Published online Jun 10, 2017. doi: 10.5306/wjco.v8.i3.178
Peer-review started: January 16, 2017
First decision: February 20, 2017
Revised: March 28, 2017
Accepted: April 18, 2017
Article in press: April 19, 2017
Published online: June 10, 2017
Processing time: 140 Days and 0.2 Hours
Because pancreatic cancer (PC) historically has had poor prognosis and five year survival rates, it has been intensely investigated. Analysis of PC incidence and biology has shown a link between different risk factors such as smoking, alcoholism, and obesity and disease progression. Important factors affecting PC include the epigenomic changes driven by DNA methylation and histone acetylation, and actions of microRNA inducing oncogenic or tumor suppressor effects. Studies have identified markers whose dysregulation seem to play important roles in PC progression. PC markers involve classical histone deacetylases (HDAC), PC stem cell (PCSC), and leptin. In this review, we discuss the role of several PC biomarkers, and the potential crosstalk between HDAC, microRNA, and leptin in PC progression. Dysregulated expression of these molecules can increase proliferation, survival, PCSC, resistance to chemotherapy and tumor angiogenesis. The potential relationships between these molecules are further analyzed using data from The Cancer Genome Atlas and crosstalk pathways generated by the Pathway Studio Platform (Ariadne Genomics, Inc.). Oncogenic miRNA21 and tumor suppressor miRNA200 have been previously linked to leptin signaling. Preliminary analysis of PC biopsies and signaling crosstalk suggests that the main adipokine leptin could affect the expression of microRNA and HDAC in PC. Data analysis suggests that HDAC-microRNA-leptin signaling crosstalk may be a new target for PC therapy.
Core tip: Pancreatic cancer has no targeted therapy. Obesity is a risk factor for pancreatic cancer, characterized by high levels of leptin. In this review, we discuss the potential crosstalk between histone deacetylases, microRNA, and leptin in disease progression. Crosstalk among these molecules increases proliferation, survival, cancer stem cells and resistance to chemotherapy. The potential relationships between these molecules are analyzed using data from the Cancer Genome Atlas and the Pathway Studio Platform. The crosstalk among these molecules could be a novel target for pancreatic cancer prevention or treatment, particularly in obese patients that show elevated levels of leptin.
- Citation: Tchio Mantho CI, Harbuzariu A, Gonzalez-Perez RR. Histone deacetylases, microRNA and leptin crosstalk in pancreatic cancer. World J Clin Oncol 2017; 8(3): 178-189
- URL: https://www.wjgnet.com/2218-4333/full/v8/i3/178.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i3.178
Pancreatic cancer (PC) is a malignant disease, which is difficult to treat. It is a silent disease that can go undetected for long periods of time; however, when diagnosed, it is often in advanced stages (III or IV)[1]. PC incidence and mortality rates vary across different racial/ethnic groups, with the highest rates found in African Americans, and the lowest in Asian Americans/Pacific Islanders. Moreover, PC incidence rate is higher in African Americans when compared to European Americans at every age[1]. Risk factors for the development of PC include tobacco usage, continuous exposure to such chemicals as dyes and pesticides, family history, age, epigenetic changes, and obesity[1-5]. The best outcomes from PC treatments are obtained after complete surgical resection, with no residual disease; this can improve 5 year survival, but only from 5% to about 20%-25%[6]. Even patients who are eligible for surgical treatment with tumor free margins often experience recurrence and eventually require palliative treatment[7,8]. Surgical resection of PC is performed on patients with locally advanced or borderline resectable tumors. Improved outcomes may be achieved with a multimodal approach, combining neo-adjuvant chemotherapy with radiation therapy and surgery. Adjuvant therapy includes 5-fluoruracil (5-FU) or capecitabine; gemcitabine induction followed by concomitant chemoradiation with either gemcitabine or 5-FU; FOLFIRINOX (an aggressive chemotherapeutic regimen, including several chemotherapeutic agents) or gemcitabine-Nab paclitaxel (albumin bound) with or without subsequent chemoradiation. For patients with metastatic PC, the treatment options are very limited. It mainly consists of palliative care (pain and nutrition management), as well as chemotherapy. The chemotherapeutic regimens for metastatic PC (gemcitabine alone or in combination with other agents for example, FOLFIRINOX, Nab-paclitaxel) have only modest results, improving the survival of these patients by only a few months[9,10].
Important factors affecting PC are the changes in the epigenome driven by DNA methylation and histone acetylation. Epigenetic changes are alterations in gene expression or cellular phenotype that occur without changes in the DNA sequence. Some of the epigenetic changes are DNA methylation and histone acetylation. This last process is characterized by the addition of acetyl groups to the lysine residues of the histones via histone acetyltransferases (HAT). Histone acetylation is essential to gene regulation, and is usually associated with the relaxed form of chromatin. Lysine residues can also be deacetylated by histone deacetylases (HDAC). These enzymes are involved in cancer progression by increasing proliferation, survival and resistance to chemotherapy of cancer cells as well as angiogenesis[11-15].
The dysregulation of microRNAs is another factor involved in cancer progression[16-18]. MicroRNAs (miRNA or miR) are noncoding endogenous RNAs that regulate protein expression. Accumulating data show important relationships between dysregulated miRNAs and cancer[16-19]. The effect that miRNAs dysregulation has on the cancer cells determines whether these molecules are considered oncogenics or tumor suppressors. Oncogenic miRNAs promote cancer development through various signaling mechanisms while tumor suppressor miRNAs have contrary effects and their expression is decreased in cancer[19,20]. There are many oncogenic microRNAs (e.g., miR21) that have been reported to play a role in cancer progression[20-23]. Furthermore, the decreased expression of tumor suppressor miR200 family has been associated with PC progression[24,25].
Obesity is one the most observed risk factors for cancer progression. Obesity is a growing pandemic, and is associated with more than 100000 incidences of various cancers in the United States, particularly breast, colon, endometrium and PC[26-28]. Obesity is characterized by the accumulation of excessive body fat, and a body mass index (BMI) value greater than 30. Obesity is also characterized by high levels of leptin, which has been consistently associated with many cancers, including PC[29-33]. Preliminary analyses suggest that leptin could affect the expression of microRNA and HDAC in PC.
Because of the absence of targeted therapies for obese PC patients, there is a need to better understand the mechanisms behind the disease progression in order to develop better treatment strategies. Thus, in this review, we will discuss the potential relationships between HDAC, microRNA, cancer stem cells, and leptin signaling in PC.
There are two types of PC - those that comprise tumors arising from the endocrine pancreatic cells and those that arise from the exocrine pancreatic cells. Cancers of the endocrine pancreas are rare and represent less than 4% of all PC cases[1]. Pancreatic Adenocarcinoma (PA) is the most common type of PC and usually begins in the ducts of the pancreatic glands. PC has been recently classified into four main subtypes based on their genomic analysis (e.g., squamous, pancreatic progenitor, immunogenic, and aberrantly differentiated endocrine exocrine or ADEX)[34].
PC squamous subtype is characterized by four core genes programs involved in inflammation, hypoxia response, metabolic reprogramming, transforming growth factor-β (TGF-β) signaling, autophagy, and upregulated expression of TP63ΔN and its target genes[34]. The pancreatic progenitor subtype is characterized by the transcriptional networks of pancreatic and duodenal homeobox-1 (PDX1), motor neuron and pancreas homeobox- 1 (MNX1), hepatocyte nuclear factor-4-α (HNF4A), hepatocyte nuclear factor-1-β (HNF1B), hepatocyte nuclear factor-1-α (HNF1A), forkhead box-A2 (FOXA2), forkhead box-A3 (FOXA3), and hairy and enhancer of split-1 (HES1) transcription factors[34,35]. The immunogenic subtype of PC is characterized by changes in the programs of immune genes that include antigen presentation, CD4+ and CD8+ T cells, and toll-like receptor and B cell signaling pathways[8]. ADEX is characterized by the upregulation of transcriptional networks of both exocrine and endocrines lineages that are important in later stages of pancreatic development and differentiation[34].
In addition, some hereditary factors play roles in the development of PC. Individuals with a high risk of developing PC can be divided into underlying gene defect, like cyclin-dependent kinase Inhibitor 2A (CDKN2A), breast cancer gene 1 and 2 (BRCA1/2), partner and localizer of BRCA2 (PALB2), and serine/threonine kinase 11 (STK11) mutations[35,36]. In a study performed by Vasen et al[36], the longterm outcome of prospective surveillance of a large group of CDKN2A/p216-Leiden carriers and, BRCA1/2 and PALB2 mutation carriers, and individuals at risk (IARs) for familial PC (FPC) was evaluated. The main goal of the study was to determine whether or not surveillance will lead to the detection of early stage PC or the detection of relevant precursors lesions (PRLs) as well as to assess if their program leads to improvements in prognosis[36]. Based on the surveillance, it was determined that PRLs were more frequent in patients with FPC than those with CDKN2A/p216-Leiden mutation[36]. The surveillance study also reveals that the resection of screen detected PC with CDKN2A/p216-Leiden mutation carriers was 75%, which is higher than that reported for patients with sporadic PC (15%-20%)[36]. Overall, the study demonstrated that the surveillance of CDNK2A mutation carriers was successful for the detection of PC at the resectable stage[36].
PC is generally diagnosed when approximately 30% of patients present a locally advanced disease[1]. Because there is no effective treatment for advanced PC, this disease should be detected in the early stages when treatment could significantly increase the percentage of patients with five years of survival. The best way to PC early diagnosis would be via the usage of screening biomarkers with high specificity and sensitivity. Currently, the most established and used biomarker is CA19-9. However, CA19-9 detection is not highly specific for PC, as it can also be detected in colorectal cancer, stomach, and biliary epithelium and chronic pancreatitis[1,37,38].
A vast array of other PC biomarkers has been investigated, but so far none are as yet widely used clinically. It has recently been shown that exosomes could potentially impact on the pathogenesis of PC through the modulation of tumor growth, microenvironment, and immune response. This suggests that exosomes could be used as biomarkers for PC[39]. An additional PC marker could be the leptin receptor, OB-R, which has been detected in PC cell lines[40]. Moreover, OB-R expression was positively correlated with the matrix metalloproteinase-13 (MMP-13) in human PC tissues. The increased expression of either OB-R or MMP-13 was significantly associated with lymph node metastasis; it also tends to be associated with the TNM stage in PC patients[40].
Likewise, it has been proposed that the detection of PC cells in blood could be used as a surrogate for PC detection[41,42]. Circulating tumor cells (CTC) could be related to metastatic and more aggressive PC disease, according to the results from an international multicenter randomized study that included 79 patients. A subgroup of PC patients was screened for CTCs before the start of the chemotherapy, and after two months of treatment. Overall, CTC detection was found in 11% of PC patients and associated with poor tumor differentiation (P = 0.04), and with shorter overall survival (RR = 2.5, P = 0.01). Therefore, CTC detection might be a new way to detect PC[38].
HDAC play a major role in the regulation of gene expression via epigenetics changes. HDAC catalyze the removal of an acetyl group, which stimulates chromatin condensation, thus suppressing transcription. Currently, 18 HDAC family members have been identified in the human genome, which are grouped into four classes (I-IV)[43]. HDAC are also classified into two major types: Sirtuins (SIRT) and classical HDAC. Classical HDAC include Classes I, II, and IV, whereas the sirtuins comprise Class III[43,44] (Table 1). HDAC classes I, II, and IV are zinc dependent metalloproteins, while class III are nicotinamide adenine dinucleotide (NAD+) dependent enzymes[43]. Class I HDAC family consists of HDAC 1, 2, 3, and 8. These enzymes are mainly located in the cellular nucleus. Class II HDAC family is divided into two groups - Classes IIA and IIB. These HDAC are mainly located in the cytoplasm, but can also be found in the nucleus, which is dependent on their phosphorylation status influencing their shuttle mechanism[43,44]. Subclass IIA HDAC family consists of HDAC 4, 5, 7, and 9; while subclass IIB consists of HDAC 6 and 10. HDAC Class IV is only made of HDAC11 that is mainly located in the nucleus. Class III is composed of SIRT1, SIRT2, SIRT3, SIRT4, SIRT5, SIRT6, and SIRT7, which are located in the nucleus, cytoplasm, and mitochondria[38]. Due to the role of HDAC in epigenetic regulations and their effect on chromatin structures, many studies have found them linked to cancer progression[13,14,45,46]. The classical HDAC have been associated with cancer progression through the increase of proliferation, survival and resistance to chemotherapy of cancer cells, and angiogenesis. More studies suggest the roles of HDAC in PC progression. The use of HDAC inhibitors is a novel avenue toward targeted therapy for PC. Several HDAC inhibitors are currently under clinical trials for cancer-targeted treatment. However, currently there are only three FDA approved HDAC inhibitor drugs available [Vorinostat or Suberoylanilide Hydroxamic Acid (SAHA), Zolina], Romidepsin (Depsipeptide, ISTODAX), and Belinostat (Beleodaq)[47-50].
| Class | Members | Cellular localization | Function in cancer[13,14,45,46,104] | Substrates[43,44,104] |
| I | HDAC1 | Nucleus | Proliferation, survival and resistance to chemoresistance | P53, E2F-1, Stat3, and androgen |
| HDAC2 | Nucleus | Proliferation and survival | Bcl-6, Stat3. YY-1, and glucocorticoid receptor | |
| HDAC3 | Nucleus | Proliferation and anti-differentiation | GATA-1, RelA, Stat3, MEF2D, YY-1, and SHP | |
| HDAC8 | Nucleus | Proliferation and anti-differentiation | ERRα, Inv (16), and CREB | |
| IIA | HDAC4 | Nucleus/cytoplasm | Angiogenesis and anti-differentiation | GCMa, GATA-1, and HP-1 |
| HDAC5 | Nucleus/cytoplasm | Anti-differentiation | Smad7, HP-1, and GCMa | |
| HDAC7 | Nucleus/cytoplasm | Angiogenesis and migration | FLAG-1, and FLAG-2 | |
| HDAC9 | Nucleus/cytoplasm | Cell survival | ATDC (TRIM29) | |
| IIB | HDAC6 | Cytoplasm | Angiogenesis and migration | Alpha-Tubulin, HSP-90, SHP, Smad7 |
| HDAC10 | Cytoplasm | Angiogenesis | HSP90 | |
| IV | HDAC11 | Nucleus/cytoplasm | Tumor immune response | OX40L |
Vorinostat was the first FDA approved anti-HDAC drug[47,49,51]. It is a hydroxamic acid based drug that inhibits Class I, II, IV HDAC by chelating the zinc cofactor. This drug shows apoptotic and anti-proliferative effects by modifying the expression of specific genes related to insulin-like growth factor-1 receptor signaling receptor (IGF-1R)[47,49,52]. The second FDA approved HDAC inhibitor drug is Romidepsin, which was effective in phase II clinical trials when used with gemcitabine for treatment of advanced PC[49,53-55]. The third FDA approved HDAC inhibitor drug is Belinostat. It showed a dose dependent growth inhibitory or pro-apoptotic effects promoting cell cycle arrest at the G0/G1 or S phase transition[56-58]. Additionally, positive results in the treatment of PC have been reported with the use of benzamide derivative HDAC inhibitor (Class I HDAC inhibitor MGCD0103) selective for Class I and IV HDAC[59]. PC cell lines treated with MGCD0103 showed dose dependent growth arrest, apoptosis, and induction of p21, which mediated cell cycle arrest in G2/M phase[59].
MicroRNAs (miRNA or miR) are noncoding endogenous RNAs of 14-24 nucleotides that have the ability to regulate protein expression at the post-transcriptional level. Many studies have found strong correlations between dysregulated microRNAs and cancer[17,18,60]. According to the effect that miRNAs dysregulation has on the cancer cells, these molecules are considered oncogenic or tumor suppressors. There are many oncogenic microRNAs, such as miR1290, miR24, miR134, miR146a, miR378, miR484, miR628-3p, miR1825[61] and miR21[20-23] that have been reported to play a role in cancer progression. It was reported that serum levels of miR1290 distinguished patients with low-stage PC from controls better than CA19-9 levels[61]. Furthermore, decreased expressions of miRNA34[62] and miR200[20] family have been associated with PC progression.
A study found that oncogenic miR21 was expressed in the early stage of PA[63]. Furthermore, knockdown of miR21 using lentiviral vectors inhibited cell proliferation in PC derived cell lines. In addition, miR21 was found to protect PC cell from apoptosis, and its knockdown resulted in the activation of mitochondrial pathway apoptosis via the downregulation of Bcl9 (a protein involved in Wnt Pathway), upregulation of Bax, and induction of Bim[63]. Targeting miR21 in vivo strongly inhibited PC growth, which led to the suggestion that simultaneous standard gemcitabine chemotherapy combined with miR21 targeting could improve the prognosis of PC
MiRNA200 family consists of five members (miR200a, b, c and miR429, and miR141)[64]. In vitro studies suggested that miR200c expression was related to low cancer invasion[20]. MiRNA200 activities include inhibition of epithelial-mesenchymal-transformation (EMT), repression of cancer stem cell (CSC) self-renewal and differentiation, modulation of cell division and apoptosis, and involvement in chemoresistance. High level of miR200c correlated to better survival rates[20]; however, miR200a and miR200b, were hypomethylated and overexpressed in PC[65]. It has been suggested that targeting miRNA200 upregulation could improve PC prognosis if used together with the chemotherapeutic drug gemcitabine[20]. Indeed, treatment of PC cells with a curcumin analoge, CDF, improved gemcitabine effects by upregulating miR200 and downregulating miR-21 expression. These effects were found together with the downregulation of Akt, cyclooxygenase-2, prostaglandin E2, vascular endothelial growth factor, and nuclear factor-κB DNA binding activity, and induction of PTEN[66].
Transcriptor factor ZFH family (ZEB1 and ZEB2)[67] represses the expression of epithelial genes. miR200 members increased Notch activation by ZEB1 that regulates the expression of Jagged1 and the mastermind-like coactivators Maml2 and Maml3.. Moreover, in PC and breast cancer cells, decreased miR200 expression was associated with increased Jag1 and ZEB1 levels[68]. Therefore, MiR200 inhibits EMT by interacting with ZEB1/2 and the Notch pathway, represses self-renewal and differentiation in CSCs, and is involved in the regulation of cell division and apoptosis[64]. In turn, ZEB1 suppresses the expression of miR200 family, which inhibits the translation of ZEB1 mRNA, resulting in the double-negative ZEB/miR200 feedback loop[69]. Additionally, in lung cancer Jagged2 inhibits the expression of miR200 family by induction of GATA transcription factors, which promotes tumor metastasis[70].
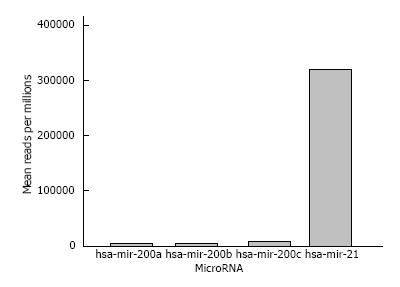
We preliminarily analyzed PC biopsies using TCGA databank[71]. Data analysis shows higher miR21 expression compared with miR200 in PC (Figure 1). These data suggest that progression of PC could be positively associated with upregulation of miR21 and downregulation of miR200.
PC is usually diagnosed in the advanced stages after distant metastasis has already occurred in most cases. PC shows high frequency of local relapse, even after surgical resection. Treatment of PC via surgery and chemotherapy has historically had little success. Patients still have a poor survival rate with chemoresistance and reoccurrence of the disease as significant factors. These features of PC could be related to the action of PC stem cell (PCSC)[72]. Cancer stem cells are a small population that have the capacity to self-renew, and generate cells with identical tumorigenic potential that could also differentiate to form the bulk of the tumor cells, thereby contributing to the formation of heterogenic cellular composition of cancers[55]. A highly tumorigenic PCSC population (CD24+CD44+ESA+) was described in PC for the first time by Li et al[73] in a xenograft human model. PCSC are resistant to chemotherapy and contribute to tumor initiation, growth and metastasis[74]. PCSC were also identified as CD133+ population that is highly resistant to standard chemotherapy. A subgroup of these cells, CD133+CXCR4+ was found to be involved in metastasis[75].
C-Met, also known as hepatocyte growth factor receptor (HGFR), is an oncogene involved in the progression of cancer. C-Met was earlier described as a potential PCSC marker[75-77]. C-Met+ PCSC showed similar tumorigenic capacity as CD24+CD44+ESA+ population[78-80]. C-Met is a heterodimer that consist of an extracellular α-chain bound through a disulphide bridge to a transmembrane β-chain[79]. It is also a tyrosine kinase found in the cell membrane. HGF ligand binding to C-Met immunoglobulin like-domain induces C-Met dimerization, leading to autophosphorylation of the two tyrosine residues within the catalytic loop[79]. Subsequently, further autophosphorylation of two more tyrosine residues occurs in the C-terminal of c-Met receptors, which provides the platform for the recruitment of other molecular factors and signal conveyors like Grb2-associated binding protein 1 (Gab1). This provides a binding site for such SH2-containing effectors as SHP2, PLCγL, STAT3, Ras GTPase, and PI3K9[79]. With the emerging evidence of c-Met as a stem cell marker, some studies were able to identify part of the c-Met cell population that also expresses CD44, CD24, CD133, and ALDH1[78,81]. However, in a study c-Met+ PCSC produced tumors in 35% of cases when compared to PCSC CD133+ (16%) and CD44+ (25%) of cases[78-80].
Notch signaling pathway is another factor that affects the maintenance of PCSC. Notch signaling pathway is a known regulator of the balance between cell self-renewal and cell differentiation. Abel et al[82] found that components of Notch signaling were upregulated in PCSC. Moreover, the inhibition of Notch signaling pathway with gamma secretase inhibitors or Hes1 shRNA in PCSC reduced the percentage of PCSC and their ability to form tumorspheres. Furthermore, these authors found that the activation of Notch signaling pathway using an exogenous peptide ligand greatly increased the percentage of PCSC and formation of tumorspheres[82].
Due to PCSC role in chemoresistance and disease reoccurrence, c-Met, CD44, CD24, CD133, and ALDH1 could potentially be used as biomarkers to detect PC progression. Moreover, developing therapies that would target PCSC markers could be adjuvant to the standard gemcitabine chemotherapy, which could improve PC survival rate[72,78].
Obesity is mainly the result of unhealthy diets and lifestyles, and has proven to be a contributing factor to higher risk and poor prognosis of cancer[1,83,84]. Several studies have examined the impact that obesity has on the overall survival rate of PC patients[84,85]. Some studies have determined that obesity in adulthood significantly shortened the overall survival of PC patients, whereas obesity at diagnosis was not associated with increased risk of death[84,85]. In another study, Sandini et al[86] assessed whether the evaluation of different body compartments and their relationships were associated with the development of major postoperative complications after pancreatoduodenectomy for cancer. It was found that the prevalence of sarcopenia (loss of muscle tissue related to aging) was 24.2%. Overall, sarcopenic obesity[86] and non-sarcopenic obesity[87] are strong predictors of major complications after pancreatoduodenectomy for cancer.
Obese PC patients have the poorest prognosis, and often develop chemoresistance. Obesity is recognized as a co-morbidity factor to cancer and there is great interest in understanding the mechanism linking this condition and cancer. In this regard, a recent study has found that obesity promoted desmoplasia associated with accelerated PC growth and impaired delivery/efficacy of chemotherapeutics through reduced perfusion in vivo[87]. Furthermore, the inhibition of angiotensin-II type-1 receptor (AT1) reversed obesity-augmented desmoplasia and PC growth and improved response to 5-FU chemotherapeutic in vivo[87]. In addition, clinical studies have shown that excess weight alters PC microenvironment to augment the crosstalk between cancer associated adipocytes, tumor associated neutrophils, and pancreatic stellate cells, which subsequently lead to increased tumor progression and survival[87].
A potential link between obesity and PC could be the major adipokine leptin. A crosstalk between leptin and Notch (an embryonic signaling pathway altered in PC) has been reported in PC lines. Moreover, leptin induces PC tumorspheres formation and expansion of PCSC[81,88]. Leptin is a small cytokine secreted by adipose tissue that is coded by the obese (ob) gene. Leptin has been the most studied adipokine since it was first cloned in 1994[89]. Leptin is an adipokine that regulates appetite, energy intake and expenditure. Leptin plays many roles, some of which involve regulation of glucose homeostasis, growth response, reproduction and immune response[90]. The level of circulating leptin is proportional to total body fat. Obese patients exhibit high circulating levels of leptin due to leptin resistance[91]. Leptin is a pleiotropic adipokine and pro-inflammatory molecule that belongs to the family of helical cytokines. It is structurally similar to interleukin (IL)-6, IL-12, IL-15, prolactin, GH, oncostatin M, and granulocyte CSF[92].
Leptin receptor, OB-R, is a product of diabetic (db) gene that shows six alternatives spliced isoforms, including a long isoform (OB-RL, OB-Rb or LEPR) with full intracellular signaling capabilities, shorter isoforms with less biological activity (OB-Rs or OB-Ra) and a soluble isoform (OB-Re or sOB-R)[93,94]. Both the long and short isoforms of OB-R are expressed in PC cell lines[4]. Moreover, PC cells secreted leptin and expressed OB-R, which indicates a leptin autocrine/paracrine signaling loop could also affect tumor progression[95]. The binding of leptin to OB-R activates a cascade of events that promotes tumor progression and cancer cell survival[3,30,31]. Leptin binding to its receptor triggers an activation cascade of several canonical (JAK2/STAT3, MAPK, PI-3K/AKT1) and non-canonical signaling pathways (p38MAK, JNK and AMPK)[29,88,96].
A nested case control study from three cohort studies of middle-aged adults showed that high pre-diagnostic circulating leptin concentrations were associated with an increased PC risk among those with longer follow-up[97]. In another study, Mendonsa et al[4] showed the contribution of obesity and leptin to PC growth by using an in vivo orthotopic murine PC model. These studies revealed the increase of tumor growth in diet-induced obese mice when compared to lean mice.
We have recently showed that leptin and Notch crosstalk could influence PC progression. Our data suggest that a functional leptin-Notch axis affects PC progression and expansion of cancer stem cells (PCSC) in PC cell lines (BxPC-3, MiaPaCa-2, Panc-1, AsPC-1) and derived tumorspheres. Leptin treatment increased cell cycle progression and proliferation, and the expression of Notch receptors, ligands and targeted molecules (Notch1-4, DLL4, JAG1, Survivin and Hey2), PCSC markers (CD24/CD44/ESA, ALDH, CD133, Oct-4), ABCB1 protein, as well as tumorsphere formation. PC has no targeted therapy and is mainly treated with chemotherapy, whose efficiency could be decreased by leptin and Notch activities. Thus, the leptin-Notch axis could be a novel therapeutic target, particularly for obese PC patients[95].
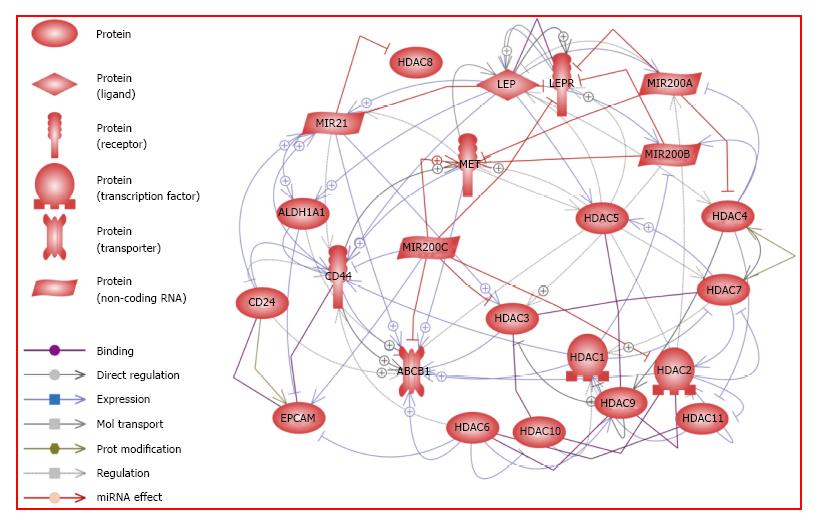
Resistance to leptin is observed in obese people who show high levels of leptin. Precise reasons explaining why some obese patients are leptin-resistant are not fully known. Some studies have suggested that leptin resistance could be due to abnormalities of the leptin molecule while others believe the resistance might be due to impairment of OB-R function or deficient leptin transport. Leptin is a known proliferation factor for cancer[3,29,31,96]. Analysis of data from Pathway Studio Platform shows that leptin signaling could promote PC through crosstalk mechanisms that involve PCSC, classical HDAC, oncogenic microRNA21, and tumor suppressor microRNA200a/b/c (Figure 2 and Supplemental Table 1).
A relationship between leptin signaling and miR21 in cutaneous wound healing was earlier reported[98]. The expression of miR21 and miR200 was previously linked to leptin hypothalamic signaling[16,99,100]. It was shown that the use of a pegylated leptin antagonist predisposed the rats to obesity and promoted leptin resistance in the both hypothalamus and liver. RT-PCR data from these studies showed that miR200 was upregulated in rats treated with leptin antagonist[16]. Additionally, miR21 (oncogenic) and miR200 (tumor suppressor) have been shown to affect PC progression[20,63]. The potential relationships between leptin signaling and miR21 and miR200a/b/c regulation in PC are shown in Figure 2. Leptin signaling is involved in the crosstalk to many important oncogenic and tumor suppressor molecules. Previous studies have determined that leptin increases the expression of PCSC markers ALDH1 and CD44. Leptin has also been found to increase the expression of miR21 while the tumor suppressors miR200a, miR200b, and miR200c decrease the expression of OB-R. Interestingly, these tumor suppressors could also interact with some of PCSC markers (Met, ABCB1, CD44), which decrease their expression. In contrast, oncogenic miR21 increases the expression of ALDH1, ABCB1 and CD44 markers. With regard to the classical HDAC, only HDAC5 and HDAC4 were reported to be directly regulated by leptin signaling (Figure 2). However, leptin signaling could indirectly affect the expression of some of HDAC via microRNA or PCSC markers. Further analysis suggests that leptin increases the expression of miR21, which, in turn, could increase the expression of HDAC3. The combined action of these factors could promote cancer proliferation and the expression of an anti-differentiation phenotype (Supplemental Table 1).
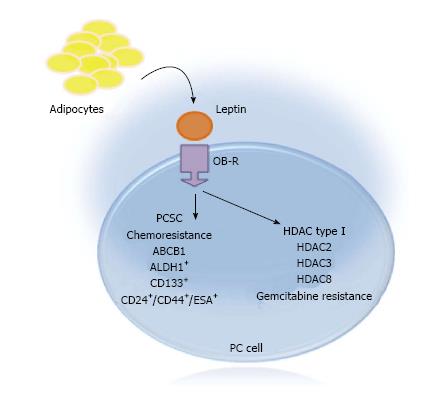
Our published data show that leptin increased PCSC populations that correlated with growth of PC tumorspheres and resistance to gemcitabine[88,95]. Furthermore, leptin induced PCSC populations (CD24+CD44+ESA+, CD133+, ALDH+) in MiaPaCa-2 PC cells. Additionally, in Panc-1 cells, leptin increased mostly CD133+ PCSC. Moreover, leptin increased ABCB1 (an ATP Binding Transporter Protein linked to chemoresistance) expression in PC tumorspheres[95]. These data suggest that leptin could play a role in the induction of PCSC and PC chemoresistance (Figure 3).
Several studies have found that classical HDAC are overexpressed in PC. Therefore, HDAC inhibition has become a potential target therapy for cancer[101,102]. Intriguingly, high expression of HDAC Class I and II in PC could be associated with obesity. It was found that the hypothalamic expression of classical HDAC was increased in obese mice fed a high fat diet[100,103]. Thus, it is possible that the increase in classical HDAC in obese mice could be related to leptin signaling. To initially explore the potential relationships between leptin signaling and HD- AC, leptin effects on HDAC expression was preliminary determined in PC tumorspheres. Results from these experiments show that leptin increased the expression of HDAC3 and HDAC8 in BxPC-3 tumorspheres (Figure 3). Furthermore, preliminary data suggest that gemcitabine decreased the expression of HDAC2, HDAC3 and HDAC8 that was reverted by leptin. This suggests that leptin could affect the expression of HDAC in PC, which might be associated with chemoresistance.
PC is an aggressive disease commonly detected in its late stages, continues to show poor prognosis, and has no targeted treatment. Surgical tumor removal is the best option to eliminate PC, but only in limited number of cases. Therefore, most PC patients are treated with chemotherapeutics, but survival rates have historically been poor. Obesity is a modifiable risk factor of PC that is characterized by inflammation and high levels of the adipokine leptin, which is a cancer proliferation factor that can also contribute to chemoresistance. Studies have identified that the dysregulation of HDAC, miR21, miR200, leptin, and PCSC could play important roles in PC progression. Previous reports showed that leptin signaling can induce PC proliferation, PCSC expand and regulate miR21, miR200, and HDAC levels. Moreover, the analysis of data from PC biopsies (Cancer Genome Atlas)[71] showed inverse expression profiles for miRNA21 and miRNA200 that suggests these molecules could be involved in PC development. Furthermore, HDAC, miRNA21/200, and leptin could have complex signaling crosstalk, according to Pathway Studio analysis. Therefore, leptin, miR21, miR200 and HDAC could be involved in PC progression. Thus, the potential crosstalk among these molecules could be a novel target for PC prevention or treatment, particularly in obese patients who show elevated levels of leptin.
We thank Dr. Gale Newman for her assistance with the Pathway Studio software.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Barreto S, Bilir C, Bramhall S S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | American Cancer Society. Pancreatic Cancer American Cancer Society, 2015. [accessed 2015 Feb 18]. Available from: http://www.cancer.org/cancer/pancreaticcancer/detailedguide/pancreatic-cancer-what-is-pancreatic-cancer. |
| 2. | Casari I, Falasca M. Diet and Pancreatic Cancer Prevention. Cancers (Basel). 2015;7:2309-2317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Dranka-Bojarowska D, Lekstan A, Olakowski M, Jablonska B, Lewinski A, Musialski P, Sobczyk W, Kapalka A, Lampe P. The assessment of serum concentration of adiponectin, leptin and serum carbohydrate antigen-19.9 in patients with pancreatic cancer and chronic pancreatitis. J Physiol Pharmacol. 2015;66:653-663. [PubMed] |
| 4. | Mendonsa AM, Chalfant MC, Gorden LD, VanSaun MN. Modulation of the leptin receptor mediates tumor growth and migration of pancreatic cancer cells. PLoS One. 2015;10:e0126686. [PubMed] |
| 5. | Shi YQ, Yang J, Du P, Xu T, Zhuang XH, Shen JQ, Xu CF. Effect of Body Mass Index on Overall Survival of Pancreatic Cancer: A Meta-Analysis. Medicine (Baltimore). 2016;95:e3305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Smyth AG. A modified miniplate for use in malar complex fractures. Br J Oral Maxillofac Surg. 1995;33:169-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Assfalg V, Hüser N, Michalski C, Gillen S, Kleeff J, Friess H. Palliative interventional and surgical therapy for unresectable pancreatic cancer. Cancers (Basel). 2011;3:652-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Fazal S, Saif MW. Supportive and palliative care of pancreatic cancer. JOP. 2007;8:240-253. [PubMed] |
| 9. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5634] [Article Influence: 402.4] [Reference Citation Analysis (1)] |
| 10. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4883] [Article Influence: 406.9] [Reference Citation Analysis (0)] |
| 11. | Fritzsche FR, Weichert W, Röske A, Gekeler V, Beckers T, Stephan C, Jung K, Scholman K, Denkert C, Dietel M. Class I histone deacetylases 1, 2 and 3 are highly expressed in renal cell cancer. BMC Cancer. 2008;8:381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 12. | Rikimaru T, Taketomi A, Yamashita Y, Shirabe K, Hamatsu T, Shimada M, Maehara Y. Clinical significance of histone deacetylase 1 expression in patients with hepatocellular carcinoma. Oncology. 2007;72:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Weichert W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett. 2009;280:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 321] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 14. | Weichert W, Röske A, Gekeler V, Beckers T, Ebert MP, Pross M, Dietel M, Denkert C, Röcken C. Association of patterns of class I histone deacetylase expression with patient prognosis in gastric cancer: a retrospective analysis. Lancet Oncol. 2008;9:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 15. | Zhang Z, Yamashita H, Toyama T, Sugiura H, Ando Y, Mita K, Hamaguchi M, Hara Y, Kobayashi S, Iwase H. Quantitation of HDAC1 mRNA expression in invasive carcinoma of the breast*. Breast Cancer Res Treat. 2005;94:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 217] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 16. | Benoit C, Ould-Hamouda H, Crepin D, Gertler A, Amar L, Taouis M. Early leptin blockade predisposes fat-fed rats to overweight and modifies hypothalamic microRNAs. J Endocrinol. 2013;218:35-47. |
| 17. | Garofalo M, Romano G, Di Leva G, Nuovo G, Jeon YJ, Ngankeu A, Sun J, Lovat F, Alder H, Condorelli G. EGFR and MET receptor tyrosine kinase-altered microRNA expression induces tumorigenesis and gefitinib resistance in lung cancers. Nat Med. 2011;18:74-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 314] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 18. | Susuki D, Kimura S, Naganuma S, Tsuchiyama K, Tanaka T, Kitamura N, Fujieda S, Itoh H. Regulation of microRNA expression by hepatocyte growth factor in human head and neck squamous cell carcinoma. Cancer Sci. 2011;102:2164-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Wang G, Chan ES, Kwan BC, Li PK, Yip SK, Szeto CC, Ng CF. Expression of microRNAs in the urine of patients with bladder cancer. Clin Genitourin Cancer. 2012;10:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Yu J, Ohuchida K, Mizumoto K, Sato N, Kayashima T, Fujita H, Nakata K, Tanaka M. MicroRNA, hsa-miR-200c, is an independent prognostic factor in pancreatic cancer and its upregulation inhibits pancreatic cancer invasion but increases cell proliferation. Mol Cancer. 2010;9:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 21. | Irani S. miRNAs Signature in Head and Neck Squamous Cell Carcinoma Metastasis: A Literature Review. J Dent (Shiraz). 2016;17:71-83. [PubMed] |
| 22. | Moriyama T, Ohuchida K, Mizumoto K, Yu J, Sato N, Nabae T, Takahata S, Toma H, Nagai E, Tanaka M. MicroRNA-21 modulates biological functions of pancreatic cancer cells including their proliferation, invasion, and chemoresistance. Mol Cancer Ther. 2009;8:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 266] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 23. | Chen L, Bourguignon LY. Hyaluronan-CD44 interaction promotes c-Jun signaling and miRNA21 expression leading to Bcl-2 expression and chemoresistance in breast cancer cells. Mol Cancer. 2014;13:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Sureban SM, May R, Lightfoot SA, Hoskins AB, Lerner M, Brackett DJ, Postier RG, Ramanujam R, Mohammed A, Rao CV. DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism. Cancer Res. 2011;71:2328-2338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 25. | Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1262] [Cited by in RCA: 1352] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 26. | Ballard-Barbash R, Hunsberger S, Alciati MH, Blair SN, Goodwin PJ, McTiernan A, Wing R, Schatzkin A. Physical activity, weight control, and breast cancer risk and survival: clinical trial rationale and design considerations. J Natl Cancer Inst. 2009;101:630-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Hursting SD, Digiovanni J, Dannenberg AJ, Azrad M, Leroith D, Demark-Wahnefried W, Kakarala M, Brodie A, Berger NA. Obesity, energy balance, and cancer: new opportunities for prevention. Cancer Prev Res (Phila). 2012;5:1260-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 28. | Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2163] [Cited by in RCA: 2420] [Article Influence: 268.9] [Reference Citation Analysis (0)] |
| 29. | Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, Styer AK, Sullivan BT, Sakamoto H, Olawaiye A, Serikawa T. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2). J Biol Chem. 2006;281:26320-26328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 30. | Gonzalez-Perez RR, Xu Y, Guo S, Watters A, Zhou W, Leibovich SJ. Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFkappaB/HIF-1alpha activation. Cell Signal. 2010;22:1350-1362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 31. | Guo S, Gonzalez-Perez RR. Notch, IL-1 and leptin crosstalk outcome (NILCO) is critical for leptin-induced proliferation, migration and VEGF/VEGFR-2 expression in breast cancer. PLoS One. 2011;6:e21467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 32. | Harbuzariu A, Daley-Brown DS, Harmon TL, Garrison RC, Beech DJ, Cason FD, Klug C, Gonzalez-Perez RR. Abstract B26: Leptin affects proliferation, stem cells and chemotherapeutic treatment outcome of pancreatic cancer: A link to health disparity. CEBP. 2016;B26. [DOI] [Full Text] |
| 33. | Tchio CIM, Harbuzariu A, Harmon TL, Beech DJ, Gonzalez-Perez RR. Abstract B31: A new approach to targeted therapy for obesity-related pancreatic adenocarcinoma. CEBP. 2016;B31. [DOI] [Full Text] |
| 34. | Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2848] [Cited by in RCA: 2552] [Article Influence: 283.6] [Reference Citation Analysis (0)] |
| 35. | Lynch HT, Lynch JF, Lanspa SJ. Familial pancreatic cancer. Cancers (Basel). 2010;2:1861-1883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Vasen H, Ibrahim I, Ponce CG, Slater EP, Matthäi E, Carrato A, Earl J, Robbers K, van Mil AM, Potjer T. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies From Three European Expert Centers. J Clin Oncol. 2016;34:2010-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 269] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 37. | Bhat K, Wang F, Ma Q, Li Q, Mallik S, Hsieh TC, Wu E. Advances in biomarker research for pancreatic cancer. Curr Pharm Des. 2012;18:2439-2451. [PubMed] |
| 38. | Bidard FC, Huguet F, Louvet C, Mineur L, Bouché O, Chibaudel B, Artru P, Desseigne F, Bachet JB, Mathiot C. Circulating tumor cells in locally advanced pancreatic adenocarcinoma: the ancillary CirCe 07 study to the LAP 07 trial. Ann Oncol. 2013;24:2057-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 39. | Robinson SM, Fan L, White SA, Charnley RM, Mann J. The role of exosomes in the pathogenesis of pancreatic ductal adenocarcinoma. Int J Biochem Cell Biol. 2016;75:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Fan Y, Gan Y, Shen Y, Cai X, Song Y, Zhao F, Yao M, Gu J, Tu H. Leptin signaling enhances cell invasion and promotes the metastasis of human pancreatic cancer via increasing MMP-13 production. Oncotarget. 2015;6:16120-16134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 41. | Rückert F, Pilarsky C, Grützmann R. Serum tumor markers in pancreatic cancer-recent discoveries. Cancers (Basel). 2010;2:1107-1124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Verma M. Pancreatic cancer biomarkers and their implication in cancer diagnosis and epidemiology. Cancers (Basel). 2010;2:1830-1837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol. 2004;338:17-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1093] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 44. | de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2200] [Cited by in RCA: 2332] [Article Influence: 106.0] [Reference Citation Analysis (0)] |
| 45. | Marquard L, Gjerdrum LM, Christensen IJ, Jensen PB, Sehested M, Ralfkiaer E. Prognostic significance of the therapeutic targets histone deacetylase 1, 2, 6 and acetylated histone H4 in cutaneous T-cell lymphoma. Histopathology. 2008;53:267-277. [PubMed] |
| 46. | Saji S, Kawakami M, Hayashi S, Yoshida N, Hirose M, Horiguchi S, Itoh A, Funata N, Schreiber SL, Yoshida M. Significance of HDAC6 regulation via estrogen signaling for cell motility and prognosis in estrogen receptor-positive breast cancer. Oncogene. 2005;24:4531-4539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 208] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 47. | Chan E, Arlinghaus LR, Cardin DB, Goff L, Berlin JD, Parikh A, Abramson RG, Yankeelov TE, Hiebert S, Merchant N. Phase I trial of vorinostat added to chemoradiation with capecitabine in pancreatic cancer. Radiother Oncol. 2016;119:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | Lassen U, Molife LR, Sorensen M, Engelholm SA, Vidal L, Sinha R, Penson RT, Buhl-Jensen P, Crowley E, Tjornelund J. A phase I study of the safety and pharmacokinetics of the histone deacetylase inhibitor belinostat administered in combination with carboplatin and/or paclitaxel in patients with solid tumours. Br J Cancer. 2010;103:12-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Mottamal M, Zheng S, Huang TL, Wang G. Histone deacetylase inhibitors in clinical studies as templates for new anticancer agents. Molecules. 2015;20:3898-3941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 512] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 50. | Tinari N, De Tursi M, Grassadonia A, Zilli M, Stuppia L, Iacobelli S, Natoli C. An epigenetic approach to pancreatic cancer treatment: the prospective role of histone deacetylase inhibitors. Curr Cancer Drug Targets. 2012;12:439-452. [PubMed] |
| 51. | Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist. 2007;12:1247-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 1026] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 52. | Sarfstein R, Bruchim I, Fishman A, Werner H. The mechanism of action of the histone deacetylase inhibitor vorinostat involves interaction with the insulin-like growth factor signaling pathway. PLoS One. 2011;6:e24468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, Caballero D, Borchmann P, Morschhauser F, Wilhelm M. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 525] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 54. | Karthik S, Sankar R, Varunkumar K, Ravikumar V. Romidepsin induces cell cycle arrest, apoptosis, histone hyperacetylation and reduces matrix metalloproteinases 2 and 9 expression in bortezomib sensitized non-small cell lung cancer cells. Biomed Pharmacother. 2014;68:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 55. | Piekarz RL, Frye R, Turner M, Wright JJ, Allen SL, Kirschbaum MH, Zain J, Prince HM, Leonard JP, Geskin LJ. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009;27:5410-5417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 561] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 56. | Chien W, Lee DH, Zheng Y, Wuensche P, Alvarez R, Wen DL, Aribi AM, Thean SM, Doan NB, Said JW. Growth inhibition of pancreatic cancer cells by histone deacetylase inhibitor belinostat through suppression of multiple pathways including HIF, NFkB, and mTOR signaling in vitro and in vivo. Mol Carcinog. 2014;53:722-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 57. | Dovzhanskiy DI, Arnold SM, Hackert T, Oehme I, Witt O, Felix K, Giese N, Werner J. Experimental in vivo and in vitro treatment with a new histone deacetylase inhibitor belinostat inhibits the growth of pancreatic cancer. BMC Cancer. 2012;12:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Wang B, Wang XB, Chen LY, Huang L, Dong RZ. Belinostat-induced apoptosis and growth inhibition in pancreatic cancer cells involve activation of TAK1-AMPK signaling axis. Biochem Biophys Res Commun. 2013;437:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Wang G, He J, Zhao J, Yun W, Xie C, Taub JW, Azmi A, Mohammad RM, Dong Y, Kong W. Class I and class II histone deacetylases are potential therapeutic targets for treating pancreatic cancer. PLoS One. 2012;7:e52095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Debernardi S, Massat NJ, Radon TP, Sangaralingam A, Banissi A, Ennis DP, Dowe T, Chelala C, Pereira SP, Kocher HM. Noninvasive urinary miRNA biomarkers for early detection of pancreatic adenocarcinoma. Am J Cancer Res. 2015;5:3455-3466. [PubMed] |
| 61. | Li A, Yu J, Kim H, Wolfgang CL, Canto MI, Hruban RH, Goggins M. MicroRNA array analysis finds elevated serum miR-1290 accurately distinguishes patients with low-stage pancreatic cancer from healthy and disease controls. Clin Cancer Res. 2013;19:3600-3610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 241] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 62. | Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, Xiang D, Desano JT, Bommer GT, Fan D. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 532] [Cited by in RCA: 543] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 63. | Sicard F, Gayral M, Lulka H, Buscail L, Cordelier P. Targeting miR-21 for the therapy of pancreatic cancer. Mol Ther. 2013;21:986-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 64. | Feng X, Wang Z, Fillmore R, Xi Y. MiR-200, a new star miRNA in human cancer. Cancer Lett. 2014;344:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 285] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 65. | Li A, Omura N, Hong SM, Vincent A, Walter K, Griffith M, Borges M, Goggins M. Pancreatic cancers epigenetically silence SIP1 and hypomethylate and overexpress miR-200a/200b in association with elevated circulating miR-200a and miR-200b levels. Cancer Res. 2010;70:5226-5237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 240] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 66. | Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, Schaffert JM, Wang Z, Philip PA, Sarkar FH. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010;70:3606-3617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 334] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 67. | Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6805] [Cited by in RCA: 7745] [Article Influence: 484.1] [Reference Citation Analysis (0)] |
| 68. | Brabletz S, Bajdak K, Meidhof S, Burk U, Niedermann G, Firat E, Wellner U, Dimmler A, Faller G, Schubert J. The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. EMBO J. 2011;30:770-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 298] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 69. | Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1287] [Cited by in RCA: 1403] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 70. | Yang Y, Ahn YH, Gibbons DL, Zang Y, Lin W, Thilaganathan N, Alvarez CA, Moreira DC, Creighton CJ, Gregory PA. The Notch ligand Jagged2 promotes lung adenocarcinoma metastasis through a miR-200-dependent pathway in mice. J Clin Invest. 2011;121:1373-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 71. | TCGA databank. Available from: http://cancergenome.nih.gov/. |
| 72. | Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2377] [Cited by in RCA: 2428] [Article Influence: 134.9] [Reference Citation Analysis (0)] |
| 73. | Li C, Lee CJ, Simeone DM. Identification of human pancreatic cancer stem cells. Methods Mol Biol. 2009;568:161-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 74. | Yin T, Wei H, Gou S, Shi P, Yang Z, Zhao G, Wang C. Cancer stem-like cells enriched in Panc-1 spheres possess increased migration ability and resistance to gemcitabine. Int J Mol Sci. 2011;12:1595-1604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 75. | Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1987] [Cited by in RCA: 2140] [Article Influence: 118.9] [Reference Citation Analysis (0)] |
| 76. | Brandes F, Schmidt K, Wagner C, Redekopf J, Schlitt HJ, Geissler EK, Lang SA. Targeting cMET with INC280 impairs tumour growth and improves efficacy of gemcitabine in a pancreatic cancer model. BMC Cancer. 2015;15:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 77. | Herreros-Villanueva M, Zubia-Olascoaga A, Bujanda L. c-Met in pancreatic cancer stem cells: therapeutic implications. World J Gastroenterol. 2012;18:5321-5323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 78. | Fitzgerald TL, McCubrey JA. Pancreatic cancer stem cells: association with cell surface markers, prognosis, resistance, metastasis and treatment. Adv Biol Regul. 2014;56:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 79. | Giordano S, Ponzetto C, Di Renzo MF, Cooper CS, Comoglio PM. Tyrosine kinase receptor indistinguishable from the c-met protein. Nature. 1989;339:155-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 366] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 80. | Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, Pasca di Magliano M, Simeone DM. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141:2218-2227.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 290] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 81. | Harbuzariu A, Mullen M, Gonzalez-Perez RR. Pancreatic Cancer and Obesity: Some Molecular Perspectives. J Carcinog Mutagen. 2016;7:1000276. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 82. | Abel EV, Kim EJ, Wu J, Hynes M, Bednar F, Proctor E, Wang L, Dziubinski ML, Simeone DM. The Notch pathway is important in maintaining the cancer stem cell population in pancreatic cancer. PLoS One. 2014;9:e91983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 83. | Arslan AA, Helzlsouer KJ, Kooperberg C, Shu XO, Steplowski E, Bueno-de-Mesquita HB, Fuchs CS, Gross MD, Jacobs EJ, Lacroix AZ. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch Intern Med. 2010;170:791-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 277] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 84. | Hendifar A, Osipov A, Khanuja J, Nissen N, Naziri J, Yang W, Li Q, Tuli R. Influence of Body Mass Index and Albumin on Perioperative Morbidity and Clinical Outcomes in Resected Pancreatic Adenocarcinoma. PLoS One. 2016;11:e0152172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 85. | Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2509] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 86. | Sandini M, Bernasconi DP, Fior D, Molinelli M, Ippolito D, Nespoli L, Caccialanza R, Gianotti L. A high visceral adipose tissue-to-skeletal muscle ratio as a determinant of major complications after pancreatoduodenectomy for cancer. Nutrition. 2016;32:1231-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 87. | Incio J, Liu H, Suboj P, Chin SM, Chen IX, Pinter M, Ng MR, Nia HT, Grahovac J, Kao S. Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov. 2016;6:852-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 331] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 88. | Lipsey CC, Harbuzariu A, Daley-Brown D, Gonzalez-Perez RR. Oncogenic role of leptin and Notch interleukin-1 leptin crosstalk outcome in cancer. World J Methodol. 2016;6:43-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 89. | Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9119] [Cited by in RCA: 8855] [Article Influence: 285.6] [Reference Citation Analysis (0)] |
| 90. | Newman G, Gonzalez-Perez RR. Leptin-cytokine crosstalk in breast cancer. Mol Cell Endocrinol. 2014;382:570-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 91. | Babaei Z, Moslemi D, Parsian H, Khafri S, Pouramir M, Mosapour A. Relationship of obesity with serum concentrations of leptin, CRP and IL-6 in breast cancer survivors. J Egypt Natl Canc Inst. 2015;27:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 92. | Daley-Brown D, Oprea-Ilies GM, Lee R, Pattillo R, Gonzalez-Perez RR. Molecular cues on obesity signals, tumor markers and endometrial cancer. Horm Mol Biol Clin Investig. 2015;21:89-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 93. | Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai CF, Tartaglia LA. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA. 1996;93:8374-8378. [PubMed] |
| 94. | Lewandowski K, Horn R, O’Callaghan CJ, Dunlop D, Medley GF, O’Hare P, Brabant G. Free leptin, bound leptin, and soluble leptin receptor in normal and diabetic pregnancies. J Clin Endocrinol Metab. 1999;84:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 95. | Harbuzariu A, Rampoldi A, Daley-Brown DS, Candelaria P, Harmon TL, Lipsey CC, Beech DJ, Quarshie A, Ilies GO, Gonzalez-Perez RR. Leptin-Notch signaling axis is involved in pancreatic cancer progression. Oncotarget. 2017;8:7740-7752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 96. | Guo S, Liu M, Wang G, Torroella-Kouri M, Gonzalez-Perez RR. Oncogenic role and therapeutic target of leptin signaling in breast cancer and cancer stem cells. Biochim Biophys Acta. 2012;1825:207-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 97. | Stolzenberg-Solomon RZ, Newton CC, Silverman DT, Pollak M, Nogueira LM, Weinstein SJ, Albanes D, Männistö S, Jacobs EJ. Circulating Leptin and Risk of Pancreatic Cancer: A Pooled Analysis From 3 Cohorts. Am J Epidemiol. 2015;182:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 98. | Pastar I, Khan AA, Stojadinovic O, Lebrun EA, Medina MC, Brem H, Kirsner RS, Jimenez JJ, Leslie C, Tomic-Canic M. Induction of specific microRNAs inhibits cutaneous wound healing. J Biol Chem. 2012;287:29324-29335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 99. | Derghal A, Djelloul M, Airault C, Pierre C, Dallaporta M, Troadec JD, Tillement V, Tardivel C, Bariohay B, Trouslard J. Leptin is required for hypothalamic regulation of miRNAs targeting POMC 3’UTR. Front Cell Neurosci. 2015;9:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 100. | Kabra DG, Pfuhlmann K, García-Cáceres C, Schriever SC, Casquero García V, Kebede AF, Fuente-Martin E, Trivedi C, Heppner K, Uhlenhaut NH. Hypothalamic leptin action is mediated by histone deacetylase 5. Nat Commun. 2016;7:10782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 101. | Aghdassi A, Sendler M, Guenther A, Mayerle J, Behn CO, Heidecke CD, Friess H, Büchler M, Evert M, Lerch MM. Recruitment of histone deacetylases HDAC1 and HDAC2 by the transcriptional repressor ZEB1 downregulates E-cadherin expression in pancreatic cancer. Gut. 2012;61:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 221] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 102. | Tanase CP, Neagu AI, Necula LG, Mambet C, Enciu AM, Calenic B, Cruceru ML, Albulescu R. Cancer stem cells: involvement in pancreatic cancer pathogenesis and perspectives on cancer therapeutics. World J Gastroenterol. 2014;20:10790-10801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 103. | Funato H, Oda S, Yokofujita J, Igarashi H, Kuroda M. Fasting and high-fat diet alter histone deacetylase expression in the medial hypothalamus. PLoS One. 2011;6:e18950. [PubMed] |
| 104. | Buglio D, Khaskhely NM, Voo KS, Martinez-Valdez H, Liu YJ, Younes A. HDAC11 plays an essential role in regulating OX40 ligand expression in Hodgkin lymphoma. Blood. 2011;117:2910-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |