Published online Dec 10, 2016. doi: 10.5306/wjco.v7.i6.425
Peer-review started: June 20, 2016
First decision: August 11, 2016
Revised: September 29, 2016
Accepted: October 25, 2016
Article in press: October 27, 2016
Published online: December 10, 2016
Processing time: 162 Days and 15.5 Hours
To investigate the potential benefit of combining the cMET inhibitor crizotinib and cisplatin we performed in vitro combination studies.
We tested three different treatment schemes in four non-small cell lung cancer (NSCLC) cell lines with a different cMET/epidermal growth factor receptor genetic background by means of the sulforhodamine B assay and performed analysis with Calcusyn.
All treatment schemes showed an antagonistic effect in all cell lines, independent of the cMET status. Despite their different genetic backgrounds, all cell lines (EBC-1, HCC827, H1975 and LUDLU-1) showed antagonistic combination indexes ranging from 1.3-2.7. These results were independent of the treatment schedule.
These results discourage further efforts to combine cMET inhibition with cisplatin chemotherapy in NSCLC.
Core tip: Targeted therapies are a valuable treatment option in non-small cell lung cancer. Several therapies have now been approved like erlotinib and gefitinib for epidermal growth factor receptor - mutant patients and crizotinib for Anaplastic Lymphoma Kinase-rearranged patients. However, resistance against these therapies eventually occurs. Combination therapy might be able to overcome or delay this resistance. Here we investigate the combination of the cMET inhibitor crizotinib with cisplatin in a panel of non-small cell lung cancer (NSCLC) cell lines with different histological and genetic backgrounds. We show that this leads to strong antagonism in all of the used cell lines. Furthermore we also link these results to the earlier in vitro and clinical results of the combination of erlotinib/gefitinib with cisplatin based chemotherapy in NSCLC.
- Citation: Van Der Steen N, Deben C, Deschoolmeester V, Wouters A, Lardon F, Rolfo C, Germonpré P, Giovannetti E, Peters GJ, Pauwels P. Better to be alone than in bad company: The antagonistic effect of cisplatin and crizotinib combination therapy in non-small cell lung cancer. World J Clin Oncol 2016; 7(6): 425-432
- URL: https://www.wjgnet.com/2218-4333/full/v7/i6/425.htm
- DOI: https://dx.doi.org/10.5306/wjco.v7.i6.425
During the last decade, targeted therapies have revolutionized the treatment for non-small cell lung cancer (NSCLC). Several epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) have been approved for patients with sensitizing mutations in EGFR[1-3]. Furthermore, several cMET inhibitors are currently under development with promising clinical benefit[4,5]. However, only a small percentage of NSCLC patients are eligible for these treatments. Thus, for the majority of NSCLC patients, cisplatin based therapy remains the standard of care treatment in first or later lines, usually in combination with pemetrexed, gemcitabine or a taxane[6-9].
cMET, with its ligand hepatocyte growth factor (HGF), is known to be activated in many tumor types, including NSCLC[10], with cMET amplification recognized as a resistance mechanism during EGFR tyrosine kinase inhibition[11]. The cMET and EGFR signaling pathways are heavily intertwined[12,13], with EGFR activation being sufficient for downstream cMET phosphorylation. The mitogen activated protein kinase (MAPK) dependent activation of cMET by EGFR takes place at different regulatory levels, with cMET transcriptional upregulation, the elongation of cMET half-life and a decrease in cMET-ubiquitylation[12]. Upon binding of HGF, the cMET receptor dimerizes and cross-phosphorylation takes place. This ultimately leads to phosphorylation of the docking sites recruiting proteins involved in the signaling of MAPK cascades, phosphoinositide 3 kinase (PI3K), signal transducer and activator of transcription 3 (STAT3) and nuclear factor-κB (NF-κB). Thus activating many oncogenic processes such as migration, invasion, and angiogenesis[14]. Two main cMET aberrations have been described, which can be used to predict sensitivity to cMET therapies: Amplification of the cMET gene[4] and cMET exon 14 skipping[5,15].
Several small molecule inhibitors and monoclonal antibodies inhibiting cMET signaling are currently being investigated in several clinical trials[16]. One of these small molecule inhibitors is crizotinib, which was originally developed as a cMET inhibitor[17] but has been approved for treatment of anaplastic lymphoma kinase (ALK)-translocated NSCLC patients[18]. Currently, crizotinib is being investigated in several clinical trials (METROS trial and the NCT02499614) for the treatment of patients with cMET-dependent NSCLC and in other cancer types where patients carry a cMET amplification[16,19].
The combination of a cMET inhibitor and cisplatin has not been investigated in NSCLC patients to date. However, in vitro studies show contradictory results where the outcome is dependent on tumor type and origin. For example, addition of the cMET ligand HGF enhanced cisplatin resistance in seven different NSCLC cell lines. This was explained by the fact that HGF binding induces cMET signaling which led to activation of focal adhesion kinase (FAK). FAK, in turn, suppressed the apoptosis inducing factor (AIF), resulting in a decreased sensitivity to cisplatin[20]. Therefore, theoretically, inhibition of cMET could possibly result in sensitization towards cisplatin. However, another study in SW620 cells, a KRAS mutated colon cancer cell line, showed that conditioned knock-down of cMET did not influence cisplatin sensitivity[21]. In contrast, ovarian cancer cell lines were sensitized towards cisplatin with the addition of HGF[22], this was established to be linked to the p38-MAPK signaling of cMET[23]. HGF pretreatment of these cells decreased the transcription of protein phosphatase 2A, thus increasing the effect of cisplatin[24].
Given the contradictory results in previous studies, more studies were warranted. Therefore, we investigated whether a combination of these compounds could result in a synergistic treatment effect in NSCLC cell lines with different cMET and EGFR genetic backgrounds.
Four NSCLC cell lines were included in this study. The HCC827 and H1975 cell lines were purchased from the American Type Culture Collection (ATCC), the EBC-1 cell line from the Japanese Collection of Research Bioresources (JCRB, Japan) and the LUDLU-1 cell line from the European Collection of Authenticated Cell Cultures (ECACC) (Figure 1 and Table 1). The EBC-1 cell line was cultured in DMEM (Invitrogen, Merelbeke, Belgium) supplemented with 10% FBS, 1% penicillin/streptomycin, L-glutamine (2 mmol/L) and sodium pyruvate (1 mmol/L). The HCC 827, H1975 and LUDLU-1 cell lines were cultured in RPMI1640 (Invitrogen) supplemented with 10% FBS, 1% penicillin/streptomycin, L-glutamine (2 mmol/L) and sodium pyruvate (1 mmol/L). Cultures were incubated at 37 °C under an atmosphere of 5% CO2. The HCC827 cell line harbors an exon 19 deletion in the ErbB1 gene[25], while the H1975 cell line has L858R and T790M mutations in the ErbB1 gene[26]. The EBC-1 cell line harbors a cMET amplification[27], while the LUDLU-1 is wild-type for both EGFR and cMET (Table 1). All cell lines were wild-type for ALK, free from mycoplasma contamination and STR profiles were checked.
| HCC827 | H1975 | EBC-1 | LUDLU | |
| Properties | ||||
| Histology | Adeno | Adeno | Squamous | Squamous |
| EGFR-status | Exon 19 deletion | L858R + T790M | Wild-type | Wild-type |
| cMET-status | Wild-type | Wild-type | Amplification | Wild-type |
| Drug sensitivity (µmol/L, IC50± SEM) | ||||
| Cisplatin | 8.39 ± 0.36 | 6.10 ± 0.07 | 16.52 ± 0.89 | 3.37 ± 0.19 |
| Crizotinib | 6.05 ± 0.11 | 4.00 ± 0.06 | 0.054 ± 0.002 | 8.12 ± 0.28 |
Cisplatin and crizotinib were purchased from Selleckchem (Huissen, The Netherlands). Cisplatin was dissolved in a sterile 0.9% NaCl solution (Fisher Scientific, Aalst, Belgium), while crizotinib was dissolved in dimethylsulfoxide (DMSO). Both were diluted in phosphate buffered saline (PBS) to the desired concentrations.
Cells were harvested from exponential phase cultures by trypsinization (Trypsin-EDTA 0.05% with phenol red, Invitrogen, Merelbeke, Belgium), counted, seeded in sterile 96-well plates and allowed to attach before treatment. Optimal seeding densities for each cell line were determined to ensure exponential growth during a 5-d or 7-d assay. For the 5-d assay the EBC-1 and HCC827 were seeded at 4500 cell/well, H1975 at 3500 cell/well and the LUDLU-1 at 8000 cell/well. For the 7-d assay the EBC-1 and HCC827 were seeded at 1500 cell/well, the H1975 at 850 cell/well and the LUDLU-1 at 4000 cell/well. Cells were incubated with cisplatin alone (0-10 μmol/L for 72 h), crizotinib alone (0-5 μmol/L for 72 h) or with a combination of both. The combination used crizotinib at a fixed concentration (IC20 or IC40), while a concentration range of cisplatin (0-10 μmol/L) was added. Cells treated with 0.1% diluted DMSO in the case of crizotinib or pure PBS in the case of cisplatin were used as controls. Three combination schedules were investigated: (1) simultaneous exposure to cisplatin and crizotinib for 72 h; (2) cisplatin for 72 h, followed by washing and crizotinib for 72 h; or (3) 72 h of crizotinib followed by washing and cisplatin for 72 h (Table 2). When crizotinib was used as first drug, the concentration was reduced in three out of the four cell lines, due to the toxic after-effect of this drug.
| Drug scheme | HCC827 | H1975 | EBC-1 | LUDLU-1 | ||||
| Criz | CI ± SEM | Criz | CI ± SEM | Criz | CI ± SEM | Criz | CI ± SEM | |
| Cisplatin + Crizotinib | 3 μmol/L | 1.58 ± 0.10 | 3 μmol/L | 1.94 ± 0.27 | 0.025 μmol/L | 2.08 ± 0.49 | 3 μmol/L | 2.65 ± 0.30 |
| 5 μmol/L | 1.54 ± 0.15 | 5 μmol/L | 1.93 ± 0.19 | 0.05 μmol/L | 1.42 ± 0.06 | 4 μmol/L | 2.71 ± 0.14 | |
| Cisplatin → Crizotinib | 3 μmol/L | 1.74 ± 0.17 | 3 μmol/L | 1.75 ± 0.30 | 0.025 μmol/L | 2.29 ± 0.53 | 3 μmol/L | 1.27 ± 0.13 |
| 5 μmol/L | 2.06 ± 0.30 | 5 μmol/L | 1.96 ± 0.14 | 0.05 μmol/L | 2.38 ± 0.56 | 4 μmol/L | 1.34 ± 0.15 | |
| Crizotinib → Cisplatin | 1 μmol/L | 2.70 ± 0.37 | 1 μmol/L | 1.58 ± 0.24 | 0.025 μmol/L | 2.08 ± 0.49 | 2 μmol/L | 1.74 ± 0.14 |
| 2 μmol/L | 2.42 ± 0.21 | 2 μmol/L | 0.95 ± 0.03 | 0.05 μmol/L | 1.42 ± 0.06 | 3 μmol/L | 1.89 ± 0.17 | |
After treatment, growth inhibition was determined by the sulforhodamine B (SRB) assay, as previously described[28]. In short, the medium was discarded and the cells were fixed with ice cold 10% Trichloric acid (Fisher Scientific, Aalst, Belgium) solution for 1 h at 4 °C. Next, the plates were washed 5 times with demineralized water. The cells were stained with 100 μL 0.1% SRB (Acros organics, Geel, Belgium) dissolved in 1% glacial acetic acid (Fisher Scientific, Aalst, Belgium) for at least 15 min and subsequently washed five times with 1% acetic acid to remove unbound stain. The plates were left to dry at room temperature and bound protein stain was solubilized with 100 μL 10 mmol/L unbuffered Tris base [tris (hydroxymethyl) aminomethane] (Fisher Scientific, Aalst, Belgium) and read at an optical density (OD) of 540 nm (IMark microplate absorbance reader, Biorad, Nazareth, Belgium)[29].
Each test was performed at least three times, unless otherwise stated. Results are presented as mean ± SEM.
To assess the IC50 value of cisplatin and crizotinib, WinNonlin software was used (Pharsight Corporation, Mountain View, CA, United States). To determine possible synergism between cisplatin and crizotinib, the combination index (CI) was calculated with the Calcusyn software of Biosoft. This program is based on the method of Chou et al[30,31] to assess whether a combination of two drugs results in an antagonistic effect (CI > 1.2), an additive effect (0.8 < CI < 1.2) or a synergistic effect (CI < 0.8). This method takes into account the fraction of affected cells of both monotherapies and compares this with the fraction of affected cells of the combination therapies.
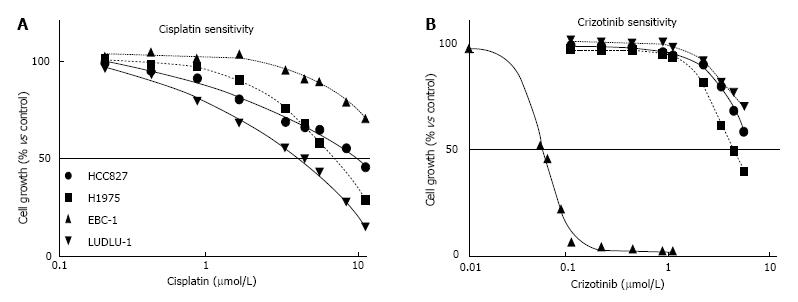
The effects of cisplatin and crizotinib monotherapy were investigated in four NSCLC cell lines (Figure 1). LUDLU-1 cells were most sensitive to cisplatin, followed by the EGFR-mutated H1975 and HCC827 cell lines. As for the cMET amplified EBC-1 cell line, concentrations up to 10 μM cisplatin induced only 30% growth inhibition and the IC50 value was determined by extrapolation (Figure 1).
EBC-1 cells were 74-150 fold more sensitive to crizotinib than the other 3 cell lines, due to the presence of a cMET amplification in these cells. The IC50 values of the HCC827 and LUDLU-1 cell line were determined by extrapolation, with the LUDLU-1 being the most resistant to crizotinib (Figure 1 and Table 1). Based on these results, we decided to use the IC20 and IC40 values of crizotinib during combination treatment (Table 2).
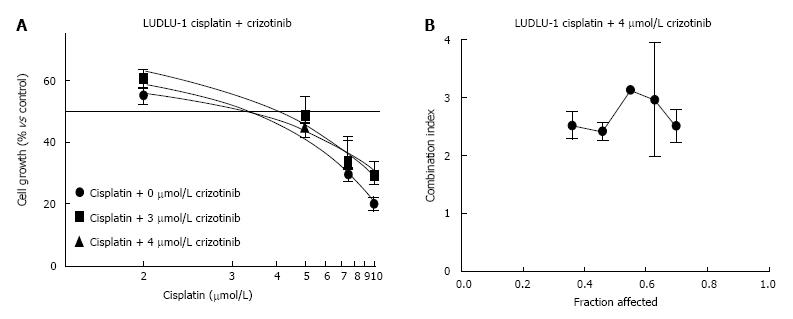
Despite their different genetic backgrounds for cMET and EGFR, all cell lines showed strong antagonism (CI ranging from 1.3 to 2.7) when crizotinib and cisplatin were combined, which was independent of the used treatment schedule (Table 2). This antagonistic effect was visible for all growth inhibition rates of the cells (Figure 2). However, for one treatment condition, i.e., crizotinib followed by cisplatin treatment in the H1975 cell line, an additive effect (CI = 1.0) could be detected. However, this combination only led to 40% growth inhibition at most and needs to be interpreted with caution.
Although both cisplatin and crizotinib are active drugs used in monotherapy for the treatment of various forms of NSCLC, the combination of both compounds was found to be antagonistic, independent of the genetic background of the investigated cell lines.
As described in literature, the high sensitivity of the EBC-1 cell line for crizotinib monotherapy can be explained by its cMET amplification, which is known to confer sensitivity to crizotinib and other cMET small molecule inhibitors[19]. In contrast, the EBC-1 cells were not sensitive to cisplatin, with an IC50 value around 16 μmol/L. Although we did not investigate common resistance mechanisms for cisplatin (such as transporters or DNA repair[32-34]) the cMET amplification might also explain the observed results, since cMET activation can induce cisplatin resistance in cell lines[20]. In contrast to the EBC-1 cells, the LUDLU-1 cells (WT EGFR, WT cMET) where the most sensitive to cisplatin but resistant to crizotinib.
When both therapies were combined, an antagonistic effect was observed in all cell lines, even in the cMET amplified EBC-1 cell line with high basal levels of cMET, independent of the treatment schedule. Previous studies suggested that the addition of HGF induced cisplatin resistance in NSCLC cell lines[20], since the activation of cMET would lead to decreased AIF levels. However, a cMET inhibitor combined with cisplatin had never been investigated previously.
Other TKIs have been known in vitro to synergize with chemotherapy, such as EGFR-inhibitors with platinum doublet chemotherapy[35-38], whereas clinical trials showed no substantial benefit when combining both drugs. Combinations of cisplatin with EGFR-TKIs, have been investigated extensively, both in vitro and in vivo. In wild-type EGFR (WT-EGFR) NSCLC cell lines, cisplatin may upregulate phosphorylated EGFR, thus sensitizing these cells to erlotinib; However, in NSCLC cell lines with sensitizing EGFR mutations, combining cisplatin with erlotinib treatment was found to be antagonistic[36]. Other studies showed that platinum analogs in combination with erlotinib led to synergistic cell death in EGFR-mutant NSCLC cell lines and xenografts[37,38]. Possible mechanisms for this synergy are a decrease in hypoxia-inducible factor 1α (HIF1α), a decrease in c-Myc or cell cycle effects[37], while also platinum-adduct formation by cisplatin was increased[38]. However, several clinical trials[8,9,39-41] combining cisplatin with EGFR-TKIs show no benefit in EGFR-WT or in EGFR-mutant patients. Furthermore, triple combinations of cisplatin, pemetrexed and gefitinib[39]; cisplatin, gemcitabine and erlotinib[40] or cisplatin, pemetrexed followed by gefitinib maintenance therapy[41] showed no or only a minor beneficial effect[42]. In contrast, studies investigating the dual combination of erlotinib and pemetrexed, showed synergism in NSCLC cell lines with different genetic backgrounds[35]. Several molecular mechanisms contributed to this synergism. Firstly, pemetrexed increased phosphorylated-EGFR, thus enhancing the effect of EGFR-blocking by erlotinib. Secondly, the combination of both drugs enhanced the reduction of Akt-phosphorylation, leading to increased apoptosis. Finally, the combination of both drugs also decreased the Thymidylate Synthase (TS) in situ activity[35], which has been correlated with increased pemetrexed sensitivity[43,44].
For many combination therapies no appropriate preclinical investigations were performed before starting clinical trials to determine whether synergism could be expected and what would be the most optimal treatment schedule. This also precludes proper patient selection. Possibly, the combination of both EGFR/cMET inhibitors with cisplatin and pemetrexed chemotherapy activates survival mechanisms that abrogate the benefit of inhibiting these receptor tyrosine kinases, although these mechanisms remain to be further investigated.
Given the intertwining of the EGFR and cMET signaling, we opted to test the same combination in EGFR mutant cell lines. These cell lines reflect the NSCLC patient populations with exon 19 deletion, L858R and T790M mutations in EGFR, cMET amplification, and different histological subtypes (adenocarcinoma and squamous cell carcinoma). Despite mimicking several clinical combinations in vitro, the results showed strong antagonism in all the tested treatment schemes.
In conclusion, we show that the combination of the cMET inhibitor crizotinib with cisplatin is moderately to strongly antagonistic in four NSCLC cell lines. This effect was independent of the cMET/EGFR genetic background, the histological subtype of the cells and the used treatment schedule. Our in vitro results suggest an antagonistic effect of combining cMET inhibition with cisplatin in NSCLC, discouraging further development of this combination in an in vivo and/or clinical setting.
During the last decade, several targeted therapies have been developed for the treatment of lung cancer, inhibiting specific receptors in cancer patients. Given the small number of patients eligible for these therapies, cisplatin based therapy still remains the standard of care treatment for most non-small cell lung cancer (NSCLC) patients. The potential benefit of combining cisplatin with targeted therapies, predominantly against the epidermal growth factor receptor (EGFR), has proved to be disappointing. To investigate the potential benefit of combining cisplatin with crizotinib, the authors have performed in vitro studies on a panel of NSCLC lines with different genetic backgrounds.
The combination of a cMET inhibitor and cisplatin has not been investigated in NSCLC patients to date. However, in vitro studies show contradictory results where the outcome is dependent on tumor type and origin. For example, addition of the cMET ligand hepatocyte growth factor (HGF) enhanced cisplatin resistance in seven different NSCLC cell lines.
In vitro studies show contradictory results where the outcome is dependent on tumor type and origin. For example, addition of the cMET ligand HGF enhanced cisplatin resistance in seven different NSCLC cell lines. However, another study in SW620 cells, a KRAS mutated colon cancer cell line, showed that conditioned knock-down of cMET did not influence cisplatin sensitivity. In contrast, ovarian cancer cell lines were sensitized towards cisplatin with the addition of HGF. HGF pretreatment of these cells decreased the transcription of protein phosphatase 2A, thus increasing the effect of cisplatin. Here the authors show that the combination of the cMET inhibitor crizotinib with cisplatin is moderately to strongly antagonistic in four NSCLC cell lines. This effect was independent of the cMET/EGFR genetic background, the histological subtype of the cells and the used treatment schedule.
The in vitro results suggest an antagonistic effect of combining cMET inhibition with cisplatin in NSCLC, discouraging further development of this combination in an in vivo and/or clinical setting.
NSCLC: Non-small cell lung cancer; EGFR: Epidermal growth factor receptor, one of the known drivers of NSCLC.
This is an interesting work that will help to understand the molecular mechanism of resistance of EGFR inhibitors and the necessity of continuing search of new investigation for the treatment of such lethal disease that is NSCLC.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: The Netherlands
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Deng B, Kawai H, Mehdi I, Neninger E S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Mok TS, D’arcangelo M, Califano R. Clinical outcomes with erlotinib in patients with epidermal growth factor receptor mutation. Drugs. 2012;72 Suppl 1:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Taron M, Ichinose Y, Rosell R, Mok T, Massuti B, Zamora L, Mate JL, Manegold C, Ono M, Queralt C. Activating mutations in the tyrosine kinase domain of the epidermal growth factor receptor are associated with improved survival in gefitinib-treated chemorefractory lung adenocarcinomas. Clin Cancer Res. 2005;11:5878-5885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 266] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 3. | Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7278] [Cited by in RCA: 7497] [Article Influence: 357.0] [Reference Citation Analysis (0)] |
| 4. | Smolen GA, Sordella R, Muir B, Mohapatra G, Barmettler A, Archibald H, Kim WJ, Okimoto RA, Bell DW, Sgroi DC. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci USA. 2006;103:2316-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 427] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 5. | Frampton GM, Ali SM, Rosenzweig M, Chmielecki J, Lu X, Bauer TM, Akimov M, Bufill JA, Lee C, Jentz D. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015;5:850-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 604] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 6. | Eberhardt WE, De Ruysscher D, Weder W, Le Péchoux C, De Leyn P, Hoffmann H, Westeel V, Stahel R, Felip E, Peters S. 2nd ESMO Consensus Conference in Lung Cancer: locally advanced stage III non-small-cell lung cancer. Ann Oncol. 2015;26:1573-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 291] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 7. | Besse B, Adjei A, Baas P, Meldgaard P, Nicolson M, Paz-Ares L, Reck M, Smit EF, Syrigos K, Stahel R. 2nd ESMO Consensus Conference on Lung Cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol. 2014;25:1475-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 199] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 8. | Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, Scagliotti G, Rosell R, Oliff I, Reeves JA. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2. J Clin Oncol. 2004;22:785-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1323] [Cited by in RCA: 1284] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 9. | Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, Natale RB, Schiller JH, Von Pawel J, Pluzanska A. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1. J Clin Oncol. 2004;22:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1324] [Cited by in RCA: 1296] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 10. | Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1153] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 11. | Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3442] [Cited by in RCA: 3669] [Article Influence: 203.8] [Reference Citation Analysis (0)] |
| 12. | Breindel JL, Haskins JW, Cowell EP, Zhao M, Nguyen DX, Stern DF. EGF receptor activates MET through MAPK to enhance non-small cell lung carcinoma invasion and brain metastasis. Cancer Res. 2013;73:5053-5065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Guo A, Villén J, Kornhauser J, Lee KA, Stokes MP, Rikova K, Possemato A, Nardone J, Innocenti G, Wetzel R. Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci USA. 2008;105:692-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 435] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 14. | Van Der Steen N, Pauwels P, Gil-Bazo I, Castañon E, Raez L, Cappuzzo F, Rolfo C. cMET in NSCLC: Can We Cut off the Head of the Hydra? From the Pathway to the Resistance. Cancers (Basel). 2015;7:556-573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Van Der Steen N, Giovannetti E, Pauwels P, Peters GJ, Hong DS, Cappuzzo F, Hirsch FR, Rolfo C. cMET Exon 14 Skipping: From the Structure to the Clinic. J Thorac Oncol. 2016;11:1423-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Garajová I, Giovannetti E, Biasco G, Peters GJ. c-Met as a Target for Personalized Therapy. Transl Oncogenomics. 2015;7:13-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Cui JJ, Tran-Dubé M, Shen H, Nambu M, Kung PP, Pairish M, Jia L, Meng J, Funk L, Botrous I. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med Chem. 2011;54:6342-6363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 711] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 18. | Ou SH, Bazhenova L, Camidge DR, Solomon BJ, Herman J, Kain T, Bang YJ, Kwak EL, Shaw AT, Salgia R. Rapid and dramatic radiographic and clinical response to an ALK inhibitor (crizotinib, PF02341066) in an ALK translocation-positive patient with non-small cell lung cancer. J Thorac Oncol. 2010;5:2044-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Ou SH, Kwak EL, Siwak-Tapp C, Dy J, Bergethon K, Clark JW, Camidge DR, Solomon BJ, Maki RG, Bang YJ. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011;6:942-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 356] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 20. | Chen JT, Huang CY, Chiang YY, Chen WH, Chiou SH, Chen CY, Chow KC. HGF increases cisplatin resistance via down-regulation of AIF in lung cancer cells. Am J Respir Cell Mol Biol. 2008;38:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Li Y, Wang J, Gao X, Han W, Zheng Y, Xu H, Zhang C, He Q, Zhang L, Li Z. c-Met targeting enhances the effect of irradiation and chemical agents against malignant colon cells harboring a KRAS mutation. PLoS One. 2014;9:e113186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Rasola A, Anguissola S, Ferrero N, Gramaglia D, Maffe A, Maggiora P, Comoglio PM, Di Renzo MF. Hepatocyte growth factor sensitizes human ovarian carcinoma cell lines to paclitaxel and cisplatin. Cancer Res. 2004;64:1744-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Coltella N, Rasola A, Nano E, Bardella C, Fassetta M, Filigheddu N, Graziani A, Comoglio PM, Di Renzo MF. p38 MAPK turns hepatocyte growth factor to a death signal that commits ovarian cancer cells to chemotherapy-induced apoptosis. Int J Cancer. 2006;118:2981-2990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Olivero M, Ruggiero T, Saviozzi S, Rasola A, Coltella N, Crispi S, Di Cunto F, Calogero R, Di Renzo MF. Genes regulated by hepatocyte growth factor as targets to sensitize ovarian cancer cells to cisplatin. Mol Cancer Ther. 2006;5:1126-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Amann J, Kalyankrishna S, Massion PP, Ohm JE, Girard L, Shigematsu H, Peyton M, Juroske D, Huang Y, Stuart Salmon J. Aberrant epidermal growth factor receptor signaling and enhanced sensitivity to EGFR inhibitors in lung cancer. Cancer Res. 2005;65:226-235. [PubMed] |
| 26. | Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, Varmus H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2470] [Cited by in RCA: 2684] [Article Influence: 134.2] [Reference Citation Analysis (0)] |
| 27. | Lutterbach B, Zeng Q, Davis LJ, Hatch H, Hang G, Kohl NE, Gibbs JB, Pan BS. Lung cancer cell lines harboring MET gene amplification are dependent on Met for growth and survival. Cancer Res. 2007;67:2081-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 274] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 28. | Pauwels B, Korst AE, de Pooter CM, Pattyn GG, Lambrechts HA, Baay MF, Lardon F, Vermorken JB. Comparison of the sulforhodamine B assay and the clonogenic assay for in vitro chemoradiation studies. Cancer Chemother Pharmacol. 2003;51:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2223] [Cited by in RCA: 2586] [Article Influence: 143.7] [Reference Citation Analysis (0)] |
| 30. | Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5258] [Cited by in RCA: 5703] [Article Influence: 139.1] [Reference Citation Analysis (0)] |
| 31. | Bijnsdorp IV, Giovannetti E, Peters GJ. Analysis of drug interactions. Methods Mol Biol. 2011;731:421-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 32. | Fink D, Zheng H, Nebel S, Norris PS, Aebi S, Lin TP, Nehmé A, Christen RD, Haas M, MacLeod CL. In vitro and in vivo resistance to cisplatin in cells that have lost DNA mismatch repair. Cancer Res. 1997;57:1841-1845. [PubMed] |
| 33. | Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, Howell SB. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther. 2005;4:1595-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 415] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 34. | Samimi G, Safaei R, Katano K, Holzer AK, Rochdi M, Tomioka M, Goodman M, Howell SB. Increased expression of the copper efflux transporter ATP7A mediates resistance to cisplatin, carboplatin, and oxaliplatin in ovarian cancer cells. Clin Cancer Res. 2004;10:4661-4669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 251] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 35. | Giovannetti E, Lemos C, Tekle C, Smid K, Nannizzi S, Rodriguez JA, Ricciardi S, Danesi R, Giaccone G, Peters GJ. Molecular mechanisms underlying the synergistic interaction of erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor, with the multitargeted antifolate pemetrexed in non-small-cell lung cancer cells. Mol Pharmacol. 2008;73:1290-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 36. | Van Schaeybroeck S, Kyula J, Kelly DM, Karaiskou-McCaul A, Stokesberry SA, Van Cutsem E, Longley DB, Johnston PG. Chemotherapy-induced epidermal growth factor receptor activation determines response to combined gefitinib/chemotherapy treatment in non-small cell lung cancer cells. Mol Cancer Ther. 2006;5:1154-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Lee JG, Wu R. Erlotinib-cisplatin combination inhibits growth and angiogenesis through c-MYC and HIF-1α in EGFR-mutated lung cancer in vitro and in vivo. Neoplasia. 2015;17:190-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Avan A, Adema AD, Hoebe EK, Huijts CM, Avan A, Veal GJ, Ruijtenbeek R, Wosikowski K, Peters GJ. Modulation of signaling enhances the efficacy of the combination of satraplatin and erlotinib. Curr Drug Targets. 2014;15:1312-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Soria JC, Wu YL, Nakagawa K, Kim SW, Yang JJ, Ahn MJ, Wang J, Yang JC, Lu Y, Atagi S. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015;16:990-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 318] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 40. | Wu YL, Lee JS, Thongprasert S, Yu CJ, Zhang L, Ladrera G, Srimuninnimit V, Sriuranpong V, Sandoval-Tan J, Zhu Y. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol. 2013;14:777-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 249] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 41. | Yang JC, Kang JH, Mok T, Ahn MJ, Srimuninnimit V, Lin CC, Kim DW, Tsai CM, Barraclough H, Altug S. First-line pemetrexed plus cisplatin followed by gefitinib maintenance therapy versus gefitinib monotherapy in East Asian patients with locally advanced or metastatic non-squamous non-small cell lung cancer: a randomised, phase 3 trial. Eur J Cancer. 2014;50:2219-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Gao G, Ren S, Li A, Xu J, Xu Q, Su C, Guo J, Deng Q, Zhou C. Epidermal growth factor receptor-tyrosine kinase inhibitor therapy is effective as first-line treatment of advanced non-small-cell lung cancer with mutated EGFR: A meta-analysis from six phase III randomized controlled trials. Int J Cancer. 2012;131:E822-E829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 43. | Shih C, Chen VJ, Gossett LS, Gates SB, MacKellar WC, Habeck LL, Shackelford KA, Mendelsohn LG, Soose DJ, Patel VF. LY231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Res. 1997;57:1116-1123. [PubMed] |
| 44. | Peters GJ, Backus HH, Freemantle S, van Triest B, Codacci-Pisanelli G, van der Wilt CL, Smid K, Lunec J, Calvert AH, Marsh S. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim Biophys Acta. 2002;1587:194-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 282] [Article Influence: 12.3] [Reference Citation Analysis (0)] |