Copyright
©2013 Baishideng Publishing Group Co.
World J Orthop. Oct 18, 2013; 4(4): 178-185
Published online Oct 18, 2013. doi: 10.5312/wjo.v4.i4.178
Published online Oct 18, 2013. doi: 10.5312/wjo.v4.i4.178
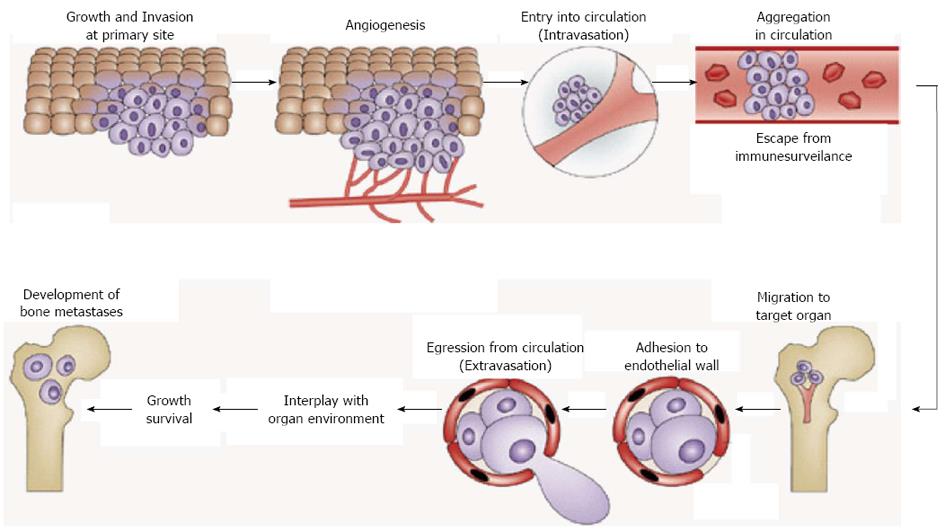
Figure 1 Steps of distant metastasis.
Distant metastasis of cancer can be divided into two broad processes including before and after cancer cell arrest in target organs. Before the arrival at distant target organs, cancer cells go through growth, invasion, angiogenesis, intravasation, escape from host immune surveillance, and migration to their target organ. Cancer cells in the circulation are called circulating tumor cells (CTCs). This process is common for all metastatic cancer cells. CTCs reached their target organ subsequently show extravasation and finally arrest in the target organ. These cancer cells are called disseminated tumor cells (DTCs). DTCs change their phenotype via interactions with bone. Establishment of the interactions with bone environments is the most critical step for disseminated breast tumor cells to develop bone metastases. This process is unique depending on the target organs. Adapted from Ref. 14 and modified.
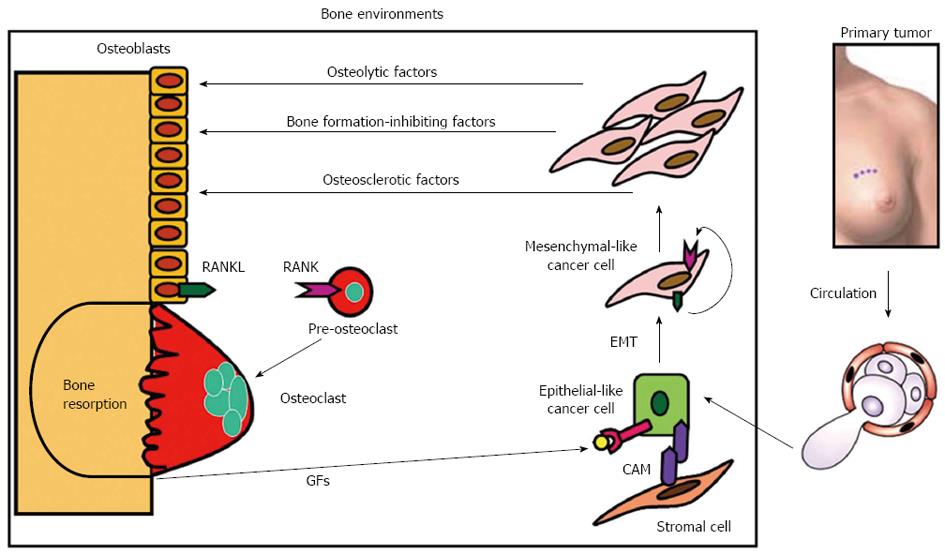
Figure 2 Vicious cycle between bone and breast cancer cells in bone metastasis.
Bone-derived growth factors (GFs) such as insulin-like growth factors (IGF) and transforming growth factor-β (TGF-β) that are continually released via osteoclastic bone resorption promote proliferation and suppress apoptosis and stimulate the production of parathyroid hormone-related protein (PTH-rP), prostaglandin E2 (PGE2) and interleukin-11 (IL-11) in metastatic breast cancer cells migrating from primary site via circulation. Bone is fertile soil for metastatic cancer cells representing the concept of “Seed and Soil” theory proposed by Paget[9]. These osteolytic factors further stimulate osteoclastic bone resorption, followed by enhanced release of bone-stored growth factors, thus establishing “vicious cycle”. Prostate cancer may produce osteosclerotic factors and multiple myeloma is shown to produce bone formation-inhibiting factors[14-16]. These osteolytic factors up-regulate the production of ligand for receptor activator of nuclear factor-κB (RANKL) in osteoblasts/stromal cells, which then interacts with its receptor receptor activator of nuclear factor-κB (RANK) in the osteoclast precursors promoting osteoclastogenesis and bone resorption. RANKL and RANK play a central role in the establishment of the vicious cycle. Metastatic breast cancer cells themselves occasionally express RANKL and RANK to develop an autocrine stimulation of carcinogenesis or tumorigenesis. RANKL/RANK expression in breast cancer cells could be a predicting indicator for subsequent occurrence of bone metastasis. Some metastatic breast cancer cells reside in stromal cell niche via cell-cell contact that is mediated by cell adhesion molecules (CAMs) and stay dormant. Metastatic breast cancer cells undergo epithelial-mesenchymal transition (EMT) by changing cell shape from epithelial to mesenchymal and acquire more aggressiveness in the presence of bone-derived TGF-β.
- Citation: Yoneda T, Tanaka S, Hata K. Role of RANKL/RANK in primary and secondary breast cancer. World J Orthop 2013; 4(4): 178-185
- URL: https://www.wjgnet.com/2218-5836/full/v4/i4/178.htm
- DOI: https://dx.doi.org/10.5312/wjo.v4.i4.178