fluid were increased in OA patients with more radiographic damage compared with patients with less severe disease. Synovial visfatin levels in grade 4 KL-scores were significantly higher than those of grade 3 KL-scores[81].
Gómez et al[49] found no association between baseline plasma resistin levels and cartilage volume loss. Plasma resistin concentrations were positively associated with the prevalence of radiographic knee OA, independently with BMI, but it was not associated with the disease progression. Interestingly, the association between resistin and the presence of radiographic knee OA was more obvious in OA patients with higher adiponectin levels[74]. Moreover, plasma resistin levels were positively associated with histologically determined grades of synovial inflammation[27]. The presence of radiographic changes such as subchondral erosion in hand OA was shown to be related with plasma resistin levels[29].
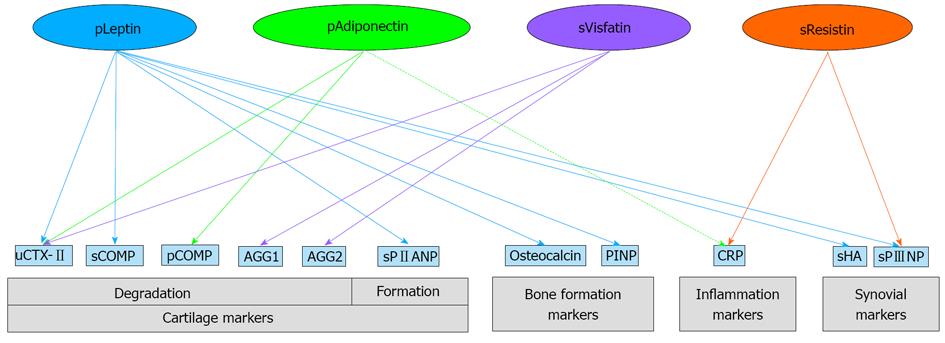
Berry et al[71] have revealed that plasma leptin was significantly associated with the level of bone formation markers, such as osteocalcin and N-terminal type I procollagen propeptide (PINP). In addition, leptin was positively associated with the cartilage biomarkers such as urine C-terminal telopeptide of type II collagen (uCTX-II), synovial cartilage oligomeric matrix protein (sCOMP), and synovial N-terminal propeptide of type IIA procollagen (sPIIANP), as well as synovial markers such as synovial hyaluronic acid (sHA) and synovial N-terminal propeptide of type III procollagen (sPIIINP) after adjustment for gender and age. However, after additional adjustment for BMI, these associations disappeared except for sPIIANP and sPIIINP. In contrast, baseline expression levels of soluble leptin receptors OB-Rb were negatively associated with 2-year changes of the cartilage formation biomarkers PIIANP and bone formation markers, osteocalcin levels.
Plasma adiponectin levels showed positive associations with markers of cartilage degradation such as uCTX-IIand plasma COMP (pCOMP), but showed negative associations with plasma high sensitivity C-reactive protein (hsCRP) levels. These associations turned stronger after adjustments for BMI. In addition, Kang et al[53] reported increased levels of collagenase-cleaved type II collagen neoepitope in supernatants of OA cartilage explants incubated with adiponectin.
Synovial visfatin concentrations also showed positive correlation with uCTX-II, and two aggrecan degradation biomarkers: aggrecan (AGG)1 and AGG2[81]. In addition, visfatin increases the release of a marker of cartilage breakdown sulfated glycosaminoglycans (s-GAG), suggesting its involvement in cartilage matrix degradation[66].
Plasma resistin concentrations were positively associated with sPIIINP and hsCRP levels[74]. In addition, A positive correlation has been found between synovial resistin levels and systemic markers of inflammation[82]. Association between adipokines and other OA biomarkers are illustrated in Figure 1.
Prevention and early diagnosis are undoubtedly important for OA management. This review demonstrates that the levels of leptin, visfatin and resistin are elevated in OA patients, suggesting the catabolic role of these adipokines. In contrast, adiponectin is upregulated in OA patients and seems to play protective roles against OA. Adipokines might be also produced in other tissues and altered adipokine levels are also contributes to a wide range of obesity-related health problems such as autoimmune diseases, cardiovascular diseases and metabolic disorders. Therefore, the use of adipokines alone may not be enough for the prediction of OA risk. Nevertheless, adipokines exhibit prominent role in OA pathophysiology and show associations with OA progression. Thus it may become possible to use adipokines as biomarkers for monitoring disease progression and following the efficiency of therapeutic interventions. In addition, the ratio of different adipokines levels or the ratio of adipokines and other biomarker levels might be used to better reflect the net effect of these molecules. Importantly, further experiments are needed to understand paradoxical relationship between adipokines and OA in both genders. However, uncertainty still remains whether adipokines could be utilized as biomarkers in clinical practice for OA.
The authors would like to thank Thomas Mabey for kindly reviewing the manuscript. The authors commemorate the 100th Anniversary of the King Chulalongkorn Memorial Hospital, Thai Red Cross Society.
P- Reviewer: Bener A S- Editor: Ma YJ L- Editor: A E- Editor: Lu YJ
| 1. | Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33:2271-2279. [PubMed] [Cited in This Article: ] |
| 2. | Salihu HM, Bonnema SM, Alio AP. Obesity: What is an elderly population growing into? Maturitas. 2009;63:7-12. [PubMed] [Cited in This Article: ] |
| 3. | Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am. 2004;42:1-9, v. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 314] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 4. | Yusuf E, Nelissen RG, Ioan-Facsinay A, Stojanovic-Susulic V, DeGroot J, van Osch G, Middeldorp S, Huizinga TW, Kloppenburg M. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis. 2010;69:761-765. [PubMed] [Cited in This Article: ] |
| 5. | Pottie P, Presle N, Terlain B, Netter P, Mainard D, Berenbaum F. Obesity and osteoarthritis: more complex than predicted! Ann Rheum Dis. 2006;65:1403-1405. [PubMed] [Cited in This Article: ] |
| 6. | Presle N, Pottie P, Dumond H, Guillaume C, Lapicque F, Pallu S, Mainard D, Netter P, Terlain B. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production. Osteoarthritis Cartilage. 2006;14:690-695. [PubMed] [Cited in This Article: ] |
| 7. | Gegout PP, Francin PJ, Mainard D, Presle N. Adipokines in osteoarthritis: friends or foes of cartilage homeostasis? Joint Bone Spine. 2008;75:669-671. [PubMed] [Cited in This Article: ] |
| 8. | de Boer TN, van Spil WE, Huisman AM, Polak AA, Bijlsma JW, Lafeber FP, Mastbergen SC. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthritis Cartilage. 2012;20:846-853. [PubMed] [Cited in This Article: ] |
| 9. | Dumond H, Presle N, Terlain B, Mainard D, Loeuille D, Netter P, Pottie P. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 2003;48:3118-3129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 378] [Cited by in F6Publishing: 361] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 10. | Gualillo O, Eiras S, Lago F, Diéguez C, Casanueva FF. Elevated serum leptin concentrations induced by experimental acute inflammation. Life Sci. 2000;67:2433-2441. [PubMed] [Cited in This Article: ] |
| 11. | Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Leibel RL. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab. 1996;81:3424-3427. [PubMed] [Cited in This Article: ] |
| 12. | Karvonen-Gutierrez CA, Harlow S. Leptin Levels Are Associated with Knee Osteoarthritis among Mid-Aged Women. Osteoarthritis Cartilage. 2012;20:S189-S190. [Cited in This Article: ] |
| 13. | Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1014] [Cited by in F6Publishing: 1028] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 14. | Oh DK, Ciaraldi T, Henry RR. Adiponectin in health and disease. Diabetes Obes Metab. 2007;9:282-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 15. | Filková M, Lisková M, Hulejová H, Haluzík M, Gatterová J, Pavelková A, Pavelka K, Gay S, Müller-Ladner U, Senolt L. Increased serum adiponectin levels in female patients with erosive compared with non-erosive osteoarthritis. Ann Rheum Dis. 2009;68:295-296. [PubMed] [Cited in This Article: ] |
| 16. | Laurberg TB, Frystyk J, Ellingsen T, Hansen IT, Jørgensen A, Tarp U, Hetland ML, Hørslev-Petersen K, Hornung N, Poulsen JH. Plasma adiponectin in patients with active, early, and chronic rheumatoid arthritis who are steroid- and disease-modifying antirheumatic drug-naive compared with patients with osteoarthritis and controls. J Rheumatol. 2009;36:1885-1891. [PubMed] [Cited in This Article: ] |
| 17. | Chen TH, Chen L, Hsieh MS, Chang CP, Chou DT, Tsai SH. Evidence for a protective role for adiponectin in osteoarthritis. Biochim Biophys Acta. 2006;1762:711-718. [PubMed] [Cited in This Article: ] |
| 18. | Distel E, Cadoudal T, Durant S, Poignard A, Chevalier X, Benelli C. The infrapatellar fat pad in knee osteoarthritis: an important source of interleukin-6 and its soluble receptor. Arthritis Rheum. 2009;60:3374-3377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | Ebina K, Fukuhara A, Ando W, Hirao M, Koga T, Oshima K, Matsuda M, Maeda K, Nakamura T, Ochi T. Serum adiponectin concentrations correlate with severity of rheumatoid arthritis evaluated by extent of joint destruction. Clin Rheumatol. 2009;28:445-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1269] [Cited by in F6Publishing: 1244] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 21. | Haider DG, Pleiner J, Francesconi M, Wiesinger GF, Müller M, Wolzt M. Exercise training lowers plasma visfatin concentrations in patients with type 1 diabetes. J Clin Endocrinol Metab. 2006;91:4702-4704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Jurdana M, Petelin A, Černelič Bizjak M, Bizjak M, Biolo G, Jenko-Pražnikar Z. Increased serum visfatin levels in obesity and its association with anthropometric/biochemical parameters, physical inactivity and nutrition. e-SPEN J. 2013;8:e59-e67. [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Chen WP, Bao JP, Feng J, Hu PF, Shi ZL, Wu LD. Increased serum concentrations of visfatin and its production by different joint tissues in patients with osteoarthritis. Clin Chem Lab Med. 2010;48:1141-1145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Gosset M, Berenbaum F, Salvat C, Sautet A, Pigenet A, Tahiri K, Jacques C. Crucial role of visfatin/pre-B cell colony-enhancing factor in matrix degradation and prostaglandin E2 synthesis in chondrocytes: possible influence on osteoarthritis. Arthritis Rheum. 2008;58:1399-1409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Klein-Wieringa IR, Kloppenburg M, Bastiaansen-Jenniskens YM, Yusuf E, Kwekkeboom JC, El-Bannoudi H, Nelissen RG, Zuurmond A, Stojanovic-Susulic V, Van Osch GJ. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann Rheum Dis. 2011;70:851-857. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Degawa-Yamauchi M, Bovenkerk JE, Juliar BE, Watson W, Kerr K, Jones R, Zhu Q, Considine RV. Serum resistin (FIZZ3) protein is increased in obese humans. J Clin Endocrinol Metab. 2003;88:5452-5455. [PubMed] [Cited in This Article: ] |
| 27. | Senolt L, Housa D, Vernerová Z, Jirásek T, Svobodová R, Veigl D, Anderlová K, Müller-Ladner U, Pavelka K, Haluzík M. Resistin in rheumatoid arthritis synovial tissue, synovial fluid and serum. Ann Rheum Dis. 2007;66:458-463. [PubMed] [Cited in This Article: ] |
| 28. | Conde J, Scotece M, Gómez R, Lopez V, Gómez-Reino JJ, Gualillo O. Adipokines and osteoarthritis: novel molecules involved in the pathogenesis and progression of disease. Arthritis. 2011;2011:203901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Choe JY, Bae J, Jung HY, Park SH, Lee HJ, Kim SK. Serum resistin level is associated with radiographic changes in hand osteoarthritis: cross-sectional study. Joint Bone Spine. 2012;79:160-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Steppan CM, Lazar MA. Resistin and obesity-associated insulin resistance. Trends Endocrinol Metab. 2002;13:18-23. [PubMed] [Cited in This Article: ] |
| 31. | Hu PF, Bao JP, Wu LD. The emerging role of adipokines in osteoarthritis: a narrative review. Mol Biol Rep. 2011;38:873-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Toussirot E, Streit G, Wendling D. The contribution of adipose tissue and adipokines to inflammation in joint diseases. Curr Med Chem. 2007;14:1095-1100. [PubMed] [Cited in This Article: ] |
| 33. | Iliopoulos D, Malizos KN, Tsezou A. Epigenetic regulation of leptin affects MMP-13 expression in osteoarthritic chondrocytes: possible molecular target for osteoarthritis therapeutic intervention. Ann Rheum Dis. 2007;66:1616-1621. [PubMed] [Cited in This Article: ] |
| 34. | Bao JP, Chen WP, Feng J, Hu PF, Shi ZL, Wu LD. Leptin plays a catabolic role on articular cartilage. Mol Biol Rep. 2010;37:3265-3272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Tang CH, Chiu YC, Tan TW, Yang RS, Fu WM. Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-kappa B pathway. J Immunol. 2007;179:5483-5492. [PubMed] [Cited in This Article: ] |
| 36. | Lago R, Gomez R, Otero M, Lago F, Gallego R, Dieguez C, Gomez-Reino JJ, Gualillo O. A new player in cartilage homeostasis: adiponectin induces nitric oxide synthase type II and pro-inflammatory cytokines in chondrocytes. Osteoarthritis Cartilage. 2008;16:1101-1109. [PubMed] [Cited in This Article: ] |
| 37. | Tong KM, Chen CP, Huang KC, Shieh DC, Cheng HC, Tzeng CY, Chen KH, Chiu YC, Tang CH. Adiponectin increases MMP-3 expression in human chondrocytes through AdipoR1 signaling pathway. J Cell Biochem. 2011;112:1431-1440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | Simopoulou T, Malizos KN, Iliopoulos D, Stefanou N, Papatheodorou L, Ioannou M, Tsezou A. Differential expression of leptin and leptin’s receptor isoform (Ob-Rb) mRNA between advanced and minimally affected osteoarthritic cartilage; effect on cartilage metabolism. Osteoarthritis Cartilage. 2007;15:872-883. [PubMed] [Cited in This Article: ] |
| 39. | Figenschau Y, Knutsen G, Shahazeydi S, Johansen O, Sveinbjörnsson B. Human articular chondrocytes express functional leptin receptors. Biochem Biophys Res Commun. 2001;287:190-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 134] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Wang SJ, Li XF, Jiang LS, Dai LY. Leptin regulates estrogen receptor gene expression in ATDC5 cells through the extracellular signal regulated kinase signaling pathway. J Cell Biochem. 2012;113:1323-1332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Liang J, Feng J, Wu WK, Xiao J, Wu Z, Han D, Zhu Y, Qiu G. Leptin-mediated cytoskeletal remodeling in chondrocytes occurs via the RhoA/ROCK pathway. J Orthop Res. 2011;29:369-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Ben-Eliezer M, Phillip M, Gat-Yablonski G. Leptin regulates chondrogenic differentiation in ATDC5 cell-line through JAK/STAT and MAPK pathways. Endocrine. 2007;32:235-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Kishida Y, Hirao M, Tamai N, Nampei A, Fujimoto T, Nakase T, Shimizu N, Yoshikawa H, Myoui A. Leptin regulates chondrocyte differentiation and matrix maturation during endochondral ossification. Bone. 2005;37:607-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 44. | Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept. 2000;92:73-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 382] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 45. | Kume K, Satomura K, Nishisho S, Kitaoka E, Yamanouchi K, Tobiume S, Nagayama M. Potential role of leptin in endochondral ossification. J Histochem Cytochem. 2002;50:159-169. [PubMed] [Cited in This Article: ] |
| 46. | Gordeladze JO, Drevon CA, Syversen U, Reseland JE. Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: Impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem. 2002;85:825-836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 273] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 47. | Mutabaruka MS, Aoulad Aissa M, Delalandre A, Lavigne M, Lajeunesse D. Local leptin production in osteoarthritis subchondral osteoblasts may be responsible for their abnormal phenotypic expression. Arthritis Res Ther. 2010;12:R20. [PubMed] [Cited in This Article: ] |
| 48. | Otero M, Gomez Reino JJ, Gualillo O. Synergistic induction of nitric oxide synthase type II: in vitro effect of leptin and interferon-gamma in human chondrocytes and ATDC5 chondrogenic cells. Arthritis Rheum. 2003;48:404-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 49. | Gómez R, Scotece M, Conde J, Gómez-Reino JJ, Lago F, Gualillo O. Adiponectin and leptin increase IL-8 production in human chondrocytes. Ann Rheum Dis. 2011;70:2052-2054. [PubMed] [Cited in This Article: ] |
| 50. | Vuolteenaho K, Koskinen A, Kukkonen M, Nieminen R, Päivärinta U, Moilanen T, Moilanen E. Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage--mediator role of NO in leptin-induced PGE2, IL-6, and IL-8 production. Mediators Inflamm. 2009;2009:345838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 51. | Griffin TM, Huebner JL, Kraus VB, Guilak F. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum. 2009;60:2935-2944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 52. | Choi HM, Lee YA, Lee SH, Hong SJ, Hahm DH, Choi SY, Yang HI, Yoo MC, Kim KS. Adiponectin may contribute to synovitis and joint destruction in rheumatoid arthritis by stimulating vascular endothelial growth factor, matrix metalloproteinase-1, and matrix metalloproteinase-13 expression in fibroblast-like synoviocytes more than proinflammatory mediators. Arthritis Res Ther. 2009;11:R161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 53. | Kang EH, Lee YJ, Kim TK, Chang CB, Chung JH, Shin K, Lee EY, Lee EB, Song YW. Adiponectin is a potential catabolic mediator in osteoarthritis cartilage. Arthritis Res Ther. 2010;12:R231. [PubMed] [Cited in This Article: ] |
| 54. | Conde J, Gomez R, Bianco G, Scotece M, Lear P, Dieguez C, Gomez-Reino J, Lago F, Gualillo O. Expanding the adipokine network in cartilage: identification and regulation of novel factors in human and murine chondrocytes. Ann Rheum Dis. 2011;70:551-559. [PubMed] [Cited in This Article: ] |
| 55. | Hao D, Li M, Wu Z, Duan Y, Li D, Qiu G. Synovial fluid level of adiponectin correlated with levels of aggrecan degradation markers in osteoarthritis. Rheumatol Int. 2011;31:1433-1437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Luo XH, Guo LJ, Yuan LQ, Xie H, Zhou HD, Wu XP, Liao EY. Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway. Exp Cell Res. 2005;309:99-109. [PubMed] [Cited in This Article: ] |
| 57. | Connor JR, Dodds RA, Emery JG, Kirkpatrick RB, Rosenberg M, Gowen M. Human cartilage glycoprotein 39 (HC gp-39) mRNA expression in adult and fetal chondrocytes, osteoblasts and osteocytes by in-situ hybridization. Osteoarthritis Cartilage. 2000;8:87-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 58. | Luo XH, Guo LJ, Xie H, Yuan LQ, Wu XP, Zhou HD, Liao EY. Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Res. 2006;21:1648-1656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 260] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 59. | Ehling A, Schäffler A, Herfarth H, Tarner IH, Anders S, Distler O, Paul G, Distler J, Gay S, Schölmerich J. The potential of adiponectin in driving arthritis. J Immunol. 2006;176:4468-4478. [PubMed] [Cited in This Article: ] |
| 60. | Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772-783. [PubMed] [Cited in This Article: ] |
| 61. | Challa TD, Rais Y, Ornan EM. Effect of adiponectin on ATDC5 proliferation, differentiation and signaling pathways. Mol Cell Endocrinol. 2010;323:282-291. [PubMed] [Cited in This Article: ] |
| 62. | Yammani RR, Loeser RF. Extracellular nicotinamide phosphoribosyltransferase (NAMPT/visfatin) inhibits insulin-like growth factor-1 signaling and proteoglycan synthesis in human articular chondrocytes. Arthritis Res Ther. 2012;14:R23. [PubMed] [Cited in This Article: ] |
| 63. | Hong EH, Yun HS, Kim J, Um HD, Lee KH, Kang CM, Lee SJ, Chun JS, Hwang SG. Nicotinamide phosphoribosyltransferase is essential for interleukin-1beta-mediated dedifferentiation of articular chondrocytes via SIRT1 and extracellular signal-regulated kinase (ERK) complex signaling. J Biol Chem. 2011;286:28619-28631. [PubMed] [Cited in This Article: ] |
| 64. | Dvir-Ginzberg M, Gagarina V, Lee EJ, Hall DJ. Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase. J Biol Chem. 2008;283:36300-36310. [PubMed] [Cited in This Article: ] |
| 65. | Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, Tilg H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748-1758. [PubMed] [Cited in This Article: ] |
| 66. | McNulty AL, Miller MR, O’Connor SK, Guilak F. The effects of adipokines on cartilage and meniscus catabolism. Connect Tissue Res. 2011;52:523-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 67. | Li Y, He X, Li Y, He J, Anderstam B, Andersson G, Lindgren U. Nicotinamide phosphoribosyltransferase (Nampt) affects the lineage fate determination of mesenchymal stem cells: a possible cause for reduced osteogenesis and increased adipogenesis in older individuals. J Bone Miner Res. 2011;26:2656-2664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 68. | Xie H, Tang SY, Luo XH, Huang J, Cui RR, Yuan LQ, Zhou HD, Wu XP, Liao EY. Insulin-like effects of visfatin on human osteoblasts. Calcif Tissue Int. 2007;80:201-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 69. | Lee JH, Ort T, Ma K, Picha K, Carton J, Marsters PA, Lohmander LS, Baribaud F, Song XY, Blake S. Resistin is elevated following traumatic joint injury and causes matrix degradation and release of inflammatory cytokines from articular cartilage in vitro. Osteoarthritis Cartilage. 2009;17:613-620. [PubMed] [Cited in This Article: ] |
| 70. | Thommesen L, Stunes AK, Monjo M, Grøsvik K, Tamburstuen MV, Kjøbli E, Lyngstadaas SP, Reseland JE, Syversen U. Expression and regulation of resistin in osteoblasts and osteoclasts indicate a role in bone metabolism. J Cell Biochem. 2006;99:824-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 71. | Berry PA, Jones SW, Cicuttini FM, Wluka AE, Maciewicz RA. Temporal relationship between serum adipokines, biomarkers of bone and cartilage turnover, and cartilage volume loss in a population with clinical knee osteoarthritis. Arthritis Rheum. 2011;63:700-707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 72. | Ku JH, Lee CK, Joo BS, An BM, Choi SH, Wang TH, Cho HL. Correlation of synovial fluid leptin concentrations with the severity of osteoarthritis. Clin Rheumatol. 2009;28:1431-1435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 73. | Koskinen A, Juslin S, Nieminen R, Moilanen T, Vuolteenaho K, Moilanen E. Adiponectin associates with markers of cartilage degradation in osteoarthritis and induces production of proinflammatory and catabolic factors through mitogen-activated protein kinase pathways. Arthritis Res Ther. 2011;13:R184. [PubMed] [Cited in This Article: ] |
| 74. | Van Spil WE, Welsing PM, Kloppenburg M, Bierma-Zeinstra SM, Bijlsma JW, Mastbergen SC, Lafeber FP. Cross-sectional and predictive associations between plasma adipokines and radiographic signs of early-stage knee osteoarthritis: data from CHECK. Osteoarthritis Cartilage. 2012;20:1278-1285. [PubMed] [Cited in This Article: ] |
| 75. | Gandhi R, Takahashi M, Virtanen C, Syed K, Davey JR, Mahomed NN. Microarray analysis of the infrapatellar fat pad in knee osteoarthritis: relationship with joint inflammation. J Rheumatol. 2011;38:1966-1972. [PubMed] [Cited in This Article: ] |
| 76. | Honsawek S, Chayanupatkul M. Correlation of plasma and synovial fluid adiponectin with knee osteoarthritis severity. Arch Med Res. 2010;41:593-598. [PubMed] [Cited in This Article: ] |
| 77. | Massengale M, Lu B, Pan JJ, Katz JN, Solomon DH. Adipokine hormones and hand osteoarthritis: radiographic severity and pain. PLoS One. 2012;7:e47860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 78. | Gandhi R, Takahashi M, Smith H, Rizek R, Mahomed NN. The synovial fluid adiponectin-leptin ratio predicts pain with knee osteoarthritis. Clin Rheumatol. 2010;29:1223-1228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 79. | Auguet T, Terra X, Porras JA, Orellana-Gavaldà JM, Martinez S, Aguilar C, Lucas A, Pellitero S, Hernández M, Del Castillo D. Plasma visfatin levels and gene expression in morbidly obese women with associated fatty liver disease. Clin Biochem. 2013;46:202-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 80. | Lago F, Dieguez C, Gómez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007;3:716-724. [PubMed] [DOI] [Cited in This Article: ] |
| 81. | Duan Y, Hao D, Li M, Wu Z, Li D, Yang X, Qiu G. Increased synovial fluid visfatin is positively linked to cartilage degradation biomarkers in osteoarthritis. Rheumatol Int. 2012;32:985-990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |