INTRODUCTION
Rheumatoid arthritis (RA) is a chronic inflammatory disorder characterized by remarkable synovial hyperplasia followed by the massive joint destruction[1,2]. Investigation into the pathogenesis of joint destruction in RA has revealed the transformed phenotype of rheumatoid synovial cells[3]. Proliferating inflammatory synovial cells lead to pannus formation that invades articular cartilage and bone[4]. Radiographic studies demonstrate that bone erosion in RA begins early in the disease, and progresses throughout its course[5,6]. Bone erosion results in severe deformity of the affected joints and impairs the normal activity of patients. Therefore, inhibiting bone destruction is one of the most challenging goals in the treatment of RA. Because the exact etiology of RA remains unknown, most treatments of RA have targeted symptoms of the disease. Non-steroidal anti-inflammatory drugs have been used to reduce the painful symptoms of the disease, but they have little effect on stopping the progression of joint destruction. Some disease-modifying anti-rheumatic drugs such as methotrexate are known to suppress joint destruction in RA[7,8]. In addition, recent clinical studies have demonstrated that various biological agents such as antibodies against inflammatory cytokines (e.g., infliximab, adalimumab and tocilizumab) or CTLA4-Ig (abatacept) not only suppress joint symptoms in RA patients but also markedly ameliorate joint destruction[9-12]. However, the bone-protective function of these drugs is still limited, and they are accompanied by severe side effects, such as infection, since they suppress a patient’s immunological reaction[13].
There is accumulating evidence that osteoclasts, primary cells responsible for bone resorption, are involved in bone destruction in RA, and recent progress in molecular biology and biochemistry has revealed the molecular mechanism of osteoclast differentiation and bone resorption. In this chapter, I would like to focus on the role of osteoclasts in bone and joint destruction in RA, the mechanism of osteoclast generation in inflammatory joint, and propose that osteoclasts can be potential targets in RA therapy.
INVOLVEMENT OF OSTEOCLASTS IN BONE DESTRUCTION IN RA
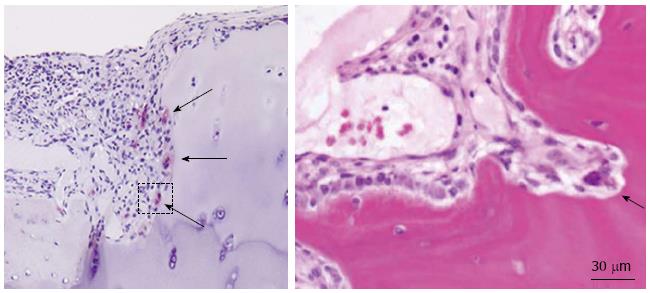
RA is characterized by proliferative pannus formation leading to erosive bone destruction originating from the interface of cartilage and bone (the bare area). Synovial tissues of RA joints produce various inflammatory cytokines, such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), which are believed to play important roles in joint destruction. The cellular mechanism of bone and cartilage destruction in RA still remains unclear, but recent studies have revealed the essential role of osteoclasts (Figure 1). Bromley et al[14] observed a number of acid phosphatase-positive multinucleated cells (chondroclasts and osteoclasts) in the erosive areas of RA joints obtained at the time of joint replacements. In collagen-induced arthritis, multinucleated giant cells were observed at the bone-pannus junctions of arthritic joints, and cells isolated from the lesions were able to differentiate into mature osteoclasts. Gravallese et al[15] also found multinucleated cells present on subchondral bone surface and in the areas of direct invasion of pannus into subchondral bone. Their important discovery was that those multinucleated cells were positive for unique markers of osteoclasts such as tartrate-resistant acid phosphatase (TRAP), cathepsin K, and calcitonin receptors, satisfying the major criteria of mature osteoclasts. Interestingly, some multinucleated cells and mononuclear cells apart from the bone surface were TRAP-positive. These findings suggest the possible role of synovial tissues for osteoclastogenesis in RA. To reveal the osteoclastogenic potential of RA synovial tissues, synovial cells from RA synovia were cultured in the presence of osteotropic factors such as 1β,25-dihydroxyvitamin D3 [1,25(OH)2D3] and macrophage colony-stimulating factor (M-CSF)[16]. After 3 wk of culture, we observed many multinucleated giant cells, which were TRAP-positive, possessed abundant calcitonin receptors, and made resorption pits on dentine slices. We also demonstrated that peripheral monocytes can differentiate into osteoclast-like cells when co-cultured with synovial fibroblasts obtained from RA synovial tissues in the presence of 1,25(OH)2D3 and M-CSF. Similar results were reported by Fujikawa et al[17]. They found that synovial macrophages isolated from RA synovial tissues can differentiate into osteoclast-like cells when co-cultured with UMR 106 rat osteoblast-like cells. These results suggest that RA synovial fibroblasts can support osteoclast differentiation from monocyte-macrophage lineage precursor cells under a suitable condition, at least in vitro.
Figure 1 Inflammatory synovial proliferation and bone erosion (arrows) by osteoclasts in rheumatoid arthritis patients.
INVOLVEMENT OF RANKL/RANK PATHWAYS IN BONE DESTRUCTION IN RA
Remarkable progress has been made in recent years in the field of osteoclast research primarily due to the finding of the receptor activator of nuclear factor κB (NF-κB) ligand (RANKL)/RANK system[18]. RANKL is a member of the TNF superfamily of cytokines, which was originally identified as a membrane-bound survival factor for dendritic cells produced by activated T cells[19]. The expression of RANKL can be also induced in osteoblasts and bone marrow stromal cells by osteotropic hormones such as 1,25(OH)2D3 and parathyroid hormone[20]. In the presence of M-CSF, RANKL can stimulate osteoclast differentiation from hematopoietic precursor cells in vitro[20]. RANKL also acts on mature osteoclasts and activates the bone-resorbing activity and survival of the cells. RANKL binds to its receptor RANK, a transmembrane receptor belonging to the TNF receptor superfamily, which is expressed in monocyte-macrophage lineage osteoclast precursor cells as well as in mature osteoclasts and dendritic cells. Binding of RANKL to RANK induces intracellular signals including NF-κB activation and c-Jun N-terminus kinase activation. The other important actor in this system is osteoprotegerin (OPG) a soluble receptor of RANKL, belonging to the TNF receptor superfamily[19]. OPG specifically binds to RANKL, and inhibits RANKL activity by preventing its binding to RANK.
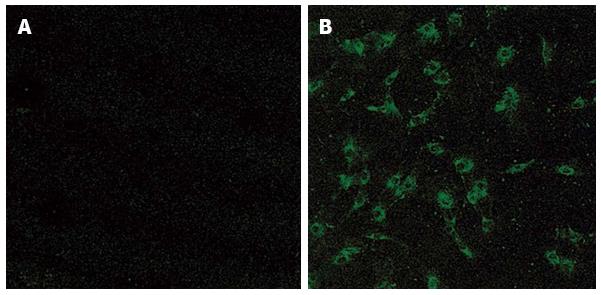
The essential role of RANKL/RANK signaling pathways in osteoclast development in vivo has been established by a series of targeted gene disruption experiments[19] comprising, the targeted disruption of either RANKL or RANK induced osteopetrosis in mice, a pathological bone disease which is characterized by an increased bone mass due to a deficiency in osteoclast differentiation[21,22]. We and another group found that mice deficient in TRAF6, a signaling molecule involved in RANK signaling, also showed osteopetrotic phenotypes. In contrast, the targeted disruption of OPG induces reduced bone mass in mice, reminiscent of osteoporosis, due to the increased number and activity of osteoclasts[18,23,24]. These results clearly demonstrate the essential role of RANKL/RANK pathways in osteoclast development and activation in vivo. The next question is whether the RANKL/RANK system is also involved in pathological bone destruction, such as in RA. We and others have revealed by Northern blotting, immunocytochemistry and in situ hybridization (Figure 2) that RANKL is highly expressed in synovial fibroblasts[12,15,25,26]. 1,25(OH)2D3 treatment increased the expression of RANKL in synovial fibroblasts and reduced the expression of OPG in the cells. RANKL expression was also detected in CD4+ T lymphocytes in RA synovial tissues by in situ hybridization. Kong et al[27] demonstrated that activated CD4+ T lymphocytes fixed with paraformaldehyde or culture supernatants from activated T cells can support osteoclast differentiation through the surface-bound and/or soluble RANKL they produce. They also showed that RANKL was expressed on the surface of activated T cells in synovial tissues of adjuvant arthritis rats[27]. These results suggest the important role of activated T lymphocytes in bone and joint destruction in RA. However, the role of T cells in osteoclast development is still controversial because activated T cells also produce many cytokines which inhibit osteoclast differentiation, such as interferon-β and IL-10. In any case, these studies indicate that RANKL produced by synovial fibroblasts and/or activated T lymphocytes in RA synovial tissues may play an essential role in osteoclast development and bone destruction in RA. Based on these findings, Kong et al[27] proposed that OPG can be a potent therapeutic agent against bone destruction in RA. Exogenous administration of recombinant OPG suppressed bone and joint destruction in rat adjuvant arthritis.
Figure 2 Immunostaining of synovial fibroblasts obtained from osteoarthritis (A) and rheumatoid arthritis (B) patients with anti-receptor activator of nuclear factor κB ligand antibody.
Reduced bone destruction in a patient with osteopetrosis and RA
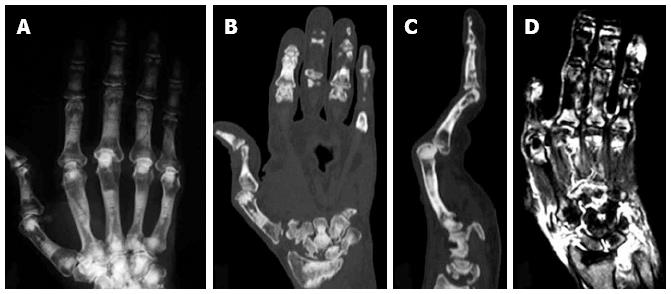
In addition to the animal studies described above, the importance of osteoclasts in bone destruction in RA was further confirmed by the clinical finding in a RA patient with osteopetrosis[28]. Osteopetrosis is an inherited disorder characterized by an increase in bone mass[29]. In humans, osteopetrosis comprises a heterogeneous group of diseases, which are classified into three major groups on the basis of inheritance, age of onset, severity, and secondary clinical features: autosomal recessive infantile malignant osteopetrosis, autosomal recessive intermediate mild osteopetrosis, and autosomal dominant adult onset benign osteopetrosis. The most frequent form of osteopetrosis, which has autosomal dominant (ADO) inheritance (incidence 5:100000), is also called Albers-Schönberg disease or ADO type II. ADO type II is characterized by vertebral endplate thickening (rugger-jersey appearance), fragile bones with multiple fractures and delayed healing. Recent studies have shown that the CLCN7 gene encoding type 7 chloride channel, which is essential for the acidification of the extracellular environment in resorption lacuna by osteoclasts, is a candidate gene for ADO type II. We recently reported a very rare case of RA associated with ADO type II. In spite of the severe inflammation and rapid progression of cartilage destruction in the patient, the progression of bone erosion was quite slow (Figure 3)[28]. These clinical findings further confirm the critical role of osteoclasts in bone destruction in RA but not in inflammation or cartilage destruction.
Figure 3 Plain X ray (A), computed tomography scan (B and C) and magnetic resonance imaging (D) of the right hand of an autosomal dominant II patient with rheumatoid arthritis.
Erosion of the carpal bones (B) and severe synovitis, as determined by the high intensity areas by T2-weighted magnetic resonance imaging images (D), were observed[14].
The mechanisms of action of aminobisphosphonate
Since osteoclasts are critically involved in bone destruction in RA, therapeutics which target osteoclasts could be good candidates for the treatment of RA. One of the most promising group of reagents which inhibit osteoclast function is bisphosphonates. Bisphosphonates (BPs), stable analogs of pyrophosphate, strongly inhibit bone resorption and have been used to treat various diseases driven by increased bone resorption, such as postmenopausal osteoporosis. Although BPs are poorly absorbed from the intestine, they are quickly deposited on the bone surface once absorbed. BPs are divided into two groups according to the structure of the side chains, a nitrogen-containing type (N-BPs) and a non-nitrogen-containing type. Non-nitrogen-containing BPs are reported to act through the intracellular accumulation of non-hydrolyzable ATP analogs that exert cytotoxic effects on OCs, while N-BPs inhibit the mevalonate pathway and prevent the post-translational prenylation of small GTP-binding proteins such as Ras, Rho, Rac and Cdc42. We recently reported that risedronate, one of the N-BPs, induced osteoclast apoptosis by suppressing the Erk pathway and increasing the expression of a pro-apoptotic Bcl family protein, Bim, while it reduced bone-resorbing activity of the cells through suppression of the Akt pathway[30].
Osteoporosis and osteoporosis-related fractures are common in RA patients[31-33] and several studies have demonstrated that bisphosphonates effectively increase bone mineral density and decrease fragile fractures in RA patients[34-36]. In spite of these strong and specific inhibitory effects of bisphosphonates on osteoclasts, only limited clinical data demonstrate the effectiveness of bisphosphonates in RA patients. Jarette et al[37]reported preliminary evidence that treatment with zoledronic acid plus methotrexate showed better results in reducing bone destruction than methotrexate alone. However, many other studies have failed to show positive effects of bisphosphonates against bone destruction in RA[38-40]. This may be because, in these studies, the treatment was initiated too late or the strength of the bisphosphonates used was not enough to treat the bone destruction in RA.
Effects of anti-RANKL antibody on bone destruction in RA
Denosumab is a fully human monoclonal antibody that specifically and avidly binds to RANKL. Previous clinical studies have demonstrated that administration 60 mg of denosumab subcutaneously every 6 mo to postmenopausal women with osteoporosis significantly reduced bone turnover markers, increased bone mineral density, and reduced osteoporosis-related fractures[41]. Because of the critical role of the RANKL-RANK system in osteoclast development and bone destruction in RA, clinical studies were conducted to analyze the effect of denosumab on RA[42-44]. Sharp et al[42]demonstrated that twice-yearly subcutaneous injections of denosumab (60 mg or 180 mg) with ongoing methotrexate treatment significantly reduced cortical bone loss in RA patients for up to 12 mo. In a phase II clinical trial, subcutaneous administration of denosumab every 6 mo to patients with active RA suppressed the progression of subchondral bone erosions and systemic bone loss, although there was no an apparent reduction of joint inflammation or joint space narrowing. In addition, denosumab treatment over 12 mo increased mean lumbar spine and hip bone mineral density and reduced bone turnover markers such as sCTx-I and P1NP compared with placebo, regardless of baseline bone mineral density or marker levels or concomitant bisphosphonate or glucocorticoid use[44]. The rate of adverse events and serious infections requiring hospitalization did not differ between patients treated with denosumab and with placebo. These clinical observations, in addition to the results of the basic studies, clearly suggest that denosumab is effective in preventing bone erosion but not cartilage destruction in RA.
CONCLUSION
The ultimate goal of the treatment of RA is to prevent the bone and joint destruction and preserve the daily activity of patients. Recent studies have revealed that osteoclasts are involved in the pathogenesis of bone and joint destruction in RA and can be a potent therapeutic target of the disease[4,45]. Therapeutics targeting osteoclast formation or function can at least ameliorate the progression of these bone changes[27,43]. However, inhibition of osteoclast function by anti-resorptive agents alone do not completely prevent bone erosion in RA in spite of their preventive effects against systemic bone loss. Therefore, the combination of anti-resorption therapy and anti-inflammatory therapy could be an ideal therapy for RA. Thus, anti-RANKL therapy in combination with the anti-inflammatory therapy is a promising strategy for RA treatment, and safe and effective therapies against RA may be expected in the near future.