Published online Aug 10, 2017. doi: 10.5306/wjco.v8.i4.351
Peer-review started: February 8, 2017
First decision: May 10, 2017
Revised: June 5, 2017
Accepted: July 7, 2017
Article in press: July 10, 2017
Published online: August 10, 2017
Processing time: 193 Days and 2.5 Hours
To report a single-center experience in rescue associating liver partition and portal vein ligation for staged hepatectomy (ALPPS), after failure of previous portal embolization. We also performed a literature review.
Between January 2014 and December 2015, every patient who underwent a rescue ALPPS procedure in Toulouse Rangueil University Hospital, France, was included. Every patient included had a project of major hepatectomy and a previous portal vein embolization (PVE) with insufficient future liver remnant to body weight ratio after the procedure. The ALPPS procedure was performed in two steps (ALPPS-1 and ALPPS-2), separated by an interval phase. ALPPS-2 was done within 7 to 9 d after ALPPS-1. To estimate the FLR, a computed tomography scan examination was performed 3 to 6 wk after the PVE procedure and 6 to 8 d after ALPPS-1. A transcystic stent was placed during ALPPS-1 and remained opened during the interval phase, in order to avoid biliary complications. Postoperative liver failure was defined using the 50-50 criteria. Postoperative complications were assessed according to the Dindo-Clavien Classification.
From January 2014 to December 2015, 7 patients underwent a rescue ALPPS procedure. Median FLR before PVE, ALPPS-1 and ALPPS-2 were respectively 263 cc (221-380), 450 cc (372-506), and 660 cc (575-776). Median FLR/BWR before PVE, ALPPS-1 and ALPPS-2 were respectively 0.4% (0.3-0.5), 0.6% (0.5-0.8), and 1% (0.8-1.2). Median volume growth of FLR was 69% (18-92) after PVE, and 45% (36-82) after ALPPS-1. The combination of PVE and ALPPS induced a growth of median initial FLR of +408 cc (254-513), leading to an increase of +149% (68-199). After ALPPS-2, 4 patients had stage I-II complications. Three patients had more severe complications (one stage III, one stage IV and one death due to bowel perforation). Two patients suffered from postoperative liver failure according to the 50/50 criteria. None of our patients developed any biliary complication during the ALPPS procedure.
Rescue ALPPS may be an alternative after unsuccessful PVE and could allow previously unresectable patients to reach surgery. Biliary drainage seems to reduce biliary complications.
Core tip: Hepatic surgery appears as the best curative option for patients with primary or secondary malignant hepatic tumors. Several strategies have been developed to avoid postoperative liver failure, such as portal vein embolization (PVE). In 2012, associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) was developed. It induces rapid and extensive hypertrophy of the future liver remnant, but with high morbidity and mortality. Therefore, some authors have suggested that ALPPS should be performed only as a “rescue”, after failed PVE. We describe our results of rescue ALPPS after failure of previous PVE and we perform a literature review.
- Citation: Maulat C, Philis A, Charriere B, Mokrane FZ, Guimbaud R, Otal P, Suc B, Muscari F. Rescue associating liver partition and portal vein ligation for staged hepatectomy after portal embolization: Our experience and literature review. World J Clin Oncol 2017; 8(4): 351-359
- URL: https://www.wjgnet.com/2218-4333/full/v8/i4/351.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i4.351
Hepatic surgery appears as the best curative option for patients with primary or secondary malignant tumors of the liver[1,2]. As complete resection of the tumor load is directly linked to overall survival, it is sometimes necessary to perform major hepatectomies in order to achieve such a goal. The main complication after major hepatectomy is liver failure. Several studies have shown that the size of the future liver remnant (FLR) is a key element[3-5], as it is directly correlated to the postoperative liver function[6,7]. In 2013, a consensus statement established a FLR cut-off above which the risk of postoperative liver failure was considered too high for safe surgery: 20% in normal liver, 30% in liver pretreated with chemotherapy, and 40% in cirrhotic liver[8]. The FLR to body weight ratio (FLR-BWR) (%) is also used as a predictive factor for hepatic dysfunction: Patients with FLR-BWR < 0.5% have a major risk of liver failure and postoperative mortality[9,10].
Several strategies have been developed to lower the risk of postoperative liver failure, such as portal vein occlusion (PVO), either by ligature (PVL) or embolization (PVE). The aim of these techniques is to decrease the portal blood flow to the ipsilateral liver, inducing atrophy of the ipsilateral liver and hypertrophy of the contralateral liver[11]. It enables previously unresectable patients to have access to a surgical treatment by achieving an appropriate FLR volume[12]. Indeed, the PVE leads to an average hypertrophy of the contralateral liver (usually the left lobe) of 40% in 4-8 wk[13]. In 2012, a new surgical technique has been developed, “associating liver partition and portal vein ligation for staged hepatectomy” (ALPPS)[14].
This procedure induces rapid and extensive hypertrophy of the FLR in two steps. During the first surgical step of the original ALPPS procedure, called the “in situ splitting”, the right portal vein is ligated (if there were no previous PVE), and the surgeon performs a transection of the hepatic parenchyma for extended right hepatectomy. The right hepatic artery, the right bile duct and the drainage veins are not ligated at this point. After the “in situ splitting”, the right extended lobe is covered by a membrane or a bag to prevent adhesions. Several variations of ALLPS were later developed such as “left ALPPS”, allowing left lobectomy, or “right ALPPS”, allowing right posterior sectoriectomy[15].
The second surgery is usually performed within 7 to 15 d after the first step. During this step, the right liver is removed, after having dissected and ligated the remaining artery, bile duct and hepatic veins[14,16].
Although promising results were published by Schnitzbauer et al[14], several studies have described high perioperative morbidity and mortality, suggesting the necessity of a better selection of patients[17-19]. Some authors have suggested that the ALPPS procedure should be performed only as a “rescue”, that is in case of insufficient liver hypertrophy after PVE[15,20-22]. Yet, very few data relative specifically to the rescue ALPPS have been published[21-24] as most of the existing articles do not focus on this particular indication.
The aim of our study was to report the outcomes of patients with primary and secondary liver tumors undergoing a rescue ALPPS procedure in our center, after failure of previous portal embolization. We also performed a literature review.
Between January 2014 and December 2015, every patient who underwent a rescue ALPPS procedure in Toulouse Rangueil University Hospital, France, was included. We evaluated the patients who could benefit from this strategy at our regional multidisciplinary team meeting, attended by senior hepatobiliary surgeons, hepatologists, oncologists, and radiologists.
The criteria for a rescue ALPPS procedure in our center were: A project of major hepatectomy, a previous PVE with insufficient liver hypertrophy after the procedure, age above 18, an absence of contraindication to surgery, and the approval of the patient after thorough information regarding the risks of the procedure.
In our center, a FLR-BWR < 0.5% was considered as a contraindication to perform major liver surgery. In case of fibrotic liver, previous chemotherapy, previous hepatectomy or multiple comorbidities, our center had higher ratio objectives. FLR-BWR around 1 was considered as optimal for major hepatic surgery. Patients who were eligible for a rescue ALPPS but did not complete the procedure were excluded from our study. All data were retrospectively collected in our local database, including patient characteristics, volumetric measurements, surgical characteristics and complications.
PVE was performed in an interventional X-ray room or in an operating theater. For all patients, we used a floor-mounted image-guided system (Innova™ IGS 520, General Electric Healthcare, United Kingdom) to perform ultrasound-guided puncture of a portal branch of the left liver lobe, usually segment III.
After a complete portography, we performed a contralateral embolization, using a mixture of 50% Lipiodol® (Guerbet, Vilepinte, France) and 50% Glubran2® (GEM SRL, Viareggio, Italy). We injected it selectively in each branch of the right portal tree, in order to occlude it. If occlusion of segment IV portal veins was necessary, it was performed after selective catheterization of the portal branches. Then, we administered 100, 250 and 400 μm microspheres (Embozene TM, Boston scientifics, Marlborough, MA, United States). This step was generally completed by 0.35 inch coils (Tornado®, Cook Medical, Bloomington, IN, United States).
After each PVE, we confirmed the complete occlusion of the right portal veins and the integrity of the left remaining ones by a final portography.
Before inclusion, each patient had a classical multi-slice computed tomography (MSCT) examination, including a portal phase.
A control CT scan examination was done 3 to 6 wk after the PVE procedure and 6 to 8 d after ALPPS-1. These examinations were performed using a 16-detector row CT scanner (Innova 411, General Electric Healthcare, United Kingdom). The acquisition parameters were: Voltage 120 KVp, intensity 650 mAs, and slice thickness 2 mm, collimation 1mm. Hepatic volumetry was evaluated on the portal phase of the MSCT examination using a semi-automatic method (Terarecon® software, Frankfort, Germany).
The ALPPS procedure was performed in two steps (ALPPS-1 and ALPPS-2), separated by an interval phase.
The surgical technique during ALPPS-1 was the following: Exploration of the abdominal cavity to look for signs of extra-hepatic metastases, which would be a contraindication to surgical resection. In case of cholangiocarcinoma, intra-hepatic metastases were also considered a contraindication; Ultrasonographic examination of the liver; Cholecystectomy; Introduction of a transcystic catheter, left in place after the first step (except for one patient who had a radiological biliary drainage); “Hanging maneuver”[25]; Splitting of the hepatic parenchyma for extended right hepatectomy, under intermittent clamping. We performed a complete parenchymal split; in case of metastases located in the FLR, wedge resections or thermoablations were performed during ALPPS-1.
Parenchymal transection was performed using Erbejet (Erbejet, RBE Elektromedizin GmbH, Waldhornlestrasse, Tubingen, Germany, ESM2 model, ref 10340-000) or ultrasonic dissector (Dissectron, Satelec Medical, ref DP 000108). The right hepatic artery, the bile duct and the hepatic veins were identified and surrounded with vessel loops to allow better identification during ALPPS-2. After in situ splitting, the two slices of the liver were covered using sheets of Tachosil with hemostatic aim. Instead of a bag, we placed COVA™ membranes (COVA+™, Biom’Up, France) around the liver, the hepatic pedicle and between the two hepatic slices. Silicone drainage was placed between the resection surfaces.
ALPPS-2 was performed within 7 to 9 d after the first step. After identifying the vascular and biliary structures, we performed the dissection and ligation of the remaining artery, the bile duct and the hepatic veins. Then the right liver was removed. For hilar cholangiocarcinoma, a Roux-en-Y hepaticojejunostomy was performed. Silicone drainage was placed near the resection surface.
Patients were hospitalized into intensive care unit during the first few days, and the transcystic stent (or the radiological biliary drain) remained opened during the interval phase, in order to avoid biliary complications. We encouraged enhanced recovery by early removal of catheter, mobilization and transfer into standard care unit.
Postoperative liver failure was defined using the 50-50 criteria[26], which associates prothrombin time (PT) < 50% and serum bilirubin (SB) > 50 μmol/L at day 5. Postoperative complications were assessed according to the Dindo-Clavien Classification[27].
Literature review was performed using PubMed, Google Scholar and the Cochrane Library Central. Articles reported were written in English and ALPPS procedures were limited to humans. The mesh terms were: “ALPPS”, “Associating liver partition and portal vein ligation for staged hepatectomy”, “Portal vein embolization”, “rescue ALPPS”, “salvage ALPPS”.
From January 2014 to December 2015, 10 patients were initially selected to undergo a rescue ALPPS procedure. Two patients had only an explorative laparotomy because their tumor was found unresectable during ALPPS-1. The third patient had more metastases in the left lobe than expected; therefore, the surgeon changed strategy during ALPPS-1 and performed a classical two-stage hepatectomy. These 3 patients were excluded from our analysis.
The characteristics of the 7 patients who underwent the rescue ALPPS procedure are detailed in Table 1. In our cohort, 4 patients had colorectal liver metastases (CRLM), and the others had cholangiocarcinoma. The 2 patients with a Bismuth-Corlette type IIIa perihilar cholangiocarcinoma (pCCA) had had a radiological biliary drainage prior to surgery. Among our 7 patients, one had a previous history of left lobectomy.
| Variable | Rescue ALPPS (n = 7) |
| Male/female gender | 4/3 |
| Age, yr (range) | 61 (53-70) |
| Body mass index (range) | 23 (21-27) |
| ASA 1-2 | 6 |
| ASA 3 | 1 |
| Colorectal liver metastases | 4 |
| Number of liver metastases (range) | 5 (2-7) |
| Size of the largest metastases, mm (range) | 45 (20-65) |
| Tumor location | |
| Right lobe ± segment IV | 3 |
| Right lobe + segment IV + left lateral segment | 1 |
| Previous colorectal resection | 3 |
| Previous hepatic resection or thermoablation | 3 |
| Preoperative chemotherapy | 4 |
| Oxaliplatin based | 4 |
| Irinotecan based | 3 |
| Angiogenesis inhibitor | 1 |
| Intra-arterial chemotherapy | 1 |
| Number of preoperative chemotherapy cycles (range) | 16 (8-25) |
| Cholangiocarcinoma | 3 |
| Perihilar/intrahepatic | 2/1 |
| Preoperative chemotherapy | 1 |
| Gemcitabine and oxaliplatin | 1 |
| No. of preoperative chemotherapy cycles | 3 |
| Portal vein embolization | 7 |
| Right lobe | 5 |
| Right lobe + segment IV | 2 |
| Comorbidity | |
| Cardiovascular | 2 |
| Pulmonary | 0 |
| Diabetes | 0 |
| Prior history of cancer | 2 |
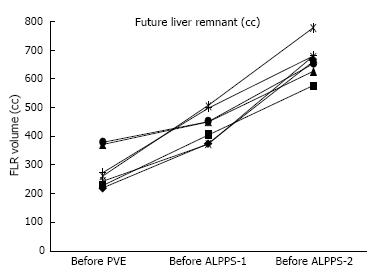
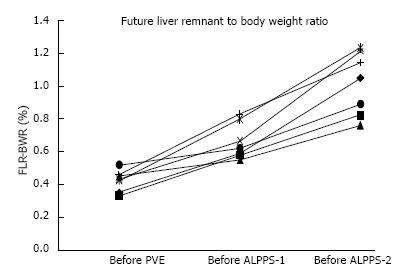
FLR and FLR/BWR volume increase among the different steps of rescue ALPPS are reported in Figures 1 and 2. Median FLR before PVE, ALPPS-1 and ALPPS-2 were respectively 263 cc (221-380), 450 cc (372-506), and 660 cc (575-776) (Figure 1). Median FLR/BWR before PVE, ALPPS-1 and ALPPS-2 were respectively 0.4% (0.3%-0.5%), 0.6% (0.5%-0.8%), and 1% (0.8%-1.2%) (Figure 2).
Median volume growth of FLR was 69% (18%-92%) after PVE, and 45% (36%-82%) after ALPPS-1. The combination of PVE and ALPPS induced a median growth of initial FLR of +408 cc (254-513), leading to a median increase of +149% (68%-199%).
Intermittent hilar or portal clamping was performed in all patients during ALPPS-1, with a median total duration of 20 min (15-35). ALPPS-1 had a median surgical duration of 240 min (180-300), and median blood losses were 750 mL (300-1000). ALPPS-2 median surgical duration was 90 min (60-120) and median blood losses were 300 mL (0-800). Six patients required blood transfusions during ALPPS-1 and 4 patients during ALPPS-2. One patient required platelet transfusion during ALPPS-2. R0 resection was completed in 6 patients. One patient with CLRM had a R1 resection (surgical margin in contact with one metastasis) (Table 2).
| Variable | Rescue ALPPS (n = 7) |
| Surgery | |
| Right trisegmentectomy extended to segment I | 4/7 |
| Right lobectomy | 1/7 |
| Right lobectomy combined with thermoablation | 2/7 |
| Days between ALPPS-1 and ALPPS-2 (range) | 7 (7-9) |
| ALPPS-1 | |
| Surgery duration ALPPS-1, min (range) | 240 (180-300) |
| Blood loss during ALPPS-1, mL (range) | 750 (300-1000) |
| Prothrombin ratio day 5, % (range) | 76 (70-85) |
| Bilirubin day 5, µmol/L (range) | 24 (15-70) |
| MELD score day 5 (range) | 10 (8-15) |
| ALPPS-2 | |
| Surgery duration ALPPS-2, min (range) | 90 (60-120) |
| Blood loss during ALPPS-2, mL (range) | 300 (0-800) |
| Prothrombin ratio day 5, % (range) | 60 (41-73) |
| Bilirubin day 5, µmol/L (range) | 43 (10-182) |
| MELD score day 5 (range) | 14 (9-21) |
| Complications | |
| Liver failure after ALPPS-1 | 0/7 |
| Liver failure after ALPPS-2 | 2/7 |
| Complications after ALPPS-1 and before ALPPS-2 | 0/7 |
| Complications after ALPPS-2 | 7/7 |
| Clavien I-II | 4/7 |
| Clavien III | 1/7 |
| Clavien IV | 1/7 |
| Clavien V | 1/7 |
| 30 d mortality | 1/7 |
| 90 d mortality | 1/7 |
| R0 resection | 6/7 |
The postoperative outcomes are detailed in Tables 2 and 3. There was no per-operative incident reported during ALPPS-1 or ALPPS-2 surgical steps, and we did not experience any complication, including biliary complications, during the interval phase between ALPPS-1 and ALPPS-2.
| Patient number | Gender | Age | Tumor | Underlying liver function | FLR/BWR before PVE, % | FLR/BWR before ALPPS-1, % | FLR/BWR before ALPPS-2, % | ALPPS-2 day 5 Bilirubin, µmol/L | ALPPS-2 day 5 PT, % | Dindo-Clavien classification | Complications(by order of appearance) |
| 1 | M | 68 | pCCA | Cholestasis | 0.35 | 0.59 | 1.05 | 182 | 45 | V | Intraoperative blood transfusion, intra-abdominal abscess, pleural effusion, death due to peritonitis caused by bowel perforation |
| 2 | M | 70 | iCCA | - | 0.33 | 0.58 | 0.82 | 43 | 60 | II | Intraoperative blood transfusion, transitory ascites |
| 3 | M | 55 | CRLM | FNH | 0.45 | 0.55 | 0.76 | 43 | 41 | II | Intraoperative blood transfusion |
| 4 | F | 66 | pCCA | Cholestasis | 0.43 | 0.66 | 1.21 | 44 | 73 | III | Transitory ascites and intra-abdominal abscess |
| 5 | F | 59 | CRLM | SOS and steatosis | 0.42 | 0.80 | 1.23 | 10 | 69 | II | Intraoperative blood transfusion, urinary infection |
| 6 | F | 53 | CRLM | Dystrophy | 0.52 | 0.62 | 0.89 | 87 | 50 | IV | Intraoperative blood transfusion, internal hemorrhage, transitory hepatic insufficiency, infected ascites, septic choc, and intra-abdominal abscess |
| 7 | M | 61 | CRLM | - | 0.46 | 0.83 | 1.14 | 13 | 67 | II | Intraoperative blood transfusion, transitory chylous ascites |
After ALPPS-2, postoperative complications occurred among all of our patients. Four patients had stage I-II complications: Ascites (n = 3), urinary infection (n = 1) or intraoperative blood transfusion (n = 6). Three patients had more serious complications. One had intra-abdominal abscess requiring radiological drainage (patient 4). Patient 6 developed a hemorrhage two hours after ALPPS-2, requiring an emergency revision surgery. A surgical clip on an arterial branch had slipped, causing massive internal bleeding. Six days later, she had septic shock, leading to another emergency revision surgery, but we could not find the cause of the septic shock. A radiological drainage was performed a few days later to drain an abdominal abscess. Afterwards, she progressively enhanced total recovery. We report one postoperative death 10 d after ALPPS-2, due to a peritonitis caused by bowel perforation (patient 1). Two patients suffered from postoperative liver failure according to the 50/50 criteria. None of our patients developed any biliary complication.
In this study, we report 7 cases of rescue ALPPS that illustrate how such a procedure can be used successfully after failure of PVE.
In our cohort, FLR volume increased despite a previously insufficient hypertrophy after PVE. The causes of insufficient volume growth of FLR after PVE are known: Technical failure during the procedure (impossibility of cannulating the portal system due to altered portal anatomy), portal vein recanalization, portal collateral development and poor quality of hepatic parenchyma[7,28,29]. In our study, 5 patients had chemotherapy before PVE, which induced histopathological damages (steatosis, sinusoidal obstruction syndrome, cholestasis, etc.), and affected the regenerative capacities of the liver after PVE.
It allowed 7 patients to reach surgery, while they were considered unresectable after PVE. Among them, 6 had R0 resection. Without the rescue ALPPS technique, they would have been considered unresectable despite PVE, and offered only palliative measures. Nonresection following PVE has been described by Abulkhir et al[7] in 2008. Their study focused on a cohort of 1088 patients undergoing PVE and showed a 15% failure rate, including inadequate hypertrophy of remnant liver in 2% of cases. These results show that while the inadequate hypertrophy of FLR after PVE must be feared, it remains infrequent. It explains the low number of patients in our study, and it also explains the scarcity of literature about rescue ALPPS performed after PVO, as is shown in our literature review (Table 4). However, the rate of insufficient FLR after PVE will probably increase in the years to come, due to more and more intensive chemotherapies, which greatly alter hepatic parenchyma. Most papers describe series of 1 to 3 cases and only 4 studies report small cohorts (9 to 11 patients) (Table 4). Therefore, the size of our cohort (7 patients) is consistent with the number of cases developed in literature.
| Rescue ALPPS after PVO (PVE/PVL/PVE + PVL) | Tumor | Days between ALPPS-1 and ALPPS-2 | FLR/BWR before PV0, % | FLR/BWR before ALPPS-1, % | FLR/BWR before ALPPS-2, % | Growth of FLR between PVO and ALPPS-1, % (range) | Growth of FLR between ALPPS-1 and ALPPS-2, % (range) | Clavien Dindo > III | 30-d mortality | |
| Conrad et al[37], 2012 | 1 (1/0/0) | CRLM | 9 | NC | NC | NC | -1 | 47 | 0/1 | 0/1 |
| Gauzolino et al[15], 2013 | 1 (1/0/0) | CRLM | 7 | NC | NC | 0.4 | NC | 26 | 0/1 | 0/1 |
| Knoefel et al[21], 2013 | 3 (3/0/0) | NC | 6 | NC | NC | NC | 462 | 652 | 1/2 | 1/2 |
| Björnsson et al[20], 2013 | 2 (2/0/0) | CRLM (n = 1) HCC (n = 1) | 9 | NC | NC | NC | NC | NC | 0/2 | NC |
| Tschuor et al[22], 2013 | 3 (1/1/1) | CRLM | 8 | NC | NC | NC | 612 | 792 | 2/3 | 0/3 |
| Vyas et al[38], 2014 | 1 (1/0/0) | Neuroendocrine metastases | 8 | 0.4 | 0.5 | 0.9 | 24 | 70 | 0/1 | 0/1 |
| Nadalin et al[39], 2014 | 2 (2/0/0) | CRLM (n = 1) | 13 | NC | 0.52 | NC | NC | NC | 1/2 | 1/2 |
| Pancreatic metastases (n = 1) | ||||||||||
| Fard-Aghaie et al[40], 2015 | 1 (1/0/0) | CRLM | 26 | NC | NC | NC | 69 | 50 | 1/1 | 1/1 |
| Alavrez et al[41], 2015 | 1 (0/0/1) | CRLM | 7 | NC | NC | NC | 38 | 65 | 1/1 | 0/1 |
| Croome et al[42], 2015 | 2 (2/0/0) | CRLM | 8 | NC | NC | NC | NC | NC | NC | NC |
| Truant et al[23], 2015 | 9 (9/0/0) | NC | 8 | NC | NC | NC | NC | NC | NC | NC |
| Björnsson et al[43], 2016 | 10 (NC) | CRLM | 8 | NC | NC | NC | NC | NC | NC | 0/10 |
| Sparrelid et al[24], 2016 | 11 (7/4/2) | CRLM | 7 | 0.31 | 0.41 | 0.71 | 271 (7-67) | 621 (19-120) | 4/11 | 0/11 |
| Ulmer et al[44], 2017 | 9 (9/0/0) | CRLM (n = 6), CCA (n = 2), others liver metastases (n = 1) | 9 | NC | NC | NC | 302 | 782 | 6/9 | 1/9 |
| Maulat, 2017 | 7 (7/0/0) | CRLM (n = 4), CCA (n = 3) | 71 | 0.41 | 0.61 | 11 | 691 | 451 | 3/7 | 1/7 |
We decided to perform ALPPS procedure for 2 patients who had a median FLR/BWR before PVE of 0.8% due to a high risk of liver failure after major hepatectomy: One patient had previous left lobectomy, wedge resections, thermoablation and neoadjuvant chemotherapy, inducing steatosis and sinusoidal obstruction syndrome. The second patient had multiple neoadjuvant chemotherapy cycles, suggesting that it was necessary to optimize its FLR volume to avoid postoperative liver failure.
In our cohort, median FLR growth between ALPPS-1 and ALPPS-2 was 45% (36%-82%), which might appear less than in the literature (70%-80%[14,18]). The impact of PVE before ALPPS might be an explanation to this result. Compared to the original ALPPS procedure, liver hypertrophy is developed in two steps: With PVE first, and then with the rescue ALPPS procedure. Therefore, it is more adequate to compare the FLR growth of the original ALPPS with the overall FLR growth of the complete rescue ALPPS procedure (from PVE to ALPPS). In our study, the median overall FLR growth of the complete rescue ALPPS procedure is 149%, which is far greater than the FLR growth induced by the “original ALPPS” described in the literature. Another factor which might explain our results regarding FLR growth between ALPPS-1 and ALPPS-2 is that the interval phase was shorter (7 d) than reported in literature: An average of 14 d was reported from 320 cases in the International ALPPS Registry by Schadde et al[30] in 2015.
Among our 7 patients, we report 43% of major complications (Clavien-Dindo > III), including one death after ALPPS-2, due to bowel perforation, which is consistent with the literature of original ALPPS[31,32]. It is important to note that rescue ALPPS after PVE does not induce more major complication than the original ALPPS. It suggests that PVE does not have any impact on the rate of complications. Surgical complications after ALPPS procedure are partly linked to inflammatory adhesions around the liver and the hepatic pedicle, inducing many dissection difficulties during ALPPS-2. Using absorbable collagen membranes (COVA™ membranes) instead of bags at the end of ALPPS-1 helped prevent these inflammatory adhesions. We also performed ALPPS-2 within 7 to 9 d after ALPPS-1, which is shorter than the interval phase duration described in literature[30]. These two factors explain why we did not experience major inflammatory adhesions during ALPPS-2. It is interesting to note that we did not have any biliary complication in our cohort. In 2015, Truant et al[23] reported that among a series of 62 patients who underwent ALPPS procedure, 25 patients (40%) had biliary fistula: 19 (31%) after ALPPS-1 and 16 (27%) after ALPPS-2. Other studies are reporting bile leakage in up to 20% of patients after ALPPS procedure[14,18,33-35]. Biliary complications are the main cause of morbidity after ALPPS, and they are much more frequent than with ordinary hepatectomies (5%). It is even one of the main criticisms of this technique, as biliary fistula is known to alter the liver regeneration capacities, increase the risk for sepsis, extend the time of hospital stay, and increase postoperative mortality[36].
Therefore, it is of great importance to prevent biliary complication. Our results suggest that the use of biliary drainage during the interval phase (with transcystic catheter or radiological biliary drainage) is a promising technique to prevent biliary complications. To our knowledge, this is the first publication describing the use of a systematic biliary drainage between ALPPS-1 and ALPPS-2.
In conclusion, our study suggests that rescue ALPPS may be an alternative after unsuccessful PVE and could allow previously unresectable patients to reach surgery. It provides an opportunity for complete resection in cases otherwise eligible only to palliative treatments. Although the rate of complications is high, the use of PVE prior to the ALPPS procedure does not seem to increase morbidity. The use of a biliary drainage during the interval phase seems a promising technique to reduce biliary complications, although further studies should be performed to confirm these results.
Hepatic surgery appears as the best curative option for patients with primary or secondary malignant tumors of the liver. The main complication after major hepatectomy is liver failure. Several studies have shown that the size of the future liver remnant (FLR) is a key element as it is directly correlated to the postoperative liver function. Several strategies have been developed to lower the risk of postoperative liver failure, such as portal vein embolization (PVE). The aim of this technique is to decrease the portal blood flow to the ipsilateral liver, inducing atrophy of the ipsilateral liver and hypertrophy of the contralateral liver. In 2012, a new surgical technique has been developed, “associating liver partition and portal vein ligation for staged hepatectomy” (ALPPS). This procedure induces rapid and extensive hypertrophy of the FLR in two steps. Several studies have described high perioperative morbidity and mortality. Therefore, some authors have suggested that ALPPS should be performed only as a “rescue”, after failed PVE.
Considering the high perioperative morbidity and mortality of ALPPS procedure, the current hotspots in this research field is the necessity of a better selection of patients and the necessity to minimize complications, and more specifically biliary complications.
Yet, very few data relative specifically to the rescue ALPPS have been published as most of the existing articles do not focus on this particular indication. Most papers describe series of 1 to 3 cases and only 4 studies report small cohorts (9 to 11 patients). Therefore, the size of the cohort (7 patients) is consistent with the number of cases developed in literature. Moreover, biliary complications are the main cause of morbidity after ALPPS, and they are much more frequent than with ordinary hepatectomies (5%). This study suggests that the use of a biliary drainage during the interval phase seems a promising technique to reduce biliary complications. To our knowledge, this is the first publication describing the use of a systematic biliary drainage between ALPPS-1 and ALPPS-2.
The results of the study suggest that in the future, ALPPS procedure should be performed only as a “rescue”, in case of insufficient liver hypertrophy after PVE. Rescue ALPPS could allow previously unresectable patients to reach surgery. It provides an opportunity for complete resection in cases otherwise eligible only to palliative treatments.
ALPPS: (Associating liver partition and portal vein ligation for staged hepatectomy) procedure was performed in two steps (ALPPS-1 and ALPPS-2), separated by an interval phase. During ALPPS-1, the surgeon performs a transection of the hepatic parenchyma. In this study, ALPPS-2 was performed within 7 to 9 d after ALPPS-1. During ALPPS-2, the right liver is removed, after having ligated the remaining artery, bile duct and hepatic veins.
The authors present a study on the interesting subject of rescue ALPPS.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Peters GH, Sandri JBL, Sturesson C, Tarazov PG S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 588] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 2. | Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 923] [Cited by in RCA: 967] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 3. | Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya T, Sugimachi K. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 326] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 4. | Shoup M, Gonen M, D’Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, Tuorto S, Blumgart LH, Fong Y. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 340] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 5. | Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, Curley SA, Vauthey JN. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 364] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 6. | Azoulay D, Castaing D, Krissat J, Smail A, Hargreaves GM, Lemoine A, Emile JF, Bismuth H. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 284] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, Habib N, Jiao LR. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 474] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 8. | Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford). 2013;15:91-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 9. | Truant S, Oberlin O, Sergent G, Lebuffe G, Gambiez L, Ernst O, Pruvot FR. Remnant liver volume to body weight ratio & gt; or =0.5%: A new cut-off to estimate postoperative risks after extended resection in noncirrhotic liver. J Am Coll Surg. 2007;204:22-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 214] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Truant S, Boleslawski E, Sergent G, Leteurtre E, Duhamel A, Hebbar M, Pruvot FR. Liver function following extended hepatectomy can be accurately predicted using remnant liver volume to body weight ratio. World J Surg. 2015;39:1193-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Madoff DC, Gaba RC, Weber CN, Clark TW, Saad WE. Portal Venous Interventions: State of the Art. Radiology. 2016;278:333-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Hemming AW, Reed AI, Howard RJ, Fujita S, Hochwald SN, Caridi JG, Hawkins IF, Vauthey JN. Preoperative portal vein embolization for extended hepatectomy. Ann Surg. 2003;237:686-691; discussion 691-693. [PubMed] [DOI] [Full Text] |
| 13. | Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, Denys A, Sauvanet A. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 429] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 14. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 934] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 15. | Gauzolino R, Castagnet M, Blanleuil ML, Richer JP. The ALPPS technique for bilateral colorectal metastases: three “variations on a theme”. Updates Surg. 2013;65:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Vivarelli M, Vincenzi P, Montalti R, Fava G, Tavio M, Coletta M, Vecchi A, Nicolini D, Agostini A, Ahmed EA. ALPPS Procedure for Extended Liver Resections: A Single Centre Experience and a Systematic Review. PLoS One. 2015;10:e0144019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Kokudo N, Shindoh J. How can we safely climb the ALPPS? Updates Surg. 2013;65:175-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Schadde E, Ardiles V, Robles-Campos R, Malago M, Machado M, Hernandez-Alejandro R, Soubrane O, Schnitzbauer AA, Raptis D, Tschuor C. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg. 2014;260:829-836; discussion 836-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 349] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 19. | Dokmak S, Belghiti J. Which limits to the “ALPPS” approach? Ann Surg. 2012;256:e6; author reply e16-e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Björnsson B, Gasslander T, Sandström P. In situ split of the liver when portal venous embolization fails to induce hypertrophy: a report of two cases. Case Rep Surg. 2013;2013:238675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Knoefel WT, Gabor I, Rehders A, Alexander A, Krausch M, Schulte am Esch J, Fürst G, Topp SA. In situ liver transection with portal vein ligation for rapid growth of the future liver remnant in two-stage liver resection. Br J Surg. 2013;100:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 22. | Tschuor Ch, Croome KP, Sergeant G, Cano V, Schadde E, Ardiles V, Slankamenac K, Clariá RS, de Santibaňes E, Hernandez-Alejandro R, Clavien PA. Salvage parenchymal liver transection for patients with insufficient volume increase after portal vein occlusion -- an extension of the ALPPS approach. Eur J Surg Oncol. 2013;39:1230-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Truant S, Scatton O, Dokmak S, Regimbeau JM, Lucidi V, Laurent A, Gauzolino R, Castro Benitez C, Pequignot A, Donckier V. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): impact of the inter-stages course on morbi-mortality and implications for management. Eur J Surg Oncol. 2015;41:674-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 24. | Sparrelid E, Gilg S, Brismar TB, Lundell L, Isaksson B. Rescue ALPPS is efficient and safe after failed portal vein occlusion in patients with colorectal liver metastases. Langenbecks Arch Surg. 2017;402:69-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Belghiti J, Guevara OA, Noun R, Saldinger PF, Kianmanesh R. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg. 2001;193:109-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 356] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 26. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828, discussion 824-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 823] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 27. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24849] [Article Influence: 1183.3] [Reference Citation Analysis (0)] |
| 28. | van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM, van Delden OM. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol. 2013;36:25-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 333] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 29. | Brouquet A, Belghiti J. Chemotherapy and its effect on liver hypertrophy: implications for portal vein embolization and resection. Semin Intervent Radiol. 2008;25:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Schadde E, Raptis DA, Schnitzbauer AA, Ardiles V, Tschuor C, Lesurtel M, Abdalla EK, Hernandez-Alejandro R, Jovine E, Machado M. Prediction of Mortality After ALPPS Stage-1: An Analysis of 320 Patients From the International ALPPS Registry. Ann Surg. 2015;262:780-785; discussion 780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 31. | Schadde E, Schnitzbauer AA, Tschuor C, Raptis DA, Bechstein WO, Clavien PA. Systematic review and meta-analysis of feasibility, safety, and efficacy of a novel procedure: associating liver partition and portal vein ligation for staged hepatectomy. Ann Surg Oncol. 2015;22:3109-3120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 32. | Adam R, Imai K, Castro Benitez C, Allard MA, Vibert E, Sa Cunha A, Cherqui D, Baba H, Castaing D. Outcome after associating liver partition and portal vein ligation for staged hepatectomy and conventional two-stage hepatectomy for colorectal liver metastases. Br J Surg. 2016;103:1521-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 33. | Cai YL, Song PP, Tang W, Cheng NS. An updated systematic review of the evolution of ALPPS and evaluation of its advantages and disadvantages in accordance with current evidence. Medicine (Baltimore). 2016;95:e3941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Takamoto T, Sugawara Y, Hashimoto T, Makuuchi M. Associating liver partition and portal vein ligation (ALPPS): Taking a view of trails. Biosci Trends. 2015;9:280-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Zhang GQ, Zhang ZW, Lau WY, Chen XP. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): a new strategy to increase resectability in liver surgery. Int J Surg. 2014;12:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Yamashita Y, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K, Shimada M, Sugimachi K. Bile leakage after hepatic resection. Ann Surg. 2001;233:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 255] [Article Influence: 10.6] [Reference Citation Analysis (2)] |
| 37. | Conrad C, Shivathirthan N, Camerlo A, Strauss C, Gayet B. Laparoscopic portal vein ligation with in situ liver split for failed portal vein embolization. Ann Surg. 2012;256:e14-e15; author reply e14-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Vyas SJ, Davies N, Grant L, Imber CJ, Sharma D, Davidson BR, Malago M, Fusai G. Failure of portal venous embolization. ALPPS as salvage enabling successful resection of bilobar liver metastases. J Gastrointest Cancer. 2014;45 Suppl 1:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Nadalin S, Capobianco I, Li J, Girotti P, Königsrainer I, Königsrainer A. Indications and limits for associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Lessons Learned from 15 cases at a single centre. Z Gastroenterol. 2014;52:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 40. | Fard-Aghaie MH, Stavrou GA, Schuetze KC, Papalampros A, Donati M, Oldhafer KJ. ALPPS and simultaneous right hemicolectomy - step one and resection of the primary colon cancer. World J Surg Oncol. 2015;13:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Alvarez FA, Ardiles V, de Santibañes M, Pekolj J, de Santibañes E. Associating liver partition and portal vein ligation for staged hepatectomy offers high oncological feasibility with adequate patient safety: a prospective study at a single center. Ann Surg. 2015;261:723-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 42. | Croome KP, Hernandez-Alejandro R, Parker M, Heimbach J, Rosen C, Nagorney DM. Is the liver kinetic growth rate in ALPPS unprecedented when compared with PVE and living donor liver transplant? A multicentre analysis. HPB (Oxford). 2015;17:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 43. | Björnsson B, Sparrelid E, Røsok B, Pomianowska E, Hasselgren K, Gasslander T, Bjørnbeth BA, Isaksson B, Sandström P. Associating liver partition and portal vein ligation for staged hepatectomy in patients with colorectal liver metastases--Intermediate oncological results. Eur J Surg Oncol. 2016;42:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Ulmer TF, de Jong C, Andert A, Bruners P, Heidenhain CM, Schoening W, Schmeding M, Neumann UP. ALPPS Procedure in Insufficient Hypertrophy After Portal Vein Embolization (PVE). World J Surg. 2017;41:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |