Published online Aug 10, 2017. doi: 10.5306/wjco.v8.i4.343
Peer-review started: October 23, 2016
First decision: January 14, 2017
Revised: April 27, 2017
Accepted: May 3, 2017
Article in press: May 5, 2017
Published online: August 10, 2017
Processing time: 292 Days and 21.7 Hours
To evaluate the clinical value and efficiency of trans-arterial chemoperfusion (TACP) in patients with liver metastases from breast cancer (BC) and colorectal cancer (CRC).
We treated 36 patients with liver metastases of BC (n = 19, 19 females) and CRC (n = 17; 8 females, 9 males) with repeated TACP. The treatment interval was 4 wk. TACP was performed with gemcitabine (1000 mg/m2) and mitomycin (10 mg/m2), administered within 1 h after positioning the catheter tip in the hepatic artery. Before treatment, the size, location, tumour volume, vascularization and number of liver tumours were evaluated using magnetic resonance imaging (MRI). Tumour response was evaluated according to the Response Evaluation Criteria in Solid Tumors guidelines.
TACP using gemcitabine and mitomycin for metastases from CRC and BC was performed without any serious side effects. The follow-up MRI showed a therapeutic response in 84.2% of the BC patients - stable disease 47.4% and partial response 36.8%. A progression was seen in 15.8%. CRC patients showed a therapeutic response in 52.9% of cases. A progression of the disease was documented in 47.1% of the patients with CRC. These data show that TACP in patients with liver metastases of BC leads to a significantly better therapeutic response compared with CRC patients (P = 0.042). The median survival time was 13.2 mo for the BC patients, which is significantly longer than for CRC patients at 9.3 mo (P = 0.001).
TACP for liver metastases of BC appears to be a safe and effective palliative treatment with improved outcomes in comparison to patients with CRC.
Core tip: Trans-arterial chemoperfusion could be an alternative treatment option for advanced stage palliative patients suffering from liver-dominant metastatic disease.
- Citation: Gruber-Rouh T, Langenbach M, Naguib NNN, Nour-Eldin NEM, Vogl TJ, Zangos S, Beeres M. Trans-arterial chemoperfusion for the treatment of liver metastases of breast cancer and colorectal cancer: Clinical results in palliative care patients. World J Clin Oncol 2017; 8(4): 343-350
- URL: https://www.wjgnet.com/2218-4333/full/v8/i4/343.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i4.343
Liver metastases are often found in malignant disease. In most cases, the appearance of liver metastases is associated with a poor prognosis of the disease. One third of all patients have metastases even at the time of the primary diagnosis of their cancer. Half of patients resected in an early tumour stage will develop metastases, especially in the liver. Currently, the surgical approach is seen as the only curative treatment for liver metastases. However, only in 20% of patients can curative surgery of their liver metastases be performed[1,2].
Here, other treatment options have to be considered, such as systemic chemotherapy, loco-regional chemotherapy or selective internal radiation therapy (SIRT). In all, the treatment of liver metastases is an interdisciplinary decision that should be discussed in an interdisciplinary tumour board.
Reasonable results have been achieved in the past using intra-arterial chemotherapy, especially in metastases of colorectal cancer (CRC), breast cancer (BC) and neuroendocrine tumours[3]. The main idea underlying the intra-arterial delivery of cytotoxic medication is that the liver predominantly derives its blood from the portal venous system, while the metastases predominantly use the arterial system for their blood supply[4]. As higher concentrations of the chemotherapeutic agent can be used, using the “first pass mechanism” of the liver, less cytotoxic medication arrives in the systemic circulation resulting in only minimal side effects.
In our study, 36 patients with unresectable, therapy-resistant advanced hepatic metastases of colorectal and breast cancer were treated with hepatic intra-arterial chemotherapy (HIC). Our palliative patient cohort consisted in most cases of patients with symptomatic disease. Gemcitabine as an antimetabolite was chosen because of its tolerable hematologic toxicity and its effect in tumour biology similar to fluorouracil (5-FU). Mitomycin C was added to the chemotherapy protocol because in previous studies at our department it has demonstrated good response rates, especially in HIC pre-treated patients[5]. Primary endpoints of our retrospective analysis were tumour response, patient survival and the time at which the maximum therapeutic effect could be observed.
The patients’ medical histories were evaluated and documented in detail. Patients were included if the liver was the only organ with metastases, except for BC patients who were included if they also had bone metastases. Patients were only included if the metastases could not be resected and other ablative treatment, e.g., radiofrequency ablation (RFA), microwave ablation (MWA) or laser-induced thermotherapy (LITT), could not be performed. All patients had undergone surgery for their primary tumour and some of the patients (n = 13) also for their liver metastases. Each patient had undergone several therapies before, which were stopped because of progression of the disease or side effects with following progressive disease. Only adult patients with an Eastern Cooperative Oncology Group (ECOG) performance score of 0 or 1 and an estimated remaining survival time of ≥ 12 wk were treated. Female patients who were pregnant or breastfeeding were excluded. A minimum of three sessions in 4-wk intervals were performed in an outpatient setting. Sufficient coagulation parameters, bone marrow, renal and hepatic function were required. These parameters in general were evaluated before each treatment session. In the case of acute infection, dysfunction of the liver, kidney or bone marrow, as well as worsening of the general condition, therapeutic intervals were extended or the therapy was discontinued (Table 1). Before treatment, the size, location, vascularization and number of the liver tumours were evaluated using contrast-enhanced magnetic resonance imaging (MRI; 1.5 T; Magnetom Symphony, Siemens, Erlangen, Germany) as a baseline evaluation. Unenhanced T1- and T2-weighted spin-echo (SE) and gradient-echo (GE) sequences, as well as contrast-enhanced T1 sequences (True Fisp, HASTE, TSE, FLASH-2D in-phase and opposed phase and dynamic sequences), were used. Tumour response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines.
| Indications |
| Unresectable liver metastases |
| Liver-dominant metastatic disease |
| Minimum of three different chemotherapies before |
| No systemic chemotherapy available |
| Symptomatic liver metastases |
| Contraindications |
| ECOG >1 |
| Tumour burden of the liver > 75% |
| Poor liver function (quick < 40%, PTT < 45 s, albumin < 2 g/dL) |
| Extensive amounts of ascites |
| Obstructive icterus (bilirubin > 3 mg/dL) |
| Acute infection |
| Myelodepression (leucocytes < 2000/mL, platelets < 100000/μL) |
| Limited kidney function (creatinine > 2 mg/dL) |
| Extensive heart insufficiency ( > NYHA II) |
All patients were informed of the risks, side effects and other therapeutic options at least 24 h before the start of therapy. Informed consent was obtained. As pre- and concomitant medication for the most common side effects pethidine (Dolantin, Sanofi-Aventis, Frankfurt, Germany), granisetron (Kevatril, Roche, Mannheim, Germany) and dexamethasone were administered. After applying local anaesthesia, a commercially available angiographic catheter was introduced through the femoral artery using the Seldinger technique. In our cases, 4 or 5F gate (Introducer II, Terumo, Eschborn, Germany) and Pigtail, Renegade (Boston Scientific, Munich, Germany), Sidewinder and Headhunter (Terumo, Eschborn, Germany) catheters were used. After an angiography of the aorta to rule out an abnormal anatomy of the vessels or atypical tumour vessels, an angiography of the upper abdomen was performed to evaluate the vascularization of the liver and the metastases. The catheter was then selectively placed in the right, the left or the common hepatic artery, depending on the tumour localization. In cases of anatomic variants or accessory hepatic arteries supplying the tumour, these arteries were selectively catheterized. Following our procedure, the two chemotherapeutic drugs were administered over 60 min using a perfusor (Perfusor, B. Braun; Melsungen, Germany). Our therapy consisted of 1000 mg/m2 gemcitabine (Gemzar, Lilly, Bad-Homburg, Germany) and 10 mg/m2 body surface mitomycin C (Mitomycin, Medac, Hamburg, Germany).
Therapy response was evaluated after the third therapy cycle according to the RECIST criteria. “Complete response” (CR) was defined as the disappearance of all tumour lesions, “partial response” (PR) as a reduction of > 30%, “stable disease” (SD) as a reduction of < 30% or a growth of < 20% and “progressive disease” (PD) as a growth of > 20% or the occurrence of new lesions; all changes were relative to the baseline imaging (Table 2). Therapeutic response was defined as “complete response”, “partial response” or “stable disease”. The trans-arterial chemoperfusion (TACP) therapy was discontinued if tumour progression had occurred. In that case, alternative therapy options were discussed in an interdisciplinary tumour board and subsequently discussed with the patient. In the case of tumour response, therapy was continued as long as tumour growth could be controlled, or until it was possible to follow up with surgery or an interventional approach to remove the remaining tumour lesions.
| Category | RECIST |
| CR | Disappearance of all tumour lesions |
| PR | Reduction of > 30% in total tumour size |
| SD | Reduction of < 30% or a growth of < 20% |
| PD | Growth of > 20% or occurrence of new lesions |
Institutional Review Board approval for this retrospective study was obtained. All statistical analyses were performed in SPSS 15.0.1 (SPSS Inc.; United States, 2006). Survival data were assessed according to the Kaplan-Meier method. Groups were compared using the χ2 test and the Cochran-Armitage trend test, as appropriate. The Mann-Whitney U test was used to evaluate tumour volumes because these data were not normally distributed. Survival times were compared with the log rank test. For each test, a P-value < 0.05 was considered to indicate a statistically significant difference.
In total, 36 patients with liver metastases of CRC (n = 17; 8 females, 9 males) and BC (n = 19, 19 females) were treated with repeated hepatic trans-arterial chemoperfusion (TACP). The median age of our patients at the start of the therapy was 60.5 years. In the patients with CRC (n = 17; 8 females, 9 males) the median age at the beginning was 64 years (range 43-84 years); in the patients with BC (n = 19, 19 females) the median age was 55 years (range 37-77). The median survival from the start of the TACP therapy in BC patients was 13.2 mo and survival from diagnosis was 75.2 mo. The median survival from the beginning of the TACP therapy in CRC patients was 9.3 mo and the median survival from diagnosis was 36.9 mo.
Defining complete response, partial response and stable disease as the overall response and progressive disease as non-response, the results are shown in Table 3.
| Carcinoma | Therapy response (CR + PR + SD) | Non responders (PD) |
| CRC | 9 (52.9) | 8 (47.1) |
| Breast-Ca | 16 (84.2) | 3 (15.8) |
Colorectal carcinoma: In all, seventeen patients in our study suffered from CRC. All patients had undergone surgery for their primary tumour and a minimum of three courses of chemotherapy. Three patients had metastases of 2-4 cm, four patients had metastases of 4-7 cm and in ten patients the metastases were larger than 7 cm. Concerning the number of liver metastases, no patient had one metastasis, two patients had two metastases, one patient had 3-4 metastases, four patients had 5-9 metastases and ten patients had multiple liver metastases.
Breast cancer: This group consisted of 19 patients, all female. All patients had undergone surgery for their primary tumour and a minimum of three courses of chemotherapy. Four patients had metastases of 2-4 cm in size, nine patients had metastases of 4-7 cm and in six patients the metastases were larger than 7 cm. Concerning the number of liver metastases in BC patients, one patient had only one metastasis (> 7 cm), one patient had two metastases, two patients had 3-4 metastases, two patients had 5-9 metastases and thirteen patients had multiple metastases. Table 4 shows the number of treatments.
Trans-arterial chemoperfusion as a palliative treatment was tolerated well by all patients. During our therapy sessions no major technical problems occurred. Concerning therapy side effects, we did not observe severe common toxicity criteria (CTC) grade III, IV or V adverse events. CTC grade I and II side effects were common in our therapy cohort. These emerged in most cases as fatigue, nausea, vomiting and reduced appetite. The typical duration of these found to be 2-6 d after TACP. Haematological adverse events such as mild thrombocytopenia (grade I and II), dropped white blood cell count and reduced Hb values were observed as well. No serious side effects occurred that require hospitalisation or any other major medical intervention or treatment. Albeit we did not record a specific survey, all patients of our cohort rated the therapy side-effects as less severe compared to previous systemic chemotherapy treatment they all had undergone before.
Overall, we had 0 CR, 9 PR, 16 SD and 11 PD (Table 5). Comparing the success rates and the therapeutic responses, the difference between the two tumour groups reached statistical significance (P = 0.042, χ2 test and P = 0.0232, Cochran-Armitage trend test). Patients with liver metastases of BC survived significantly longer compared to patients with CRC (median 13.2 mo vs 9.3 mo, P = 0.001 - log rank test).
| Carcinoma | Partial response | Stable disease | Progressive disease | Total |
| Colon | 2 | 7 | 8 | 17 |
| Breast | 7 | 9 | 3 | 19 |
Comparing the overall response (OR = CR, PR and SD) vs PD for each tumour group, there was a significant difference between BC (OR = 16 patients, 84.2%) and CRC (OR = 9 patients, 52.9%). The difference reached statistical significance (P = 0.042, χ2 test), which means that our treatment is more effective in BC patients than in CRC patients.
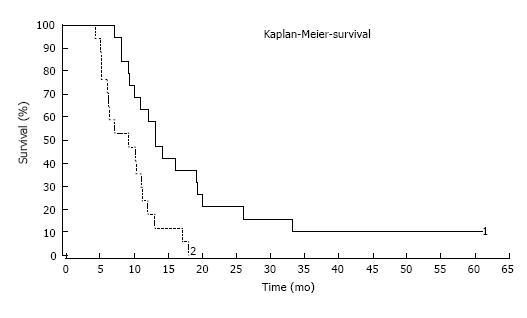
Median survival time after the first HIC session was 9.3 mo, and after initial diagnosis of the primary tumour 36.9 mo (Figure 1). We found no CR, two PR, seven SD and eight PD after the third cycle of TACP. However, none of the CRC patients are now alive, which might be due to the palliative setting of our study.
The median survival after the first HIC session was 13.2 mo (Figure 1), after initial diagnosis of the primary tumour 75.2 mo. We found no CR, seven PR, nine SD and three PD after the third cycle of TACP. Two patients with PR were treated by LITT and microwave ablation to treat their last remaining metastasis and they are both alive today - 55 and 61 mo after the first therapy with TACP.
The main idea for this study was that in many cases of CRC or BC, liver metastases are a main factor influencing survival. In recent years, many advances in therapy have been achieved. Without therapy, the median survival time with liver metastases of CRC is about 7.5 mo[6]. In BC, the time is about 6 mo[7,8]. The treatment of liver metastases is nowadays normally an interdisciplinary approach involving various departments, such as surgery, gynaecology, oncology and radiology. The standard therapy for liver metastases is still surgery, with the most promising outcome and the best long-term survival considering isolated liver metastases as a curable disease. Interventional radiological techniques, such as RFA, LITT and MWA have also been used as curative treatments of liver metastases. The limitations of such resection or ablative therapies are local spreading of tumours and unfavourable anatomical tumour localization[9]. Systemic chemotherapy can be viewed as standard in advanced disease. Nowadays, the systemic therapy regime consists of combinations of 5-FU, folinic acid, oxaliplatin, irinotecan, capecitabine and monoclonal antibodies bevacizumab or cetuximab. However, in general, such treatments are not suitable for all patients because of co-morbidities, major problems with the heart, liver or the kidneys (together with the tumour) or other disease. Loco-regional treatments can be an alternative to such general treatment. The loco-regional intra-arterial application of anti-tumour medication has now been an object of research for decades. In several studies, high tumour response rates have been achieved using this technique, but this does not necessarily lead to improved survival. In our study, this observation can be confirmed (overall response rate 69.5%; median survival 11 mo). The patients enrolled in this study were all in palliative care, they had all undergone surgery for their primary tumour and three courses of intravenous chemotherapy. Most patients had a high number of lesions concerning the liver (29 of 36 patients had more than 5 lesions and therefore disseminated liver disease), they were all multiply pre-treated and therapy-resistant patients. Gemcitabine has not yet demonstrated high activity in CRC, but it has a more favourable toxicity profile compared to other cytostatic drugs and is well known in our institute as a treatment for palliative therapy[5].
Without therapy, the median survival time of patients with CRC liver metastases is between 3.8 to 21 mo[6,10,11]. The five-year survival rate is 3%-6.1%[6,8]. In liver metastases of BC, the median survival time is often less than 10 mo[12,13].
Currently, there are many different therapeutic strategies used for the treatment of liver metastases. Therapy for liver metastases now tends to be an interdisciplinary approach involving different clinical partners. For modern oncological therapy, concepts such as quality of life and the side effects of a therapy are increasingly important. These aspects are even more important in a palliative situation or when the malignant disease progresses. The gold standard therapy for liver metastases is the surgical approach, but this is only possible for 25% of patients with liver metastases with a curative intention[1,6,8,14]. If a complete R0 resection of the liver metastases is possible, this leads to 5-year survival rates in 10%-49% of patients and a median survival of up to 84 mo[6,15-17]. With adjuvant systemic chemotherapy, the 5-year survival time can be improved from 47.8% to 51.2%[18]. In a cohort study, the median survival time of the group treated with adjuvant chemotherapy (5-FU/FS) was 62 mo vs 46 mo in the control group[19]. The FOLFOX regimen is often used as an adjuvant chemotherapy protocol[20].
The indication for a surgical approach for metastases of BC is only given in patients with isolated liver metastases. However, only around 3%-5% of all BC patients show isolated metastases of the liver. With R0 resection of liver metastases in BC patients, 5-year survival rates of 33%-40% can be attained compared with R1 resection, depending on patient selection criteria[21]. In metastasized BC, chemotherapy is often used. Current therapy regimes are normally based on anthracycline or taxan chemotherapy protocols[22]. In some combination therapy studies, a median survival time of 10.3-24 mo and a 5-year survival rate of 18% maximum have been attained[23,24].
Many patients, even if their tumour is progressive, suffer from a liver-dominant metastatic disease. The side effects of loco-regional chemotherapy are often better tolerated by patients, which might be due to the first pass effect of the chemotherapy in the liver[25,26]. Side effects are very rare during intra-arterial chemotherapy. For this reason, the therapy is performed on an outpatient basis[27]. We had no severe side effects (no CTC > 3). Intra-arterial chemotherapy remains a palliative treatment. In our study, we have shown a good response rate of 69.4%. In those patients, we achieved a partial response or stable disease after three courses of HIC. Especially in patients treated for liver metastases from BC, good tumour control was attained after the third session of TACP. In contrast to the stable disease or partial response in 84.2% of BC patients, the rate was only 52.9% among CRC patients, a statistically significant difference (P = 0.042, χ2 test).
Thus, our results are similar to those of other studies. In 1999 a meta-analysis was published that showed a better response to HIC than systemic chemotherapy (41% vs 14%, P-value < 0.001) and a better median survival time (15 mo vs 11 mo, P-value < 0.009)[28]. Another study showed median survival in a group of patients receiving systemic chemotherapy of 20 mo compared to a median survival of 24.4 mo among patients treated with intra-arterial regional chemotherapy. Tumour response after systemic chemotherapy was 24% compared to 47% treated by intra-arterial chemotherapy[29]. The only limitation of this study was extrahepatic tumour progression, which the regional approach stopped for only 7.7 mo. The systemic approach in contrast stopped such progression for around 14.8 mo median. Intrahepatic tumour progression was better in the intra-arterial group (9.8 mo vs 7.3 mo). In recent years, more chemotherapeutic drugs have become available for intra-arterial chemotherapy. It has been documented that oxaliplatin, folinic acid and 5-FU intra-arterially administered (via a port system) attained a median survival time of 36.1 mo. The 2- and 3-year survival rates were 62% and 52% respectively[30].
The chemotherapy used in our study is normally used for the treatment of pancreatic cancer or BC[31]. For BC, gemcitabine is often administered, especially in second or third-line therapy[22]. In our institute, we have had good results using this combination[5].
In the treatment of liver metastases of CRC, many studies have shown that loco-regional treatment using intra-arterial chemotherapy is very promising[32-34]. Currently, for liver metastases of BC, intra-arterial chemotherapy is only rarely used[35].
If response to intra-arterial chemotherapy is documented, a repetition of the treatment is reasonable and generally the therapy can be repeated an unlimited number of time. This can lead to longer median survival and fewer side effects[29,31,36]. However, it is still a palliative therapy: Metastases can only be reduced and normally no general necrosis can be achieved. Nonetheless, intra-arterial chemotherapy in combination with local ablative procedures or with other therapeutic procedures is increasingly being used, for example in SIRT[37,38]. One study showed good response rates in primary and secondary liver tumours using combined TACE and LITT in a neoadjuvant setting[39]. Other promising studies have shown good response rates in combination with SIRT. This therapy might show good response rates especially in palliative care, without serious side effects[37,38,40].
Our results show that in the palliative care setting, a rather good response rate can be achieved using intra-arterial chemotherapy for liver metastases. However, perhaps the dosage of gemcitabine (1000 mg/m2) was too low, or we should have used some embolization material in combination with our therapy protocol. In comparison to another study from our institute, we increased the mitomycin dosage to 10 mg/m2 without serious side effects, but the effect was not as high as we expected[5]. Our palliative therapy should at least make the patient feel better, improve quality of life and suppress the symptoms of the disease. To achieve this, we used chemotherapy based on gemcitabine as, among the cytostatic drugs available, it has a good toxicity profile and provides clinical benefits. Based on the promising observations at our institute concerning the use of embolization material and SIRT, we aim to see what these therapy options will bring in further studies and we intend to use embolization material, other cytotoxic drugs and SIRT in earlier tumour stages.
In conclusion, our data indicate that repeated hepatic intra-arterial chemotherapy for liver metastases, especially of breast cancer appears to be a safe and effective palliative treatment with significantly improved outcomes in comparison to patients with colorectal cancer [χ2 test P-value < 0.05 (= 0.042); statistically significant]. We observed good tumour response rates; indeed, although our treatment intention was palliative, two patients are still alive. In those two patients, suffering from breast cancer, an interventional ablative approach was performed following the intra-arterial chemotherapy to destroy their remaining lesions.
Our survival data lie within what we expected from the literature and our experience at the institute in the past. Selective and super-selective intra-arterial chemotherapy using gemcitabine and mitomycin for metastases from colorectal and breast cancer was performed without any serious side effects (CTC < 3). This is most likely due to the relatively low toxicity profile of gemcitabine and the loco-regional drug application, which resulted in lower systemic drug levels and lower side effects. More studies in the field of palliative care need to be undertaken to evaluate clearly the role of intra-arterial chemotherapy in the oncological therapy regime. Based on our findings to date, we think that this technique is an important therapy option that should be considered when a patient becomes palliative.
In malignant disease liver metastases can often be found. Currently, the surgical approach is seen as the only curative treatment for liver metastases. However, only in 20% of patients can curative surgery of their liver metastases be performed. Here other treatment options have to be considered, such as systemic chemotherapy, locoregional chemotherapy, selective internal radiation therapy. Intraarterial chemotherapy showed reasonable results in the past, especially in metastases of colorectal cancer (CRC), breast cancer (BC) and neuroendocrine tumors. As higher concentrations of the chemotherapeutic agent can be used and, using the “first pass mechanism” of the liver, less cytotoxic medication arrives in the systemic circulation with only minimal side effects. Thuas, leading to the research question: To evaluate loco-regional chemoperfusion of liver metastases for tumor response, survival rate and therapy effect.
Loco-regional chemoperfusion is not in daily practise for tumor patients so far. However, the authors wanted to add this therapy as an additional tool for palliative cancer treatment, to open up this treatment as additional option to think of.
In this study, the median survival time of CRC patients after the first hepatic intra-arterial chemotherapy (HIC) session was 9.3 mo, and after initial diagnosis of the primary tumor 36.9 mo. In breast cancer patients, the median survival time after first HIC session was 13.2 mo, and after initial diagnosis of the primary tumor 75.2 mo. This strengthened the authors’ idea to keep this therapy as another option in mind.
This study suggests that loco-regional chemotherapy is useful in a palliative setting to treat liver dominant metastases without serious side effects.
The scientific question proposed in the manuscript were the results achieved with intra-arterial hepatic chemotherapy in 36 patients suffering from unresectable and therapy-resistant advanced and hepatically metastasized CRC and BC tumor response. It is a promising study to add another tool to the basket of palliative patient treatment; however, large population trials would be valuable in the future.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Stanojevic GZ S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Bechstein WO, Golling M. [Standard surgical resection of colorectal liver metastases]. Chirurg. 2005;76:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | de Jong KP. Review article: Multimodality treatment of liver metastases increases suitability for surgical treatment. Aliment Pharmacol Ther. 2007;26 Suppl 2:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Vogl TJ, Zangos S, Balzer JO, Thalhammer A, Mack MG. [Transarterial chemoembolization of liver metastases: Indication, technique, results]. Rofo. 2002;174:675-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Vogl TJ, Zangos S, Eichler K, Yakoub D, Nabil M. Colorectal liver metastases: regional chemotherapy via transarterial chemoembolization (TACE) and hepatic chemoperfusion: an update. Eur Radiol. 2007;17:1025-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Vogl TJ, Zangos S, Eichler K, Selby JB, Bauer RW. Palliative hepatic intraarterial chemotherapy (HIC) using a novel combination of gemcitabine and mitomycin C: results in hepatic metastases. Eur Radiol. 2008;18:468-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Grundmann RT, Hermanek P, Merkel S, Germer CT, Grundmann RT, Hauss J, Henne-Bruns D, Herfarth K, Hermanek P, Hopt UT. [Diagnosis and treatment of colorectal liver metastases - workflow]. Zentralbl Chir. 2008;133:267-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Lehnert T, Golling M. [Indications and outcome of liver metastases resection]. Radiologe. 2001;41:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Ott R, Wein A, Hohenberger W. [Liver metastases--primary or multimodal therapy?]. Chirurg. 2001;72:887-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Vogl T, Mack M, Straub R, Zangos S, Woitaschek D, Eichler K, Engelmann K. [Thermal ablation of liver metastases. Current status and prospects]. Radiologe. 2001;41:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Nordlinger B, Peschaud F, Malafosse R. Resection of liver metastases from colorectal cancer--how can we improve results? Colorectal Dis. 2003;5:515-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Wyld L, Gutteridge E, Pinder SE, James JJ, Chan SY, Cheung KL, Robertson JF, Evans AJ. Prognostic factors for patients with hepatic metastases from breast cancer. Br J Cancer. 2003;89:284-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Selzner M, Morse MA, Vredenburgh JJ, Meyers WC, Clavien PA. Liver metastases from breast cancer: long-term survival after curative resection. Surgery. 2000;127:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 151] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Schlag PM, Benhidjeb T, Kilpert B. [Principles of curative resection of liver metastases]. Chirurg. 1999;70:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Scheele J, Altendorf-Hofmann A, Grube T, Hohenberger W, Stangl R, Schmidt K. [Resection of colorectal liver metastases. What prognostic factors determine patient selection?]. Chirurg. 2001;72:547-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Tanaka K, Shimada H, Ohta M, Togo S, Saitou S, Yamaguchi S, Endo I, Sekido H. Procedures of choice for resection of primary and recurrent liver metastases from colorectal cancer. World J Surg. 2004;28:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Antoniou A, Lovegrove RE, Tilney HS, Heriot AG, John TG, Rees M, Tekkis PP, Welsh FK. Meta-analysis of clinical outcome after first and second liver resection for colorectal metastases. Surgery. 2007;141:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, Belghiti J, Piedbois P, Guimbaud R, Nordlinger B. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976-4982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 414] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 19. | Parks R, Gonen M, Kemeny N, Jarnagin W, D’Angelica M, DeMatteo R, Garden OJ, Blumgart LH, Fong Y. Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents. J Am Coll Surg. 2007;204:753-761; discussion 761-763. [PubMed] |
| 20. | André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2653] [Cited by in RCA: 2732] [Article Influence: 130.1] [Reference Citation Analysis (0)] |
| 21. | Kollmar O, Moussavian MR, Richter S, Bolli M, Schilling MK. Surgery of liver metastasis in gynecological cancer - indication and results. Onkologie. 2008;31:375-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Schmid P, Possinger K. [Chemotherapy for metastatic breast cancer]. Zentralbl Gynakol. 2006;128:318-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Schmid P, Krocker J, Schulz CO, Michniewicz K, Dieing A, Eggemann H, Heilmann V, Blohmer JU, Sezer O, Elling D. Primary chemotherapy with gemcitabine, liposomal doxorubicin and docetaxel in patients with locally advanced breast cancer: results of a phase I trial. Anticancer Drugs. 2005;16:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Sledge GW, Neuberg D, Bernardo P, Ingle JN, Martino S, Rowinsky EK, Wood WC. Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: an intergroup trial (E1193). J Clin Oncol. 2003;21:588-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 516] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 25. | Collins JM. Pharmacologic rationale for regional drug delivery. J Clin Oncol. 1984;2:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 278] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Lubienski A, Simon M, Lubienski K, Gellissen J, Hoffmann RT, Jakobs TF, Helmberger T. [Update on chemoinfusion and chemoembolization treatments]. Radiologe. 2007;47:1097-1106, 1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Vogl TJ, Schwarz W, Eichler K, Hochmuth K, Hammerstingl R, Jacob U, Scheller A, Zangos S, Heller M. Hepatic intraarterial chemotherapy with gemcitabine in patients with unresectable cholangiocarcinomas and liver metastases of pancreatic cancer: a clinical study on maximum tolerable dose and treatment efficacy. J Cancer Res Clin Oncol. 2006;132:745-755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Link KH, Kornmann M, Formentini A, Leder G, Sunelaitis E, Schatz M, Pressmar J, Beger HG. Regional chemotherapy of non-resectable liver metastases from colorectal cancer - literature and institutional review. Langenbecks Arch Surg. 1999;384:344-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, Weeks JC, Sigurdson ER, Herndon JE, Zhang C. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol. 2006;24:1395-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 286] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 30. | Del Freo A, Fiorentini G, Sanguinetti F, Muttini MP, Pennucci C, Mambrini A, Pacetti P, Della Seta R, Lombardi M, Torri T. Hepatic arterial chemotherapy with oxaliplatin, folinic acid and 5-fluorouracil in pre-treated patients with liver metastases from colorectal cancer. In Vivo. 2006;20:743-746. [PubMed] |
| 31. | Vogl TJ, Zangos S, Heller M, Hammerstingl RM, Böcher E, Jacob U, Bauer RW. [Transarterial chemoperfusion with gemcitabine and mitomycin C in pancreatic carcinoma: results in locally recurrent tumors and advanced tumor stages]. Rofo. 2007;179:1181-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Kelly RJ, Kemeny NE, Leonard GD. Current strategies using hepatic arterial infusion chemotherapy for the treatment of colorectal cancer. Clin Colorectal Cancer. 2005;5:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Kemeny N. Management of liver metastases from colorectal cancer. Oncology (Williston Park). 2006;20:1161-1176, 1179; discussion 1179-1180, 1185-1186. [PubMed] |
| 34. | Hildebrandt B, Pech M, Nicolaou A, Langrehr JM, Kurcz J, Bartels B, Miersch A, Felix R, Neuhaus P, Riess H. Interventionally implanted port catheter systems for hepatic arterial infusion of chemotherapy in patients with colorectal liver metastases: a Phase II-study and historical comparison with the surgical approach. BMC Cancer. 2007;7:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Camacho LH, Kurzrock R, Cheung A, Barber DF, Gupta S, Madoff DC, Wallace MJ, Kim EE, Curley SA, Hortobagyi GN. Pilot study of regional, hepatic intra-arterial paclitaxel in patients with breast carcinoma metastatic to the liver. Cancer. 2007;109:2190-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Spangenberg HC, Mohr L, Blum HE. [Regional therapy of liver tumors]. Internist (Berl). 2007;48:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Hoffmann RT, Jakobs TF, Kubisch CH, Stemmler HJ, Trumm C, Tatsch K, Helmberger TK, Reiser MF. Radiofrequency ablation after selective internal radiation therapy with Yttrium90 microspheres in metastatic liver disease-Is it feasible? Eur J Radiol. 2010;74:199-205. [PubMed] |
| 38. | Sato KT, Lewandowski RJ, Mulcahy MF, Atassi B, Ryu RK, Gates VL, Nemcek AA, Barakat O, Benson A, Mandal R. Unresectable chemorefractory liver metastases: radioembolization with 90Y microspheres--safety, efficacy, and survival. Radiology. 2008;247:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 39. | Vogl TJ, Mack MG, Balzer JO, Engelmann K, Straub R, Eichler K, Woitaschek D, Zangos S. Liver metastases: neoadjuvant downsizing with transarterial chemoembolization before laser-induced thermotherapy. Radiology. 2003;229:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Jakobs TF, Hoffmann RT, Tatsch K, Trumm C, Reiser MF, Helmberger TK. [Developments and perspectives in radioablative techniques]. Radiologe. 2007;47:1083-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |