Published online Apr 10, 2016. doi: 10.5306/wjco.v7.i2.234
Peer-review started: June 15, 2015
First decision: September 17, 2015
Revised: October 29, 2015
Accepted: November 24, 2015
Article in press: November 25, 2015
Published online: April 10, 2016
Processing time: 300 Days and 12 Hours
The incidence of multifocal (MF) and multicentric (MC) carcinomas varies widely among clinical studies, depending on definitions and methods for pathological sampling. Magnetic resonance imaging is increasingly used because it can help identify additional and conventionally occult tumors with high sensitivity. However, false positive lesions might incorrectly influence treatment decisions. Therefore, preoperative biopsies must be performed to avoid unnecessary surgery. Most studies have shown higher lymph node involvement rates in MF/MC tumors than in unifocal tumors. However, the rate of local recurrences is usually low after breast conservative treatment (BCT) of MC/MF tumors. It has been suggested that BCT is a reasonable option for MC/MF tumors in women aged 50-69 years, with small tumors and absence of extensive ductal carcinoma in situ. A meta-analysis showed an apparent decreased overall survival in MC/MF tumors but data are controversial. Surgery should achieve both acceptable cosmetic results and negative margins, which requires thorough preoperative radiological workup and localization of lesions. Boost radiotherapy techniques must be evaluated since double boosts might result in increased toxicity, namely fibrosis. In conclusion, BCT is feasible in selected patients with MC/MF but the choice of surgery must be discussed in a multidisciplinary team comprising at least radiologists, surgeons and radiotherapists.
Core tip: Multicentric and multifocal breast tumors should be identified preoperatively in order to adapt surgical treatment. They might be associated with more frequent lymph node involvement and worse prognosis but in most studies, the rates of local recurrence are low and similar to those of unifocal tumors. Breast conservative treatment is a reasonable option in selected patients (age 50-69 years, small tumors and absence of extensive ductal carcinoma in situ). Postoperative radiotherapy, and especially boost radiotherapy must be discussed and evaluated due to the risk of increased toxicity in case of double boost.
- Citation: Houvenaeghel G, Tallet A, Jalaguier-Coudray A, Cohen M, Bannier M, Jauffret-Fara C, Lambaudie E. Is breast conservative surgery a reasonable option in multifocal or multicentric tumors? World J Clin Oncol 2016; 7(2): 234-242
- URL: https://www.wjgnet.com/2218-4333/full/v7/i2/234.htm
- DOI: https://dx.doi.org/10.5306/wjco.v7.i2.234
Multicentric (MC: At least 2 invasive tumors in 2 different quadrants) or multifocal (MF: At least 2 invasive tumors in the same quadrant) carcinoma can be diagnosed preoperatively or in resected specimens[1,2]. The frequency of these tumors ranges from 4% to 75%[3-10]. This large variability results from differences in the definitions used and the methods of pathologic sampling[11,12]. With continuous advances in preoperative imaging, the rate of MF and MC tumors is increasing[13-15].
Conservative surgery with radiotherapy has been widely accepted as an alternative to mastectomy in the management of early stage breast cancer[16,17], with a long-term local recurrence rate of approximately 15%-20%[16-28]. The diagnosis of multifocality may influence breast cancer management, particularly with regard to the choice of surgery. Conservative treatment as an alternative to mastectomy in patients with synchronous ipsilateral breast cancer is controversial and no consensus exists. MF/MC breast cancer is generally considered as a contraindication for conservative surgery because of concerns about an increased risk of local recurrence[29-32]. According to some reports, the local recurrence rate in MF/MC breast cancer after breast conservative therapy (BCT) is higher than that of unifocal tumors[31,33]. This is the main reason for excluding BCT for MF/MC breast cancer[34]. Moreover, poor cosmetic results, due to large resections, are also evoked. Therefore, many surgeons continue to perform mastectomy in patients with MF/MC breast cancer.
In contrast, extensive data have confirmed an excellent local control after BCT for unifocal breast cancer[16,17,35-43]. At the 12-year follow-up of the National Surgical Adjuvant Breast and Bowel Project B-06 trial, the cumulative incidence of ipsilateral breast recurrence was only 10% in the group treated by lumpectomy and breast irradiation[36].
The effectiveness of boost radiation treatment to decrease local recurrence has been established in a randomized trial by Bartelink et al[44]. However, in MF/MC breast cancer, the influence of boost radiation has been poorly reported. From a surgical point of view, BCT with negative margins and acceptable aesthetic outcome can be achieved if tumour foci are close enough to be resected as a single specimen[45].
One study has shown a significant association between positive surgical margins and failure of attempted BCT in the case of MF tumors[46].
This review will focus on the issue of conservative surgery with radiotherapy in the management of patients with MF/MC breast cancer.
MC carcinomas are defined by the presence of at least two invasive tumors in two different quadrants of the breast or in the same quadrant but at least 5 cm apart[1]. MF carcinomas are defined by the presence of several invasive tumors in the same quadrant of the breast or in different quadrants if the distance between foci is below 5 cm.
Multiple tumors are defined by the presence of synchronous, distinct, invasive tumors in the same breast, and comprise MC and MF carcinomas. They can be discovered in two different settings: (1) Preoperative diagnosis of at least 2 different invasive tumors, based on clinical and/or radiological findings; and (2) Histological diagnosis when pathological examination of surgical specimens shows several foci, while the tumor was considered as unifocal based on preoperative workup.
However, various situations must be considered according to the localization of multiple tumors in the different quadrants of the breast and to the distance from the nipple-areola complex[47].
In the meta-analysis published by Vera-Badillo et al[3], including 67557 patients, the rate of MC/MF tumors was 9.5% (6434 patients). In the EORTC 10981-22023 AMAROS trial, MF tumors of the same quadrant were included after 2008 and represented 33% of cases (342/1026)[4].
However, the prevalence of MC/MF tumors varies from 5% to 44% in published series[4-8], depending on the definition used, the method of histological examination of mastectomy specimens and the type of imaging used for diagnosis (Table 1).
| Ref. | Yr | MF/MC (n) | MF/MC (%) |
| NIH et al[81] | 1986 | 342 | 9 |
| Vlastos et al[82] | 2000 | 60 | 21 |
| 1Katz et al[83] | 2001 | 149 | 14 |
| Andea et al[62] | 2002 | 101 | 18 |
| 1Pedersen et al[84] | 2004 | 158 | 17 |
| EBCTCG[85] | 2005 | 1187 | 6 |
| Coombs et al[8] | 2005 | 94 | 11 |
| 1Litton et al[86] | 2007 | 58 | 19 |
| 1Joergensen et al[87] | 2008 | 945 | 13 |
| 1Cabioglu et al[88] | 2009 | 147 | 11 |
| 1Yerushalmi et al[11] | 2009 | 1554 | 6.1 |
| 1Weissenbacher et al[65] | 2010 | 288 | 5 |
| 1Tot et al[89] | 2011 | 148 | 30 |
| Tot et al[90] | 2011 | 225 | 44 |
| Rezo et al[91] | 2011 | 141 | 17 |
| 1Ustaalioglu et al[2] | 2012 | 107 | 15.4 |
| 1Lynch et al[63] | 2012 | 942 | 24 |
| 1Yerushalmi et al[5] | 2012 | 1187 | 6 |
| 1Chung et al[66] | 2012 | 164 | 14 |
| Meretoja et al[92] | 2012 | 206 | 20.6 |
| 1Pekar et al[93] | 2013 | 153 | 34 |
| Wolters et al[64] | 2013 | 1862 | 20.8 |
| Lynch et al[63] | 2013 | 906 | 24 |
| Hilton et al[94] | 2013 | 202 | 15 |
| van der Heiden-van der Loo et al[95] | 2013 | 1729 | 13.1 |
| Vera-Badillo et al[3] | 2014 | 6565 | 9.7 |
Mammography and ultrasound are the standard imaging tests for the diagnosis of breast cancer, and are also used to determine the extent of the disease within the affected breast. Because of its high sensitivity in breast cancer diagnosis and screening, magnetic resonance imaging (MRI) is being increasingly evaluated and used for preoperative local staging of breast cancer. Several multicenter trials showed that, in women with newly diagnosed breast cancer, MRI helped identify additional, conventionally occult lesions in 15%-27% of cases[48-51]. In addition, MR helped identify unsuspected synchronous cancer in the opposite breast in 3%-6% of women with a recent diagnosis of unilateral breast cancer[51]. However, the impact of breast MRI on breast cancer management is debated, due to a large number of additional benign lesions that could be detected and incorrectly influence clinical decisions[52,53]. Indeed, one of the major limitations of breast MRI is that false-positive enhancement may appear in benign lesions, resulting in a relatively low specificity[49]. If additional suspicious findings are identified, preoperative biopsies must be performed to limit the number of unnecessary wider excisions or mastectomies[54].
Although the meta-analysis of Vera-Bardillo et al[3] did not show differences in the rate of lymph node involvement, all the other studies demonstrated a higher rate in MC/MF tumors compared to unifocal tumors, with a mean difference of 10% to 20%.
The studies that reported lymph node detection showed the positivity of sentinel nodes in 42% to 59% of cases[4,55-62]. The main hypothesis to explain this higher rate is that the global tumor volume, that includes all MC/MF tumors, is usually more important than that of unifocal tumors. However, in MC/MF carcinomas, tumor size is determined by the largest index lesion regardless of the number and size of other lesions, which does not take into account the cumulative tumor volume.
In the EORTC 10981-22023 AMAROS trial, sentinel node involvement for MF and unifocal tumors respectively was the following: Macrometastases 61% (105/171) and 57% (109/192) (NS), micrometastases 30% (52/171) and 29% (55/192) (NS), isolated tumor cells 8% (13/171) and 14% (27/192) (P = 0.05)[4].
The rate of local recurrences for MC/MF tumors in case of conservative treatment is low, except in the 3 oldest studies (Table 2), and similar to that observed after conservative treatment of unifocal tumors. As for unifocal tumors, this rate depends on selection criteria, particularly resection in negative margins, age over 35 or 40 years and tumor phenotype (hormone receptors and HER2 status).
| Ref. | Yr | Patients | MF or MC | Median follow-up (mo) | Local recurrences, % |
| Leopold et al[32] | 1989 | 10 | MF/MC | 64 | 40 |
| Kurtz et al[31] | 1990 | 61 | MF/MC | 71 | 25 |
| Wilson et al[33] | 1993 | 13 | MF | 72 | 25 |
| Hartsell et al[71] | 1994 | 27 | MC | 53 | 3.7 |
| Nos et al[72] | 1999 | 56 | MF | 60 | 11 |
| Cho et al[73] | 2002 | 15 | MF/MC | 76 | 0 |
| Kaplan et al[74] | 2003 | 36 | MF/MC | 45 | 3 |
| Okumura et al[75] | 2004 | 34 | MF/MC | 58 | 0 |
| Oh et al[96] | 2006 | 97 | MF/MC | 66 | 6 |
| Gentilini et al[76] | 2008 | 476 | MF/MC | 73 | 5 |
| Lim et al[97] | 2009 | 147 | MF | 59 | 2 |
| Bauman et al[98] | 2010 | 22 | MF/MC | 42 | 4.5 |
| Chung et al[66] | 2012 | 164 | MF | 112 | 6.1 |
| Yerushalmi et al[5] | 2012 | 300 | MF/MC | 95 | 5.51 |
| Lynch et al[63] | 2013 | 256 | MF | 52 | 1.95 |
| Kadioğlu et al[99] | 2014 | 237 | MF | 46 | 5.2 |
| Kadioğlu et al[99] | 2014 | 36 | MC | 46 | 2 |
In the study by Lynch et al[63], published in 2013, the rate of local recurrences was determined for unifocal tumors (n = 2816) and for MC (n = 233) or MF (n = 673) tumors according to treatment, namely 256 BCT, 466 mastectomies without radiotherapy and 184 mastectomies followed by radiotherapy (PMRT). After a median follow-up of 52 mo, the rate of locoregional control was 99%, 96% and 98% for MF, MC and unifocal tumors respectively (P = 0.44). Subgroup analyses showed similar results for the three treatment strategies (BCT, mastectomy without radiotherapy or PMRT). In multivariate analysis, multicentricity/multifocality was not associated with decreased locoregional control. The authors concluded that BCT was a valid option for MC/MF carcinomas of the breast and that the presence of MC/MF alone is not an indication of PMRT.
In the study by Yerushalmi et al[5], local recurrence rate was determined after a median follow-up of 7.9 years and the authors compared the outcome of 11983 BCT (11683 unifocal tumors and 300 MC/MF tumors), and 7771 mastectomies (6884 unifocal tumors and 887 MC/MF tumors)[5]. One fourth of MC/MF patients had BCT (300/1187). MC/MF patients who benefited from BCT were aged 50 to 69 years, they had no extensive ductal carcinoma in situ (DCIS) and they had smaller tumors. Cumulative local recurrence rate at 10 years was 1) for BCT 4.6% (95%CI: 4.1, 5) in unifocal tumors vs 5.5% (95%CI: 2.6, 9.9) in MC/MF tumors, P = 0.76, 2) for mastectomies 5.8% (95%CI: 5.2, 6.5) for unifocal tumors vs 6.5% (95%CI: 4.7, 8.7) in MC/MF tumors, P = 0.77. In multivariate analysis, MC/MF was not significantly associated with recurrence or poor survival. In an additional matched analysis, recurrence rates were similar for MC/MF and unifocal tumors (P = 0.6). The authors concluded that BCT is a reasonable option in selected cases of MC/MF tumors, in particular in women aged 50-69 years, with small size tumors (< 1 cm) without extensive DCIS.
Wolters et al[64] compared recurrence free survival and overall survival in 8935 patients with 7073 unifocal tumors (79.2%), 1398 MF tumors (15.6%) and 464 MC tumors (5.2%). They did not show any difference in RFS (1) in MF tumors (T1/T2 treated according to guidelines) for BCT (n = 623) vs mastectomy (n = 319): HR = 1.25, (95%CI: 0.83-1.88), P = 0.284, and (2) in MC tumors after adjustment on tumor size in case of negative margins, for BCT (n = 60) vs mastectomy (n = 217): HR = 1.19, (95%CI: 0.48-2.97), P = 0.7 and vs mastectomy + PMRT, HR = 1.23, (95%CI: 0.51-3.00), P = 0.64.
In a study on 288 unifocal tumors matched with 288 MC/MF tumors the presence of MC/MF was significantly associated with decreased OS (P = 0.016), increased local recurrences (P = 0.001) and development of metastases (P = 0.038)[65].
In the study by Wolters et al[64], after adjustment on age, tumor size, grade and nodal status, no difference was shown in RFS or OS in patients who received adjuvant therapy according to guidelines in MC or MF tumors compared to unifocal tumors: (1) For MC carcinomas, no difference in RFS [HR = 0.88, (95%CI: 0.67-1.16), P = 0.35] and in OS [HR = 1.08, (95%CI: 0.85-1.36), P = 0.54]; and (2) for MF carcinomas, no difference in RFS [HR = 1.05, (95%CI: 0.89-1.24), P = 0.597] and in OS [HR = 0.92, (95%CI: 0.78-1.08), P = 0.28].
In the meta-analysis of Vera-Badillo et al[3], the impact on survival of MF/MC tumors was compared to that of unifocal tumors from 22 studies and 67557 patients (6565 MF/MC et 62326 unifocal tumors. In multivariate analysis, MC/MF tumors were associated with decreased OS (HR = 1.65, 95%CI: 1.07-2.52; P = 0.02), but the difference was not statistically significant in RFS (HR = 1.96, 95%CI: 0.94-4.12; P = 0.07). The authors concluded that MC/MF tumors seem to be associated with worse prognosis; however, the heterogeneity between studies did not allow an accurate determination of the real risk (one study alone, that differs from other studies, determined the shorter OS[66]).
In MC/MF carcinomas, the localization of tumors is of utmost importance to determine the type of resection allowing both favorable cosmetic results and negative margins. Types of incision and resections are determined according to the localization of tumors, the breast size, the degree of ptosis, the areola size and the distance from areola. In the last decade with the introduction of oncoplastic techniques, the surgical approach of MC tumors have changed. Oncoplastic techniques are therefore particularly adapted and valuable in this situation, achieving negative margins and a good cosmetic results better than conventional BCS; a schematic cartography of various possible situations and resection techniques[47] and a classification quadrant per quadrant atlas for many oncoplastic surgical procedures were proposed[67]. This strategy was applied to a consecutive series of 175 women with breast cancer who required mammoplasty, including 27 patients (15.4%) with MF tumors[68]. This study has confirmed that oncoplastic surgery techniques for breast cancer are associated with a low reoperation rate, a low risk of delay to adjuvant therapy and good cosmetic results. In an another study, Clough et al[69] reported 17.2% (10/58) of positive margins after oncoplastic surgery for MF breast cancer, without significant difference with positive margins rate after oncoplastic surgery for unifocal tumor (10.6%: 23/217).
Radiological workup and preoperative tracking are essential to perform appropriate resection with negative margins. The orientation and the identification of resection margins on surgical specimens, that sometimes have complex shapes and localizations, must be accurate and requires the collaboration of surgeons and pathologists. A completion resection might be necessary.
The benefits of a boost to the tumor bed have been demonstrated for invasive breast cancer treated with conservative surgery. However, extended boost, and more specifically boost fields for two locations in the breast, should be thoroughly evaluated because of possible toxicity and side effects, particularly fibrosis[70]. A preoperative consultation with radiotherapists should be proposed, if not recommended, when different boost fields are considered.
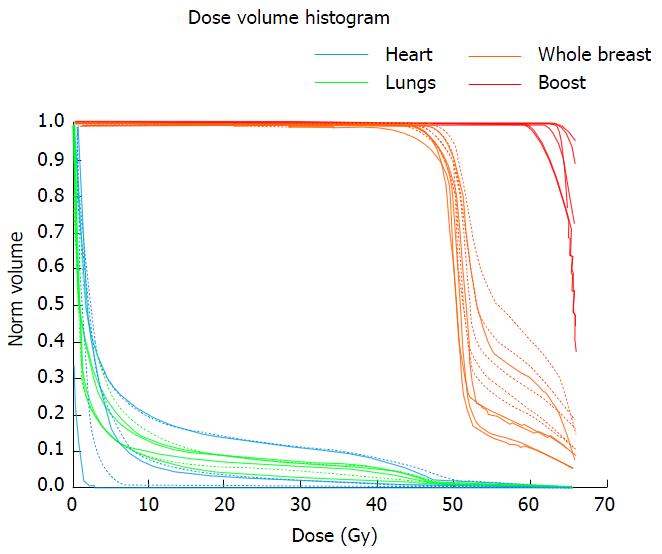
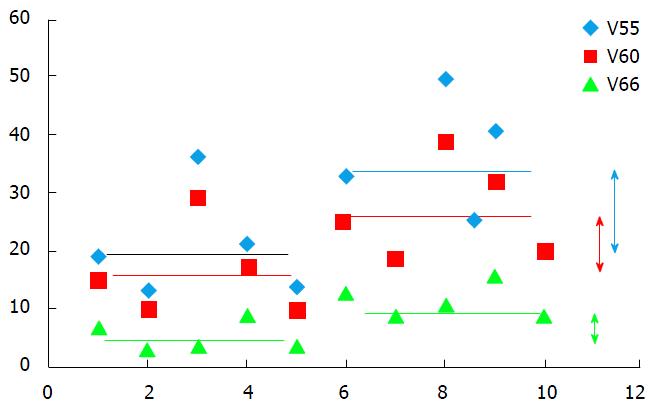
Fifteen studies have reported the outcomes of patients with multiple ipsilateral synchronous breast cancer treated with BCT followed by whole-breast irradiation (WBI)[11,31-33,66,71-79]. Most of these studies included patients treated for MF disease rather than MC disease and the patients were mostly operated with a single incision and therefore a single field designed for the boost[31-33,71,72]. BCT through double lumpectomy for MC disease raises the question of the safety of a double boost, regarding particularly the cosmetic result. Adding a boost after 50 Gy WBI increases the 10-year rate of severe fibrosis from 1.6% to 4.4% and of moderate fibrosis from 13% to 26%[80]. Increasing the volume of the boost may increase this risk resulting in a poor cosmetic outcome, which is however the goal of BCT. This is the reason why we conducted a dosimetric study to assess the volume of breast receiving an increased dose, in patients treated in a classical manner (50 Gy-whole-breast + 16 Gy-single boost) and in patients treated with a double boost. The dose levels investigated were 110% and 120% of the prescribed dose (V55 and V60), and V66 as 66 Gy was the dose prescribed to each boost volume. Adding a second boost resulted in a 14%-increase of the volume of breast receiving more than 55 Gy, (from 19% to 33%), a 10%-increase of the volume of breast receiving more than 60 Gy, (from 15% to 25%) and a 2 Gy-increase in the mean dose received by the ipsilateral whole breast (Figures 1 and 2). The clinical significance of this increased dose is unknown but is expected to be real and deserves evaluation. An alternative could be an intraoperative boost, which would allow the preservation of the surrounding structures (normal tissues).
Conservative treatment is a reasonable option in selected cases of MF or MC tumors. Radiological workup and preoperative evaluation of all tumor sites are essential. A multidisciplinary discussion should be mandatory, especially for distant localizations, involving above all surgeons, radiologists and radiotherapists.
The selection of patients with low risk of recurrence might be determined on the following criteria[11,47,76]: (1) Technical feasibility, acceptable planed cosmetic result; (2) patient’s choice after information about the risk of a new resection or mastectomy in cases of positive margins; (3) age > 40 years or > 50 years, absence of DCIS; (4) size of the largest lesion < 20 mm; and (5) feasibility of radiotherapy, including boost.
P- Reviewer: Johnson N, Rubio IT S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | van la Parra RF, de Roos WK, Contant CM, Bavelaar-Croon CD, Barneveld PC, Bosscha K. A prospective validation study of sentinel lymph node biopsy in multicentric breast cancer: SMMaC trial. Eur J Surg Oncol. 2014;40:1250-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Ustaalioglu BO, Bilici A, Kefeli U, Şeker M, Oncel M, Gezen C, Gumus M, Demirelli F. The importance of multifocal/multicentric tumor on the disease-free survival of breast cancer patients: single center experience. Am J Clin Oncol. 2012;35:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Vera-Badillo FE, Napoleone M, Ocana A, Templeton AJ, Seruga B, Al-Mubarak M, AlHashem H, Tannock IF, Amir E. Effect of multifocality and multicentricity on outcome in early stage breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;146:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Donker M, Straver ME, van Tienhoven G, van de Velde CJ, Mansel RE, Litière S, Werutsky G, Duez NJ, Orzalesi L, Bouma WH. Comparison of the sentinel node procedure between patients with multifocal and unifocal breast cancer in the EORTC 10981-22023 AMAROS Trial: identification rate and nodal outcome. Eur J Cancer. 2013;49:2093-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Yerushalmi R, Tyldesley S, Woods R, Kennecke HF, Speers C, Gelmon KA. Is breast-conserving therapy a safe option for patients with tumor multicentricity and multifocality? Ann Oncol. 2012;23:876-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Gallager HS, Martin JE. The study of mammary carcinoma by mammography and whole organ sectioning. Early observations. Cancer. 1969;23:855-873. [PubMed] |
| 7. | Holland R, Veling SH, Mravunac M, Hendriks JH. Histologic multifocality of Tis, T1-2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer. 1985;56:979-990. [PubMed] |
| 8. | Coombs NJ, Boyages J. Multifocal and multicentric breast cancer: does each focus matter? J Clin Oncol. 2005;23:7497-7502. [PubMed] |
| 9. | Fowble B, Yeh IT, Schultz DJ, Solin LJ, Rosato EF, Jardines L, Hoffman J, Eisenberg B, Weiss MC, Hanks G. The role of mastectomy in patients with stage I-II breast cancer presenting with gross multifocal or multicentric disease or diffuse microcalcifications. Int J Radiat Oncol Biol Phys. 1993;27:567-573. [PubMed] |
| 10. | Eeles R, Knee G, Jhavar S, Mangion J, Ebbs S, Gui G, Thomas S, Coppen M, A’hern R, Gray S. Multicentric breast cancer: clonality and prognostic studies. Breast Cancer Res Treat. 2011;129:703-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Yerushalmi R, Kennecke H, Woods R, Olivotto IA, Speers C, Gelmon KA. Does multicentric/multifocal breast cancer differ from unifocal breast cancer? An analysis of survival and contralateral breast cancer incidence. Breast Cancer Res Treat. 2009;117:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Egan RL. Multicentric breast carcinomas: clinical-radiographic-pathologic whole organ studies and 10-year survival. Cancer. 1982;49:1123-1130. [PubMed] |
| 13. | Wilkinson LS, Given-Wilson R, Hall T, Potts H, Sharma AK, Smith E. Increasing the diagnosis of multifocal primary breast cancer by the use of bilateral whole-breast ultrasound. Clin Radiol. 2005;60:573-578. [PubMed] |
| 14. | Sardanelli F, Giuseppetti GM, Panizza P, Bazzocchi M, Fausto A, Simonetti G, Lattanzio V, Del Maschio A. Sensitivity of MRI versus mammography for detecting foci of multifocal, multicentric breast cancer in Fatty and dense breasts using the whole-breast pathologic examination as a gold standard. AJR Am J Roentgenol. 2004;183:1149-1157. [PubMed] |
| 15. | Houssami N, Ciatto S, Macaskill P, Lord SJ, Warren RM, Dixon JM, Irwig L. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol. 2008;26:3248-3258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 593] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 16. | Fisher B, Redmond C, Poisson R, Margolese R, Wolmark N, Wickerham L, Fisher E, Deutsch M, Caplan R, Pilch Y. Eight-year results of a randomized clinical trial comparing total mastectomy and lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1989;320:822-828. [PubMed] |
| 17. | Veronesi U, Saccozzi R, Del Vecchio M, Banfi A, Clemente C, De Lena M, Gallus G, Greco M, Luini A, Marubini E. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med. 1981;305:6-11. [PubMed] |
| 18. | Haffty BG, Goldberg NB, Fischer D, McKhann C, Beinfield M, Weissberg JB, Carter D, Gerald W. Conservative surgery and radiation therapy in breast carcinoma: local recurrence and prognostic implications. Int J Radiat Oncol Biol Phys. 1989;17:727-732. [PubMed] |
| 19. | Solin LJ, Fowble B, Martz KL, Goodman RL. Definitive irradiation for early stage breast cancer: The University of Pennsylvania experience. Int J Radiat Oncol Biol Phys. 1988;14:235-242. [PubMed] |
| 20. | Stotter AT, McNeese MD, Ames FC, Oswald MJ, Ellerbroek NA. Predicting the rate and extent of locoregional failure after breast conservation therapy for early breast cancer. Cancer. 1989;64:2217-2225. [PubMed] |
| 21. | Recht A, Silen W, Schnitt SJ, Connolly JL, Gelman RS, Rose MA, Silver B, Harris JR. Time-course of local recurrence following conservative surgery and radiotherapy for early stage breast cancer. Int J Radiat Oncol Biol Phys. 1988;15:255-261. [PubMed] |
| 22. | Kurtz JM, Amalric R, Brandone H, Ayme Y, Jacquemier J, Pietra JC, Hans D, Pollet JF, Bressac C, Spitalier JM. Local recurrence after breast-conserving surgery and radiotherapy. Frequency, time course, and prognosis. Cancer. 1989;63:1912-1917. [PubMed] |
| 23. | Fourquet A, Campana F, Zafrani B, Mosseri V, Vielh P, Durand JC, Vilcoq JR. Prognostic factors of breast recurrence in the conservative management of early breast cancer: a 25-year follow-up. Int J Radiat Oncol Biol Phys. 1989;17:719-725. [PubMed] |
| 24. | Harris JR, Recht A, Schnitt S, Connolly J, Silver B, Come S, Henderson IC. Current status of conservative surgery and radiotherapy as primary local treatment for early carcinoma of the breast. Breast Cancer Res Treat. 1985;5:245-255. [PubMed] |
| 25. | Harris JR, Recht A, Amalric R, Calle R, Clark RM, Reid JG, Spitalier JM, Vilcoq JR, Hellman S. Time course and prognosis of local recurrence following primary radiation therapy for early breast cancer. J Clin Oncol. 1984;2:37-41. [PubMed] |
| 26. | Haffty BG, Fischer D, Rose M, Beinfield M, McKhann C. Prognostic factors for local recurrence in the conservatively treated breast cancer patient: a cautious interpretation of the data. J Clin Oncol. 1991;9:997-1003. [PubMed] |
| 27. | Clark RM, Wilkinson RH, Mahoney LJ, Reid JG, MacDonald WD. Breast cancer: a 21 year experience with conservative surgery and radiation. Int J Radiat Oncol Biol Phys. 1982;8:967-979. [PubMed] |
| 28. | Mate TP, Carter D, Fischer DB, Hartman PV, McKhann C, Merino M, Prosnitz LR, Weissberg JB. A clinical and histopathologic analysis of the results of conservation surgery and radiation therapy in stage I and II breast carcinoma. Cancer. 1986;58:1995-2002. [PubMed] |
| 29. | Danoff BF, Haller DG, Glick JH, Goodman RL. Conservative surgery and irradiation in the treatment of early breast cancer. Ann Intern Med. 1985;102:634-642. [PubMed] |
| 30. | Winchester DP, Cox JD. Standards for diagnosis and management of invasive breast carcinoma. American College of Radiology. American College of Surgeons. College of American Pathologists. Society of Surgical Oncology. CA Cancer J Clin. 1998;48:83-107. [PubMed] |
| 31. | Kurtz JM, Jacquemier J, Amalric R, Brandone H, Ayme Y, Hans D, Bressac C, Spitalier JM. Breast-conserving therapy for macroscopically multiple cancers. Ann Surg. 1990;212:38-44. [PubMed] |
| 32. | Leopold KA, Recht A, Schnitt SJ, Connolly JL, Rose MA, Silver B, Harris JR. Results of conservative surgery and radiation therapy for multiple synchronous cancers of one breast. Int J Radiat Oncol Biol Phys. 1989;16:11-16. [PubMed] |
| 33. | Wilson LD, Beinfield M, McKhann CF, Haffty BG. Conservative surgery and radiation in the treatment of synchronous ipsilateral breast cancers. Cancer. 1993;72:137-142. [PubMed] |
| 34. | Veronesi U. NIH consensus meeting on early breast cancer. Eur J Cancer. 1990;26:843-844. [PubMed] |
| 35. | Fisher B, Anderson S. Conservative surgery for the management of invasive and noninvasive carcinoma of the breast: NSABP trials. National Surgical Adjuvant Breast and Bowel Project. World J Surg. 1994;18:63-69. [PubMed] |
| 36. | Fisher B, Anderson S, Redmond CK, Wolmark N, Wickerham DL, Cronin WM. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1995;333:1456-1461. [PubMed] |
| 37. | Clark RM, McCulloch PB, Levine MN, Lipa M, Wilkinson RH, Mahoney LJ, Basrur VR, Nair BD, McDermot RS, Wong CS. Randomized clinical trial to assess the effectiveness of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer. J Natl Cancer Inst. 1992;84:683-689. [PubMed] |
| 38. | Haffty BG, Goldberg NB, Rose M, Heil B, Fischer D, Beinfield M, McKhann C, Weissberg JB. Conservative surgery with radiation therapy in clinical stage I and II breast cancer. Results of a 20-year experience. Arch Surg. 1989;124:1266-1270. [PubMed] |
| 39. | Jacobson JA, Danforth DN, Cowan KH, d’Angelo T, Steinberg SM, Pierce L, Lippman ME, Lichter AS, Glatstein E, Okunieff P. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med. 1995;332:907-911. [PubMed] |
| 40. | Mansfield CM, Komarnicky LT, Schwartz GF, Rosenberg AL, Krishnan L, Jewell WR, Rosato FE, Moses ML, Haghbin M, Taylor J. Ten-year results in 1070 patients with stages I and II breast cancer treated by conservative surgery and radiation therapy. Cancer. 1995;75:2328-2336. [PubMed] |
| 41. | Sarrazin D, Lê M, Rouëssé J, Contesso G, Petit JY, Lacour J, Viguier J, Hill C. Conservative treatment versus mastectomy in breast cancer tumors with macroscopic diameter of 20 millimeters or less. The experience of the Institut Gustave-Roussy. Cancer. 1984;53:1209-1213. [PubMed] |
| 42. | Stehlin JS, de Ipolyi PD, Greeff PJ, Gutierrez AE, Hardy RJ, Dahiya SL. A ten year study of partial mastectomy for carcinoma of the breast. Surg Gynecol Obstet. 1987;165:191-198. [PubMed] |
| 43. | Recht A. Selection of patients with early stage invasive breast cancer for treatment with conservative surgery and radiation therapy. Semin Oncol. 1996;23:19-30. [PubMed] |
| 44. | Bartelink H, Horiot JC, Poortmans P, Struikmans H, Van den Bogaert W, Barillot I, Fourquet A, Borger J, Jager J, Hoogenraad W. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med. 2001;345:1378-1387. [PubMed] |
| 45. | Schwartz GF, Veronesi U, Clough KB, Dixon JM, Fentiman IS, Heywang-Köbrunner SH, Holland R, Hughes KS, Margolese R, Olivotto IA. Proceedings of the Consensus Conference on Breast Conservation, April 28 to May 1, 2005, Milan, Italy. Cancer. 2006;107:242-250. [PubMed] |
| 46. | Kurniawan ED, Wong MH, Windle I, Rose A, Mou A, Buchanan M, Collins JP, Miller JA, Gruen RL, Mann GB. Predictors of surgical margin status in breast-conserving surgery within a breast screening program. Ann Surg Oncol. 2008;15:2542-2549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 47. | Patani N, Carpenter R. Oncological and aesthetic considerations of conservational surgery for multifocal/multicentric breast cancer. Breast J. 2008;16:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Liberman L, Morris EA, Kim CM, Kaplan JB, Abramson AF, Menell JH, Van Zee KJ, Dershaw DD. MR imaging findings in the contralateral breast of women with recently diagnosed breast cancer. AJR Am J Roentgenol. 2003;180:333-341. [PubMed] |
| 49. | Liberman L, Morris EA, Dershaw DD, Abramson AF, Tan LK. MR imaging of the ipsilateral breast in women with percutaneously proven breast cancer. AJR Am J Roentgenol. 2003;180:901-910. [PubMed] |
| 50. | Houssami N, Turner R, Morrow M. Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg. 2013;257:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 217] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 51. | Kuhl CK. Current status of breast MR imaging. Part 2. Clinical applications. Radiology. 2007;244:672-691. [PubMed] |
| 52. | Turnbull L, Brown S, Harvey I, Olivier C, Drew P, Napp V, Hanby A, Brown J. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet. 2010;375:563-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 437] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 53. | Peters NH, van Esser S, van den Bosch MA, Storm RK, Plaisier PW, van Dalen T, Diepstraten SC, Weits T, Westenend PJ, Stapper G. Preoperative MRI and surgical management in patients with nonpalpable breast cancer: the MONET - randomised controlled trial. Eur J Cancer. 2011;47:879-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 225] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 54. | Sardanelli F, Boetes C, Borisch B, Decker T, Federico M, Gilbert FJ, Helbich T, Heywang-Köbrunner SH, Kaiser WA, Kerin MJ. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer. 2010;46:1296-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 660] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 55. | Goyal A, Newcombe RG, Mansel RE, Chetty U, Ell P, Fallowfield L, Kissin M, Sibbering M. Sentinel lymph node biopsy in patients with multifocal breast cancer. Eur J Surg Oncol. 2004;30:475-479. [PubMed] |
| 56. | Ozmen V, Muslumanoglu M, Cabioglu N, Tuzlali S, Ilhan R, Igci A, Kecer M, Bozfakioglu Y, Dagoglu T. Increased false negative rates in sentinel lymph node biopsies in patients with multi-focal breast cancer. Breast Cancer Res Treat. 2002;76:237-244. [PubMed] |
| 57. | Meretoja TJ, Leidenius MH, Heikkilä PS, Joensuu H. Sentinel node biopsy in breast cancer patients with large or multifocal tumors. Ann Surg Oncol. 2009;16:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Bergkvist L, Frisell J, Liljegren G, Celebioglu F, Damm S, Thörn M. Multicentre study of detection and false-negative rates in sentinel node biopsy for breast cancer. Br J Surg. 2001;88:1644-1648. [PubMed] |
| 59. | Kumar R, Jana S, Heiba SI, Dakhel M, Axelrod D, Siegel B, Bernik S, Mills C, Wallack M, Abdel-Dayem HM. Retrospective analysis of sentinel node localization in multifocal, multicentric, palpable, or nonpalpable breast cancer. J Nucl Med. 2003;44:7-10. [PubMed] |
| 60. | Tousimis E, Van Zee KJ, Fey JV, Hoque LW, Tan LK, Cody HS, Borgen PI, Montgomery LL. The accuracy of sentinel lymph node biopsy in multicentric and multifocal invasive breast cancers. J Am Coll Surg. 2003;197:529-535. [PubMed] |
| 61. | Spillane AJ, Brennan ME. Accuracy of sentinel lymph node biopsy in large and multifocal/multicentric breast carcinoma--a systematic review. Eur J Surg Oncol. 2011;37:371-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Andea AA, Wallis T, Newman LA, Bouwman D, Dey J, Visscher DW. Pathologic analysis of tumor size and lymph node status in multifocal/multicentric breast carcinoma. Cancer. 2002;94:1383-1390. [PubMed] |
| 63. | Lynch SP, Lei X, Hsu L, Meric-Bernstam F, Buchholz TA, Zhang H, Hortobágyi GN, Gonzalez-Angulo AM, Valero V. Breast cancer multifocality and multicentricity and locoregional recurrence. Oncologist. 2013;18:1167-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 64. | Wolters R, Wöckel A, Janni W, Novopashenny I, Ebner F, Kreienberg R, Wischnewsky M, Schwentner L. Comparing the outcome between multicentric and multifocal breast cancer: what is the impact on survival, and is there a role for guideline-adherent adjuvant therapy? A retrospective multicenter cohort study of 8,935 patients. Breast Cancer Res Treat. 2013;142:579-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 65. | Weissenbacher TM, Zschage M, Janni W, Jeschke U, Dimpfl T, Mayr D, Rack B, Schindlbeck C, Friese K, Dian D. Multicentric and multifocal versus unifocal breast cancer: is the tumor-node-metastasis classification justified? Breast Cancer Res Treat. 2010;122:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 66. | Chung AP, Huynh K, Kidner T, Mirzadehgan P, Sim MS, Giuliano AE. Comparison of outcomes of breast conserving therapy in multifocal and unifocal invasive breast cancer. J Am Coll Surg. 2012;215:137-146; discussion 146-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Clough KB, Kaufman GJ, Nos C, Buccimazza I, Sarfati IM. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol. 2010;17:1375-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 416] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 68. | Clough KB, Ihrai T, Oden S, Kaufman G, Massey E, Nos C. Oncoplastic surgery for breast cancer based on tumour location and a quadrant-per-quadrant atlas. Br J Surg. 2012;99:1389-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 69. | Clough KB, Gouveia PF, Benyahi D, Massey EJ, Russ E, Sarfati I, Nos C. Positive Margins After Oncoplastic Surgery for Breast Cancer. Ann Surg Oncol. 2015;22:4247-4253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 70. | Khan SA. The many questions that surround multicentric and multifocal breast cancer. Breast J. 2010;16:219-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 71. | Hartsell WF, Recine DC, Griem KL, Cobleigh MA, Witt TR, Murthy AK. Should multicentric disease be an absolute contraindication to the use of breast-conserving therapy? Int J Radiat Oncol Biol Phys. 1994;30:49-53. [PubMed] |
| 72. | Nos C, Bourgeois D, Darles C, Asselain B, Campana F, Zafrani B, Durand JC, Clough K. [Conservative treatment of multifocal breast cancer: a comparative study]. Bull Cancer. 1999;86:184-188. [PubMed] |
| 73. | Cho LC, Senzer N, Peters GN. Conservative surgery and radiation therapy for macroscopically multiple ipsilateral invasive breast cancers. Am J Surg. 2002;183:650-654. [PubMed] |
| 74. | Kaplan J, Giron G, Tartter PI, Bleiweiss IJ, Estabrook A, Smith SR. Breast conservation in patients with multiple ipsilateral synchronous cancers. J Am Coll Surg. 2003;197:726-729. [PubMed] |
| 75. | Okumura S, Mitsumori M, Yamauchi C, Kawamura S, Oya N, Nagata Y, Hiraoka M, Kokubo M, Mise K, Kodama H. Feasibility of breast-conserving therapy for macroscopically multiple ipsilateral breast cancer. Int J Radiat Oncol Biol Phys. 2004;59:146-151. [PubMed] |
| 76. | Gentilini O, Botteri E, Rotmensz N, Da Lima L, Caliskan M, Garcia-Etienne CA, Sosnovskikh I, Intra M, Mazzarol G, Musmeci S. Conservative surgery in patients with multifocal/multicentric breast cancer. Breast Cancer Res Treat. 2009;113:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 77. | Kapoor NS, Chung A, Huynh K, Giuliano AE. Preliminary results: double lumpectomies for multicentric breast carcinoma. Am Surg. 2012;78:1345-1348. [PubMed] |
| 78. | Zervoudis S, Iatrakis G, Mares P, Boileau L, Grammatikakis I, Evangelinakis N, Daures JP, Leteuff I, Avgoulea A, Stefos T. Breast conserving surgery in multicentric breast cancer, preliminary data of our experience. Eur J Gynaecol Oncol. 2014;35:530-534. [PubMed] |
| 79. | Neri A, Marrelli D, Megha T, Bettarini F, Tacchini D, De Franco L, Roviello F. “Clinical significance of multifocal and multicentric breast cancers and choice of surgical treatment: a retrospective study on a series of 1158 cases”. BMC Surg. 2015;15:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 80. | Bartelink H, Horiot JC, Poortmans PM, Struikmans H, Van den Bogaert W, Fourquet A, Jager JJ, Hoogenraad WJ, Oei SB, Wárlám-Rodenhuis CC. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol. 2007;25:3259-3265. [PubMed] |
| 81. | National Institutes of Health Consensus Development Conference Statement: Adjuvant Chemotherapy for Breast Cancer. September 9-11, 1985. CA Cancer J Clin. 1985;36:42-47. [PubMed] |
| 82. | Vlastos G, Rubio IT, Mirza NQ, Newman LA, Aurora R, Alderfer J, Buzdar AU, Singletary SE. Impact of multicentricity on clinical outcome in patients with T1-2, N0-1, M0 breast cancer. Ann Surg Oncol. 2000;7:581-587. [PubMed] |
| 83. | Katz A, Strom EA, Buchholz TA, Theriault R, Singletary SE, McNeese MD. The influence of pathologic tumor characteristics on locoregional recurrence rates following mastectomy. Int J Radiat Oncol Biol Phys. 2001;50:735-742. [PubMed] |
| 84. | Pedersen L, Gunnarsdottir KA, Rasmussen BB, Moeller S, Lanng C. The prognostic influence of multifocality in breast cancer patients. Breast. 2004;13:188-193. [PubMed] |
| 85. | Early Breast Cancer Trialists’ Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687-1717. [PubMed] |
| 86. | Litton JK, Eralp Y, Gonzalez-Angulo AM, Broglio K, Uyei A, Hortobagyi GN, Arun B. Multifocal breast cancer in women & lt; or =35 years old. Cancer. 2007;110:1445-1450. [PubMed] |
| 87. | Joergensen LE, Gunnarsdottir KA, Lanng C, Moeller S, Rasmussen BB. Multifocality as a prognostic factor in breast cancer patients registered in Danish Breast Cancer Cooperative Group (DBCG) 1996-2001. Breast. 2008;17:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 88. | Cabioglu N, Ozmen V, Kaya H, Tuzlali S, Igci A, Muslumanoglu M, Kecer M, Dagoglu T. Increased lymph node positivity in multifocal and multicentric breast cancer. J Am Coll Surg. 2009;208:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 89. | Tot T, Gere M, Pekár G, Tarján M, Hofmeyer S, Hellberg D, Lindquist D, Chen TH, Yen AM, Chiu SY. Breast cancer multifocality, disease extent, and survival. Hum Pathol. 2011;42:1761-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 90. | Tot T, Pekár G. Multifocality in “basal-like” breast carcinomas and its influence on lymph node status. Ann Surg Oncol. 2011;18:1671-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 91. | Rezo A, Dahlstrom J, Shadbolt B, Rodins K, Zhang Y, Davis AJ. Tumor size and survival in multicentric and multifocal breast cancer. Breast. 2011;20:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | Meretoja TJ, Leidenius MH, Heikkilä PS, Boross G, Sejben I, Regitnig P, Luschin-Ebengreuth G, Žgajnar J, Perhavec A, Gazic B. International multicenter tool to predict the risk of nonsentinel node metastases in breast cancer. J Natl Cancer Inst. 2012;104:1888-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 93. | Pekar G, Hofmeyer S, Tabár L, Tarján M, Chen TH, Yen AM, Chiu SY, Hellberg D, Gere M, Tot T. Multifocal breast cancer documented in large-format histology sections: long-term follow-up results by molecular phenotypes. Cancer. 2013;119:1132-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 94. | Hilton JF, Bouganim N, Dong B, Chapman JW, Arnaout A, O’Malley F, Gelmon KA, Yerushalmi R, Levine MN, Bramwell VH. Do alternative methods of measuring tumor size, including consideration of multicentric/multifocal disease, enhance prognostic information beyond TNM staging in women with early stage breast cancer: an analysis of the NCIC CTG MA.5 and MA.12 clinical trials. Breast Cancer Res Treat. 2013;142:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 95. | van der Heiden-van der Loo M, Schaapveld M, Ho VK, Siesling S, Rutgers EJ, Peeters PH. Outcomes of a population-based series of early breast cancer patients with micrometastases and isolated tumour cells in axillary lymph nodes. Ann Oncol. 2013;24:2794-2801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 96. | Oh JL, Dryden MJ, Woodward WA, Yu TK, Tereffe W, Strom EA, Perkins GH, Middleton L, Hunt KK, Giordano SH. Locoregional control of clinically diagnosed multifocal or multicentric breast cancer after neoadjuvant chemotherapy and locoregional therapy. J Clin Oncol. 2006;24:4971-4975. [PubMed] |
| 97. | Lim W, Park EH, Choi SL, Seo JY, Kim HJ, Chang MA, Ku BK, Son B, Ahn SH. Breast conserving surgery for multifocal breast cancer. Ann Surg. 2009;249:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 98. | Bauman L, Barth RJ, Rosenkranz KM. Breast conservation in women with multifocal-multicentric breast cancer: is it feasible? Ann Surg Oncol. 2010;17 Suppl 3:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 99. | Kadioğlu H, Yücel S, Yildiz S, Bozkurt S, Ersoy YE, Sağlam E, Müslümanoğlu M. Feasibility of breast conserving surgery in multifocal breast cancers. Am J Surg. 2014;208:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |