Copyright
©2013 Baishideng Publishing Group Co.
World J Orthop. Oct 18, 2013; 4(4): 178-185
Published online Oct 18, 2013. doi: 10.5312/wjo.v4.i4.178
Published online Oct 18, 2013. doi: 10.5312/wjo.v4.i4.178
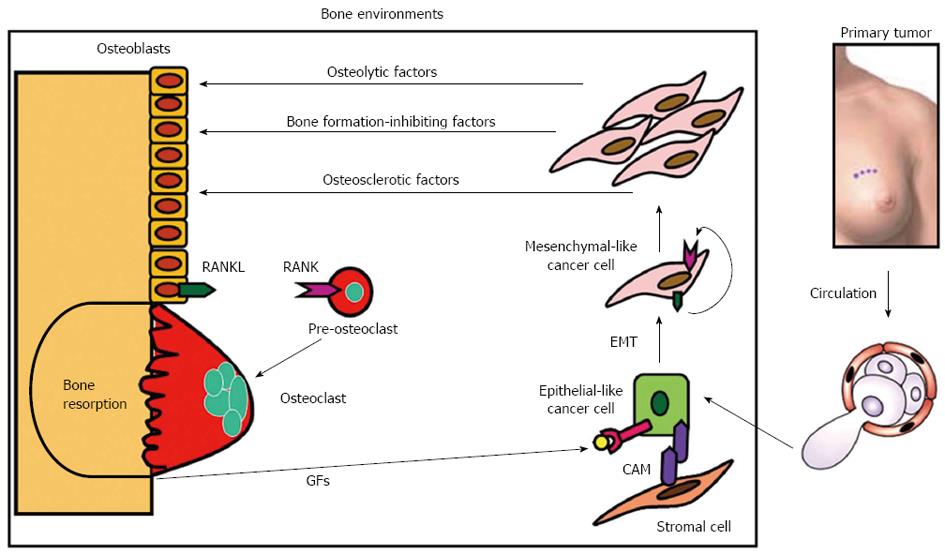
Figure 2 Vicious cycle between bone and breast cancer cells in bone metastasis.
Bone-derived growth factors (GFs) such as insulin-like growth factors (IGF) and transforming growth factor-β (TGF-β) that are continually released via osteoclastic bone resorption promote proliferation and suppress apoptosis and stimulate the production of parathyroid hormone-related protein (PTH-rP), prostaglandin E2 (PGE2) and interleukin-11 (IL-11) in metastatic breast cancer cells migrating from primary site via circulation. Bone is fertile soil for metastatic cancer cells representing the concept of “Seed and Soil” theory proposed by Paget[9]. These osteolytic factors further stimulate osteoclastic bone resorption, followed by enhanced release of bone-stored growth factors, thus establishing “vicious cycle”. Prostate cancer may produce osteosclerotic factors and multiple myeloma is shown to produce bone formation-inhibiting factors[14-16]. These osteolytic factors up-regulate the production of ligand for receptor activator of nuclear factor-κB (RANKL) in osteoblasts/stromal cells, which then interacts with its receptor receptor activator of nuclear factor-κB (RANK) in the osteoclast precursors promoting osteoclastogenesis and bone resorption. RANKL and RANK play a central role in the establishment of the vicious cycle. Metastatic breast cancer cells themselves occasionally express RANKL and RANK to develop an autocrine stimulation of carcinogenesis or tumorigenesis. RANKL/RANK expression in breast cancer cells could be a predicting indicator for subsequent occurrence of bone metastasis. Some metastatic breast cancer cells reside in stromal cell niche via cell-cell contact that is mediated by cell adhesion molecules (CAMs) and stay dormant. Metastatic breast cancer cells undergo epithelial-mesenchymal transition (EMT) by changing cell shape from epithelial to mesenchymal and acquire more aggressiveness in the presence of bone-derived TGF-β.
- Citation: Yoneda T, Tanaka S, Hata K. Role of RANKL/RANK in primary and secondary breast cancer. World J Orthop 2013; 4(4): 178-185
- URL: https://www.wjgnet.com/2218-5836/full/v4/i4/178.htm
- DOI: https://dx.doi.org/10.5312/wjo.v4.i4.178