Published online Dec 20, 2018. doi: 10.5306/wjco.v9.i8.180
Peer-review started: August 4, 2018
First decision: September 3, 2018
Revised: September 20, 2018
Accepted: November 27, 2018
Article in press: November 27, 2018
Published online: December 20, 2018
Processing time: 140 Days and 9.9 Hours
Cellular senescence is a form of permanent cell cycle arrest that can be triggered by a variety of cell-intrinsic and extrinsic stimuli, including telomere shortening, DNA damage, oxidative stress, and exposure to chemotherapeutic agents and ionizing radiation. Although the induction of apoptotic cell death is a desirable outcome in cancer therapy, mutations and/or deficiencies in the apoptotic signaling pathways have been frequently identified in many human cancer types, suggesting the importance of alternative apoptosis-independent therapeutic approaches for cancer treatment. A growing body of evidence has documented that senescence induction in tumor cells is a frequent response to many anticancer modalities including cyclin-dependent kinases 4/6 small molecule inhibitor-based targeted therapeutics and T helper-1 cytokine-mediated immunotherapy. This review discusses the recent advances and clinical relevance of therapy-induced senescence in cancer treatment.
Core tip: Both in vitro and in vivo studies have revealed that senescence induction in human cancer cells is a prominent response to chemotherapy and irradiation. A senescent phenotype has been detected in clinical tumor samples of breast cancer patients following preoperative neoadjuvant chemotherapy. Immunotherapy-induced senescence of cancer cells contributes to tumor regression in vivo. The induction of cancer cell senescence appears to be a major mechanism of action of cyclin-dependent kinases 4/6 small molecule inhibitor-based targeted therapy. Collectively, these preclinical and clinical observations have demonstrated an important role for senescence induction in cancer treatment.
- Citation: Qin S, Schulte BA, Wang GY. Role of senescence induction in cancer treatment. World J Clin Oncol 2018; 9(8): 180-187
- URL: https://www.wjgnet.com/2218-4333/full/v9/i8/180.htm
- DOI: https://dx.doi.org/10.5306/wjco.v9.i8.180
Cellular senescence is an anti-proliferative program that restrains tumorigenesis via limiting the propagation and transformation of aging and damaged cells[1,2]. Senescence can be induced by a variety of cell-intrinsic and extrinsic stimuli, including telomere shortening, DNA damage, oxidative stress, chemotherapeutic agent treatment, radiation exposure, and the activation of oncogenes such as Ras[3-6]. Senescent cells are in a state of irreversible cell cycle arrest and are characterized by a flat and enlarged morphology, elevated senescence-associated β-galactosidase activity, and the activation of the p53-p21 and p16-Rb signaling pathways[2,7]. Additional characteristics of senescence cells include the presence of senescence-associated heterochromatic foci and the senescence-associated secretory phenotype[8,9]. Traditionally, senescence induction is considered to be an important mechanism of cancer prevention and cellular aging[10]. However, numerous recent studies have revealed that senescence is a prominent solid tumor response to therapy in which cancer cells evade apoptosis and instead enter into a stable and prolonged cell cycle arrest[3,5]. Furthermore, targeted therapeutics and cancer immunotherapies also have been shown to cause cancer regression via the induction of senescence in tumor cells[11,12]. These findings underscore the significant implications of senescence induction in cancer treatment.
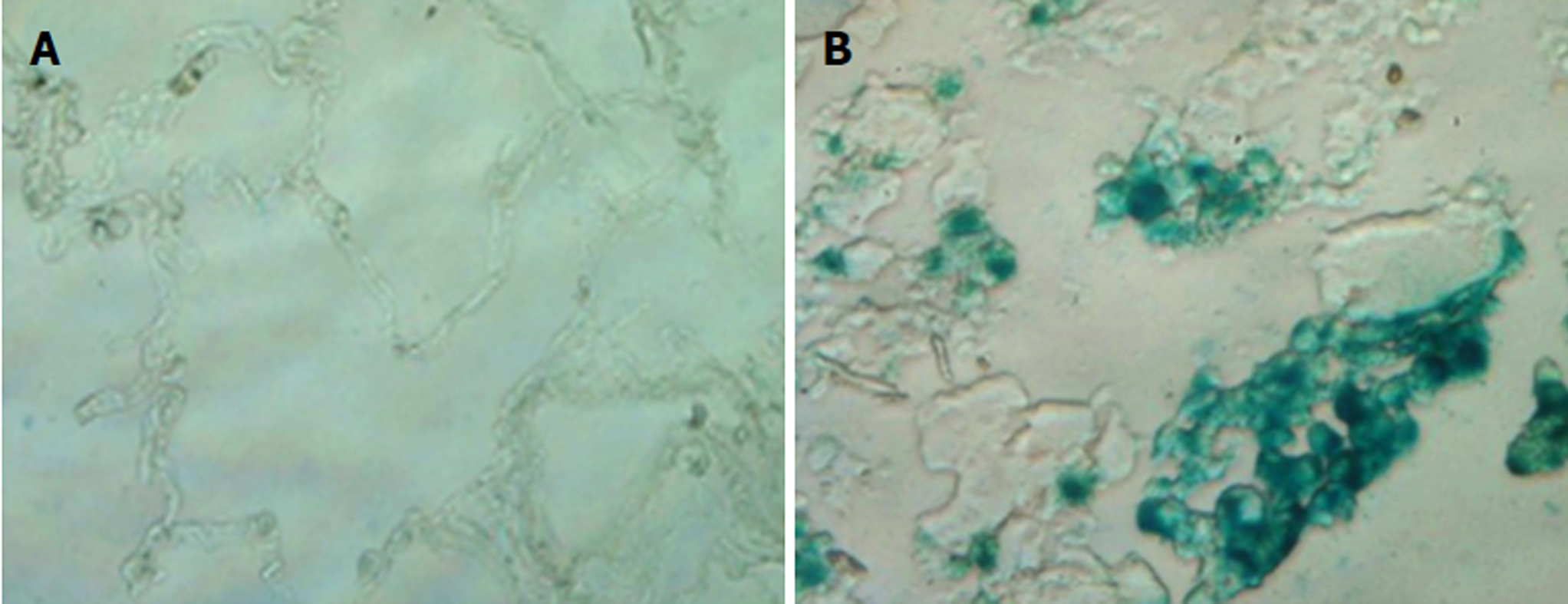
Cellular senescence is a state of permanent cell growth arrest that often has been included with apoptosis as one of the terminal outcomes of cancer treatment. It is well-established that different classes of chemotherapeutic agents and ionizing radiation (IR) induce senescent-like phenotypes in tumor cells both in vitro and in vivo[2,5,11,13]. Our recent studies indicate that resveratrol induces premature senescence in lung cancer cells via promoting reactive oxygen species (ROS)-mediated DNA damage[14]. Inactivation of Myc results in tumor regression through induction of senescence but not apoptosis in hepatocellular carcinoma and lymphoma cells[15]. In addition, it has been reported that the restoration of p53 promotes tumor regression in vivo via induction of senescence in tumor cells[16,17]. More importantly, Schmitt et al[13] showed that senescence induction contributes directly to the outcome of chemotherapy in vivo. In agreement with this finding, there is evidence that the senescent phenotype is present in clinical tumor samples of breast cancer patients after undergoing preoperative neoadjuvant chemotherapy[18]. Moreover, our recent studies found that the number of senescent cells is markedly increased in tumor samples of lung cancer patients as compared to normal lung tissues (Figure 1). However, the clinical relevance of the presence of senescent cells in tumor samples has yet to be determined.
Notably, human tumors may harbor various types of defects in the apoptotic signaling pathways (e.g., loss of p53 and overexpression of BCL2) and, as a result, are resistant to apoptosis-based anticancer therapies[19,20]. In these conditions, a senescence-targeted strategy is likely to be a more effective and practical than traditional apoptosis-inducing approaches. A welcome benefit to this approach is that therapy-induced senescence can be achieved using much lower doses of therapy than those required to induce apoptosis[2,14]. Compared to the traditional apoptosis-inducing strategies, this low dose approach would significantly reduce the side effects of anticancer therapy and thus improve the quality of life for cancer patients.
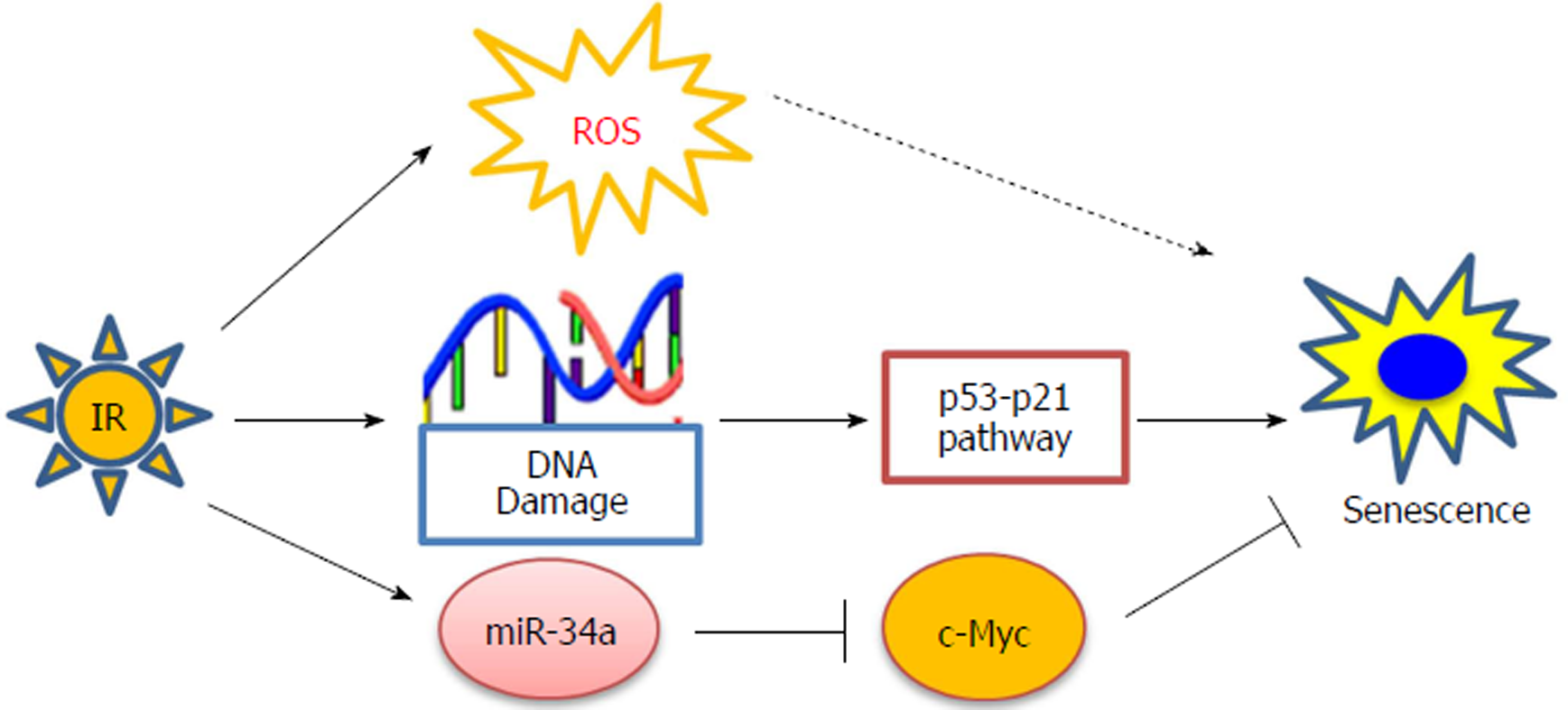
Radiotherapy is used in over 50% of patients during the course of cancer treatment and is effective both as a curative modality and for palliation[21]. However, many epithelial-derived tumors including lung cancer have been shown to be resistant to radiation-induced apoptosis[6,20]. Consistent with these observations, our recent studies have demonstrated that IR primarily induces premature senescence rather than apoptosis in human non-small cell lung cancer (NSCLC) cells[6]. Subsequent mechanistic studies revealed that the p53-p21 signaling pathway plays a critical role in modulating IR-induced senescence in NSCLC cells (Figure 2). More importantly, we showed that pharmacologic activation of p53 by Nutilin-3 sensitizes NSCLC cells to radiation by enhancing IR-induced senescence[6]. These results suggest that pharmacological promotion of senescence induction can be exploited as a novel and effective therapeutic approach to improve the efficacy of lung cancer radiotherapy.
MicroRNA-34a (miR-34a) has been shown to be a p53 responsive miRNA that is involved in regulating senescence induction[22-24]. Our recent studies showed that the expression of miRNA-34a was increased substantially in human NSCLC cells after exposure to IR[25]. Moreover, we found that treatment with synthetic miR-34a mimics enhances the anti-cancer effects of irradiation by promoting senescence induction via targeting the Myc oncoprotein in NSCLC cells (Figure 2). These findings not only provide new insights into the mechanisms by which IR induces senescence in lung cancer cells but also support the hypothesis that pharmacological manipulation of senescence induction should be explored as a new therapeutic strategy for improving the outcome of cancer radiotherapy.
The cyclin-dependent kinases (CDKs) are a large family of serine-threonine kinases that play a pivotal role in regulating cell cycle progression. Commitment to cell cycle entry occurs during the G1 phase, when CDK4 and CDK6 form active complexes with one of the three D-type cyclins (D1, D2, or D3). Cyclin D-CDK4/6 complexes promote G1/S transition by phosphorylating the Retinoblastoma tumor suppressor (Rb), which in turn releases its suppression of the E2F transcription factor, resulting in initiation of E2F-dependent gene transcription and DNA synthesis. CDK4 and CDK6 are overexpressed in the majority of human cancers and they presumably promote tumorigenesis by suppressing senescence in cancer cells[11,26,27]. Both preclinical studies and clinic trials have demonstrated the therapeutic potential of CDK4/6 small molecule inhibitors against several solid tumors[28-31]. Recently, a CDK4/6-specific inhibitor, palbociclib (also known as PD0332991], was approved by the FDA for the treatment of advanced estrogen receptor-positive breast cancer[32].
It is well established that CDK4/6 inhibitors suppress tumor growth by the induction of senescence in various type of cancer cells[11,26,27,32]. Mechanistic studies have revealed that CDK4/6 suppress tumor cell senescence via phosphorylating and activating the Forkhead Box M1 (FOXM1) transcription factor, and that activation of FOXM1 may inhibit the induction of senescence in tumor cells by repressing ROS-mediated oxidative stress[33]. However, the precise mechanisms whereby FOXO1 controls ROS and oxidative stress remain unclear. Promyelocytic leukemia (PML) acts as a tumor suppressor by inducing senescence in response to oncogenic stress[34,35]. Interestingly, Acevedo et al[36] showed that CDK4/6 suppress PML-induced senescence in tumor cells, suggesting that CDK4/6 inhibitors such as PD0332991 may induce senescence in cancer cells by blocking CDK4/6-mediated suppression of PML’s senescence-inducing function. Moreover, CDK4/6 inhibition has been shown to be efficacious against therapy-resistant HER2 positive breast cancers[37]. These results imply that although some tumors may be resistant to apoptosis-inducing treatments, it is likely that they are still responsive to senescence-targeted therapies.
Aurora kinases are a family of serine/threonine mitotic kinases that regulate a diverse set of mitotic processes including spindle formation, centrosome segregation, checkpoint activation and kinetochore-microtubule connections[38,39]. Aurora A kinase (AurA) is located on 20q13.2, a chromosomal region that is frequently amplified in many human cancers. Overexpression of AurA correlates with poor clinical outcomes in patients with hormone-related cancers[40]. Aurora kinase inhibitors (AKIs) are a promising class of drugs for cancer treatment. Several small molecule AKIs including MK-0457, PHA-739358 and MLN8237 have been investigated in clinical trials for the treatment of human cancers[41-43].
Alisertib (MLN8237) is an orally bioavailable, second-generation selective inhibitor of Aurora kinases which binds to AurA and prevents its phosphorylation and activation[44]. It has been shown that senescence is likely a terminal outcome of AurA inhibition and that pharmacological inhibition of AurA induces senescence in colon cancer cells both in vitro and in vivo[45]. AurA is overexpressed in gliomas and treatment with Alisertib induces senescence and differentiation in glioblastoma cells[46]. Moreover, recent studies have revealed that p53 and p73 tumor suppressors play a critical role in modulating tumor cell responses to AKIs. Tentler et al[47] reported that Alisertib treatment resulted in apoptosis in human triple-negative breast cancer (TNBC) cells with functional p53 and p73, whereas it induced senescence in cells lacking p53 and p73. These findings suggest that AKI may induce growth arrest in TNBC through a p53- and p73-independent mechanism. Nevertheless, further studies are warranted to better understand the in-depth mechanisms whereby AKIs induce senescence in human tumor cells.
Immunotherapy has shown promising efficacy against human cancers both in preclinical studies and in clinical trials[48-50]. T helper-1 (Th1) cells play a critical role in mediating the antitumor adoptive immune response[51,52]. Th1 T-cell infiltration is associated with better outcomes and Th1 cytokines are more effective at promoting the antitumor functions of CD40L-activated macrophages as compared to Th2 cytokines[53]. Moreover, Müller-Hermelink et al[54] showed that treatment with T antigen (Tag)-specific Th1 cells induced tumor dormancy and doubled the survival of tumor-bearing mice by arresting tumor growth, without detectable evidence of tumor cell cytotoxicity, necrosis or apoptosis. These findings suggest that other alternative non-cytotoxic mechanisms are likely involved in Th1- and Th1 cytokine-mediated immunotherapy. In agreement with this idea, it has been shown that treatment with Th1 cytokines, IFN-γ and tumor necrosis factor (TNF) induces senescence in cancer cells[12].
It has been reported that cancer immunotherapy causes tumor growth arrest and regression without any clear evidence of tumor cell death or cellular toxicity[55,56]. Moreover, it has been shown that immunotherapy-induced tumor growth arrest was associated with an increase in interferon (IFN)-γ-producing CD4+ Th1 cells, but not CD8+ cytotoxic T lymphocytes[55-57]. These observations suggest that senescence induction may contribute to the outcome of immunotherapy. In agreement with this, Braumüller et al[12] showed that Th1 immunotherapy arrests tumor progression through IFN-γ- and TNF-induced cancer cell senescence in vivo. Their subsequent mechanistic studies revealed that TNFR1 signaling is required for Th1 immunotherapy-induced tumor cell senescence and that activation of the p16-Rb pathway is involved in senescence induction[12]. In line with these findings, it has been shown recently that CDK4/6 inhibition-induced tumor cell senescence promotes antitumor immunity in preclinical models[58,59]. Furthermore, there is evidence that IL-12 inhibits the growth of human sarcoma cells by senescence induction[60]. Taken together, these results highlight a novel link between cancer immunotherapy and the induction of senescence in tumor cells.
Given the fact that many human cancers may harbor different defects in the apoptotic signaling pathways, and thus are inherently resistant to chemoradiotherapy-induced apoptosis, there is a critical need for the development of innovative apoptosis-independent approaches for cancer therapeutics. The induction of tumor cell senescence has been well-established as a prominent therapeutic response of cancer cells to chemotherapy, radiation, small-molecule inhibitor-based targeted therapeutics and immunotherapy. These results underscore the important implications of senescence induction in cancer treatment. Although senescent cells were detected in clinical tumor samples of cancer patients, the clinical significance and applications of therapy-induced senescence remain incompletely understood. It is still unclear if senescence markers in patient tumor samples will be useful for prognosis prediction and/or therapeutic efficacy evaluation in the clinic. In addition, further studies, particularly clinical investigations, are necessary to better elucidate the clinical relevance and significance of therapy-induced senescence in the treatment of cancer.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chiang TA, Wei HF S- Editor: Wang XJ L- Editor: A E- Editor: Bian YN
| 1. | Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1418] [Cited by in RCA: 1988] [Article Influence: 180.7] [Reference Citation Analysis (0)] |
| 2. | Ewald JA, Desotelle JA, Wilding G, Jarrard DF. Therapy-induced senescence in cancer. J Natl Cancer Inst. 2010;102:1536-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 636] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 3. | He S, Sharpless NE. Senescence in Health and Disease. Cell. 2017;169:1000-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 1250] [Article Influence: 156.3] [Reference Citation Analysis (0)] |
| 4. | Hydbring P, Bahram F, Su Y, Tronnersjö S, Högstrand K, von der Lehr N, Sharifi HR, Lilischkis R, Hein N, Wu S. Phosphorylation by Cdk2 is required for Myc to repress Ras-induced senescence in cotransformation. Proc Natl Acad Sci USA. 2010;107:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Chang BD, Swift ME, Shen M, Fang J, Broude EV, Roninson IB. Molecular determinants of terminal growth arrest induced in tumor cells by a chemotherapeutic agent. Proc Natl Acad Sci USA. 2002;99:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 231] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Luo H, Yount C, Lang H, Yang A, Riemer EC, Lyons K, Vanek KN, Silvestri GA, Schulte BA, Wang GY. Activation of p53 with Nutlin-3a radiosensitizes lung cancer cells via enhancing radiation-induced premature senescence. Lung Cancer. 2013;81:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363-9367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5281] [Cited by in RCA: 5769] [Article Influence: 192.3] [Reference Citation Analysis (0)] |
| 8. | Narita M, Nũnez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1626] [Cited by in RCA: 1701] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 9. | Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1029] [Cited by in RCA: 1298] [Article Influence: 108.2] [Reference Citation Analysis (0)] |
| 10. | Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dörken B, Jenuwein T, Schmitt CA. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 884] [Cited by in RCA: 934] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 11. | Rader J, Russell MR, Hart LS, Nakazawa MS, Belcastro LT, Martinez D, Li Y, Carpenter EL, Attiyeh EF, Diskin SJ. Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin Cancer Res. 2013;19:6173-6182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 308] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 12. | Braumüller H, Wieder T, Brenner E, Aßmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 563] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 13. | Schmitt CA, Fridman JS, Yang M, Lee S, Baranov E, Hoffman RM, Lowe SW. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 2002;109:335-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 781] [Cited by in RCA: 803] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 14. | Luo H, Yang A, Schulte BA, Wargovich MJ, Wang GY. Resveratrol induces premature senescence in lung cancer cells via ROS-mediated DNA damage. PLoS One. 2013;8:e60065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 15. | Wu CH, van Riggelen J, Yetil A, Fan AC, Bachireddy P, Felsher DW. Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation. Proc Natl Acad Sci USA. 2007;104:13028-13033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 332] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 16. | Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1709] [Cited by in RCA: 1934] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 17. | Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, Jacks T. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1302] [Cited by in RCA: 1408] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 18. | te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002;62:1876-1883. [PubMed] |
| 19. | Mori S, Ito G, Usami N, Yoshioka H, Ueda Y, Kodama Y, Takahashi M, Fong KM, Shimokata K, Sekido Y. p53 apoptotic pathway molecules are frequently and simultaneously altered in nonsmall cell lung carcinoma. Cancer. 2004;100:1673-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Yin DX, Schimke RT. BCL-2 expression delays drug-induced apoptosis but does not increase clonogenic survival after drug treatment in HeLa cells. Cancer Res. 1995;55:4922-4928. [PubMed] |
| 21. | Ngiow SF, McArthur GA, Smyth MJ. Radiotherapy complements immune checkpoint blockade. Cancer Cell. 2015;27:437-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Hermeking H. p53 enters the microRNA world. Cancer Cell. 2007;12:414-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 409] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 23. | Tazawa H, Tsuchiya N, Izumiya M, Nakagama H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci USA. 2007;104:15472-15477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 758] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 24. | Wang Y, Scheiber MN, Neumann C, Calin GA, Zhou D. MicroRNA regulation of ionizing radiation-induced premature senescence. Int J Radiat Oncol Biol Phys. 2011;81:839-848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | He X, Yang A, McDonald DG, Riemer EC, Vanek KN, Schulte BA, Wang GY. MiR-34a modulates ionizing radiation-induced senescence in lung cancer cells. Oncotarget. 2017;8:69797-69807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Yoshida A, Lee EK, Diehl JA. Induction of Therapeutic Senescence in Vemurafenib-Resistant Melanoma by Extended Inhibition of CDK4/6. Cancer Res. 2016;76:2990-3002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 27. | Klein ME, Dickson MA, Antonescu C, Qin LX, Dooley SJ, Barlas A, Manova K, Schwartz GK, Crago AM, Singer S. PDLIM7 and CDH18 regulate the turnover of MDM2 during CDK4/6 inhibitor therapy-induced senescence. Oncogene. 2018;37:5066-5078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Jansen VM, Bhola NE, Bauer JA, Formisano L, Lee KM, Hutchinson KE, Witkiewicz AK, Moore PD, Estrada MV, Sánchez V. Kinome-Wide RNA Interference Screen Reveals a Role for PDK1 in Acquired Resistance to CDK4/6 Inhibition in ER-Positive Breast Cancer. Cancer Res. 2017;77:2488-2499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 29. | Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375:1925-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1472] [Cited by in RCA: 2006] [Article Influence: 222.9] [Reference Citation Analysis (0)] |
| 30. | Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1200] [Cited by in RCA: 1432] [Article Influence: 130.2] [Reference Citation Analysis (0)] |
| 31. | Vijayaraghavan S, Karakas C, Doostan I, Chen X, Bui T, Yi M, Raghavendra AS, Zhao Y, Bashour SI, Ibrahim NK. CDK4/6 and autophagy inhibitors synergistically induce senescence in Rb positive cytoplasmic cyclin E negative cancers. Nat Commun. 2017;8:15916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 32. | Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer Discov. 2016;6:353-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 712] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 33. | Anders L, Ke N, Hydbring P, Choi YJ, Widlund HR, Chick JM, Zhai H, Vidal M, Gygi SP, Braun P. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell. 2011;20:620-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 443] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 34. | Ferbeyre G. PML a target of translocations in APL is a regulator of cellular senescence. Leukemia. 2002;16:1918-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Pearson M, Carbone R, Sebastiani C, Cioce M, Fagioli M, Saito S, Higashimoto Y, Appella E, Minucci S, Pandolfi PP. PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature. 2000;406:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 537] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 36. | Acevedo M, Vernier M, Mignacca L, Lessard F, Huot G, Moiseeva O, Bourdeau V, Ferbeyre G. A CDK4/6-Dependent Epigenetic Mechanism Protects Cancer Cells from PML-induced Senescence. Cancer Res. 2016;76:3252-3264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Goel S, Wang Q, Watt AC, Tolaney SM, Dillon DA, Li W, Ramm S, Palmer AC, Yuzugullu H, Varadan V. Overcoming Therapeutic Resistance in HER2-Positive Breast Cancers with CDK4/6 Inhibitors. Cancer Cell. 2016;29:255-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 347] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 38. | Lioutas A, Vernos I. Aurora A kinase and its substrate TACC3 are required for central spindle assembly. EMBO Rep. 2013;14:829-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Marumoto T, Honda S, Hara T, Nitta M, Hirota T, Kohmura E, Saya H. Aurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cells. J Biol Chem. 2003;278:51786-51795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 293] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 40. | Das K, Lorena PD, Ng LK, Shen L, Lim D, Siow WY, Narasimhan K, Teh M, Choolani M, Putti TC. Aurora-A expression, hormone receptor status and clinical outcome in hormone related cancers. Pathology. 2010;42:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Falchook GS, Bastida CC, Kurzrock R. Aurora Kinase Inhibitors in Oncology Clinical Trials: Current State of the Progress. Semin Oncol. 2015;42:832-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 42. | Dickson MA, Mahoney MR, Tap WD, D’Angelo SP, Keohan ML, Van Tine BA, Agulnik M, Horvath LE, Nair JS, Schwartz GK. Phase II study of MLN8237 (Alisertib) in advanced/metastatic sarcoma. Ann Oncol. 2016;27:1855-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 43. | Fathi AT, Wander SA, Blonquist TM, Brunner AM, Amrein PC, Supko J, Hermance NM, Manning AL, Sadrzadeh H, Ballen KK. Phase I study of the aurora A kinase inhibitor alisertib with induction chemotherapy in patients with acute myeloid leukemia. Haematologica. 2017;102:719-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 44. | Manfredi MG, Ecsedy JA, Meetze KA, Balani SK, Burenkova O, Chen W, Galvin KM, Hoar KM, Huck JJ, LeRoy PJ. Antitumor activity of MLN8054, an orally active small-molecule inhibitor of Aurora A kinase. Proc Natl Acad Sci USA. 2007;104:4106-4111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 355] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 45. | Huck JJ, Zhang M, McDonald A, Bowman D, Hoar KM, Stringer B, Ecsedy J, Manfredi MG, Hyer ML. MLN8054, an inhibitor of Aurora A kinase, induces senescence in human tumor cells both in vitro and in vivo. Mol Cancer Res. 2010;8:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | Lehman NL, O’Donnell JP, Whiteley LJ, Stapp RT, Lehman TD, Roszka KM, Schultz LR, Williams CJ, Mikkelsen T, Brown SL. Aurora A is differentially expressed in gliomas, is associated with patient survival in glioblastoma and is a potential chemotherapeutic target in gliomas. Cell Cycle. 2012;11:489-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 47. | Tentler JJ, Ionkina AA, Tan AC, Newton TP, Pitts TM, Glogowska MJ, Kabos P, Sartorius CA, Sullivan KD, Espinosa JM. p53 Family Members Regulate Phenotypic Response to Aurora Kinase A Inhibition in Triple-Negative Breast Cancer. Mol Cancer Ther. 2015;14:1117-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 48. | Dobrzanski MJ, Rewers-Felkins KA, Quinlin IS, Samad KA, Phillips CA, Robinson W, Dobrzanski DJ, Wright SE. Autologous MUC1-specific Th1 effector cell immunotherapy induces differential levels of systemic TReg cell subpopulations that result in increased ovarian cancer patient survival. Clin Immunol. 2009;133:333-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol. 2017;14:655-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 758] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 50. | Rosemblit C, Datta J, Lowenfeld L, Xu S, Basu A, Kodumudi K, Wiener D, Czerniecki BJ. Oncodriver inhibition and CD4+ Th1 cytokines cooperate through Stat1 activation to induce tumor senescence and apoptosis in HER2+ and triple negative breast cancer: implications for combining immune and targeted therapies. Oncotarget. 2018;9:23058-23077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Datta J, Xu S, Rosemblit C, Smith JB, Cintolo JA, Powell DJ Jr, Czerniecki BJ. CD4(+) T-Helper Type 1 Cytokines and Trastuzumab Facilitate CD8(+) T-cell Targeting of HER2/neu-Expressing Cancers. Cancer Immunol Res. 2015;3:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Thakur A, Schalk D, Sarkar SH, Al-Khadimi Z, Sarkar FH, Lum LG. A Th1 cytokine-enriched microenvironment enhances tumor killing by activated T cells armed with bispecific antibodies and inhibits the development of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2012;61:497-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Luheshi N, Davies G, Poon E, Wiggins K, McCourt M, Legg J. Th1 cytokines are more effective than Th2 cytokines at licensing anti-tumour functions in CD40-activated human macrophages in vitro. Eur J Immunol. 2014;44:162-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Müller-Hermelink N, Braumüller H, Pichler B, Wieder T, Mailhammer R, Schaak K, Ghoreschi K, Yazdi A, Haubner R, Sander CA. TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell. 2008;13:507-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 240] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 55. | Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 819] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 56. | Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 11786] [Article Influence: 785.7] [Reference Citation Analysis (0)] |
| 57. | Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698-2703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 755] [Cited by in RCA: 735] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 58. | Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, Chhabra S, Huang W, Liu H, Aref AR. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov. 2018;8:216-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 537] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 59. | Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, Khan N, Ubellacker JM, Xie S, Metzger-Filho O. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548:471-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 700] [Cited by in RCA: 1077] [Article Influence: 134.6] [Reference Citation Analysis (0)] |
| 60. | Schilbach K, Alkhaled M, Welker C, Eckert F, Blank G, Ziegler H, Sterk M, Müller F, Sonntag K, Wieder T. Cancer-targeted IL-12 controls human rhabdomyosarcoma by senescence induction and myogenic differentiation. Oncoimmunology. 2015;4:e1014760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |