Published online Jun 10, 2018. doi: 10.5306/wjco.v9.i3.42
Peer-review started: February 7, 2018
First decision: March 7, 2018
Revised: April 18, 2018
Accepted: May 23, 2018
Article in press: May 23, 2018
Published online: June 10, 2018
Processing time: 119 Days and 12.2 Hours
The functional impact of modifications of cellular RNAs, including mRNAs, miRNAs and lncRNAs, is a field of intense study. The role of such modifications in cancer has started to be elucidated. Diverse and sometimes opposite effects of RNA modifications have been reported. Some RNA modifications promote, while others decrease the growth and invasiveness of cancer. The present manuscript reviews the current knowledge on the potential impacts of N6-Methyladenosine, Pseudouridine, Inosine, 2’O-methylation or methylcytidine in cancer’s RNA. It also highlights the remaining questions and provides hints on research avenues and potential therapeutic applications, whereby modulating dynamic RNA modifications may be a new method to treat cancer.
Core tip: The present manuscript reviews the current knowledge on RNA modifications in cancer. The potential impacts of N6-Methyladenosine, Pseudouridine, Inosine, 2’O-methylation or methylcytidine in cancer’s RNA is presented and discussed. The review also highlights the remaining questions and provides hints on research avenues and potential therapeutic applications, whereby modulating dynamic RNA modifications may be a new method to treat cancer.
- Citation: Tusup M, Kundig T, Pascolo S. Epitranscriptomics of cancer. World J Clin Oncol 2018; 9(3): 42-55
- URL: https://www.wjgnet.com/2218-4333/full/v9/i3/42.htm
- DOI: https://dx.doi.org/10.5306/wjco.v9.i3.42
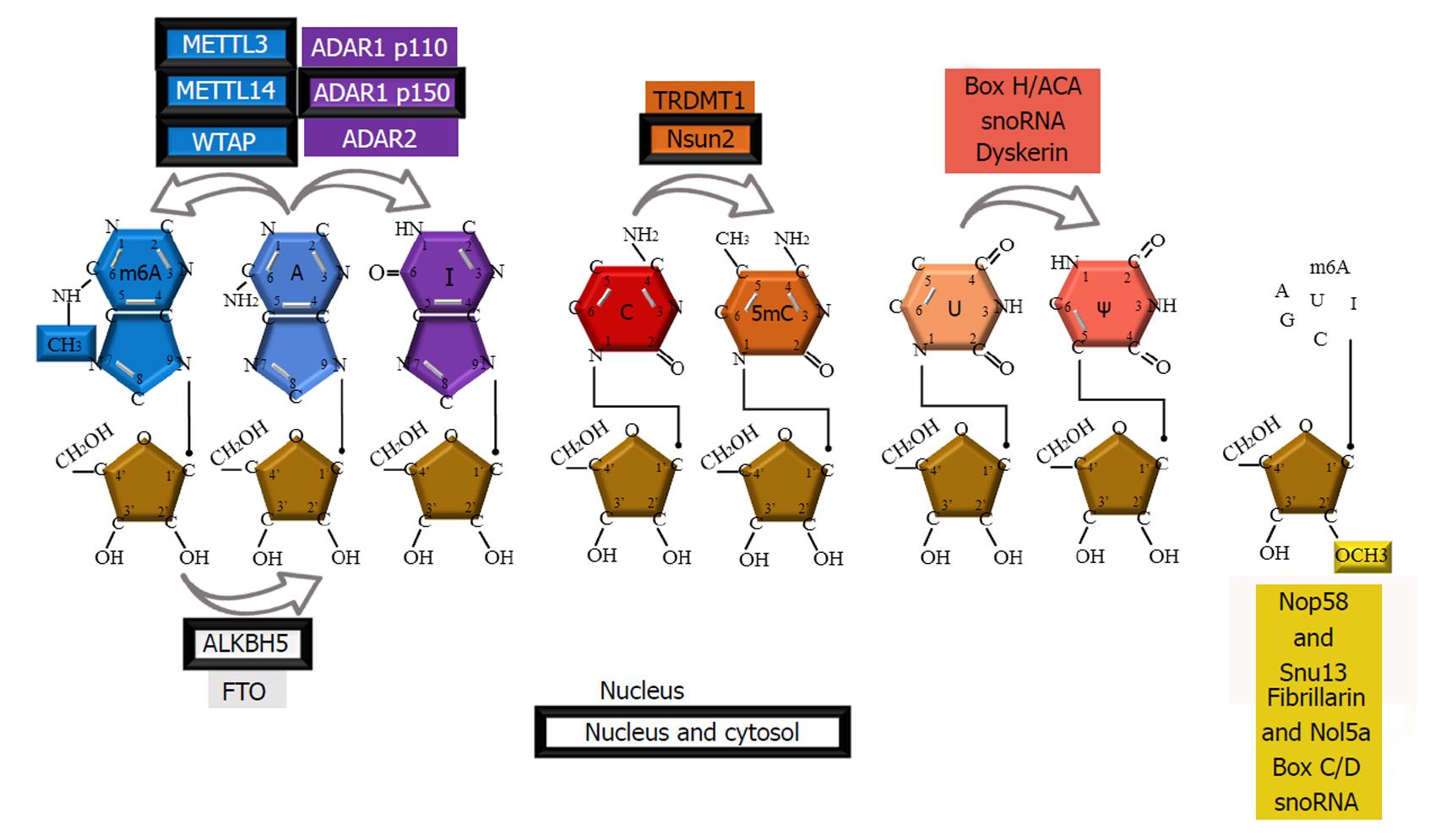
Diverse and abundant modifications are introduced posttrancriptionally in cellular RNAs during their maturation. These modifications are made on canonical A, C, G, and U residues, and their formation is catalyzed by numerous specific enzymes or RNA-protein complexes (RNPs). Ribonucleotide residues can bear single or multiple modifications on the purine/pyrimidine ring and/or ribose. To date, over one hundred RNA modifications have been identified and listed in dedicated databases (http://mods.rna.albany.edu/; http://modomics.genesilico.pl)[1,2]. These naturally occurring modified nucleosides play various structural and functional roles in different types of RNAs: Transfer RNAs (tRNAs), ribosomal RNAs (rRNAs), messenger RNAs (mRNAs), small nuclear RNAs (snRNAs), microRNAs (miRNAs) and long non-coding RNAs (lncRNAs). The most widespread RNA modifications are base or ribose methylations, deamination of adenosine to inosine and isomerization of uridine into pseudouridine. Over the past decades these modifications have been studied in the context of malignancies. Frequently, a modification is found to have pro-cancer or anti-cancer effects depending on the type of RNA, the location of the modification and, most importantly, the cell type and context (e.g., hypoxia). This review presents the current knowledge on the potential link between RNA modifications and cancer by systematically addressing the “pro-cancer”, “anti-cancer” and mixed effects of RNA modifications. Since such a relationship has been reported for only some abundant modifications and for modifications for which a detection method is available [N6-Methyladenosine (m6A), 5-Methylcytidine (m5C), 2’O-mN, Ψ and I], the present review will focus on these modifications (Figure 1).
Serendipitously discovered during the characterization of the mRNA 5’ cap, methylation of the exocyclic nitrogen of adenosine, named m6A, is by far the most abundant mRNA modification, occurring on an average of three sites per mRNA[3-5]. Recent technological advances have facilitated m6A profiling across eukaryotes, including humans, mice[6], yeasts[7], and plants[8,9], indicating that m6A is a conserved but dynamic modification. m6A has also been identified in rRNA[10], tRNA[11], snRNA[12], miRNA[13] and lncRNA[14].
M6A patterns are attributed to the consensus RRACH sequence (A is methylated; R = A or G; H = A, C, or U; and the first nucleotide next to m6A from the 5’ end most frequently is G), with preferential distribution near mRNA stop codons and 3’ untranslated regions (UTRs) and within long internal exons. Additionally, the m6A sites are conserved between human and mouse embryonic stem cells (ESCs) and somatic cells. However, distinct m6A patterns can also be detected among different species or cells at different developmental stages[4,7,15,16]. Some m6A signatures are tissue specific[4], and are altered in response to different stimuli[17], pointing to the potential role of m6A in regulating diverse cellular processes. m6A dynamics are assigned to the complex m6A enzymatic machineries, comprising m6A “writers”, “readers” and “erasers”. Although a plethora of studies suggest crucial and versatile roles of m6A and its machineries, its roles in cancer that have recently emerged are contradictory and require further investigation.
“Writers” is a term given to enzymes that are part of the methyltransferase complex that introduces m6A. Components of this complex are methyltransferase-like 3 (METTL3)[18], METTL14[19], Wilms tumor 1-associated protein (WTAP)[20] and KIAA1429[21].
METTL3 protein levels were found to be elevated in lung adenocarcinoma cell lines compared to healthy tissue[22]. Depletion of METTL3 was shown to result in the inhibition of cancer cell growth, decreased invasive ability of cancer cells and increased cell apoptosis in the same study. Additionally, METTL3 was shown to function as an m6A-binding protein (“reader”) in a specific subset of m6A-modified mRNAs, where it recruits eIF3 during translation initiation and therefore promotes translation. Expression of several oncogenes, including the mRNA of epidermal growth factor receptor (EGFR) and the Hippo pathway effector transcriptional co-activator with the PDZ-binding motif (TAZ) protein, was found to be promoted upon METTL3 recognition[22].
Similarly, in acute myeloid leukemia (AML), mRNA levels of METTL3 and METTL14 are significantly higher than in most cancers[23]. METTL3 depletion in MOLM13 caused differentiation and increased apoptosis, suggesting that high m6A levels may play a role in sustaining undifferentiated leukemic cells in AML[23] (Table 1).
| RNA modifications | High in cancer | Low in cancer |
| m6A | Lung adenocarcinoma[22], AML[23] | HER2 overexpressing subtypes breast cancer[35], t(11q23)/MLL-rearranged, t(15;17)/PML-RARA, FLT3-ITD, and/or NPM1-mutated AMLs (ASB2 and RARA)[36], GBM (FOXM1)[39], breast cancer (NANOG)[40] |
| 2’O-methylation | Breast cancer [51,67], primary and metastatic prostate cancers[58], squamous cell cervical carcinoma[57] | |
| Ψ | Leukemia, lymphoma, multiple myeloma[84-86] | |
| Inosine | BLCA, BRCA, COAD, HNSC, LUAD, THCA[87,88], NSCLC (NEIL1[94], AZIN1[103], miR-381[94]), SCLC (AZIN1)[87], HCC (AZIN1[101], FLNB[90]), GC[91], ESCC (FLNB)[92], cervical cancer[89], CRC (RHOQ)[109], AML (PTPN6)[114] | KIRP, KICH[87,88], Breast cancer (Gabra3)[118], Gastric cancer (PODXL)[91], Glioblastoma (GluR-B)[119], onco miR-21, miR-221, miR-222[128], ESCC (IGFBP7)[120], Glioma (miR-376a*)[129], Melanoma[130] (miR-455-5p)[132] |
| 5mC | Circulating tumor cells in lung cancer[137] |
Two m6A “erasers” have been described: Demethylases fat mass and obesity-associated protein (FTO) and alkB homolog 5 (ALKBH5)[24,25].
Single nucleotide polymorphisms within FTO known to be involved in the development of obesity in genome-wide association studies have been associated with the risk of developing diverse cancer types: Lung cancer, kidney cancer, high-grade prostate cancer, endometrial cancer, pancreatic cancer, pancreatic cancer in patients with type 2 diabetes, and breast cancer[26-33]. All these cancer types share a single SNP (rsrs9939609): The obesity-associated SNP in intron 1 of the FTO gene. This SNP was shown to increase primary transcript levels of the FTO gene, suggesting a gain-of-function mutation in cancers associated with this SNP[34].
In human epidermal growth factor receptor type 2 (HER2)-overexpressing subtypes of breast cancer, FTO is highly expressed in comparison to other breast cancer subtypes[35]. Contrary to the studies of high m6A levels in AML discussed in the previous chapter, low m6A levels have also been reported in AML subtypes. FTO expression can be upregulated by certain oncogenic proteins (e.g., mixed lineage leukemia (MLL)-fusion proteins, promyelocytic leukemia/retinoic acid receptor alpha (PML-RARA), fms-related tyrosine kinase 3-internal tandem duplication (FLT3-ITD), and nucleophosmin 1 (NPM1) mutant), and dataset analysis of human AML confirmed that FTO was expressed at significantly high levels in t(11q23)/MLL-rearranged, t(15;17)/PML-RARA, FLT3-ITD, and/or NPM1-mutated AMLs[36]. Overexpression of FTO reduces m6A levels in ankyrin repeat and SOCS box containing 2 (ASB2) and retinoic acid receptor alpha (RARA) mRNA transcripts. It has been shown that the loss of m6A markings reduces mRNA stability, resulting in the partial repression of ASB2 and RARA expression in AML cells. In four different AML cohorts, ASB2 and RARA exhibit a significant inverse correlation with FTO expression. ASB2 and RARA are upregulated during normal hematopoiesis and are important regulators of all-trans-retinoic acid (ATRA)-induced differentiation of leukemia cells. Through regulating the expression of such targets, FTO inhibits ATRA-induced AML cell differentiation. Both gain- and loss-of-function studies of FTO in leukemic cell models showed an oncogenic role of FTO in these AML subtypes[36]. However, recent studies have suggested that FTO acts as a demethylase of N6-2’O-dimethyladenosine in mRNA 5’ caps, having only minor effects on m6A[37]. Thus, the role of FTO in AML might be independent of m6A.
Recently, both FTO and ALKBH5 have been found to play similar roles in glioblastoma stem cells (GSCs) and their tumorigenesis[38]. These studies shed light on their crucial roles in the regulation of mRNA m6A levels for maintaining GSC growth, self-renewal, and tumor development. Enhanced growth and self-renewal of GSCs in vitro were detected upon the depletion of METTL3 or METTL14, resulting in reduced mRNA m6A levels, and promoted the ability of GSCs to form brain tumors in vivo. Accordingly, treatment with the FTO inhibitor MA2, the ethyl ester form of meclofenamic acid, increased mRNA m6A levels and suppressed GSC growth in vitro and GSC-initiated tumorigenesis, ultimately prolonging the survival of GSC-engrafted mice.
In a similar study, the authors checked the expression levels of m6A regulators in available datasets for glioblastoma multiforme (GBM) and discovered elevated expression of m6A demethylase ALKBH5 that correlated with poor clinical outcomes for GBM patients[39]. Stable knockdowns in cultured human GSCs showed that the loss of ALKBH5 decreases GSC proliferation and reduces the expression of the stemness markers Nestin, Sox2, Nanog, and Oct4, which are normally expressed in GSCs. In rescue experiments, wild-type, but not catalytically inactive, ALKBH5 recover the phenotype, suggesting that it plays a role in stemness maintenance and that the proliferation of GSCs is solely based on demethylation activity. Moreover, these authors examined the expression of transcription factor FOXM1 (forkhead box m1), which is known to play a pivotal role in regulating GSC proliferation, self-renewal, and tumorigenicity, and found that it depends on ALKBH5 demethylating activity. All these findings were based on m6A hyper erasing, which opens new possibilities for promising targeted treatments in glioblastoma (Table 1).
It has been reported that the hypoxia-inducible factors (HIFs) HIF-1α and HIF-2α activate ALKBH5 gene transcription under hypoxic conditions in breast cancer cells, thus inducing m6A demethylation. This demethylation was shown to stabilize NANOG mRNA and promote the breast cancer stem cell (BCSC) phenotype. Depletion of ALKBH5 in hypoxic breast cancer cells was identified as an effective strategy to decrease NANOG expression and limit the presence of BCSCs in vivo[40] (Table 1).
The primary microRNA (pri-miRNA) junction region between the hairpin stem and the flanking single-stranded RNA was found to be abundant in m6A consensus motifs. The recognition of the junction regions is mediated by Dicer, followed by the recruitment of the ribonuclease Drosha (the microprocessor complex), which cleaves the RNA duplex to yield the premiRNA product. Depletion of HNRNPA2B1 (a nuclear “reader”) or METTL3 knockdown in HEK293 and MDA-MB-231 cells resulted in a significant reduction in the expression levels of the mature forms of a number of m6A-marked miRNAs. The tumor-suppressor miRNA let-7 was significantly reduced upon the depletion of METTL3 possibly due to diminished Dicer binding to pri-miRNAs, thus preventing the formation of mature miRNAs. However, these METTL3-depletion experiments also showed a decrease in the expression of onco-miRNAs, such as miR-221 and miR-222[13,41]. Taken together, the presence of m6A affects diverse pri-miRNA and mature miRNA subpopulations, but its relevance in the context of cancer still needs to be investigated.
Methylation of the 2’-hydroxyl group of ribose is one of the predominant internal modifications of rRNA and snRNA[10,42]. This modification is also found in tRNA and mRNA, mostly at the first and second nucleotides in Cap1 and Cap2 structures, respectively.
Introducing 2’O-methylation on ribose is mediated by complexes of guide RNA and proteins named small nucleolar ribonucleoprotein (snoRNP) complexes or by methyltransferases: Human cap1 and 2, 2’-O-ribose methyltransferase, hMTr1 and hMTr2[43-45]. snoRNP complexes consist of Fibrillarin (the catalytic component in humans, also known as Nop1p in yeast), Nol5a (Nop56p), Nop58 and Snu13 subunits[46-48], which are guided by C/D Box snoRNAs to the appropriate base[49,50].
Tumor suppressor p53 and Fibrillarin seem to be linked[51]. Knockdown of p53 in cellular models of breast and colon cancer resulted in the overexpression of Fibrillarin at both the mRNA and protein levels. It is suggested that tumorigenesis associated to mutated p53 promotes an increase in the methylation status of rRNAs, which alters their ribozyme activity, thus affecting their translation fidelity and rate. Through the methylation of rRNA, Fibrillarin stimulates the translation of cancer-promoting proteins: (1) Insulin-like growth factor 1 receptor (IGF1R), which plays a role in tumor progression, cell survival, and the response to chemotherapy (reviewed by Pollak et al[52]); (2) c-Myc, a pleiotropic pro-oncogene (reviewed by Dang et al [53]); (3) Fibroblast growth factor 1/2 (FGF1/2), which is involved in epithelial-mesenchymal transition[54]; and (4) Vascular endothelial growth factor A (VEGFA), which acts in tumoral angiogenesis[51,55].
Translation of these proteins relies on internal ribosome entry site (IRES) in the mRNA, which is a 5’ cap-independent translation mechanism that may be used in specific conditions. The inhibition of rRNA methylation was shown to impair IRES translation initiation by perturbing the association of the 40S and 60S subunits[56]. Therefore, it is conceivable that enhanced ribosomal methylation increases the translation of IRES-containing mRNAs. Nevertheless, clinical analysis shows that a high level of Fibrillarin in primary breast tumors is associated with poor survival, independent of other biological markers[51]. Elevated expression levels of Fibrillarin were previously reported in primary and metastatic prostate cancers and in squamous cell cervical carcinoma (Table 1)[57,58].
NOL5A gene was found to be overexpressed in Bu–rkitt’s lymphoma-associated c-Myc mutants[59], and human NOP58 mRNA levels were found to be elevated in metastatic melanoma lesions[60].
Contrary to Fibrillarin’s indirect promotion of IRES-driven translation, in MCF-7, a breast cancer cell line, Fibrillarin knockdown resulted in the accumulation of p53, possibly affecting the UTR of the p53 mRNA and increasing IRES-driven de novo synthesis[61]. These studies suggest a complex interplay between p53 and Fibrillarin, while IRES-dependent translation is not exclusively stimulated by increased rRNA methylation.
SnoRNA expression profiles were investigated in endometrial, lung and prostate cancers, as well as in glioma and chronic lymphocytic leukemia. High-throughput screening of snoRNAs in cancerous versus normal tissues underlined their overexpression or underexpression as common molecular events in tumorigenesis, with the former being more pronounced than the latter[62-66]. Analysis of blood serum has shown the possibility of detecting snoRNAs in breast cancer patient samples and the associated upregulation of a specific snoRNA, U6, in active disease[67]. Therefore, profiling snoRNAs with their respective RNA 2’O methylation modification signatures might be used as a noninvasive biomarker in the diagnosis and prognosis of cancer.
The fifth base, known as pseudouridine (Ψ)[68], is one of the most abundant nucleotide modifications present in all three life domains[2]. After its initial detection in rRNA and tRNA, pseudouridine was detected in mRNA, lncRNA, and snRNAs, such as U2 snRNA and snoRNA[69,70]. Introducing Ψ in eukaryotic RNA can be mediated through guide RNA-dependent H/ACA BOX snoRNA pseudouridine synthases (PUSs) or guide RNA-independent PUSs. A recent review by Penzo et al[71] reports on the functional roles of pseudouridines and related human pathologies.
Only low Ψ levels have been reported in cancer tissues/cells; thus, this chapter will contain only a section titled “low Ψ levels in cancer”. Surprisingly, elevated levels of circulating Ψ have been measured in the body fluids of cancer patients, but its role and origin are not well defined, so this finding will not be further discussed here.
The highly conserved protein dyskerin is the human PUS that catalyzes the pseudouridylation of snoRNPs that assemble during the transcription of guide H/ACA RNA. Mutations in the Dkc1 gene coding for dyskerin can be found in the X-linked form of dyskeratosis congenita (DC). DC is a rare, inherited disorder that is characterized by mucocutaneous abnormalities and bone marrow failure. DC can be inherited as an X-linked recessive, autosomal dominant or autosomal recessive disease[72]. Although the absence of dyskerin, which results in the loss of pseudouridine in rRNA, was suggested as a primary cause of DC, a recent study assigned telomerase dysfunction as the primary cause of DC[73]. Namely, mutations in H/ACA-resembling domains in the RNA component of telomerase RNP, which are required for telomerase accumulation, stability, 3’ end processing and function, are associated with an autosomal form of DC[74-76].
In patients with DC, a higher predisposition to cancer has been reported, although low mutational frequency in the DKC1 gene was shown in primary tumors[77]. This predisposition might be a synergistic outcome of impaired pseudouridylation. Most likely, the dysregulation of rRNA pseudouridylation precedes disease onset, as studies in hypomorphic Dkc1-mutant mice suggest. A specific defect of the internal ribosome entry site also occurs upon DKC1 loss, causing a specific defect in the translation of some IRES-containing mRNAs. Ribosomes that lack pseudouridine modifications show a direct impairment in binding to IRES elements[78]. Consequently, in hypomorphic DKC-1 mice, cap-dependent translation of mRNA is not compromised, but translation of IRES-containing mRNAs, including the tumor suppressors p27 and p53, is perturbed[79-82], resulting in a higher incidence of cancer development in these mice. Thus, this impaired translation of tumor suppressor mRNA might also be a driver of cancer in DC patients. Moreover, recent identification of Ψ in mRNA[83] brings an additional level of complexity and regulation of the expression of target RNAs.
In hematological cancers, such as leukemias, lymphoma and multiple myeloma, downregulation of specific subsets of dyskerin-associated H/ACA snoRNAs has been demonstrated[84-86] (Table 1). Thus, lower pseudouridylation levels are a widespread feature of cancer.
Inosine is an RNA modification resulting from the hydrolytic deamination of adenosine catalyzed by adenosine deaminase enzymes acting on double-stranded RNA (ADAR) or adenosine deaminase acting on transfer RNA (ADAT), which are families known to function in A-to-I RNA editing. Enzymes of the ADAR family are catalytically active ADAR1, ADAR2 and ADAR3, which still has an unknown function.
ADARs introduce inosine in coding and non-coding RNAs and have drastic impacts on the cellular transcriptome and translatome. The hypo- or hyper-editome has been associated with diverse types of cancer. The role of ADAT in cancer has not been reported.
Most frequently editing locations are long, partially complementary RNAs formed from inverted non-coding repeats, such as Arthrobacter luteus (Alu) and long interspersed element (LINE) located in mRNA UTRs and introns. Two major studies have investigated RNA-editing patterns in tumors versus normal tissues. Each of the studies employed RNA-Seq datasets from The Cancer Genome Atlas (TCGA) project (https://cancergenome.nih.gov/) and compared them to reference datasets of editing sites. High-confidence RNA editing sites are annotated in the Rigorously Annotated Database of A-to-I RNA Editing (RADAR, http://rnaedit.com/), where one study focused on detecting Alu and non-Alu RNA editing events in 17 cancers, whereas the other study focused on Alu RNA editing events in 9 different cancers[87,88].
In general, elevated Alu editing activity in tumors compared to matched normal tissues was found in bladder urothelial carcinoma (BLCA), breast invasive carcinoma (BRCA), colon adenocarcinoma (COAD), head and neck squamous cell carcinoma (HNSC), lung adenocarcinoma (LUAD) and thyroid carcinoma (THCA). This hyperediting of Alu was attributed to ADAR1, whose expression levels matched in all these types of cancer, except COAD. Similarly, a study by Han et al[87] where more patient samples and non-Alu edited sequences were included, confirmed hyperediting in BLCA, BRCA, HNSC, LUAD, THCA compared to normal tissues. Again, increased editing levels correlated with the mRNA levels of ADAR1.
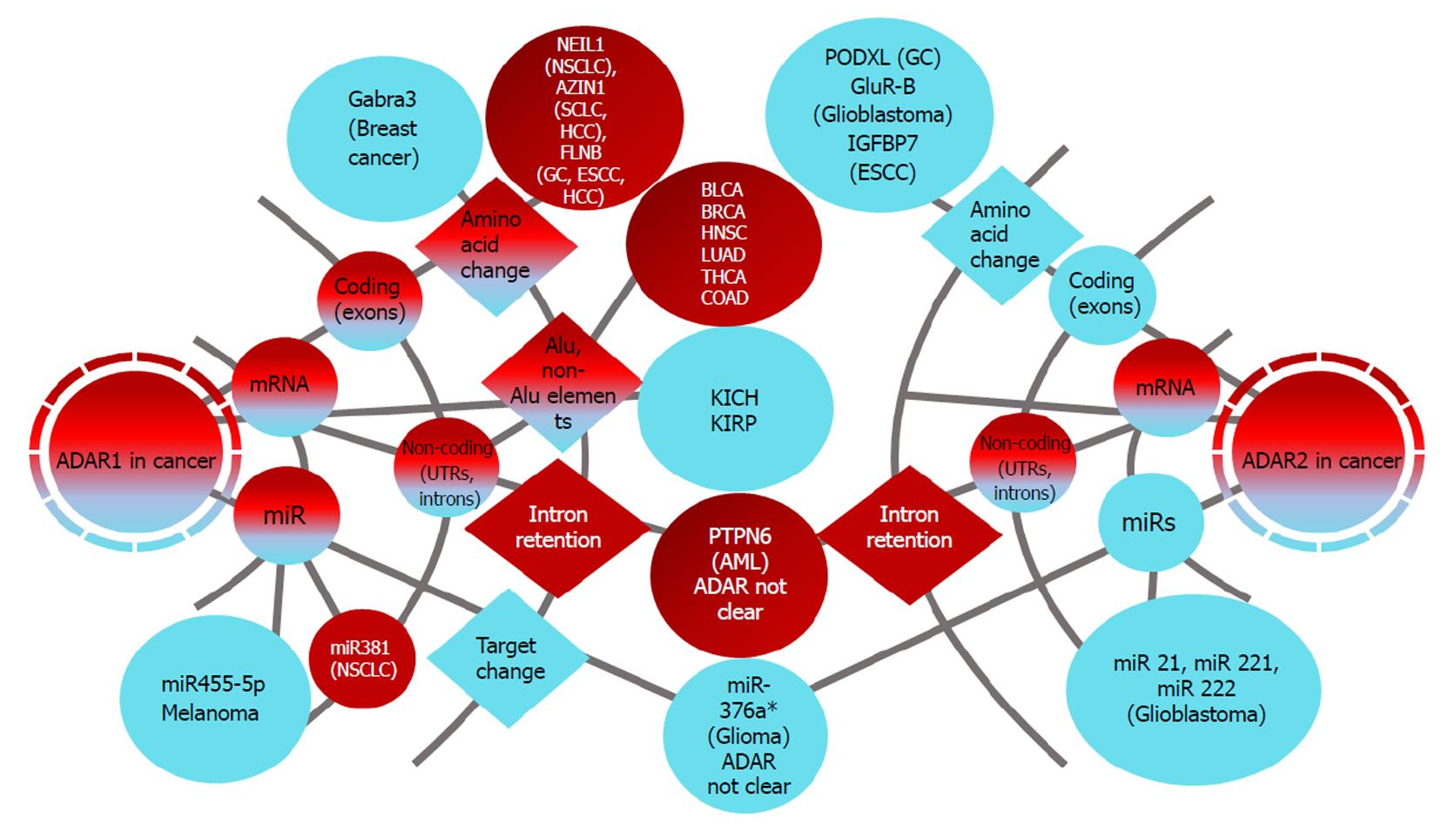
Increased ADAR-1 levels were reported in non-small cell lung cancer (NSCLC), hepatocellular carcinoma (HCC), esophageal cell carcinoma (ESCC), gastric (GC) and cervical cancer, suggesting that tight regulation of editing levels might have implications in cancer development and that ADAR1 might act as an oncogene[89-92] (Table 1 and Figure 2).
Recoding editing: In non-small cell lung cancer samples, ADAR1 gene amplification was shown to increase the editing of the DNA base excision repair glycosylase enzyme NEI-like protein 1 (NEIL1). Pre-mRNA editing of NEIL-1 causes a lysine to arginine (K242R) change in the lesion recognition loop of the protein. The edited NEIL1 protein removes thymine glycol from duplex DNA at a lower rate compared to the unedited form, while repair of the guanidinohydantoin lesion is enhanced by edited NEIL1[93]. In overexpression experiments, transfection of edited NEIL1 enhanced the growth of A459 cells in comparison with the transfection of unedited transcripts. Thus, increased recoding editing of NEIL1 as a proposed target of ADAR1 could contribute to the phenotype of lung cancer cells[94].
AZIN1 (encoding antizyme inhibitor 1) is edited by ADAR1, which has increased expression levels in HCC and was found to positively correlate with AZIN1 editing frequency. AZIN1 is an antizyme inhibitor whose activity is crucial in limiting cellular proliferation. Antizyme binds and induces the degradation of the growth-promoting proteins ornithine decarboxylase (ODC) and cyclin D1 (CCND1)[95,96]. AZIN1 is homologous to ODC and has a greater binding affinity to antizyme compared to ODC. Binding of AZIN1 to antizyme prevents the degradation of ODC[97]. Thereby, AZIN1 acts as an oncogene by inhibiting the tumor-suppressor activities of antizyme[96]. AZIN1 expression was found to be substantially elevated in cancers of the prostate, brain, breast and liver, and gene expression data have identified alterations in the AZIN1-to-antizyme ratio in many human cancers, confirming its role in promoting growth[98-100]. In HCC, increased A-I editing of the AZIN1 transcript introduces serine-to-glycine substitution at residue 367 in the protein. This recoding editing is associated with conformational changes and translocation from the nucleus to the cytoplasm and results in a higher-binding affinity to antizyme and greater protein stability, thus promoting cell proliferation. AZIN1 editing increases during the progression from primary liver cancer and cirrhosis to advanced HCC with recurrence and metastasis, suggesting its use as a prognostic marker[101]. It is plausible that similar editing events occur in other types of cancer, as has been confirmed in esophageal squamous cell carcinoma (ESCC) and breast cancer[87,92,102]. Recently, tumorigenesis of NSCLC was also attributed to high levels of AZIN1 editing[103]. It has been reported that AZIN1 editing levels correlate with sensitivity to drug treatment in cancer cell lines. Cancer cells lines with increased levels of AZIN1 editing showed more sensitivity to some of the chemotherapies used in small cell lung cancer (SCLC): Paclitaxel, irinotecan, and topotecan[87].
Filamin B (FLNB) is an actin cross-linking protein and, together with filamin A, it forms homo- and heterodimers mediating orthogonal branching of actin filaments[104]. Filamin B is known to be a target for editing[105], and interestingly, one recoding editing event was shown to be increased in two types of cancer. In HCC, ADAR1 and ADAR2 were both reported to mediate FLNB transcript editing in codon 2269, resulting in the amino acid change Met→Val. Increased editing of FLNB compared to matched non-tumor liver tissues has been closely associated with HCC pathogenesis from normal to clinically verified HCC. In this study, ADAR1 levels were shown to be increased, while ADAR2 levels were decreased in HCC samples compared to non-tumor liver tissues[90]. The same recoding editing was found in ESCC, where unlike HCC, only ADAR1 was responsible for this hyperediting. FLNB hyperediting correlated with ADAR1 levels in ESCC samples[92]. The functional role of FLNB editing is not known.
Ras homolog family member Q (RHOQ) belongs to a family of Rho GTPases that are known to be intracellular signaling molecules regulating the actin cytoskeleton and thereby cellular functions, such as cell polarity, migration, and vesicular trafficking. Rho GTPases are present as either an active GTP-bound form or an inactive GDP-bound form[106]. Activation of Rho GTPases is implicated in the development and progression of many types of human malignancies, including CRC[107]. RhoQ has been most extensively studied for its central role in insulin-stimulated GLUT4 transport in adipocytes[108]. Amino acid substitution of asparagine with serine (N136S) in the edited RhoQ was identified in colorectal cancer (CRC). The ADAR responsible has not been identified. This editing was suggested to change RhoQ protein–protein interactions and induce increased levels of RhoQ binding of GTP, causing actin cytoskeletal reorganization and increased invasion potential without affecting proliferation in CRC cell lines. Moreover, edited RHOQ was associated with recurrence of CRC when present in the tumor[109].
Protein tyrosine phosphatase non-receptor type 6 (PTPN6) is a cytoplasmic protein expressed in hematopoietic cell development, proliferation and the receptor-mediated mitogenic signaling pathway[110,111]. In bone marrow mononuclear cells (BMMCs) of patients with acute myeloid leukemia, a novel PTPN6 transcript retaining intron 3 has been identified. This transcript arises from an alternative splicing reaction where editing-mediated deamination of A7866 in intron 3 erases this branch formation site, making it invisible to the splicing machinery. The ADAR responsible has not been identified. It is suggested that this retention results in the translation of a nonfunctional protein where the intron 3-encoded sequence is located in the N-terminal Src homology 2 (SH2) domain. PTPN6 binding with partner proteins, such as proto-oncogene receptor tyrosine kinase-c-Kit[112], and its self-inhibition of phosphatase activity occurs via its N terminal domain[113]. All this information suggests that its deregulation ultimately leads to uncontrolled hematopoietic growth and function. The tumor-specific editing seen in AML might correlate with the clinical course of the disease since low levels of intron-retaining transcripts in patient BMMCs at remission compared to those at diagnosis suggest that editing promotes tumorigenesis[114].
High miRNA editing levels in cancer:ADAR1 gene amplification in NSCLC demonstrated ADAR1 overexpression in patients with early-stage lung cancer, underlining its potential oncogenic role in this cancer. Increased levels of ADAR1 corresponded with edited miR-381 levels in NSCLC. Overexpression of edited miR-381 in NSCLC possibly contributes to stemness and chemoresistance[94].
Low levels of mRNA open reading frame editing in cancer: The same correlation but in the opposite direction was shown in hypoedited cancers, such as kidney chromophobe (KICH) and kidney renal papillary cell carcinoma (KIRP), with ADAR1 mRNA paired editing levels. The ADAR2 levels checked in both studies showed a complex expression pattern but no matching editing levels (Table 1 and Figure 2)[87,88]. The role of ADAR1 in breast cancer is not fully understood. A recent study reported high ADAR1 expression in half of the examined triple-negative-cancer patients[115]. Conversely, it has been proposed that ADAR1 prevents tumor progression by editing the transcript coding for the alpha-3 subunit of gamma-aminobutyric acid type A (Gabra3).
The chloride-permeable gamma-aminobutyric acid type A (GABAA) receptors are crucial mediators of fast inhibitory neurotransmission in the central nervous system[116]. The Gabra3 transcript undergoes recoding editing of isoleucine to methionine (I/M) in the third transmembrane region. This substitution was found to affect GABAA surface presentation and its cellular trafficking[117]. In addition to being normally expressed in normal neuronal tissues, Gabra3 has been identified in breast cancer, where its high expression inversely correlates with breast cancer survival. ADAR1-edited Gabra3 was found in non-invasive breast cancer cell lines and was linked with the protein kinase B (Akt) pathway. A proposed mechanism for the non-invasive phenotype is that Gabra3 editing reduces its surface expression and indirectly prevents Akt activation, thereby preventing cell proliferation and invasiveness. Thus, the unedited form of Gabra3 in breast cancer is suggested to promote tumor progression, invasion and metastatic potential[118].
Lower ADAR2 levels are recognized in gastric cancer, glioblastoma, HCC and ESCC[91,119,120] (Figure 2). ADAR2 levels were found to correlate with changes in podocalyxin-like (PODXL) and GluR-B functions. The PODXL RNA editing event is an amino acid substitution from histidine (His) to arginine (Arg) at codon 241. This editing in the gastric cancer cell line MKN28 was shown to prevent increased growth rates and invasive capability compared to cells with the unedited form. Moreover, recoding editing of a single position located in the channel-pore-loop domain in GluR subunit B (GluR-B) (the Q/R-site) from Gln to Arg results in a channel that is impermeable to Ca2+[121]. Tight regulation of editing is essential for the adequate function of this channel. Hypoedited PODXL and GluR-B with altered functions are associated with gastric cancer and malignant glioblastoma, respectively. Consequently, a tumor-suppressor role has been attributed to ADAR2[91,119].
In ESCC, it has been reported that ADAR2 promotes apoptosis by editing and stabilizing insulin-like growth factor-binding protein 7 (IGFBP7) RNA. IGFBP7 is a secreted factor binding to and interfering with the activation of IGF1R. Through receptor occupation, IGFBP7 blocks downstream phosphatidylinositol 3-kinase (PI3K)-AKT signaling, resulting in the inhibition of protein synthesis and cell apoptosis[122]. IGFBP7 was previously reported to be an apoptotic promoter in prostate cancer[123], colorectal cancer[124] and breast cancer[125]. The editing site in IGFBP7 is at position 284 of the coding sequence, and codon 95 is changed from AAG (lysine) to AIG, which is read as AGG (arginine) (K95R). This editing was shown to protect IGFBP7 against matriptase proteolysis in ESCC culture and xenografts, thus enabling the proapoptotic function of IGFBP7. ADAR2 is known to be downregulated in ESCC, and its upregulation induces apoptosis in ESCC cell lines in vitro, suggesting that IGFBP7 under editing may promote tumorigenesis in esophageal squamous cell carcinoma[120].
Low miRNA editing levels in cancer: ADAR2 rescue in glioblastoma cells was shown to inhibit cell proliferation and migration, confirming its possible tumor-suppressor role[126]. This anti-tumor effect might be explained through the regulation of onco-miRNAs in glioblastoma. Three particularly investigated onco-miRNAs, miR-221, miR-222 and miR-21, are overexpressed in glioblastoma[127]. ADAR2 can edit miR-222/221 and miR-21 precursors and decrease the expression of the corresponding mature onco-miRNAs in the normal mouse brain and in different lines. Decreased levels of ADAR2 identified in glioblastoma probably push the balance of onco-miRNA/tumor-suppressor miRNA towards increased expression of onco-miRNAs, such as miR-221, miR-222 and miR-21, thereby supporting tumor progression[128].
In the human brain, the miR-376 cluster encodes 4 pri-miRs that give rise to 5 distinct mature miRNAs, which are subjected to specific A-to-I RNA editing on 9 adenosines. In noninvasive U87 glioma cells, the expression of the unedited miR-376a* was shown to promote aggressive tumor migration and invasion of these cells both in vitro and in vivo. The editing reaction missing in the GBM cell lines generally occurs in the seed region of pri-miR-376a1 at the +9 site, ultimately giving rise to mature edited miR-376a*. The absence of this editing changes the specific targets of the miRNA. It has been identified that non edited miR-376a*, through its binding to 3’ UTR, has a novel target, RAP2A, which is a member of the RAS oncogene family with an unknown function. However, the non edited miR-376a* targeting of RAP2A is unable to target the autocrine motility factor receptor (AMFR), resulting in its upregulation and possibly contributing to increased migration and invasiveness of glioma cells[129].
Melanoma is the most aggressive type of skin cancer. It has been reported that there is a significant decrease in ADAR1 expression in approximately 65% of metastatic melanoma specimens compared to melanocytes[130] (Table 1 and Figure 2). ADAR-1 transcripts were found to be targeted by miR-17 and miR-432, thus decreasing ADAR1 expression. Both miR-17 and miR-432 were identified to be overexpressed in melanoma possibly due to the amplification of encoding genes[130]. However, studies suggest that ADAR1 insufficiencies contribute to the enhancement of proliferation of melanoma cells through editing the independent regulation of miRNA biogenesis. miRNA-455-5p was identified as a target of ADAR1 in low-metastatic melanoma cells but not in highly metastatic cell lines. ADAR1 was shown to edit pri-miR-455-5p at +2 and +17 positions. This editing probably results in the reduction of the processing of pri-miRNA by Dicer or Drosha by lowering the binding affinity. However, it is also possible that ADAR1 binds to Dicer since the amount of miR-455-5p bound to Dicer and Drosha was inversely correlated with ADAR1 expression. ADAR1 was shown to form a complex with Dicer through protein-protein interactions[131]. In this study, the authors gave a model of RNA editing in the context of melanoma progression and metastasis, where cAMP responses element binding (CREB) downregulates ADAR1 and gives rise to non-edited miR-455-5p. Expression of miR-455-5p suppresses the tumor suppressor gene cytoplasmic polyadenylation element-binding protein 1 (CPEB1), resulting in growth promotion and metastasis in melanoma cells[132].
Cytosine base methylation - m5C has been identified in rRNA, tRNA and recently in mRNAs and is particularly enriched in untranslated regions and near Argonaute-binding regions[133]. The enzymes responsible for the introduction of m5C are members of the DNA methyltransferase homolog (Dnmt2) and the NOP2/Sun (NSun 2 and 4) RNA methyltransferase family[134-136]. The role of these enzymes in the methylating activities of tumorigenesis is currently unknown. However, in circulating tumor cells from lung cancer patients, increased RNA m5C levels were shown compared to those in whole blood cells[137]. Further investigation of the role of m5C in cancer is required.
Tight regulation of the writing, reading and eventual erasing of RNA modifications is essential for RNA metabolism. Misbalanced expression of the enzymes responsible for introducing, and in some cases removing, these modifications are considered a possible signature for specific types of cancer (Table 1). Considering the broad effect of RNA modifications on tumor cell biology, future methylome, pseudome and editome studies will shed light on those relatively unexplored epitranscriptomic mechanisms in tumors. Those studies will pave the way for the development of anti-cancer drugs that could act by steering RNA modifications.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Switzerland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Trere D S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
| 1. | Cantara WA, Crain PF, Rozenski J, McCloskey JA, Harris KA, Zhang X, Vendeix FA, Fabris D, Agris PF. The RNA Modification Database, RNAMDB: 2011 update. Nucleic Acids Res. 2011;39:D195-D201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 613] [Cited by in RCA: 665] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 2. | Machnicka MA, Milanowska K, Osman Oglou O, Purta E, Kurkowska M, Olchowik A, Januszewski W, Kalinowski S, Dunin-Horkawicz S, Rother KM. MODOMICS: a database of RNA modification pathways-2013 update. Nucleic Acids Res. 2013;41:D262-D267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 769] [Cited by in RCA: 823] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 3. | Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci USA. 1974;71:3971-3975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 974] [Cited by in RCA: 1353] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 4. | Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149:1635-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2265] [Cited by in RCA: 3192] [Article Influence: 245.5] [Reference Citation Analysis (0)] |
| 5. | Rana AP, Tuck MT. Analysis and in vitro localization of internal methylated adenine residues in dihydrofolate reductase mRNA. Nucleic Acids Res. 1990;18:4803-4808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2600] [Cited by in RCA: 3644] [Article Influence: 280.3] [Reference Citation Analysis (0)] |
| 7. | Schwartz S, Agarwala SD, Mumbach MR, Jovanovic M, Mertins P, Shishkin A, Tabach Y, Mikkelsen TS, Satija R, Ruvkun G. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell. 2013;155:1409-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 509] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 8. | Li Y, Wang X, Li C, Hu S, Yu J, Song S. Transcriptome-wide N6-methyladenosine profiling of rice callus and leaf reveals the presence of tissue-specific competitors involved in selective mRNA modification. RNA Biol. 2014;11:1180-1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 9. | Luo GZ, MacQueen A, Zheng G, Duan H, Dore LC, Lu Z, Liu J, Chen K, Jia G, Bergelson J. Unique features of the m6A methylome in Arabidopsis thaliana. Nat Commun. 2014;5:5630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 336] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 10. | Maden BE. The numerous modified nucleotides in eukaryotic ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1990;39:241-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 289] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Iwanami Y, Brown GM. Methylated bases of transfer ribonucleic acid from HeLa and L cells. Arch Biochem Biophys. 1968;124:472-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Bringmann P, Lührmann R. Antibodies specific for N6-methyladenosine react with intact snRNPs U2 and U4/U6. FEBS Lett. 1987;213:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Alarcón CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;519:482-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 1091] [Article Influence: 109.1] [Reference Citation Analysis (0)] |
| 14. | Liu N, Parisien M, Dai Q, Zheng G, He C, Pan T. Probing N6-methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA. RNA. 2013;19:1848-1856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 421] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 15. | Geula S, Moshitch-Moshkovitz S, Dominissini D, Mansour AA, Kol N, Salmon-Divon M, Hershkovitz V, Peer E, Mor N, Manor YS. Stem cells. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science. 2015;347:1002-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1253] [Article Influence: 125.3] [Reference Citation Analysis (0)] |
| 16. | Batista PJ, Molinie B, Wang J, Qu K, Zhang J, Li L, Bouley DM, Lujan E, Haddad B, Daneshvar K. m(6)A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell. 2014;15:707-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 983] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 17. | Bodi Z, Zhong S, Mehra S, Song J, Graham N, Li H, May S, Fray RG. Adenosine Methylation in Arabidopsis mRNA is Associated with the 3' End and Reduced Levels Cause Developmental Defects. Front Plant Sci. 2012;3:48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 227] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 18. | Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. 1997;3:1233-1247. [PubMed] |
| 19. | Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1590] [Cited by in RCA: 2526] [Article Influence: 210.5] [Reference Citation Analysis (0)] |
| 20. | Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1617] [Cited by in RCA: 1820] [Article Influence: 165.5] [Reference Citation Analysis (0)] |
| 21. | Schwartz S, Mumbach MR, Jovanovic M, Wang T, Maciag K, Bushkin GG, Mertins P, Ter-Ovanesyan D, Habib N, Cacchiarelli D. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5' sites. Cell Rep. 2014;8:284-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 983] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 22. | Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m(6)A Methyltransferase METTL3 Promotes Translation in Human Cancer Cells. Mol Cell. 2016;62:335-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 1200] [Article Influence: 133.3] [Reference Citation Analysis (0)] |
| 23. | Vu LP, Pickering BF, Cheng Y, Zaccara S, Nguyen D, Minuesa G, Chou T, Chow A, Saletore Y, MacKay M. The N6-methyladenosine (m6A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med. 2017;23:1369-1376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 905] [Cited by in RCA: 960] [Article Influence: 120.0] [Reference Citation Analysis (0)] |
| 24. | Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885-887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2803] [Cited by in RCA: 3094] [Article Influence: 221.0] [Reference Citation Analysis (0)] |
| 25. | Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vågbø CB, Shi Y, Wang WL, Song SH. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1763] [Cited by in RCA: 2685] [Article Influence: 206.5] [Reference Citation Analysis (0)] |
| 26. | Brennan P, McKay J, Moore L, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D. Obesity and cancer: Mendelian randomization approach utilizing the FTO genotype. Int J Epidemiol. 2009;38:971-975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | da Cunha PA, de Carlos Back LK, Sereia AF, Kubelka C, Ribeiro MC, Fernandes BL, de Souza IR. Interaction between obesity-related genes, FTO and MC4R, associated to an increase of breast cancer risk. Mol Biol Rep. 2013;40:6657-6664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Delahanty RJ, Beeghly-Fadiel A, Xiang YB, Long J, Cai Q, Wen W, Xu WH, Cai H, He J, Gao YT. Association of obesity-related genetic variants with endometrial cancer risk: a report from the Shanghai Endometrial Cancer Genetics Study. Am J Epidemiol. 2011;174:1115-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Kaklamani V, Yi N, Sadim M, Siziopikou K, Zhang K, Xu Y, Tofilon S, Agarwal S, Pasche B, Mantzoros C. The role of the fat mass and obesity associated gene (FTO) in breast cancer risk. BMC Med Genet. 2011;12:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 30. | Lewis SJ, Murad A, Chen L, Davey Smith G, Donovan J, Palmer T, Hamdy F, Neal D, Lane JA, Davis M. Associations between an obesity related genetic variant (FTO rs9939609) and prostate cancer risk. PLoS One. 2010;5:e13485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Lin Y, Ueda J, Yagyu K, Ishii H, Ueno M, Egawa N, Nakao H, Mori M, Matsuo K, Kikuchi S. Association between variations in the fat mass and obesity-associated gene and pancreatic cancer risk: a case-control study in Japan. BMC Cancer. 2013;13:337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Lurie G, Gaudet MM, Spurdle AB, Carney ME, Wilkens LR, Yang HP, Weiss NS, Webb PM, Thompson PJ, Terada K, Setiawan VW, Rebbeck TR, Prescott J, Orlow I, O'Mara T, Olson SH, Narod SA, Matsuno RK, Lissowska J, Liang X, Levine DA, Le Marchand L, Kolonel LN, Henderson BE, Garcia-Closas M, Doherty JA, De Vivo I, Chen C, Brinton LA, Akbari MR; Australian National Endometrial Cancer Study Group; Epidemiology of Endometrial Cancer Consortium (E2C2), Goodman MT. The obesity-associated polymorphisms FTO rs9939609 and MC4R rs17782313 and endometrial cancer risk in non-Hispanic white women. PLoS One. 2011;6:e16756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Tang H, Wei P, Duell EJ, Risch HA, Olson SH, Bueno-de-Mesquita HB, Gallinger S, Holly EA, Petersen GM, Bracci PM. Genes-environment interactions in obesity- and diabetes-associated pancreatic cancer: a GWAS data analysis. Cancer Epidemiol Biomarkers Prev. 2014;23:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Berulava T, Horsthemke B. The obesity-associated SNPs in intron 1 of the FTO gene affect primary transcript levels. Eur J Hum Genet. 2010;18:1054-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 35. | Tan A, Dang Y, Chen G, Mo Z. Overexpression of the fat mass and obesity associated gene (FTO) in breast cancer and its clinical implications. Int J Clin Exp Pathol. 2015;8:13405-13410. [PubMed] |
| 36. | Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, Huang H, Nachtergaele S, Dong L, Hu C. FTO Plays an Oncogenic Role in Acute Myeloid Leukemia as a N6-Methyladenosine RNA Demethylase. Cancer Cell. 2017;31:127-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 871] [Cited by in RCA: 1162] [Article Influence: 145.3] [Reference Citation Analysis (0)] |
| 37. | Mauer J, Luo X, Blanjoie A, Jiao X, Grozhik AV, Patil DP, Linder B, Pickering BF, Vasseur JJ, Chen Q. Reversible methylation of m6Am in the 5' cap controls mRNA stability. Nature. 2017;541:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 840] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 38. | Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, Sun G, Lu Z, Huang Y, Yang CG. m6A RNA Methylation Regulates the Self-Renewal and Tumorigenesis of Glioblastoma Stem Cells. Cell Rep. 2017;18:2622-2634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 689] [Cited by in RCA: 982] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 39. | Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, Chen Y, Sulman EP, Xie K, Bögler O. m6A Demethylase ALKBH5 Maintains Tumorigenicity of Glioblastoma Stem-like Cells by Sustaining FOXM1 Expression and Cell Proliferation Program. Cancer Cell. 2017;31:591-606.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 1136] [Article Influence: 142.0] [Reference Citation Analysis (0)] |
| 40. | Zhang C, Samanta D, Lu H, Bullen JW, Zhang H, Chen I, He X, Semenza GL. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m6A-demethylation of NANOG mRNA. Proc Natl Acad Sci USA. 2016;113:E2047-E2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 776] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 41. | Alarcón CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell. 2015;162:1299-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 1182] [Article Influence: 118.2] [Reference Citation Analysis (0)] |
| 42. | Reddy R, Busch H. Small nuclear RNAs: RNA sequences, structure, and modifications. Structure and function of major and minor small nuclear ribonucleoprotein particles. Verlag Berlin Heidelberg: Springer 1988; 1-37. [DOI] [Full Text] |
| 43. | Smietanski M, Werner M, Purta E, Kaminska KH, Stepinski J, Darzynkiewicz E, Nowotny M, Bujnicki JM. Structural analysis of human 2'-O-ribose methyltransferases involved in mRNA cap structure formation. Nat Commun. 2014;5:3004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 44. | Bélanger F, Stepinski J, Darzynkiewicz E, Pelletier J. Characterization of hMTr1, a human Cap1 2'-O-ribose methyltransferase. J Biol Chem. 2010;285:33037-33044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 45. | Werner M, Purta E, Kaminska KH, Cymerman IA, Campbell DA, Mittra B, Zamudio JR, Sturm NR, Jaworski J, Bujnicki JM. 2'-O-ribose methylation of cap2 in human: function and evolution in a horizontally mobile family. Nucleic Acids Res. 2011;39:4756-4768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 46. | Schimmang T, Tollervey D, Kern H, Frank R, Hurt EC. A yeast nucleolar protein related to mammalian fibrillarin is associated with small nucleolar RNA and is essential for viability. EMBO J. 1989;8:4015-4024. [PubMed] |
| 47. | Lafontaine DL, Tollervey D. Nop58p is a common component of the box C+D snoRNPs that is required for snoRNA stability. RNA. 1999;5:455-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 136] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 48. | Marmier-Gourrier N, Cléry A, Senty-Ségault V, Charpentier B, Schlotter F, Leclerc F, Fournier R, Branlant C. A structural, phylogenetic, and functional study of 15.5-kD/Snu13 protein binding on U3 small nucleolar RNA. RNA. 2003;9:821-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Balakin AG, Smith L, Fournier MJ. The RNA world of the nucleolus: two major families of small RNAs defined by different box elements with related functions. Cell. 1996;86:823-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 352] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 50. | Kiss-László Z, Henry Y, Bachellerie JP, Caizergues-Ferrer M, Kiss T. Site-specific ribose methylation of preribosomal RNA: a novel function for small nucleolar RNAs. Cell. 1996;85:1077-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 641] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 51. | Marcel V, Ghayad SE, Belin S, Therizols G, Morel AP, Solano-Gonzàlez E, Vendrell JA, Hacot S, Mertani HC, Albaret MA. p53 acts as a safeguard of translational control by regulating fibrillarin and rRNA methylation in cancer. Cancer Cell. 2013;24:318-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 250] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 52. | Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1039] [Cited by in RCA: 1043] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 53. | Dang CV. MYC on the path to cancer. Cell. 2012;149:22-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1984] [Cited by in RCA: 2590] [Article Influence: 199.2] [Reference Citation Analysis (0)] |
| 54. | Billottet C, Elkhatib N, Thiery JP, Jouanneau J. Targets of fibroblast growth factor 1 (FGF-1) and FGF-2 signaling involved in the invasive and tumorigenic behavior of carcinoma cells. Mol Biol Cell. 2004;15:4725-4734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69 Suppl 3:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 953] [Cited by in RCA: 1214] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 56. | Basu A, Das P, Chaudhuri S, Bevilacqua E, Andrews J, Barik S, Hatzoglou M, Komar AA, Mazumder B. Requirement of rRNA methylation for 80S ribosome assembly on a cohort of cellular internal ribosome entry sites. Mol Cell Biol. 2011;31:4482-4499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Choi YW, Kim YW, Bae SM, Kwak SY, Chun HJ, Tong SY, Lee HN, Shin JC, Kim KT, Kim YJ. Identification of differentially expressed genes using annealing control primer-based GeneFishing in human squamous cell cervical carcinoma. Clin Oncol (R Coll Radiol). 2007;19:308-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Koh CM, Gurel B, Sutcliffe S, Aryee MJ, Schultz D, Iwata T, Uemura M, Zeller KI, Anele U, Zheng Q. Alterations in nucleolar structure and gene expression programs in prostatic neoplasia are driven by the MYC oncogene. Am J Pathol. 2011;178:1824-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 59. | Cowling VH, Turner SA, Cole MD. Burkitt's lymphoma-associated c-Myc mutations converge on a dramatically altered target gene response and implicate Nol5a/Nop56 in oncogenesis. Oncogene. 2014;33:3519-3527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Nakamoto K, Ito A, Watabe K, Koma Y, Asada H, Yoshikawa K, Shinomura Y, Matsuzawa Y, Nojima H, Kitamura Y. Increased expression of a nucleolar Nop5/Sik family member in metastatic melanoma cells: evidence for its role in nucleolar sizing and function. Am J Pathol. 2001;159:1363-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Su H, Xu T, Ganapathy S, Shadfan M, Long M, Huang TH, Thompson I, Yuan ZM. Elevated snoRNA biogenesis is essential in breast cancer. Oncogene. 2014;33:1348-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 62. | Gao L, Ma J, Mannoor K, Guarnera MA, Shetty A, Zhan M, Xing L, Stass SA, Jiang F. Genome-wide small nucleolar RNA expression analysis of lung cancer by next-generation deep sequencing. Int J Cancer. 2015;136:E623-E629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 63. | Jha P, Agrawal R, Pathak P, Kumar A, Purkait S, Mallik S, Suri V, Chand Sharma M, Gupta D, Suri A. Genome-wide small noncoding RNA profiling of pediatric high-grade gliomas reveals deregulation of several miRNAs, identifies downregulation of snoRNA cluster HBII-52 and delineates H3F3A and TP53 mutant-specific miRNAs and snoRNAs. Int J Cancer. 2015;137:2343-2353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 64. | Berquet L, Valleron W, Grgurevic S, Quelen C, Zaki O, Quillet-Mary A, Davi F, Brousset P, Bousquet M, Ysebaert L. Small nucleolar RNA expression profiles refine the prognostic impact of IGHV mutational status on treatment-free survival in chronic lymphocytic leukaemia. Br J Haematol. 2016;172:819-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Ravo M, Cordella A, Rinaldi A, Bruno G, Alexandrova E, Saggese P, Nassa G, Giurato G, Tarallo R, Marchese G. Small non-coding RNA deregulation in endometrial carcinogenesis. Oncotarget. 2015;6:4677-4691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 66. | Martens-Uzunova ES, Jalava SE, Dits NF, van Leenders GJ, Møller S, Trapman J, Bangma CH, Litman T, Visakorpi T, Jenster G. Diagnostic and prognostic signatures from the small non-coding RNA transcriptome in prostate cancer. Oncogene. 2012;31:978-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 67. | Appaiah HN, Goswami CP, Mina LA, Badve S, Sledge GW Jr, Liu Y, Nakshatri H. Persistent upregulation of U6:SNORD44 small RNA ratio in the serum of breast cancer patients. Breast Cancer Res. 2011;13:R86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 68. | COHN WE. 5-Ribosyl uracil, a carbon-carbon ribofuranosyl nucleoside in ribonucleic acids. Biochim Biophys Acta. 1959;32:569-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 119] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 69. | Schwartz S, Bernstein DA, Mumbach MR, Jovanovic M, Herbst RH, León-Ricardo BX, Engreitz JM, Guttman M, Satija R, Lander ES. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell. 2014;159:148-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 767] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 70. | Kim NK, Theimer CA, Mitchell JR, Collins K, Feigon J. Effect of pseudouridylation on the structure and activity of the catalytically essential P6.1 hairpin in human telomerase RNA. Nucleic Acids Res. 2010;38:6746-6756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 71. | Penzo M, Guerrieri AN, Zacchini F, Treré D, Montanaro L. RNA Pseudouridylation in Physiology and Medicine: For Better and for Worse. Genes (Basel). 2017;8. [PubMed] |
| 72. | Walne AJ, Dokal I. Advances in the understanding of dyskeratosis congenita. Br J Haematol. 2009;145:164-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 73. | Thumati NR, Zeng XL, Au HH, Jang CJ, Jan E, Wong JM. Severity of X-linked dyskeratosis congenita (DKCX) cellular defects is not directly related to dyskerin (DKC1) activity in ribosomal RNA biogenesis or mRNA translation. Hum Mutat. 2013;34:1698-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 811] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 75. | Jády BE, Bertrand E, Kiss T. Human telomerase RNA and box H/ACA scaRNAs share a common Cajal body-specific localization signal. J Cell Biol. 2004;164:647-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 206] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 76. | Mitchell JR, Cheng J, Collins K. A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3' end. Mol Cell Biol. 1999;19:567-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 391] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 77. | Penzo M, Casoli L, Ceccarelli C, Treré D, Ludovini V, Crinò L, Montanaro L. DKC1 gene mutations in human sporadic cancer. Histol Histopathol. 2013;28:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 78. | Jack K, Bellodi C, Landry DM, Niederer RO, Meskauskas A, Musalgaonkar S, Kopmar N, Krasnykh O, Dean AM, Thompson SR. rRNA pseudouridylation defects affect ribosomal ligand binding and translational fidelity from yeast to human cells. Mol Cell. 2011;44:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 79. | Montanaro L, Calienni M, Bertoni S, Rocchi L, Sansone P, Storci G, Santini D, Ceccarelli C, Taffurelli M, Carnicelli D. Novel dyskerin-mediated mechanism of p53 inactivation through defective mRNA translation. Cancer Res. 2010;70:4767-4777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 80. | Bellodi C, Krasnykh O, Haynes N, Theodoropoulou M, Peng G, Montanaro L, Ruggero D. Loss of function of the tumor suppressor DKC1 perturbs p27 translation control and contributes to pituitary tumorigenesis. Cancer Res. 2010;70:6026-6035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 81. | Yoon A, Peng G, Brandenburger Y, Zollo O, Xu W, Rego E, Ruggero D. Impaired control of IRES-mediated translation in X-linked dyskeratosis congenita. Science. 2006;312:902-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 331] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 82. | Bellodi C, Kopmar N, Ruggero D. Deregulation of oncogene-induced senescence and p53 translational control in X-linked dyskeratosis congenita. EMBO J. 2010;29:1865-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 83. | Carlile TM, Rojas-Duran MF, Zinshteyn B, Shin H, Bartoli KM, Gilbert WV. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature. 2014;515:143-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 610] [Cited by in RCA: 792] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 84. | Ronchetti D, Todoerti K, Tuana G, Agnelli L, Mosca L, Lionetti M, Fabris S, Colapietro P, Miozzo M, Ferrarini M. The expression pattern of small nucleolar and small Cajal body-specific RNAs characterizes distinct molecular subtypes of multiple myeloma. Blood Cancer J. 2012;2:e96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 85. | Valleron W, Ysebaert L, Berquet L, Fataccioli V, Quelen C, Martin A, Parrens M, Lamant L, de Leval L, Gisselbrecht C. Small nucleolar RNA expression profiling identifies potential prognostic markers in peripheral T-cell lymphoma. Blood. 2012;120:3997-4005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 86. | Valleron W, Laprevotte E, Gautier EF, Quelen C, Demur C, Delabesse E, Agirre X, Prósper F, Kiss T, Brousset P. Specific small nucleolar RNA expression profiles in acute leukemia. Leukemia. 2012;26:2052-2060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 87. | Han L, Diao L, Yu S, Xu X, Li J, Zhang R, Yang Y, Werner HMJ, Eterovic AK, Yuan Y. The Genomic Landscape and Clinical Relevance of A-to-I RNA Editing in Human Cancers. Cancer Cell. 2015;28:515-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 417] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 88. | Paz-Yaacov N, Bazak L, Buchumenski I, Porath HT, Danan-Gotthold M, Knisbacher BA, Eisenberg E, Levanon EY. Elevated RNA Editing Activity Is a Major Contributor to Transcriptomic Diversity in Tumors. Cell Rep. 2015;13:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 262] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 89. | Chen Y, Wang H, Lin W, Shuai P. ADAR1 overexpression is associated with cervical cancer progression and angiogenesis. Diagn Pathol. 2017;12:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 90. | Chan TH, Lin CH, Qi L, Fei J, Li Y, Yong KJ, Liu M, Song Y, Chow RK, Ng VH. A disrupted RNA editing balance mediated by ADARs (Adenosine DeAminases that act on RNA) in human hepatocellular carcinoma. Gut. 2014;63:832-843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 182] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 91. | Chan TH, Qamra A, Tan KT, Guo J, Yang H, Qi L, Lin JS, Ng VH, Song Y, Hong H. ADAR-Mediated RNA Editing Predicts Progression and Prognosis of Gastric Cancer. Gastroenterology. 2016;151:637-650.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 92. | Qin YR, Qiao JJ, Chan TH, Zhu YH, Li FF, Liu H, Fei J, Li Y, Guan XY, Chen L. Adenosine-to-inosine RNA editing mediated by ADARs in esophageal squamous cell carcinoma. Cancer Res. 2014;74:840-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 93. | Yeo J, Goodman RA, Schirle NT, David SS, Beal PA. RNA editing changes the lesion specificity for the DNA repair enzyme NEIL1. Proc Natl Acad Sci USA. 2010;107:20715-20719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 94. | Anadón C, Guil S, Simó-Riudalbas L, Moutinho C, Setien F, Martínez-Cardús A, Moran S, Villanueva A, Calaf M, Vidal A. Gene amplification-associated overexpression of the RNA editing enzyme ADAR1 enhances human lung tumorigenesis. Oncogene. 2016;35:4422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 95. | Fujita K, Murakami Y, Hayashi S. A macromolecular inhibitor of the antizyme to ornithine decarboxylase. Biochem J. 1982;204:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 96. | Newman RM, Mobascher A, Mangold U, Koike C, Diah S, Schmidt M, Finley D, Zetter BR. Antizyme targets cyclin D1 for degradation. A novel mechanism for cell growth repression. J Biol Chem. 2004;279:41504-41511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 97. | Mangold U. Antizyme inhibitor: mysterious modulator of cell proliferation. Cell Mol Life Sci. 2006;63:2095-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 98. | Olsen RR, Zetter BR. Evidence of a role for antizyme and antizyme inhibitor as regulators of human cancer. Mol Cancer Res. 2011;9:1285-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 99. | van Duin M, van Marion R, Vissers K, Watson JE, van Weerden WM, Schröder FH, Hop WC, van der Kwast TH, Collins C, van Dekken H. High-resolution array comparative genomic hybridization of chromosome arm 8q: evaluation of genetic progression markers for prostate cancer. Genes Chromosomes Cancer. 2005;44:438-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 100. | Chin SF, Teschendorff AE, Marioni JC, Wang Y, Barbosa-Morais NL, Thorne NP, Costa JL, Pinder SE, van de Wiel MA, Green AR. High-resolution aCGH and expression profiling identifies a novel genomic subtype of ER negative breast cancer. Genome Biol. 2007;8:R215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 242] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 101. | Chen L, Li Y, Lin CH, Chan TH, Chow RK, Song Y, Liu M, Yuan YF, Fu L, Kong KL. Recoding RNA editing of AZIN1 predisposes to hepatocellular carcinoma. Nat Med. 2013;19:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 418] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 102. | Fumagalli D, Gacquer D, Rothé F, Lefort A, Libert F, Brown D, Kheddoumi N, Shlien A, Konopka T, Salgado R. Principles Governing A-to-I RNA Editing in the Breast Cancer Transcriptome. Cell Rep. 2015;13:277-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 103. | Hu X, Chen J, Shi X, Feng F, Lau KW, Chen Y, Chen Y, Jiang L, Cui F, Zhang Y. RNA editing of AZIN1 induces the malignant progression of non-small-cell lung cancers. Tumour Biol. 2017;39:1010428317700001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 104. | Popowicz GM, Schleicher M, Noegel AA, Holak TA. Filamins: promiscuous organizers of the cytoskeleton. Trends Biochem Sci. 2006;31:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 235] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 105. | Li JB, Levanon EY, Yoon JK, Aach J, Xie B, Leproust E, Zhang K, Gao Y, Church GM. Genome-wide identification of human RNA editing sites by parallel DNA capturing and sequencing. Science. 2009;324:1210-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 439] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 106. | Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1530] [Cited by in RCA: 1478] [Article Influence: 86.9] [Reference Citation Analysis (0)] |
| 107. | Leve F, Morgado-Díaz JA. Rho GTPase signaling in the development of colorectal cancer. J Cell Biochem. 2012;113:2549-2559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 108. | Kanzaki M. Insulin receptor signals regulating GLUT4 translocation and actin dynamics. Endocr J. 2006;53:267-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 109. | Han SW, Kim HP, Shin JY, Jeong EG, Lee WC, Kim KY, Park SY, Lee DW, Won JK, Jeong SY. RNA editing in RHOQ promotes invasion potential in colorectal cancer. J Exp Med. 2014;211:613-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 110. | Yi T, Mui AL, Krystal G, Ihle JN. Hematopoietic cell phosphatase associates with the interleukin-3 (IL-3) receptor beta chain and down-regulates IL-3-induced tyrosine phosphorylation and mitogenesis. Mol Cell Biol. 1993;13:7577-7586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 237] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 111. | Yi TL, Cleveland JL, Ihle JN. Protein tyrosine phosphatase containing SH2 domains: characterization, preferential expression in hematopoietic cells, and localization to human chromosome 12p12-p13. Mol Cell Biol. 1992;12:836-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 224] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 112. | Yi T, Ihle JN. Association of hematopoietic cell phosphatase with c-Kit after stimulation with c-Kit ligand. Mol Cell Biol. 1993;13:3350-3358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 172] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 113. | Pei D, Wang J, Walsh CT. Differential functions of the two Src homology 2 domains in protein tyrosine phosphatase SH-PTP1. Proc Natl Acad Sci USA. 1996;93:1141-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 113] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 114. | Beghini A, Ripamonti CB, Peterlongo P, Roversi G, Cairoli R, Morra E, Larizza L. RNA hyperediting and alternative splicing of hematopoietic cell phosphatase (PTPN6) gene in acute myeloid leukemia. Hum Mol Genet. 2000;9:2297-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 129] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 115. | Song IH, Kim YA, Heo SH, Park IA, Lee M, Bang WS, Park HS, Gong G, Lee HJ. ADAR1 expression is associated with tumour-infiltrating lymphocytes in triple-negative breast cancer. Tumour Biol. 2017;39:1010428317734816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 116. | Farrant M, Kaila K. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Prog Brain Res. 2007;160:59-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 277] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 117. | Daniel C, Wahlstedt H, Ohlson J, Björk P, Ohman M. Adenosine-to-inosine RNA editing affects trafficking of the gamma-aminobutyric acid type A (GABA(A)) receptor. J Biol Chem. 2011;286:2031-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 118. | Gumireddy K, Li A, Kossenkov AV, Sakurai M, Yan J, Li Y, Xu H, Wang J, Zhang PJ, Zhang L. The mRNA-edited form of GABRA3 suppresses GABRA3-mediated Akt activation and breast cancer metastasis. Nat Commun. 2016;7:10715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 149] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 119. | Maas S, Patt S, Schrey M, Rich A. Underediting of glutamate receptor GluR-B mRNA in malignant gliomas. Proc Natl Acad Sci USA. 2001;98:14687-14692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 304] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 120. | Chen YB, Liao XY, Zhang JB, Wang F, Qin HD, Zhang L, Shugart YY, Zeng YX, Jia WH. ADAR2 functions as a tumor suppressor via editing IGFBP7 in esophageal squamous cell carcinoma. Int J Oncol. 2017;50:622-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 121. | Higuchi M, Single FN, Köhler M, Sommer B, Sprengel R, Seeburg PH. RNA editing of AMPA receptor subunit GluR-B: a base-paired intron-exon structure determines position and efficiency. Cell. 1993;75:1361-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 500] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 122. | Evdokimova V, Tognon CE, Benatar T, Yang W, Krutikov K, Pollak M, Sorensen PH, Seth A. IGFBP7 binds to the IGF-1 receptor and blocks its activation by insulin-like growth factors. Sci Signal. 2012;5:ra92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 123. | Mutaguchi K, Yasumoto H, Mita K, Matsubara A, Shiina H, Igawa M, Dahiya R, Usui T. Restoration of insulin-like growth factor binding protein-related protein 1 has a tumor-suppressive activity through induction of apoptosis in human prostate cancer. Cancer Res. 2003;63:7717-7723. [PubMed] |
| 124. | Ruan W, Xu E, Xu F, Ma Y, Deng H, Huang Q, Lv B, Hu H, Lin J, Cui J. IGFBP7 plays a potential tumor suppressor role in colorectal carcinogenesis. Cancer Biol Ther. 2007;6:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 125. | Benatar T, Yang W, Amemiya Y, Evdokimova V, Kahn H, Holloway C, Seth A. IGFBP7 reduces breast tumor growth by induction of senescence and apoptosis pathways. Breast Cancer Res Treat. 2012;133:563-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 126. | Galeano F, Rossetti C, Tomaselli S, Cifaldi L, Lezzerini M, Pezzullo M, Boldrini R, Massimi L, Di Rocco CM, Locatelli F. ADAR2-editing activity inhibits glioblastoma growth through the modulation of the CDC14B/Skp2/p21/p27 axis. Oncogene. 2013;32:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 127. | Karsy M, Arslan E, Moy F. Current Progress on Understanding MicroRNAs in Glioblastoma Multiforme. Genes Cancer. 2012;3:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 128. | Tomaselli S, Galeano F, Alon S, Raho S, Galardi S, Polito VA, Presutti C, Vincenti S, Eisenberg E, Locatelli F. Modulation of microRNA editing, expression and processing by ADAR2 deaminase in glioblastoma. Genome Biol. 2015;16:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 129. | Choudhury Y, Tay FC, Lam DH, Sandanaraj E, Tang C, Ang BT, Wang S. Attenuated adenosine-to-inosine editing of microRNA-376a* promotes invasiveness of glioblastoma cells. J Clin Invest. 2012;122:4059-4076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 130. | Nemlich Y, Greenberg E, Ortenberg R, Besser MJ, Barshack I, Jacob-Hirsch J, Jacoby E, Eyal E, Rivkin L, Prieto VG. MicroRNA-mediated loss of ADAR1 in metastatic melanoma promotes tumor growth. J Clin Invest. 2013;123:2703-2718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 131. | Ota H, Sakurai M, Gupta R, Valente L, Wulff BE, Ariyoshi K, Iizasa H, Davuluri RV, Nishikura K. ADAR1 forms a complex with Dicer to promote microRNA processing and RNA-induced gene silencing. Cell. 2013;153:575-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 291] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 132. | Shoshan E, Mobley AK, Braeuer RR, Kamiya T, Huang L, Vasquez ME, Salameh A, Lee HJ, Kim SJ, Ivan C. Reduced adenosine-to-inosine miR-455-5p editing promotes melanoma growth and metastasis. Nat Cell Biol. 2015;17:311-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 201] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 133. | Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, Parker BJ, Suter CM, Preiss T. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012;40:5023-5033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 619] [Cited by in RCA: 751] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 134. | Metodiev MD, Spåhr H, Loguercio Polosa P, Meharg C, Becker C, Altmueller J, Habermann B, Larsson NG, Ruzzenente B. NSUN4 is a dual function mitochondrial protein required for both methylation of 12S rRNA and coordination of mitoribosomal assembly. PLoS Genet. 2014;10:e1004110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 233] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 135. | Frye M, Watt FM. The RNA methyltransferase Misu (NSun2) mediates Myc-induced proliferation and is upregulated in tumors. Curr Biol. 2006;16:971-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 136. | Goll MG, Kirpekar F, Maggert KA, Yoder JA, Hsieh CL, Zhang X, Golic KG, Jacobsen SE, Bestor TH. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science. 2006;311:395-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 777] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 137. | Huang W, Qi CB, Lv SW, Xie M, Feng YQ, Huang WH, Yuan BF. Determination of DNA and RNA Methylation in Circulating Tumor Cells by Mass Spectrometry. Anal Chem. 2016;88:1378-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |