Published online Jun 10, 2017. doi: 10.5306/wjco.v8.i3.203
Peer-review started: February 10, 2017
First decision: March 27, 2017
Revised: April 3, 2017
Accepted: April 23, 2017
Article in press: April 25, 2017
Published online: June 10, 2017
Processing time: 125 Days and 0.2 Hours
Hepatocellular carcinoma (HCC) is one of the most common malignant diseases worldwide and comes third in cancer-related mortality. Although there is a broad spectrum of treatment options to choose from, only a few patients are eligible candidates to receive a curative therapy according to their stage of disease, and thus palliative treatment is implemented in the majority of the patients suffering from liver cancer. Sorafenib, a multikinase inhibitor, is the only currently approved agent for systemic therapy in patients with advanced stage HCC and early stage liver disease. It has been shown to improve the overall survival, but with various side effects, while its cost is not negligible. Sorafenib has been in the market for a decade and has set the stage for personalized targeted therapy. Its role during this time has ranged from monotherapy to neoadjuvant and adjuvant treatment with surgical resection, liver transplantation and chemoembolization or even in combination with other chemotherapeutic agents. In this review our aim is to highlight in depth the current position of Sorafenib in the armamentarium against HCC and how that has evolved over time in its use either as a single agent or in combination with other therapies.
Core tip: Hepatocellular carcinoma (HCC) is an aggressive and invasive malignancy. Curative options, such as resection and liver transplantation, are limited to only a few patients, who are suitable candidates. Sorafenib is the only approved systemic treatment in HCC, especially for advanced tumor stage and early stage liver disease. Recent findings suggest that it may also be helpful in carefully selected decompensated patients. Its adjuvant role is yet to be proven with more promising results. The combination of Sorafenib with other chemotherapy agents has shown improved efficacy and safety. We aim to present the evolution of Sorafenib’s use over the last decade.
- Citation: Ziogas IA, Tsoulfas G. Evolving role of Sorafenib in the management of hepatocellular carcinoma. World J Clin Oncol 2017; 8(3): 203-213
- URL: https://www.wjgnet.com/2218-4333/full/v8/i3/203.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i3.203
Hepatocellular carcinoma (HCC), the most common primary malignant neoplasm of the liver (85%-90%)[1], is the sixth most frequent cancer in the world and the third cause of cancer-related[2]. Cirrhosis is the stage of chronic liver disease characterized by disrupted architecture of the liver, therefore resulting in its dysfunction over the time. Regardless of the cause leading to cirrhosis, it is a major condition predisposing to a malignant transformation of the liver eventually leading to HCC[3]. Nowadays, the incidence of HCC is increasing rapidly owing to the large number of people suffering from cirrhosis, mainly caused by hepatitis B and C virus infection, as well as due to longer survival among cirrhotic patients[1].
Equally important to the presence and stage of cirrhosis, is the stage of the HCC, as any treatment that will follow will be in accordance to that. Specifically, surgical resection, ablation and liver transplantation are the only acceptable potentially curative options, but as it turns out, despite screening and frequent follow-ups, only 40%-60% of cirrhotic patients are diagnosed with very early or early stage HCC, therefore being eligible for curative treatment[4,5]. Unfortunately, most patients are diagnosed with more advanced stage HCC, i.e., portal vein invasion and/or extrahepatic spread or general symptoms attributed to cancer, unresponsive to such modalities. As a result alternative treatment combinations and algorithms including embolization, chemotherapy, radiotherapy, molecular target therapy or immunotherapy are constantly being generated in order to improve the overall survival (OS) of such patients[4,6,7].
In particular, an aspect of systemic therapy tends to focus on an important characteristic of HCC, its angiogenesis, by developing antiangiogenic drugs that impede the formation of new blood vessels, thus inhibiting the proliferation and growth of the liver tumor[8]. Sorafenib, an antiangiogenic drug, is the first and currently the only chemotherapeutic regimen approved as a palliative type of treatment in advanced stage HCC[9]. This review describes the general characteristics of Sorafenib, its current place in the clinician’s therapeutic armamentarium, as well as the clinical results of the evolving role of Sorafenib when combined or compared to other treatment modalities.
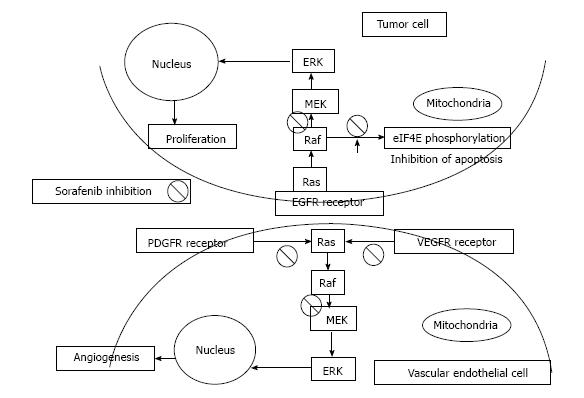
As stated above, HCC is a tumor with abundant vasculature and high heterogeneity, especially when it comes to the various signaling pathways involved[10]. One of the key pathways involved in the growth and proliferation of HCC is the Raf/MEK/ERK mitogen-activated protein (MAP) kinase cascade, which shows particularly increased activity[11]. This over-activation is mainly achieved by the combined action of hepatitis virus proteomics and growth factors, with platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) playing a critical role and highlighting the linkage between angiogenesis and HCC development[12-14]. Sorafenib (Nexavar, BAY 43-9006), a biaryl urea, is an oral multikinase inhibitor of the serine/threonine-kinases (c-RAF and BRAF), therefore blocking the Raf/MEK/ERK pathway, and of the vascular endothelial growth factor receptor 2 (VEGFR2), VEGFR3, platelet-derived growth factor receptor (PDGFR), FLT3, Ret, and c-KIT[15]. Moreover, it has been shown to result in apoptosis in various human tumor cell lines, independently of its involvement in the Raf/MEK/ERK pathway, by: (1) down-regulating an anti-apoptotic protein, the myeloid cell leukemia-1 (Mcl-1), member of the Bcl-2 family; and (2) inhibiting the phosphorylation of eukaryotic translation initiation factor 4E (eIF4E), which normally, when phosphorylated, promotes the expression of oncogenic genes[16]. According to this rationale, Sorafenib is an effective drug against not only the tumor compartment, but also the formation of new vessels[17,18]. It’s mechanism of action is illustrated in Figure 1.
This therapeutic action was firstly assessed in an uncontrolled phase 2 clinical trial of 137 patients with advanced and unresectable HCC, not having received any prior systemic therapy and with Child Pugh (CP) A or B cirrhosis[19]. The dosage administered was 400 mg orally twice a day in 4-wk cycles with a partial response of 2.2%, a minor response of 5.8% and a 33.6% of the patients reporting non progressive disease for at least 16 wk. Some other major data reported were the 4.2-mo median time to progression (TTP) and the 9.2-mo OS, while CP A and B patients showed only negligible differences regarding the pharmacokinetics[19].
Such positive results could not but be followed by the international phase 3, randomized, double-blind, placebo-controlled “Sorafenib HCC Assessment Randomized Protocol” (SHARP) clinical trial[9]. For this purpose, 602 patients with advanced stage HCC, Eastern Cooperative Oncology Group (ECOG) performance status from 0 to 2, CP A liver disease and without any preceding systemic treatment, were randomized either for Sorafenib, same dosage as in phase 2, or for placebo. According to the data reported, Sorafenib resulted in a median OS of 10.7 mo vs the 7.9 mo of the placebo, as well as in a median TTP of 24 wk compared to 12 wk of the placebo. Also, although the median TTP based on radiologic findings was 5.5 mo in the Sorafenib arm compared to 2.8 mo in the placebo arm, there was again no complete response, while the partial response was limited[9]. In spite of the positive clinical effects and the improvement in OS, Sorafenib was assessed within the frontiers of advanced stage HCC, but very early stage liver disease. This leads to many questions regarding its potential place in the treatment of patients with both advanced HCC and liver disease.
On the other hand, nobody claimed that Sorafenib was harmless. The SHARP trial, as a phase 3 study, except for the effectiveness, also reported details about some possible adverse effects, which were more frequent in the Sorafenib group compared to the placebo one (80% vs 52%, respectively). The most commonly described toxicities were grade 1 and 2 regarding the severity, i.e., weight loss, anorexia, diarrhea, changes in voice, hand-foot skin reaction, rash or desquamation and hair loss[9]. Some of these toxicities led to drug discontinuation (Sorafenib 11% vs placebo 5%)[9]. Another important study, the Sorafenib Italian Assessment (SOFIA) trial, showed that intervening by down-dosing at the appropriate time might be beneficial regarding an improved toxicity-tolerance rate and an increased OS[20].
Moreover, significant findings from the routine clinical practice were presented by Sacco et al[21], who stated that when Sorafenib is administered early at a low dose, especially in patients characterized as high-risk, it may be easier to render the patients compliant to the continuation of the therapy and for the drug to be well-tolerated. As a result, Sorafenib may induce some harmful events, mostly minor, which can be better tolerated by adjusting the dosage.
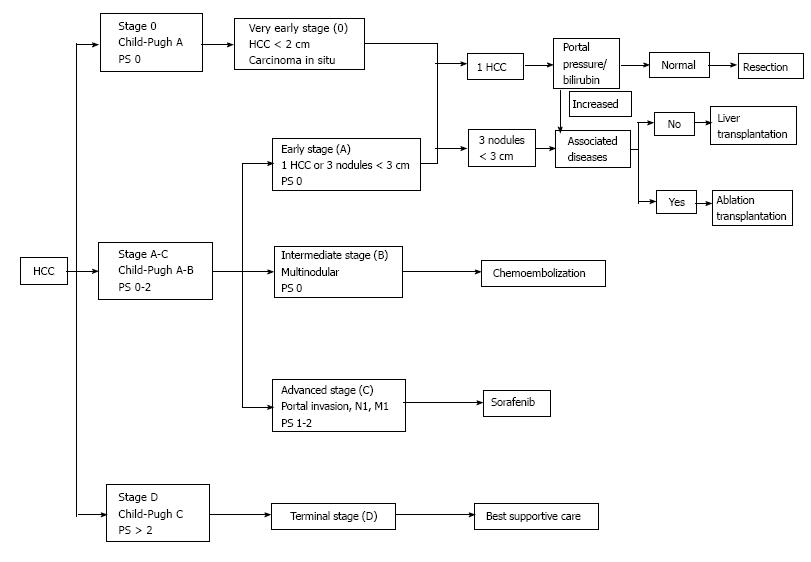
According to the European Association for the Study of the Liver (EASL) - European Organisation for Research and Treatment of Cancer (EORTC) guidelines (2012), Sorafenib is currently the only standard systemic treatment for HCC[6]. Its use is approved since 2007 upon the publication of the results of two studies: (1) the SHARP trial[9], conducted in the United States of America and Europe; and (2) the Sorafenib Asia-Pacific (Sorafenib-AP) trial[22], conducted in South Korea, China and Taiwan, which both showed an increased OS and a reduced risk of mortality in patients treated with Sorafenib. However, the aforementioned guidelines[6] highlight that Sorafenib is recommended only in patients with early stage liver disease - Child-Pugh A - and advanced stage HCC - Barcelona - Clinic Liver Cancer (BCLC) stage C - or as an adjuvant therapy combined with loco-regional treatment options. Sorafenib’s current place in the treatment algorithm, in accordance with the BCLC staging system for HCC, is presented in Figure 2[4,23].
As mentioned above, the results of systemic monotherapy with Sorafenib were encouraging according to a phase 2 trial[19] and two phase 3 trials (SHARP[9] and Sorafenib-AP[22]). There was general agreement that Sorafenib has a great impact in increasing the OS, even though in the phase 2 study 28% of the patients, who had CP B cirrhosis, showed a shorter median OS of 3.2 mo and could tolerate the treatment for only 1.8 mo. Also the incidence of ascites, encephalopathy and advanced hyperbilirubinemia is higher in advanced liver disease[24]. Interestingly, a phase 1 study, assessing the use of Sorafenib in patients with higher Child-Pugh class, underlined its link with the dose-limiting rises in serum bilirubin concentration[25]. Therefore, treatment guidelines[7] recommend taking bilirubin into consideration when adjusting the dose of Sorafenib. In addition, a post-marketing trial (GIDEON)[26] has shown equivalent results regarding safety and dosing strategy regardless of the Child-Pugh score. On the other hand, several studies evaluating the role of Sorafenib among the different stages of liver function reserve, reported a decreased response in advanced CP class, while liver-specific toxicities were independent of the liver cirrhosis stage[27-29].
On the whole, a systematic review has shown that in a male elderly population with advanced HCC and CP A cirrhosis, Sorafenib monotherapy can yield a statistically significant, yet clinically insignificant, increase in OS, time to tumor progression and disease control rate[30]. Besides, the cumulative data underline the decrease response of HBV-infected patients when compared to HCV, while patients with worse level of cirrhosis tend to display a more prominent Sorafenib-driven toxicity[30].
A study published in 2017 analysing the SEER-Medicare database, reported that elderly patients with advanced stage HCC may survive longer if treated with Sorafenib vs placebo (150.5 d vs 62 d, respectively), while the most remarkable factor associated with increased mortality was treatment taking place in an urban setting, although this survival effect was found to be neither prolonged, nor cost-effective in decompensated patients[31]. Currently, a randomized controlled phase 3 study - the B Child Patient-Optimization of Sorafenib Treatment (BOOST) study - is ongoing so as to evaluate the safety and efficacy of Sorafenib in CP B patients and is going to provide helpful information regarding the treatment of patients with decompensated disease[32]. However, reality is that for most patients Sorafenib is only one of the treatments that they receive, thus rendering it essential to review the adjuvant role of Sorafenib within the spectrum of other therapies.
Currently, surgical resection remains the treatment of choice for HCC, when it is associated with solitary masses and the hepatic remnant can maintain liver function[6]. Recently, there has been great interest concerning the down-staging of advanced HCC in order to make surgical resection even more efficient. One way to accomplish that is by taking advantage of Sorafenib’s use as a neoadjuvant treatment. In fact, a study has reported the incidence of Sorafenib-driven tumor necrosis, when used pre-operatively, therefore making resection an applicable treatment modality for a previously unresectable HCC tumor[33]. Moreover, the use of Sorafenib before surgery was not found to lead to any intra- or post-operative side-effects[34].
However, it is unclear whether Sorafenib could also be efficacious as an adjuvant therapy post-operatively. Specifically, a phase 3 study (STORM) evaluating its use after resection or ablation showed that Sorafenib is not superior to placebo when it comes to OS, recurrence-free survival or time to recurrence[35]. Unfortunately, many patients enrolled in this study could not tolerate the standard dose used[35]. These results are against incorporating Sorafenib in the guidelines as an appropriate adjuvant treatment option after resection[6].
Another curative treatment, especially for patients within the Milan criteria is orthotopic liver transplantation[36]. The challenges involved in liver transplantation, such as graft availability, have led to the increased use of grafts, including split grafts or those from living donors or from marginal donors. However, sometimes the delay between joining the waiting list and actually having a liver transplant may be quite significant, leading to patients dropping off the list[37]. Consequently, those patients with HCC waiting for a liver donor for at least six months are recommended to receive the so called “bridging therapy”, which mainly consists of locoregional treatment approaches, such as radiofrequency ablation (RFA) or transarterial chemoembolization (TACE)[6]. The rationale of “bridging” is entirely understandable when trying to prevent tumor progression in cirrhotic patients with HCC, who patiently wait a suitable donor organ to become available. An alternative strategy is down-staging of HCC patients outside the Milan criteria, in an effort to make them eligible for transplantation. A legitimate question is whether Sorafenib has an adjuvant role in this endeavor.
This neoadjuvant use of Sorafenib for down-staging comes with little evidence not demonstrating any significant advantages, even though some cases seem to accomplish reduction in the tumor boarder, down-staging and therefore allowing the patient to be added to the waiting list[38,39]. All-in-all, Sorafenib has shown a safe profile, when used before transplantation, with insignificant post-operative negative events[40,41]. Besides, the Sorafenib-driven hypoxia, because of its antiangiogenic effects, is thought to result in alterations in molecular mechanisms and growth factors, thus allowing the tumor to develop resistance and become more invasive or even metastatic[42]. Until more convincing data is reported from large clinical trials, the use of Sorafenib in this setting should be limited to investigational protocols.
On the other hand, the post-operative adjuvant use of Sorafenib has proven to be inefficient (STORM trial)[35], but when it comes to post-transplantation, results may be different. Specifically, a lot of studies agree with the fact that the use of Sorafenib, either concomitantly with mammalian target of rapamycin (mTOR) inhibitors or without them, can improve the survival when used for recurrent disease after liver transplantation, with the disadvantage of some drug-induced toxicity leading to a decrease in the dosage or even cessation of treatment[43-50]. As a matter of fact, Sorafenib has also resulted in complete remission of recurrent HCC after liver transplantation[51]. In general its use in this setting is thought to be safe[52].
Alltogether, current evidence is not favorable regarding the adjuvant use of sorafenib either pre- or post-transplantation and more research on this particular topic needs to take place, especially in the form of randomized controlled trials[53].
Current guidelines suggest the implementation of transarterial TACE in patients with intermediate stage HCC, consisting of multiple nodules, presenting without symptoms, invasion of the vessels or metastases and without advanced liver disease[6]. Although TACE can be helpful and efficient in this particular group of patients by improving survival[4], it is classified as a palliative option because it cannot achieve complete necrosis of the tumor and is associated with increased recurrent disease and tumor proliferation[54]. This tumor growth is also promoted by the ischemic area appearing after treatment with TACE, and owes its existence to the overexpression of certain growth factors, with VEGF playing a major role[55,56]. VEGF’s place in this equation lies on the side of tumor progression and metastasis and thus Sorafenib can be the ideal agent to deal with this process and impede angiogenesis, while simultaneously supplementing the promising action of TACE by eliminating the possibilities of future proliferation or recurrence[57].
Some phase 2 studies[58,59] evaluating the concurrent use of TACE and Sorafenib in patients with HCC not amenable to resection have shown a fairly safe profile for this combination with encouraging results regarding the efficacy and toxicity. When this duet was compared to TACE plus placebo in intermediate stage HCC on the background of HCV infection, it greatly improved time to tumor progression, without any unforeseen adverse events[60]. The comparison mentioned above was also assessed in a meta-analysis of six studies (1254 patients) reassuring that TACE plus sorafenib in either intermediate or advanced stage HCC patients can increase OS, time to tumor progression, as well as objective response rate, while the risk of side effects is also high[61]. Other recent meta-analyses, however, evaluating the marriage of Sorafenib and TACE for unresectable HCC showed an improvement in time to tumor progression, but not in OS[62,63].
It should also be mentioned that Sorafenib has been assessed in combination with drug-eluting beads (DEB)-TACE, an alternative method of delivering regional chemotherapy with minimal systemic exposure, for the management of both intermediate and advanced stage HCC. The results proved the increased efficacy and safety of this strategy[57].
Another important issue is that of the time of TACE and Sorafenib administration, for which three different options have been suggested: (1) TACE is followed by antiangiogenic therapy; (2) continuous antiangiogenic treatment interrupted only for the moment of TACE administration; and (3) continuous antiangiogenic therapy with no interruption at the moment of TACE administration[64]. Although the first two options are superior regarding the risk of bleeding, which is reduced, the third eliminates the possibilities of VEGF increase after TACE.
In general, it appears that Sorafenib plus TACE can lead to improved clinical results, especially regarding the intermediate stage HCC, mainly consisting of a highly heterogeneous group of patients for whom the overall approach is still to be defined based on several ongoing studies[57,65].
Locoregional treatment modalities can be efficient when it comes to HCC, but up to a point. Radiofrequency and microwave ablation trigger hypoxia and consequently hypoxia-induced angiogenesis, thus increasing the possibility of HCC recurrence. This process is primarily mediated by the hypoxia-inducible factor (HIF)-1a/vascular endothelial growth factor-A (VEGF-A) pathway, which can be impeded by Sorafenib[66]. Therefore, Sorafenib has been shown to limit the tumor’s invasive nature in vitro, a result of the cobalt chloride’s increase of the expression of HIF-1a, and to reduce proliferation and promote apoptosis in HCC cells[66].
2-Methoxyestradiol (2ME2), an inactive end product of estrogen metabolism, has recently been proven to have an antitumor effect by inhibiting proliferation and angiogenesis and by promoting apoptosis in many cancer types and especially in HCC[67]. The most important mechanism 2ME2 acts is through the inhibition of HIF-1 and the down-regulation of the HIF-driven VEGF expression[68]. It has been shown that 2ME2 comes up with synergistic effects in combination with Sorafenib in accordance to HCC suppression and antiangiogenesis, effects mostly driven be HIF-1 and -2 deregulation[69].
mTOR, a protein kinase, plays a key role in cell growth, proliferation, angiogenesis and metabolism in several cancers, including HCC[70]. It represents the target of rapamycin and its analogues, as well as Everolimus and Sirolimus, which present with an antitumor profile through the down-regulation of hypoxia-inducible factor, thus resulting in low VEGF and PDGF expression.
Everolimus has been evaluated in a phase 1/2 study in patients with advanced stage HCC, who were previously treated with systemic therapy, and has shown encouraging results in terms of tolerability and efficacy[71]. However, when Everolimus was combined with Sorafenib, again in a phase 1 trial, so that its maximum tolerated dose (MTD) could be determined, the results were disappointing regarding its efficacy in the MTD[72]. In addition, a randomized clinical trial (EVOLVE-1)[73] assessing the use of Everolimus in patients with advanced HCC, who presented with tumor progression during or after taking Sorafenib or who showed limited tolerability towards Sorafenib, reported no increase in OS.
On the other hand, the significant immunosuppressive role of mTOR inhibitors has been used in combination with Sorafenib vs Sorafenib alone in cases of post-transplantation late recurrent HCC, thus highlighting the broadening of the horizons in the treatment options against HCC towards the direction of personalized molecular targeted therapy[74]. Besides, cohort studies[46,49] assessing the combination of Sorafenib and mTOR inhibitors in the same disease context showed improved survival, but with some serious adverse events.
As a result, it is suggested that further studies are carried out, so as to evaluate the combination of mTOR inhibitors with Sorafenib in terms of achieving the maximum possible synergy and the minimum possible toxicity overlap.
Despite the blockade of the Raf/MEK/ERK cascade by Sorafenib, HCC has remarkable compensation through the over-expression of several other pro-survival pathways. The phosphoinositide 3-kinase (PI3K)/AKT pathway comes into play here as one of those and data state that it can render the tumor less susceptible to Sorafenib[75]. Thus, synergy may result from the combination of Sorafenib with a PI3K/AKT inhibitor, such as PKI-587 which simultaneously blocks the mTOR pathway, and this significant additive inhibitory effect has been proven in liver cancer stem cell patterns[76].
The complexity of the molecular mechanisms involved in the multistep process of tumor growth in HCC has been shown to incorporate mutations in the Wnt/β-catenin pathway as well[77]. Therefore, it is possible that the Wnt/β-catenin pathway represents a novel target for systemic treatment in HCC and as such it may also show an additive effect when used concurrently with Sorafenib. Indeed, not only has Sorafenib been able to down-regulate this pathway in different models[78], but also FH535, a Wnt/β-catenin inhibitor, was found to impede tumor growth of HCC and hepatoblastoma[79,80]. Moreover, when Sorafenib was combined with FH535, their synergistic effect on inhibiting the proliferation of HCC was more significant[81,82].
The MAPK/ERK kinases (MEK) 1 and 2 can be consequently activated if a Ras mutation shows up, as they are found downstream in the RAS cascade, the activation of which can therefore provide proliferative and antiapoptotic capabilities to the tumor. This “vertical” type of inhibition totally differs from the “parallel” blockade previously described in the mTOR inhibition, in which two unconnected cascades are concurrently inhibited[83]. Interestingly, MEK inhibitors, such as Refametinib (BAY 869766) which is an allosteric MEK 1/2 inhibitor, have proven their efficacy in preclinical HCC models[84]. When combined with Sorafenib in a phase 2 trial, Refametinib was found efficacious, especially in case of Ras mutations, and was well-tolerated[85].
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway plays an important part as a signal transduction cascade, with several proteins of the STAT family participating in cell growth, immunity and survival[86]. The one with the most significant role in oncogenesis is STAT3[87]. This STAT3 protein is key in modulating sensitization of HCC in recombinant tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), an antitumor drug with encouraging efficacy[88]. When Sorafenib was combined with TRAIL, it decreased the expression of STAT3 and proteins involved in its actions, thus rendering, the previously resistant to TRAIL, HCC susceptible to TRAIL-induced apoptosis[88]. Besides, Sorafenib targets STAT3 in a kinase-independent manner in patients with HCC[88,89].
In addition, the SH2 domain-containing tyrosine phosphatases family (SHP-1 and SHP-2), which are included in the family of protein tyrosine phosphatases (PTP), consist of two Src Homology (SH) 2 domains just as their name indicates[90]. These phosphatases dephosphorylate STAT3, leading to a significant decrease in its activation[91] and as a result they represent a potential target for systemic treatment of HCC. In fact, SHP-1 is a target of Sorafenib and through conformational modifications and signaling pathways, in which STAT3 is also involved, Sorafenib can also exhibit its anti-HCC effect[92]. However, we have already experienced the evolution of Sorafenib through its derivatives, such as SC-43 and SC-40, which are potent SHP-1 agonists and have proven to be superior to Sorafenib for the management of HCC[92]. Another novel derivative of Sorafenib, SC-59, when combined with radiotherapy has also shown to be superior to Sorafenib for treating HCC and its actions are mediated through STAT3 inhibition[93]. Last but not least, the synergistic combination of Sorafenib with SC-43, through their SHP-1 agonist effects, has been found efficacious, as it decreased tumor size and improved survival in preclinical models[94].
Data from preclinical models indicate that dietary phytochemicals with anti-inflammatory, antioxidant and antineoplastic characteristics may reduce the risk of HCC.
Curcumin is a yellow polyphenol derived from turmeric and has been shown to be protective against HCC caused by aflatoxins in mice[95]. Due to its solubility issues, polymeric nanoparticle formations of curcumin (NFC) have been developed and it is reported that the use of NFC alone or in combination with Sorafenib presents with remarkable findings regarding the suppression of tumor proliferation and invasiveness of HCC, as well as that of lung metastases[96].
Resveratrol is also a dietary polyphenol, mostly present in grapes, berries, peanuts and red wine, and has appeared as a promising chemopreventive agent against liver cancer[97]. The combination of Resveratrol and Sorafenib can lead to apoptosis and reduced tumor growth in HCC mice by fighting the diverted metabolic phenotype of aerobic glycolysis[98].
Indole-3-carbinol (I3C), found in cruciferous vegetables, is also one of the phytochemicals that have recently emerged with antineoplastic and antiangiogenic properties[99]. Specifically, its combined use with sorafenib has shown synergy by increasing the latter’s cytotoxicity and antiangiogenic properties, by promoting cell cycle arrest and apoptosis, as well as by reducing the expression of p-Akt, HIF-1a, VEGF and EGFR in HCC cells[99].
Several antiangiogenic drugs with the same antiangiogenic capabilities as Sorafenib have been developed over time for the management of HCC, mostly as second-line systemic therapy agents.
In case of failure to respond to Sorafenib, patients with HCC can be treated with another multikinase inhibitor, Regorafenib[100]. The addition of a fluorine atom in the central phenyl ring of Sorafenib transforms Regorafenib into an agent with increased potency[101]. A phase 2 study evaluating Regorafenib for intermediate or advanced HCC in patients that had previously received Sorafenib reported encouraging results, such as an OS of 13.8 mo, a safety profile similar to Sorafenib and no deaths attributed to Regorafenib[102]. Recently, in July 2016, at the ESMO World Congress on Gastrointestinal Cancer in Barcelona findings from a phase 3 trial (RESORCE, NCT01774344) assessing Regorafenib in HCC patients, who received prior therapy with Sorafenib, exhibited a remarkable increase in median OS for those treated with Regorafenib vs those receiving placebo as a second-line agent after radiologic progression under Sorafenib (10.6 vs 7.8)[103].
Almost a decade has passed with numerous clinicians and scientists getting negative results in trials for systemic therapy in HCC patients, while the RESORCE trial is the only one after the SHARP trial to come forward with positive findings. The most important causes of those negative results are: (1) the heterogeneity among the HCC patients recruited and the lack of selection criteria based on molecular patterns; and (2) the imbalance between adverse events and tolerable dosage vs anticancer efficiency and drug potency of the tested agents. Current advances in medicine and biology will improve our knowledge regarding the different and complex molecular mechanisms and driving mutations involved in this vast heterogeneity of this unique and multidimensional type of cancer and will guide us towards the right direction of conducting successful trials in the near future [104].
Sorafenib represents a type of medicinal revolution, therefore making antiangiogenesis drugs a feasible choice when it comes to dealing with cancer and opening the road for personalized targeted therapy. Currently, Sorafenib is the only accepted treatment for systemic therapy, as it has shown to increase the OS in patients suffering from advanced HCC, but with liver disease of early stage with tolerable adverse effects. Recently, studies show that Sorafenib is also safe in patients with advanced liver disease as well, but neither adequately efficient, nor cost-effective. Thus, ongoing studies (i.e., BOOST trial) are going to define its role in decompensated population in the future and up until then, patient selection in patients treated with Sorafenib is critical.
All-in-all, Sorafenib has evolved through time by being evaluated in several treatment protocols either as a neoadjuvant or as an adjuvant agent. Its use prior to or after liver transplantation has demonstrated a range of some minor advantages to even complete remission of recurrence, while preserving an acceptable safety profile. Still, a lot of research is needed in this field, as Sorafenib’s role post-resection was not that much promising, while its combination with TACE showed encouraging results. Overall, understanding the molecular mechanisms of HCC and Sorafenib, as well as those resulting from the implementation of other treatment methods, will guide us to the future development of combinations involving Sorafenib, agents with higher efficacy that derive from Sorafenib or even second-line agents that will complement the therapeutic role that Sorafenib could not accomplish by itself.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: El-Shemi AG, Ungtrakul T, Vetvicka V, Yamagata M S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4259] [Article Influence: 236.6] [Reference Citation Analysis (2)] |
| 2. | Ursino S, Greco C, Cartei F, Colosimo C, Stefanelli A, Cacopardo B, Berretta M, Fiorica F. Radiotherapy and hepatocellular carcinoma: update and review of the literature. Eur Rev Med Pharmacol Sci. 2012;16:1599-1604. [PubMed] |
| 3. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1784] [Article Influence: 85.0] [Reference Citation Analysis (2)] |
| 4. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 639] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 6. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4511] [Article Influence: 347.0] [Reference Citation Analysis (2)] |
| 7. | Kudo M, Trevisani F, Abou-Alfa GK, Rimassa L. Hepatocellular Carcinoma: Therapeutic Guidelines and Medical Treatment. Liver Cancer. 2016;6:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Taketomi A. Clinical trials of antiangiogenic therapy for hepatocellular carcinoma. Int J Clin Oncol. 2016;21:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10222] [Article Influence: 601.3] [Reference Citation Analysis (2)] |
| 10. | Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 1101] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 11. | Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, Lee JS, Factor VM, Thorgeirsson SS. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130:1117-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 548] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 12. | Stöckl L, Berting A, Malkowski B, Foerste R, Hofschneider PH, Hildt E. Integrity of c-Raf-1/MEK signal transduction cascade is essential for hepatitis B virus gene expression. Oncogene. 2003;22:2604-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Giambartolomei S, Covone F, Levrero M, Balsano C. Sustained activation of the Raf/MEK/Erk pathway in response to EGF in stable cell lines expressing the Hepatitis C Virus (HCV) core protein. Oncogene. 2001;20:2606-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Gollob JA, Wilhelm S, Carter C, Kelley SL. Role of Raf kinase in cancer: therapeutic potential of targeting the Raf/MEK/ERK signal transduction pathway. Semin Oncol. 2006;33:392-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 251] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 15. | Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099-7109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2966] [Cited by in RCA: 3139] [Article Influence: 149.5] [Reference Citation Analysis (0)] |
| 16. | Rahmani M, Davis EM, Bauer C, Dent P, Grant S. Apoptosis induced by the kinase inhibitor BAY 43-9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. J Biol Chem. 2005;280:35217-35227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 17. | Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851-11858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1201] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 18. | Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27:55-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 416] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 19. | Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293-4300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 907] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 20. | Iavarone M, Cabibbo G, Piscaglia F, Zavaglia C, Grieco A, Villa E, Cammà C, Colombo M. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54:2055-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 299] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 21. | Sacco R, Bargellini I, Ginanni B, Bertini M, Faggioni L, Federici G, Romano A, Bertoni M, Metrangolo S, Altomare E. Long-term results of sorafenib in advanced-stage hepatocellular carcinoma: what can we learn from routine clinical practice? Expert Rev Anticancer Ther. 2012;12:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4631] [Article Influence: 272.4] [Reference Citation Analysis (0)] |
| 23. | Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 1102] [Article Influence: 100.2] [Reference Citation Analysis (1)] |
| 24. | Abou-Alfa GK, Amadori D, Santoro A, Figer A, De Greve J, Lathia C, Voliotis D, Anderson S, Moscovici M, Ricci S. Safety and Efficacy of Sorafenib in Patients with Hepatocellular Carcinoma (HCC) and Child-Pugh A versus B Cirrhosis. Gastrointest Cancer Res. 2011;4:40-44. [PubMed] |
| 25. | Miller AA, Murry DJ, Owzar K, Hollis DR, Kennedy EB, Abou-Alfa G, Desai A, Hwang J, Villalona-Calero MA, Dees EC. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol. 2009;27:1800-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 26. | Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JF, de Guevara LL, Papandreou C. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): second interim analysis. Int J Clin Pract. 2014;68:609-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 27. | Pressiani T, Boni C, Rimassa L, Labianca R, Fagiuoli S, Salvagni S, Ferrari D, Cortesi E, Porta C, Mucciarini C. Sorafenib in patients with Child-Pugh class A and B advanced hepatocellular carcinoma: a prospective feasibility analysis. Ann Oncol. 2013;24:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 28. | Hollebecque A, Cattan S, Romano O, Sergent G, Mourad A, Louvet A, Dharancy S, Boleslawski E, Truant S, Pruvot FR. Safety and efficacy of sorafenib in hepatocellular carcinoma: the impact of the Child-Pugh score. Aliment Pharmacol Ther. 2011;34:1193-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Pinter M, Sieghart W, Graziadei I, Vogel W, Maieron A, Königsberg R, Weissmann A, Kornek G, Plank C, Peck-Radosavljevic M. Sorafenib in unresectable hepatocellular carcinoma from mild to advanced stage liver cirrhosis. Oncologist. 2009;14:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Xie B, Wang DH, Spechler SJ. Sorafenib for treatment of hepatocellular carcinoma: a systematic review. Dig Dis Sci. 2012;57:1122-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 31. | Parikh ND, Marshall VD, Singal AG, Nathan H, Lok AS, Balkrishnan R, Shahinian V. Survival and cost-effectiveness of sorafenib therapy in advanced hepatocellular carcinoma: An analysis of the SEER-Medicare database. Hepatology. 2017;65:122-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 32. | National Cancer Institute, Naples. Sorafenib in First-line Treatment of Advanced B Child Hepatocellular Carcinoma (BOOST). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01405573. |
| 33. | Irtan S, Chopin-Laly X, Ronot M, Faivre S, Paradis V, Belghiti J. Complete regression of locally advanced hepatocellular carcinoma induced by sorafenib allowing curative resection. Liver Int. 2011;31:740-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Barbier L, Fuks D, Pessaux P, Muscari F, Le Treut YP, Faivre S, Belghiti J. Safety of liver resection for hepatocellular carcinoma after sorafenib therapy: a multicenter case-matched study. Ann Surg Oncol. 2013;20:3603-3609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 777] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 36. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5293] [Article Influence: 182.5] [Reference Citation Analysis (0)] |
| 37. | Freeman RB, Edwards EB, Harper AM. Waiting list removal rates among patients with chronic and malignant liver diseases. Am J Transplant. 2006;6:1416-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 38. | Vagefi PA, Hirose R. Downstaging of hepatocellular carcinoma prior to liver transplant: is there a role for adjuvant sorafenib in locoregional therapy? J Gastrointest Cancer. 2010;41:217-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Golse N, Radenne S, Rode A, Ducerf C, Mabrut JY, Merle P. Liver Transplantation After Neoadjuvant Sorafenib Therapy: Preliminary Experience and Literature Review. Exp Clin Transplant. 2016; May 17; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Frenette CT, Boktour M, Burroughs SG, Kaseb A, Aloia TA, Galati J, Gaber AO, Monsour H, Ghobrial RM. Pre-transplant utilization of sorafenib is not associated with increased complications after liver transplantation. Transpl Int. 2013;26:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Saidi RF, Shah SA, Rawson AP, Grossman S, Piperdi B, Bozorgzadeh A. Treating hepatocellular carcinoma with sorafenib in liver transplant patients: an initial experience. Transplant Proc. 2010;42:4582-4584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2319] [Cited by in RCA: 2259] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 43. | Sposito C, Mariani L, Germini A, Flores Reyes M, Bongini M, Grossi G, Bhoori S, Mazzaferro V. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol. 2013;59:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 44. | Waghray A, Balci B, El-Gazzaz G, Kim R, Pelley R, Narayanan Menon KV, Estfan B, Romero-Marrero C, Aucejo F. Safety and efficacy of sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation. Clin Transplant. 2013;27:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Zavaglia C, Airoldi A, Mancuso A, Vangeli M, Viganò R, Cordone G, Gentiluomo M, Belli LS. Adverse events affect sorafenib efficacy in patients with recurrent hepatocellular carcinoma after liver transplantation: experience at a single center and review of the literature. Eur J Gastroenterol Hepatol. 2013;25:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Gomez-Martin C, Bustamante J, Castroagudin JF, Salcedo M, Garralda E, Testillano M, Herrero I, Matilla A, Sangro B. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2012;18:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 47. | Kim R, El-Gazzaz G, Tan A, Elson P, Byrne M, Chang YD, Aucejo F. Safety and feasibility of using sorafenib in recurrent hepatocellular carcinoma after orthotopic liver transplantation. Oncology. 2010;79:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Yoon DH, Ryoo BY, Ryu MH, Lee SG, Hwang S, Suh DJ, Lee HC, Kim TW, Ahn CS, Kim KH. Sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Jpn J Clin Oncol. 2010;40:768-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Staufer K, Fischer L, Seegers B, Vettorazzi E, Nashan B, Sterneck M. High toxicity of sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Transpl Int. 2012;25:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | de'Angelis N, Landi F, Nencioni M, Palen A, Lahat E, Salloum C, Compagnon P, Lim C, Costentin C, Calderaro J. Role of Sorafenib in Patients With Recurrent Hepatocellular Carcinoma After Liver Transplantation. Prog Transplant. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Lee HY, Yang KH, Choi BH, Park YM, Yoon KT, Ryu JH, Chu CW. Complete Regression of Recurrent Advanced Hepatocellular Carcinoma After Liver Transplantation in Response to Sorafenib Treatment: A Case Report. Transplant Proc. 2016;48:247-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Vitale A, Boccagni P, Kertusha X, Zanus G, D’Amico F, Lodo E, Pastorelli D, Ramirez Morales R, Lombardi G, Senzolo M. Sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation? Transplant Proc. 2012;44:1989-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Castelli G, Burra P, Giacomin A, Vitale A, Senzolo M, Cillo U, Farinati F. Sorafenib use in the transplant setting. Liver Transpl. 2014;20:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Alba E, Valls C, Dominguez J, Martinez L, Escalante E, Lladó L, Serrano T. Transcatheter arterial chemoembolization in patients with hepatocellular carcinoma on the waiting list for orthotopic liver transplantation. AJR Am J Roentgenol. 2008;190:1341-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 55. | Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10:2878-2882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 205] [Cited by in RCA: 228] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 56. | Shim JH, Park JW, Kim JH, An M, Kong SY, Nam BH, Choi JI, Kim HB, Lee WJ, Kim CM. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99:2037-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 57. | Cabibbo G, Tremosini S, Galati G, Mazza G, Gadaleta-Caldarola G, Lombardi G, Antonucci M, Sacco R. Transarterial chemoembolization and sorafenib in hepatocellular carcinoma. Expert Rev Anticancer Ther. 2014;14:831-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 58. | Park JW, Koh YH, Kim HB, Kim HY, An S, Choi JI, Woo SM, Nam BH. Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatol. 2012;56:1336-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 59. | Chung YH, Han G, Yoon JH, Yang J, Wang J, Shao GL, Kim BI, Lee TY, Chao Y. Interim analysis of START: Study in Asia of the combination of TACE (transcatheter arterial chemoembolization) with sorafenib in patients with hepatocellular carcinoma trial. Int J Cancer. 2013;132:2448-2458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 60. | Sansonno D, Lauletta G, Russi S, Conteduca V, Sansonno L, Dammacco F. Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: a randomized clinical trial. Oncologist. 2012;17:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 61. | Zhang L, Hu P, Chen X, Bie P. Transarterial chemoembolization (TACE) plus sorafenib versus TACE for intermediate or advanced stage hepatocellular carcinoma: a meta-analysis. PLoS One. 2014;9:e100305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 62. | Liu L, Chen H, Wang M, Zhao Y, Cai G, Qi X, Han G. Combination therapy of sorafenib and TACE for unresectable HCC: a systematic review and meta-analysis. PLoS One. 2014;9:e91124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 63. | Wang G, Liu Y, Zhou SF, Qiu P, Xu L, Wen P, Wen J, Xiao X. Sorafenib combined with transarterial chemoembolization in patients with hepatocellular carcinoma: a meta-analysis and systematic review. Hepatol Int. 2016;10:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 64. | Strebel BM, Dufour JF. Combined approach to hepatocellular carcinoma: a new treatment concept for nonresectable disease. Expert Rev Anticancer Ther. 2008;8:1743-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 65. | Montella L, Palmieri G, Addeo R, Del Prete S. Hepatocellular carcinoma: Will novel targeted drugs really impact the next future? World J Gastroenterol. 2016;22:6114-6126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Xu M, Zheng YL, Xie XY, Liang JY, Pan FS, Zheng SG, Lü MD. Sorafenib blocks the HIF-1α/VEGFA pathway, inhibits tumor invasion, and induces apoptosis in hepatoma cells. DNA Cell Biol. 2014;33:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 67. | El Naga RN, El-Demerdash E, Youssef SS, Abdel-Naim AB, El-Merzabani M. Cytotoxic effects of 2-methoxyestradiol in the hepatocellular carcinoma cell line HepG2. Pharmacology. 2009;84:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 68. | Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW, Giannakakou P. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 559] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 69. | Ma L, Li G, Zhu H, Dong X, Zhao D, Jiang X, Li J, Qiao H, Ni S, Sun X. 2-Methoxyestradiol synergizes with sorafenib to suppress hepatocellular carcinoma by simultaneously dysregulating hypoxia-inducible factor-1 and -2. Cancer Lett. 2014;355:96-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 70. | Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1467] [Cited by in RCA: 1551] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 71. | Zhu AX, Abrams TA, Miksad R, Blaszkowsky LS, Meyerhardt JA, Zheng H, Muzikansky A, Clark JW, Kwak EL, Schrag D. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer. 2011;117:5094-5102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 72. | Finn RS, Poon RT, Yau T, Klümpen HJ, Chen LT, Kang YK, Kim TY, Gomez-Martin C, Rodriguez-Lope C, Kunz T. Phase I study investigating everolimus combined with sorafenib in patients with advanced hepatocellular carcinoma. J Hepatol. 2013;59:1271-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 73. | Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, Poon RT, Blanc JF, Vogel A, Chen CL. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 486] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 74. | Bhoori S, Toffanin S, Sposito C, Germini A, Pellegrinelli A, Lampis A, Mazzaferro V. Personalized molecular targeted therapy in advanced, recurrent hepatocellular carcinoma after liver transplantation: a proof of principle. J Hepatol. 2010;52:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 75. | Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Koch A, Evers BM. PI-103 and sorafenib inhibit hepatocellular carcinoma cell proliferation by blocking Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Anticancer Res. 2010;30:4951-4958. [PubMed] |
| 76. | Gedaly R, Galuppo R, Musgrave Y, Angulo P, Hundley J, Shah M, Daily MF, Chen C, Cohen DA, Spear BT. PKI-587 and sorafenib alone and in combination on inhibition of liver cancer stem cell proliferation. J Surg Res. 2013;185:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 77. | Lee JS. The mutational landscape of hepatocellular carcinoma. Clin Mol Hepatol. 2015;21:220-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 78. | Lachenmayer A, Alsinet C, Savic R, Cabellos L, Toffanin S, Hoshida Y, Villanueva A, Minguez B, Newell P, Tsai HW. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin Cancer Res. 2012;18:4997-5007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 244] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 79. | Handeli S, Simon JA. A small-molecule inhibitor of Tcf/beta-catenin signaling down-regulates PPARgamma and PPARdelta activities. Mol Cancer Ther. 2008;7:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 80. | Liu J, Li G, Liu D, Liu J. FH535 inhibits the proliferation of HepG2 cells via downregulation of the Wnt/β-catenin signaling pathway. Mol Med Rep. 2014;9:1289-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Galuppo R, Maynard E, Shah M, Daily MF, Chen C, Spear BT, Gedaly R. Synergistic inhibition of HCC and liver cancer stem cell proliferation by targeting RAS/RAF/MAPK and WNT/β-catenin pathways. Anticancer Res. 2014;34:1709-1713. [PubMed] |
| 82. | Vilchez V, Turcios L, Marti F, Gedaly R. Targeting Wnt/β-catenin pathway in hepatocellular carcinoma treatment. World J Gastroenterol. 2016;22:823-832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 201] [Cited by in RCA: 247] [Article Influence: 27.4] [Reference Citation Analysis (2)] |
| 83. | Rixe O, Fojo T. Is cell death a critical end point for anticancer therapies or is cytostasis sufficient? Clin Cancer Res. 2007;13:7280-7287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 84. | Schmieder R, Puehler F, Neuhaus R, Kissel M, Adjei AA, Miner JN, Mumberg D, Ziegelbauer K, Scholz A. Allosteric MEK1/2 inhibitor refametinib (BAY 86-9766) in combination with sorafenib exhibits antitumor activity in preclinical murine and rat models of hepatocellular carcinoma. Neoplasia. 2013;15:1161-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 85. | Lim HY, Heo J, Choi HJ, Lin CY, Yoon JH, Hsu C, Rau KM, Poon RT, Yeo W, Park JW. A phase II study of the efficacy and safety of the combination therapy of the MEK inhibitor refametinib (BAY 86-9766) plus sorafenib for Asian patients with unresectable hepatocellular carcinoma. Clin Cancer Res. 2014;20:5976-5985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 86. | Kamran MZ, Patil P, Gude RP. Role of STAT3 in cancer metastasis and translational advances. Biomed Res Int. 2013;2013:421821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 297] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 87. | Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 1416] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 88. | Chen KF, Tai WT, Liu TH, Huang HP, Lin YC, Shiau CW, Li PK, Chen PJ, Cheng AL. Sorafenib overcomes TRAIL resistance of hepatocellular carcinoma cells through the inhibition of STAT3. Clin Cancer Res. 2010;16:5189-5199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 89. | Tai WT, Cheng AL, Shiau CW, Huang HP, Huang JW, Chen PJ, Chen KF. Signal transducer and activator of transcription 3 is a major kinase-independent target of sorafenib in hepatocellular carcinoma. J Hepatol. 2011;55:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 90. | Hung MH, Tai WT, Shiau CW, Chen KF. Downregulation of signal transducer and activator of transcription 3 by sorafenib: a novel mechanism for hepatocellular carcinoma therapy. World J Gastroenterol. 2014;20:15269-15274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 91. | Wu C, Guan Q, Wang Y, Zhao ZJ, Zhou GW. SHP-1 suppresses cancer cell growth by promoting degradation of JAK kinases. J Cell Biochem. 2003;90:1026-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 92. | Tai WT, Shiau CW, Chen PJ, Chu PY, Huang HP, Liu CY, Huang JW, Chen KF. Discovery of novel Src homology region 2 domain-containing phosphatase 1 agonists from sorafenib for the treatment of hepatocellular carcinoma. Hepatology. 2014;59:190-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 93. | Huang CY, Tai WT, Hsieh CY, Hsu WM, Lai YJ, Chen LJ, Shiau CW, Chen KF. A sorafenib derivative and novel SHP-1 agonist, SC-59, acts synergistically with radiotherapy in hepatocellular carcinoma cells through inhibition of STAT3. Cancer Lett. 2014;349:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 94. | Chao TI, Tai WT, Hung MH, Tsai MH, Chen MH, Chang MJ, Shiau CW, Chen KF. A combination of sorafenib and SC-43 is a synergistic SHP-1 agonist duo to advance hepatocellular carcinoma therapy. Cancer Lett. 2016;371:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 95. | Soni KB, Lahiri M, Chackradeo P, Bhide SV, Kuttan R. Protective effect of food additives on aflatoxin-induced mutagenicity and hepatocarcinogenicity. Cancer Lett. 1997;115:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 77] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 96. | Hu B, Sun D, Sun C, Sun YF, Sun HX, Zhu QF, Yang XR, Gao YB, Tang WG, Fan J. A polymeric nanoparticle formulation of curcumin in combination with sorafenib synergistically inhibits tumor growth and metastasis in an orthotopic model of human hepatocellular carcinoma. Biochem Biophys Res Commun. 2015;468:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 97. | Bishayee A, Politis T, Darvesh AS. Resveratrol in the chemoprevention and treatment of hepatocellular carcinoma. Cancer Treat Rev. 2010;36:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 98. | Dai W, Wang F, Lu J, Xia Y, He L, Chen K, Li J, Li S, Liu T, Zheng Y. By reducing hexokinase 2, resveratrol induces apoptosis in HCC cells addicted to aerobic glycolysis and inhibits tumor growth in mice. Oncotarget. 2015;6:13703-13717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 99. | Abdelmageed MM, El-Naga RN, El-Demerdash E, Elmazar MM. Indole-3- carbinol enhances sorafenib cytotoxicity in hepatocellular carcinoma cells: A mechanistic study. Sci Rep. 2016;6:32733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 100. | Ravi S, Singal AK. Regorafenib: an evidence-based review of its potential in patients with advanced liver cancer. Core Evid. 2014;9:81-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 101. | Trojan J, Waidmann O. Role of regorafenib as second-line therapy and landscape of investigational treatment options in advanced hepatocellular carcinoma. J Hepatocell Carcinoma. 2016;3:31-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 102. | Bruix J, Tak WY, Gasbarrini A, Santoro A, Colombo M, Lim HY, Mazzaferro V, Wiest R, Reig M, Wagner A. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412-3419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 193] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 103. | Bruix J, Merle P, Granito A, Huang Y-H, Bodoky G, Yokosuka O, Rosmorduc O, Gerolami R, Masi G, Paul JR. Efficacy and safety of regorafenib versus placebo in patients with hepatocellular carcinoma (HCC) progressing on sorafenib: Results of the international, randomized phase 3 resorce trial. Ann Oncol. 2016;27:ii1-ii3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 104. | Brizzi MP, Pignataro D, Tampellini M, Scagliotti GV, Di Maio M. Systemic treatment of hepatocellular carcinoma: why so many failures in the development of new drugs? Expert Rev Anticancer Ther. 2016;16:1053-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |