Published online Dec 10, 2015. doi: 10.5306/wjco.v6.i6.299
Peer-review started: May 31, 2015
First decision: August 11, 2015
Revised: September 8, 2015
Accepted: October 23, 2015
Article in press: October 27, 2015
Published online: December 10, 2015
Processing time: 194 Days and 21.6 Hours
AIM: To investigate the mechanism of action of lipophilic antidepressant fluoxetine (FLX) in representative molecular subtypes of breast cancer.
METHODS: The anti-proliferative effects and mechanistic action of FLX in triple-negative (SUM149PT) and luminal (T47D and Au565) cancer cells and non-transformed MCF10A were investigated. Reverse phase protein microarray (RPPM) was performed with and without 10 μmol/L FLX for 24 and 48 h to determine which proteins are significantly changed. Viability and cell cycle analysis were also performed to determine drug effects on cell growth. Western blotting was used to confirm the change in protein expression examined by RPPM or pursue other signaling proteins.
RESULTS: The FLX-induced cell growth inhibition in all cell lines was concentration- and time-dependent but less pronounced in early passage MCF10A. In comparison to the other lines, cell growth reduction in SUM149PT coincided with significant induction of endoplasmic reticulum (ER) stress and autophagy after 24 and 48 h of 10 μmol/L FLX, resulting in decreased translation of proteins along the receptor tyrosine kinase/Akt/mammalian target of rapamycin pathways. The increase in autophagy marker, cleaved microtubule-associated protein 1 light chain 3, in SUM149PT after 24 h of FLX was likely due to increased metabolic demands of rapidly dividing cells and ER stress. Consequently, the unfolded protein response mediated by double-stranded RNA-dependent protein kinase-like ER kinase resulted in inhibition of protein synthesis, growth arrest at the G1 phase, autophagy, and caspase-7-mediated cell death.
CONCLUSION: Our study suggests a new role for FLX as an inducer of ER stress and autophagy, resulting in death of aggressive triple negative breast cancer SUM149PT.
Core tip: Our study demonstrates for the first time the complex but selective actions of Food and Drug Administration-approved, well-tolerated antidepressant drug known as fluoxetine (FLX) in malignant triple negative breast cancer (TNBC) cells. The significant reduction in cell growth of inflammatory TNBC line SUM149PT was a consequence of unfolded protein response induced by FLX and subsequent induction of autophagy and mitochondrial apoptosis, demonstrating the intricate crosstalk between endoplasmic reticulum and mitochondria in response to cellular stress. Combination of low dose FLX with existing regimen for TNBC may provide dual benefit of alleviating psychological distress, including depression and anxiety, and inducing death in aggressive tumor cells.
- Citation: Bowie M, Pilie P, Wulfkuhle J, Lem S, Hoffman A, Desai S, Petricoin E, Carter A, Ambrose A, Seewaldt V, Yu D, Ibarra Drendall C. Fluoxetine induces cytotoxic endoplasmic reticulum stress and autophagy in triple negative breast cancer. World J Clin Oncol 2015; 6(6): 299-311
- URL: https://www.wjgnet.com/2218-4333/full/v6/i6/299.htm
- DOI: https://dx.doi.org/10.5306/wjco.v6.i6.299
A major roadblock to effective breast cancer therapy is development of de novo or acquired resistance. Triple-negative breast cancer, which lacks the expression of steroid estrogen and progesterone receptors as well as overexpressed HER2, accounts for 15%-20% of all breast cancers. Majority of triple negative breast cancers (TNBCs) are basal-like, among the most aggressive types, likely to develop chemotherapy resistance, and lack suitable targeted therapeutics[1]. Resistance to apoptosis is often the mechanism by which these cancers evade death. Thus, an alternative approach to trigger cell death is greatly needed.
Autophagy is an example of alternative mechanism of cell death. However, this evolutionarily conserved process in response to metabolic stress typically leads to cell survival. Autophagy is a process in which damaged or long-lived proteins and organelles are encapsulated in double-membraned vesicles called autophagosomes, targeted for lysosomal degradation, and released into the cytosol as intermediate metabolites for nutrient recycling and ATP production[2]. While evidence has been limited, autophagic cell death has been shown in cells with deficient apoptotic proteins[3,4], upregulated mitochondrial cell death protein BNIP3[5], and deficient tumor suppressor Von Hippel-Lindau[6]. The pro-death function of autophagy is believed to be due to prolonged digestion of cellular components or selective digestion of survival (over death) factors.
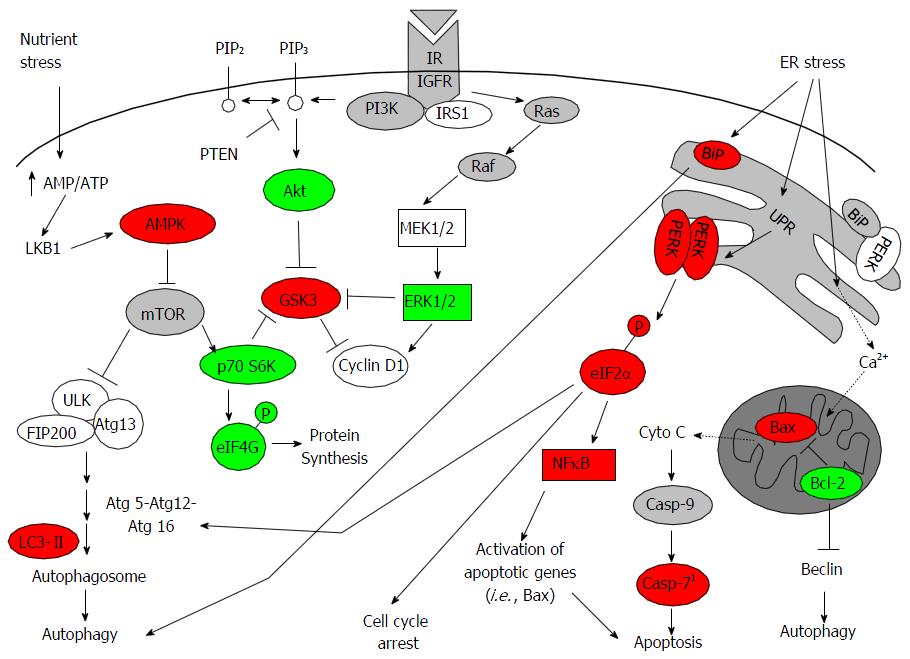
NFκB regulates diverse cellular processes in response to numerous stimuli, including unfolded protein response (UPR) as a result of oxidative and metabolic stress[7,8]. UPR is induced when there is a buildup of unfolded, misfolded or damaged proteins within the endoplasmic reticulum [i.e., endoplasmic reticulum (ER) stress]. The goal of UPR is to stop general protein synthesis but allow selective synthesis of ER chaperones, such as binding immunoglobulin protein (BiP), to restore balance[9]. ER stress can directly induce autophagy through upregulation of BiP, which is required for autophagosome formation[10]. The repressive effect of BiP on UPR signal transducers, such as double-stranded RNA-dependent protein kinase-like ER kinase (PERK), inositol-requiring enzyme 1 alpha and activating transcription factor 6, is released during ER stress[11]. If proper protein folding capacity is not restored, then all three arms of UPR induce CCAAT/enhancer binding protein-homologous protein (CHOP) and growth arrest and DNA damage 34 (GADD34) to stimulate apoptosis. In some situations, autophagy is induced to promote cell survival by removal of accumulated ubiquitinylated proteins and aggregates[12]. Together, these studies demonstrate the integration of signals from autophagy, ER stress/UPR, and apoptosis in regulating cell survival or cell death.
The anti-cancer properties of widely used antidepressants, specifically the selective serotonin reuptake inhibitors (SSRIs), have received attention in the last two decades. Fluoxetine (FLX) was the first approved SSRI for depression, and it is still used today across a diverse population, including in many cancer patients, for the treatment of anxiety and/or depression. FLX is a well-tolerated drug with a mild side effect profile, safe in overdose, and almost no associated withdrawal symptoms, even when compared to other SSRIs[13]. Like most SSRIs, FLX blocks the reuptake of serotonin (5-HT) at the pre-synaptic membrane, enhancing the actions of 5-HT on serotonin receptors at the post-synaptic neuron[14]. But FLX is known to have various off-target interactions, resulting in modulation of cancer cell growth. For example, a single study in rodents suggested that FLX stimulates malignant cell growth[15]. However, multiple epidemiological studies have shown no association between SSRI use and breast cancer risk[16,17]. Previous studies have shown FLX-induced cell death in a variety of malignant cell lines, including those originating from the prostate, colon, lung, ovary, breast, brain, and the immune system[3,18-24]. One study has implicated the inhibition of the extracellular signal regulated kinase 1 and 2 (ERK1/2) as a potential consequence of FLX’s anti-tumor effect[20]. However, the exact role of FLX in modulating ERK1/2 pathway in breast cancer subtypes is currently unknown.
In this study, distinct molecular subtypes of breast-derived cell lines, including triple-negative (SUM149PT) and luminal (T47D and Au565) breast cancer cells as well as non-transformed MCF10A, were evaluated for their response to FLX exposure in regards to protein expression of key components of signaling pathways that mediate cell growth, survival and death. Our study demonstrates that the cell growth inhibition in rapidly dividing TNBC SUM149PT is due to ER and metabolic stress that leads to decreased translation of proteins along the RTK/Akt/mTOR and MEK/ERK pathways. Excessive ER stress and autophagy induced by FLX in SUM149PT eventually leads to cell death mediated by executioner caspase-7. Given this proposed anti-proliferative mechanism and safety profile, FLX may prove an ideal part of a targeted regimen against TNBC in future in vivo and clinical studies.
Tissue cell culture media, FBS, horse serum, and 2X Tris-glycine SDS loading buffer were obtained from Life Technologies. Insulin, hydrocortisone, epidermal growth factor (EGF), FLX, MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide], and bovine serum albumin were from Sigma. Cholera toxin was obtained from Calbiochem. Mammary epithelial growth medium bullet kit was purchased from Lonza. Tissue protein extraction reagent (T-PER), bicinchoninic acid (BCA) assay, and SuperSignal West Dura chemiluminescent substrate were from Thermo Fisher Scientific. The complete protease and phosphatase (PhosSTOP) inhibitors were obtained from Roche Applied Science. Primary antibodies for Western blotting were as follows: LC3B (ab48394) from Abcam; ERK1/2 T202/Y204 (4370), AMPKα T172 (2535), p70 S6 Kinase (p70 S6K) T389 (9234), LC3B (3868), BiP (3177), PERK (5683), eIF2α Ser-51 (3398), PARP (9542), μ-Calpain (2556) from Cell Signaling Technology; β-actin (sc-47778), GADD34 (sc-8327), GADD153/CHOP (sc-575) from Santa Cruz Biotechnology; caspase-12 (PRS3195) from Sigma-Aldrich.
Most breast cancer cell lines were obtained from American Type Culture Collection (Manassas, VA). T47D cells were cultured in alpha MEM prepared as previously described[25]. SUM149PT cells were originally obtained from Dr. Stephen Ethier (Karmanos Cancer Institute, Detroit, MI) and are commercially available (Asterand, Detroit, MI). SUM149PT cells were maintained in Ham’s F12 supplemented with 5% FBS, 5 mg/mL insulin, and 1 mg/mL hydrocortisone. Au565, BT474, and lapatinib-resistant BT474 (R-BT474) cell lines were maintained in RPMI 1640 supplemented with 10% FBS and 2 mmol/L L-glutamine. R-BT474 cell line was kindly provided by Dr. Neil Spector (Duke University Medical Center, Durham, NC). MCF10A lines were cultured in two different media. MCF10A late passage cells were maintained in HuMEC complete media, while MCF10A early passage cells (generous gift of Dr. David Beebe, University of Wisconsin, Madison, WI) were grown in DMEM-F12 supplemented with 5% horse serum, 20 ng/mL EGF, 0.5 μg/mL hydrocortisone, 100 ng/mL cholera toxin, and 10 μg/mL insulin. Normal human mammary epithelial cells (HMEC) were obtained from Lonza and grown in HuMEC complete media. DKAT cell line is unique to our laboratory and maintained in supplemented MEBM[26]. All cell lines were maintained in a humidified atmosphere of 5% CO2 at 37 °C and have been authenticated by DNA fingerprinting at the Duke University Cell Culture Facility.
The aforementioned cell lines were grown in 100 mm dishes for 24 h, followed by addition of FLX at a final concentration of 10 μmol/L and cell harvest after 24 h and 48 h of treatments. The indicated FLX concentration was previously tested in another breast cancer cell line[22] and served as a starting point for our proteomic study. Untreated (control) cells were run in parallel. This experiment was performed at least three different times Briefly, adherent cells were washed twice in cold 1 × PBS and lysed directly in dishes on ice with modified T-PER buffer as previously described[27]. Following centrifugation at 3000 g for 5 min at 4 °C, each supernatant was transferred to clean microcentrifuge tubes. After determining the total protein content by BCA protein assay, samples were diluted in 2 × Tris-glycine SDS sample buffer with 2.5% 2-mercaptoethanol up to 2 mg/mL and boiled for 8 min. Samples were spun briefly and then stored at -80 °C until they were shipped in dry ice to George Mason University where subsequent lysate printing in triplicate, immunostaining, and reverse phase protein microarray (RPPM) analysis were performed. For this study, we examined the expression of 79 phosphorylated, total, and cleaved proteins that are thought to play a role in breast cancer cell proliferation, survival, apoptosis, and metastasis. Enumerated are some antibodies used in the experiments: Akt S473 (9271), ERK1/2 T202/Y204 (9101), GSK-3α/β S21/S9 (9331), AMPKβ1 S108 (4181), mTOR S2448 (2971), p70 S6K T389 (9205), eukaryotic translation initiation factor 4G (eIF4G) S1108 (2441), NFκB p65 S536 (3031), Bax (2772), Bcl-2 S70 (2827), cleaved Casp-7 D198 (9491), cleaved Casp-3 D175 (9661), E-cadherin (4065), Vimentin (3295), Snail (4719), and SAPK/JNK T183/Y185 (9251) from Cell Signaling Technology; GSK-3α/β Y279/Y216 (44-604) from BioSource; IκBα S32/S36 (551818) from BD.
Cell viability or growth was measured by the MTT assay. Cells were seeded in triplicate at 1.8 × 104 per well in 1 mL complete media in 12-well plates and grown at 37 °C for 24 h. Subsequently, 10 μmol/L FLX was added in the cell media and incubated at the indicated time points. Day 0 reading was done at the same time as treatment was added. The MTT assay was carried out as follows: MTT solution was added at a final concentration of 0.5 mg/mL and incubated in the dark at 37 °C for 2 h. The reaction was stopped by adding Solubilization solution (95% DMSO/5% 1 × PBS), and absorbance values were determined at 560 nm on the Modulus microplate reader (Turner Biosystems). All MTT assays were performed at least two independent times.
Cell lines were seeded in 100 mm dishes at 2.6 × 105, followed by treatment with and without fluoxetine after 24 h. Cells were grown at the indicated FLX concentration and time points, harvested and lysed in radio-immmunoprecipitation assay buffer[28] containing phosphatase and protease inhibitor cocktails, and centrifuged at 14000 rpm for 10 min. The resulting supernatants (whole cell lysates) were assayed via BCA to determine total protein content and stored at -80 °C until use. Cell lysates were solubilized in reducing sample buffer, boiled, electrophoresed on Bis-Tris gel (Life Technologies), and transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories) The membranes were blocked and incubated with primary antibodies overnight at 4 °C, washed 3 × in TBS with 0.1% Tween 20, incubated with horseradish peroxidase-conjugated secondary antibody, and detected by enhanced chemiluminescence.
Cells were seeded at 2.6 × 105 in 100 mm dishes. After 24 h, FLX was added at a final concentration of 10 μmol/L. Cells were harvested at indicated time points after treatment. Briefly, floating cells were retained and combined with trypsinized cells. Cells were spun down, washed with 1 × PBS, fixed in ice-cold 70% ethanol. Propidium iodide (PI) staining was performed as follows. Briefly, ethanol was removed and the cell pellets were resuspended in 1 × PBS containing 15-25 μg RNase A (Roche) and incubated for 30 min at 37 °C. PI stain (2 mg/mL) was added to each sample at a final concentration of 100 μg/mL. Cells were sorted on a BD FACSCalibur using CellQuest software.
Data were analyzed with SAS Enterprise Guide 5.1 software (Cary, NC) and represented as the mean ± standard error. Two-sample t-test was used when comparing control and treated groups. To identify which proteins were differentially expressed in cell lines as a result of FLX treatment, changes in protein levels due to treatment were evaluated as percentages relative to control via 1-way ANOVA with Tukey adjustment for multiple comparison. P-values < 0.05 were considered statistically significant. Unsupervised hierarchical clustering analysis of the log 2-transformed proteomic data was carried out using the Ward method in JMP v5.1 (SAS Institute). GraphPad Prism 6 (San Diego, CA) was used to fit curves to concentration-dependent and time-dependent cell viability (growth) data, and the IC50 values were determined from these generated curves.
While the anti-proliferative and apoptotic effects of FLX in various malignant cell lines have been demonstrated, there appears to be no common signaling pathway(s) modulated by this drug. Given the heterogeneity of breast cancer, we hypothesized that the mechanism of action of FLX would be different for each subtype of breast cell line. Here, we examined the expression levels of several proteins encompassing the broad growth factor-mediated signaling and apoptotic pathways by high-throughput RPPM. We performed RPPM on basal normal (HMEC-15 and late passage MCF10A), triple-negative (SUM149PT and DKAT), luminal A (T47D), luminal B (BT-474 and R-BT474), and HER2+ (Au565) cell lines. The unsupervised hierarchical clustering analysis segregated the samples into distinct clusters of luminal (R-BT474, BT474, Au565, T47D) and basal-like cell lines (HMEC, MCF10A, SUM149PT, and DKAT) (Figure S1), which are consistent with the gene expression analysis of breast cancer cell lines[29]. There was also a distinct clustering of the HER2+ cell lines (R-BT474, BT474, and Au565) as previously described[30]. In contrast, there was no clear partitioning of samples into treatment groups (i.e., control vs FLX).
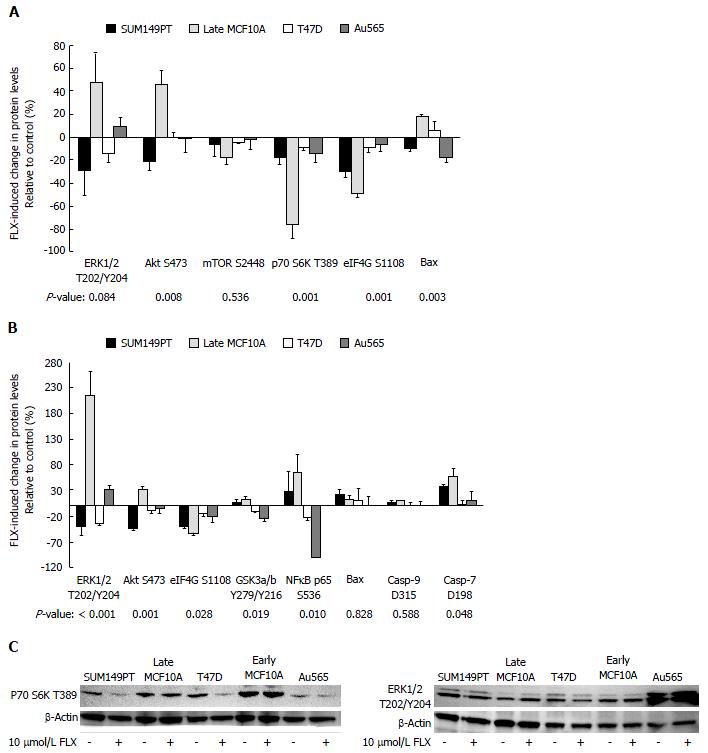
Next, we determined the effects of 10 μmol/L FLX treatment on protein expression changes across cell lines by one-way ANOVA. Here, we limit our statistical analysis to triple-negative SUM149PT, luminal T47D, HER2+ Au565, and normal late passage (Late) MCF10A. Given the mixed population of HMEC-15 (i.e., cobblestone vs large, flattened morphology) that we encountered during RPPM lysate preparation, Late MCF10A data were used in our analysis. As Figure 1A indicates, the FLX-induced changes in protein levels of Akt S473, p70 S6K T389, and eIF4G S1108 were significantly different across cell lines after 24 h. These proteins are components of the RTK/Akt/mTOR pathway, which plays a vital role in cell proliferation, growth, and survival.
The Akt S473 levels in SUM149PT consistently decreased after 24 h and 48 h of FLX treatment, while the expression in Late MCF10A cells increased (Figure 1). For both T47D and Au565 cells, treatment resulted in no change in baseline Akt S473 at 24 h, but a small decrease in protein levels after 48 h. Interestingly, SUM149PT showed an increase in activated glycogen synthase kinase 3 (GSK3)α/β Y279/Y216 after 48 h of treatment. This effect could be explained by a decrease in activity of Akt, which would otherwise inactivate GSK3. In contrast, the increased GSK3α/β Y279/Y216 levels in Late MCF10A may be attributed to some other mechanism, which remains to be determined.
Downstream of Akt are important regulators of protein synthesis, namely the p70 S6K and eIF4G. In all cell lines, FLX induced a decrease in both proteins at 24 h, suggesting inhibition of translation (Figure 1A). Although not statistically significant, the activation of master regulator of cell growth, mTOR S2448, was inhibited by FLX in all cell lines, which is consistent with decreased p70 S6K activity. The translational inhibition was sustained up to 48 h as evidenced by a decrease in eIF4G S1108 (Figure 1B) as well as p70 S6K T389 by Western blot (Figure 1C).
To date, only few studies have shown FLX-mediated inhibition of ERK, and this effect appears to be cell type-dependent[20,31]. Here, we showed that after 48 h, FLX had different effects on ERK1/2 T202/Y204 level of each breast cell line (Figure 1B). SUM149PT and T47D showed inhibition of ERK1/2 after 48 h of treatment, while Late MCF10A and Au565 showed ERK1/2 activation, which we also confirmed by Western blot (Figure 1C).
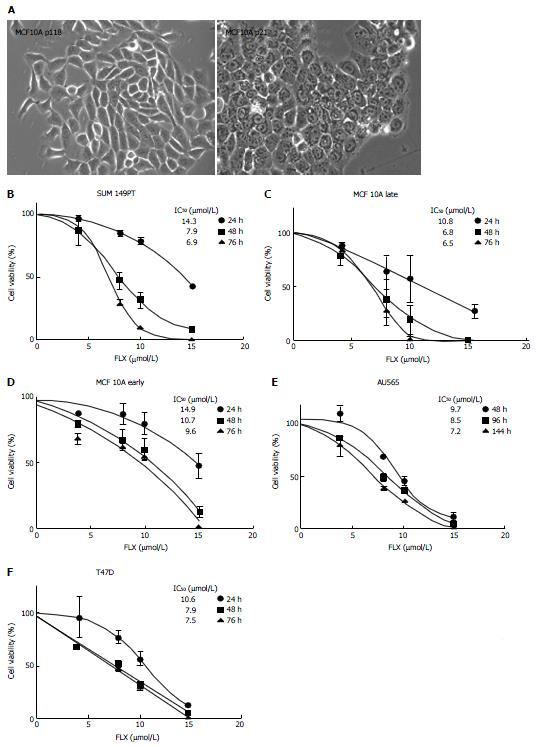
Several groups have shown that FLX treatment can lead to growth inhibition or death of cancer cells, although not much is known about its effect on breast cancer subtypes. We assessed the effect of FLX on cell viability or growth of the aforementioned cell lines, using various concentrations at different times. Although MCF10A originated from the same mastectomy fibrocystic diseased tissue, several variation of this cell line exist[32]. Initially, we obtained the Late MCF10A cells, which are spindle in shape (Figure 2A). A lower passaged MCF10A cells (Early MCF10A) were also obtained, and the derived epithelial cells have cobblestone morphology. In comparison to untreated (control) cells, FLX treatment reduces cell growth in a time-dependent and dose-dependent manner, with the biggest changes in IC50 occurring between 24 h and 48 h for most cell lines (Figure 2B-F). At 48 h, IC50 ranges from 6.8-10.7 μmol/L across cell lines. In our subsequent analysis and experiments, we used a fixed dose of 10 μmol/L to assess the mechanism of action of FLX.
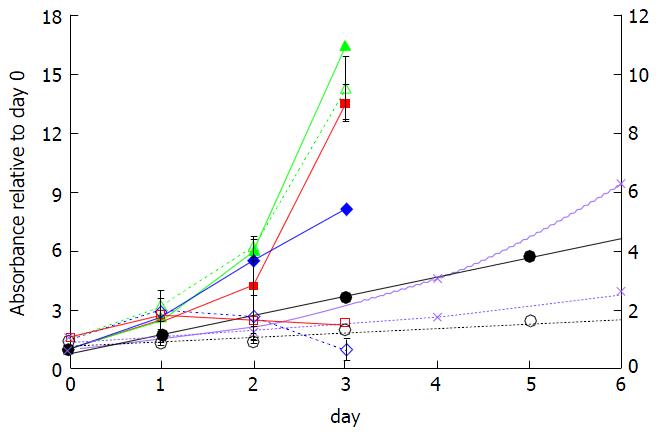
In comparison to control cells, the FLX-treated SUM149PT and Late MCF10A showed a decreased ability to reduce MTT to formazan crystals, suggesting significant cell growth inhibition by 48 h (Figure 3, red and blue solid lines vs red and blue dotted lines). In contrast, the treated Early MCF10A continued to grow albeit at a slower rate than control cells (green dotted vs solid lines) even after 48 h of treatment. The FLX-treated T47D and Au565 cells also showed cell growth reduction, which was greater than treated Early MCF10A (Figure 3, compare black and purple dotted lines vs green dotted lines). The effects of FLX on T47D and Au565 over time suggest that the drug is acting as a cytostatic rather than cytotoxic agent.
In this study, we showed that both rapidly dividing SUM149PT and Late MCF10A cells are most sensitive to FLX-induced cell growth inhibition (Figure 3). The decreased protein synthesis in both cell lines at 24 h of FLX treatment (Figure 1, p70 S6K and eIF4G) suggests altered energy metabolism, which can contribute to cell growth inhibition and even cell death.
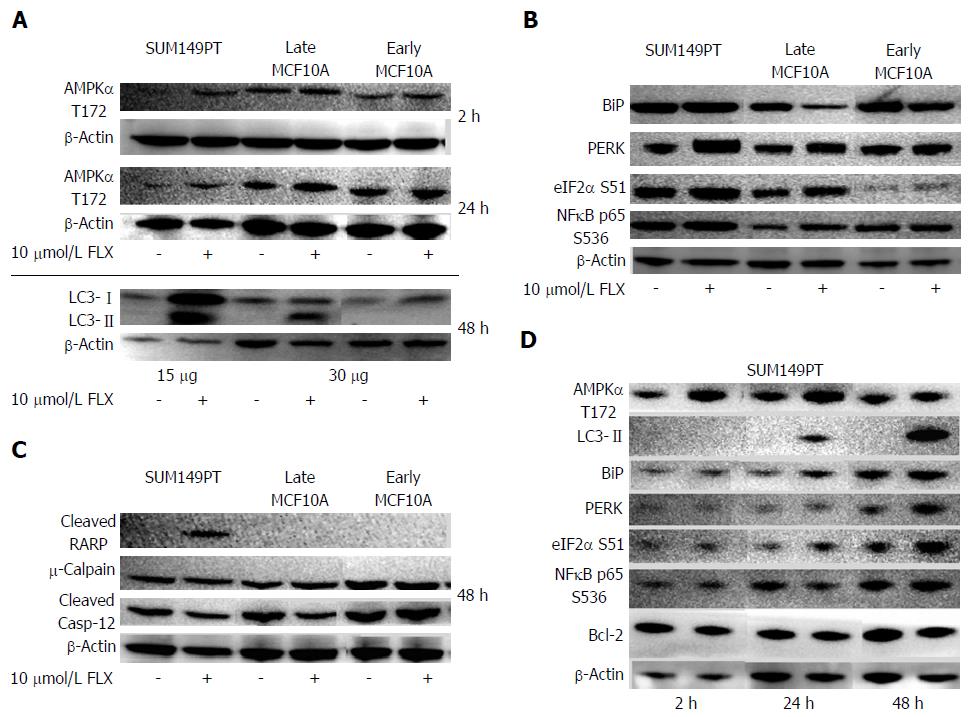
Our RPPM data suggested that mTOR activity was inhibited by FLX by 24 h (Figure 1A). Inhibition of mTOR is mediated by adenosine monophosphate kinase (AMPK) during low cellular energy status or stress, which is then followed by autophagy[2,9,33]. We confirmed by Western blot that the central metabolic sensor AMPK was activated in FLX-treated SUM149PT and Late MCF10A as early as 2 h and up to 24 h, suggesting metabolic stress in these cells (Figure 4A). Next, we examined the expression of cleaved microtubule-associated protein 1 light chain 3 (LC3-II), which is required for autophagosome transport and maturation as well as a well-accepted monitor of autophagy[2,9]. After 24 h of FLX treatment, only SUM149PT showed increased level of LC3-II (data not shown). By 48 h, the LC3-II level in SUM149PT was significantly elevated compared to Late MCF10A (Figure 4A). Meanwhile, autophagy was not induced in Early MFCF10A.
Induced cellular stress results in orchestration of several processes that dictate whether cells live or die. These processes include autophagy, ER stress, and apoptosis. The link between these processes has been elucidated only in the past 9 years. An important regulator and sensor of ER stress is BiP, which maintains proper protein folding and helps restore misfolded proteins[34]. In our study, only SUM149PT showed an apparent increase in BiP following exposure to FLX (Figure 4B). But UPR was induced in both FLX-treated SUM149PT and Late MCF10A, as indicated by an increase in PERK-mediated activation of translation initiation factor eIF2α at S51. Activation of eIF2α has been shown to increase NFκB activity as well as inhibit protein synthesis[35,36]. Both RPPM and Western blot data indicated an increase in NFκB activity in SUM149PT and Late MCF10A after 48 h of FLX treatment (Figure 1B and 4B). The increased eIF2α S51 levels in both cell lines were also consistent with the inhibition of eIF4G-mediated protein synthesis (Figure 1B). Meanwhile, the Early MCF10A did not show further increase in BiP, PERK, eIF2α S51, and NFκB p65 S536 (Figure 4C), suggesting no appreciable UPR in these normal cells with FLX treatment.
Although previous study has linked the activation of NFκB to autophagy through modulation of essential autophagy gene Beclin-1[37], our study did not show a change in basal Beclin-1 protein levels with FLX treatment (data not shown). This suggests that Beclin-1 expression in mammary epithelial cells is not dependent on NFκB activation. However, a plausible link between UPR and autophagy induction in FLX-treated SUM149PT is BiP, which has been shown to be necessary in the maturation step of autophagic vesicle (Figure 5), downstream of Beclin-mediated membrane nucleation[9,10].
Cells undergoing prolonged autophagy and ER stress eventually succumb to death. Our RPPM data indicated an increase in pro-apoptotic Bax in Late MCF10A after 24 h of FLX (Figure 1A) as well as activated caspase-7 at 48 h in both SUM149PT and Late MCF10A (Figure 1B), suggesting that both cell lines undergo apoptosis. We confirmed caspase-7 activity by monitoring PARP cleavage, which was significant only in SUM149PT after 48 h of FLX treatment (Figure 4C). The lack of cleaved PARP in treated Late MCF10A does not necessarily correlate with inactive caspase-7. Rather, PARP cleavage site is either modified in this particular cell line or inaccessible by the antibody used in the Western blot analysis. Meanwhile, FLX did not induce apoptosis in Early MCF10A.
During ER stress, calcium released into the cytoplasm may enter the mitochondria to induce the intrinsic pathway to apoptosis[38]. Few members of the caspase family have been implicated in ER stress conditions. ER-resident procaspase-12 is cleaved or activated by protease calpain in response to calcium release[39]. Another study suggested that translocation of caspase-7 from the cytoplasm to the ER can cleave caspase-12 and mediate cell death[40]. In our study, there were no significant changes in calpain and cleaved caspase-12 levels in any of the cell lines with FLX treatment (Figure 4C). This data suggests that the observed FLX-induced cell death in SUM149PT and Late MCF10A is mediated through the mitochondrial apoptotic pathway.
To closely determine the effects of FLX treatment in the aggressive TNBC line SUM149PT, the protein levels of the aforementioned regulators of ER stress, UPR, autophagy, and apoptosis were monitored at different times by Western blots. As Figure 4D indicated, the metabolic sensor AMPK was activated as early as 2 h of FLX treatment. Modest but increased expression levels of BiP, eIF2α S51, and LC3-II at 24 h indicated concurrent induction of UPR and autophagy, which were sustained up to 48 h. As a consequence of excessive ER stress, cell death mediated by mitochondrial apoptosis ensues. The increase in caspase-7 activation, as monitored by PARP cleavage (Figure 4C), coincided with a decrease in anti-apoptotic Bcl-2 level (Figure 4D). In FLX-treated SUM149PT, we would expect that the negative regulation of Beclin by Bcl-2 at the ER surface would be negligible and further support autophagy induction, given the role of Beclin in membrane nucleation[38].
Although FLX-treated SUM149PT showed UPR at 48 h (Figure 4D), we could not detect levels of CHOP and GADD34 that are known to promote apoptosis as a result of prolonged ER stress (data not shown). The absence of these proteins suggests instability, as previously shown in mouse embryonic fibroblasts (MEFs) that have been treated with classical inducers of ER stress, such as thapsigargin (TG) and tunicamycin (TM)[41]. In this study, Rutkowski et al[41] demonstrated that CHOP was rapidly degraded with a half-life of 4 h or less, while BiP expression was robust with a half-life of about 48 h. Lack of CHOP expression in the time points tested in our study was not surprising. Given the high dose of FLX (10 μmol/L) used in SUM149PT compared to the low dose of TG (2.5 nmol/L) or TM (30 nmol/L) in MEFs suggests that FLX is a less potent inducer of UPR than either TG or TM. Comparison of CHOP and GADD34 protein levels in an in vivo model treated with either classical UPR inducers or FLX will be an important future direction of this study.
Cells that undergo UPR are subjected to global translation repression and cell cycle arrest[42]. To determine which phase of the cell cycle is modulated by 10 μmol/L FLX treatment, we performed FACS analysis on cells that undergo significant UPR and apoptosis. T47D cells were used for comparison, given the cytostatic (vs cytotoxic) effect of FLX in this cell line (Figure 3). As Table 1 indicates, the proportion of SUM149PT and Late MCF10A cells entering the DNA synthesis (S) and mitosis (G2/M) phase decreased significantly with time, along with an increase in the growth (G1) phase. Meanwhile, FLX treatment in T47D did not change any phase of the cell cycle. Together, these results suggest that FLX-induced UPR in SUM149PT and Late MCF10A is associated with G1 arrest, which is consistent with previous studies that described the effect of FLX in colon (HT29), breast (MDA-MB-231), and cervical (SiHa) cancer cell lines[20,22].
| SUM149PT Control | SUM149PT 10 μmol/L FLX | Late MCF10A Control | Late MCF10A 10 μmol/L FLX | T47D Control | T47D 10 μmol/L FLX | |
| 24 h | ||||||
| Sub G | 1.1 | 1.72 | 0.66 | 1.772 | 1.86 | 1.63 |
| (0.34)1 | (0.55) | (0.13) | (0.2) | (0.26) | (0.18) | |
| G1 | 39.6 | 62.412 | 60.26 | 79.892 | 56.03 | 59.21 |
| (0.74) | (1.02) | (1.59) | (0.71) | (2.05) | (0.55) | |
| S | 34.64 | 20.182 | 23.78 | 10.752 | 21.14 | 18.26 |
| (1.28) | (1.11) | (1.16) | (0.43) | (1.12) | (1.45) | |
| G2/M | 25.26 | 16.122 | 15.8 | 7.862 | 21.44 | 21.39 |
| (0.79) | (0.3) | (0.65) | (0.13) | (0.98) | (1.32) | |
| 48 h | ||||||
| Sub G | 1.91 | 6.67 | 0.41 | 10.263 | 1.7 | 2.66 |
| (0.42) | (2.47) | (0.05) | (0.52) | (0.32) | (0.39) | |
| G1 | 46.79 | 67.733 | 65.48 | 79.773 | 57.52 | 62.35 |
| (1.94) | (1.16) | (0.93) | (1.3) | (2.01) | (1.31) | |
| S | 29.69 | 14.012 | 22.24 | 6.593 | 22.33 | 18.07 |
| (2.79) | (0.84) | (0.6) | (0.72) | (1.54) | (1.19) | |
| G2/M | 22.24 | 11.902 | 12.31 | 3.583 | 18.95 | 17.32 |
| (1.08) | (2.64) | (0.43) | (0.13) | (0.24) | (0.29) |
In the present study, we showed that the treatment of TNBC line SUM149PT with antidepressant fluoxetine induces autophagy (Figure 4A) with concomitant decrease in cell growth (Figures 2 and 3). The activation of AMPK, but a decrease in Akt and ERK activation, are likely to contribute to FLX-mediated cytotoxic autophagy in this cell line as early as 24 h (Figures 1, 2, and 4A). In contrast, the autophagy in Late MCF10A after 48 h of treatment may be dependent on ERK activation as previously reported for breast cell lines[43,44]. However, unlike those cells with survival advantage, Late MCF10A growth was significantly inhibited. Whether or not this growth inhibition is due to nuclear translocation of ERK to promote p53 upregulation and subsequent apoptosis, as has been suggested in some models[45], remains to be determined.
Both SUM149PT and Late MCF10A are rapidly dividing cells (Figure 3) and have increased metabolic demands. The FLX-induced decrease in protein synthesis mediated by p70 S6K or eIF4G is likely to contribute to metabolic stress, thereby promoting autophagy in these cells. Given that the spindle-shaped Late MCF10A used in our study may have undergone some biochemical and/or genetic changes due to continual passaging, we acquired a different MCF10A line that has not been passaged extensively and shows normal cobblestone morphology (i.e., Early MCF10A). The 48 h FLX treatment did not induce autophagy (Figure 4A) but reduced cell growth in Early MCF10A (Figure 2) at a smaller proportion (40.1%) compared to Late MCF10A (81.3%) and SUM149PT (68.2%).This result is consistent with chemical-induced autophagy that is selective for only transformed mammary epithelial cells[44]. In preliminary testing of another TNBC line, MDA-MB-231, we found that the FLX-induced cytotoxicity in these cells was also associated with autophagy induction (data not shown). To our knowledge, this study is the first report of FLX-induced autophagy that results in significant growth inhibition of aggressive TNBC line.
In addition, the observed FLX-induced autophagy may be the result of ER stress and subsequent induction of UPR, consisting of PERK-dependent phosphorylation of eIF2α (Figure 4B), which can lead to translation inhibition of IκBα and subsequently NFκB activation[35,36]. Our RPPM data indicated residual IKK activity, as measured by IκBα S32/S36, even after 48 h of FLX treatment (data not shown), which promotes proteasomal-mediated degradation of IκBα, followed by NFκB translocation to the nucleus and subsequent activation. The FLX-induced NFκB activation in SUM149PT (Figure 1B), as determined by post hoc analysis of ANOVA, was significant compared to slowly dividing T47D and Au565 cells (P < 0.05), but comparable to Late MCF10A (P > 0.05). The elevated NFκB activation during ER stress is expected to increase selective transcription of stress response genes[8]. The identities of those genes in our preclinical study have yet to be determined.
The cytotoxic effect of FLX in SUM149PT and Late MCF10A is associated with cell cycle arrest at the G1 phase as early as 24 h (Table 1). This is consistent with the inhibition of global protein translation as a result of ER stress. In an attempt to mitigate such stress, autophagy is induced in rapidly dividing SUM149PT and Late MCF10A. When each cell’s basal metabolic machinery could no longer be maintained for survival, cell death ensues. In our study, prolonged stress and sustained autophagy leads to activation of caspase-7 (Figures 1B and 4C), which has been shown in previous studies to be an effector of ER stress-induced cell death[40]. As suggested by others[39,40,46], we cannot rule out that the Ca2+ release from the ER during UPR may promote apoptosis, perhaps through activation of a different calpain isoform and subsequent proteolytic processing of a caspase other than caspase-12. Moreover, ER-localized Bcl-2 has been shown to regulate the amount of Ca2+ released from the ER to cytosol during stress[38]. In SUM149PT, the decreased Bcl-2 after 48 h of FLX treatment further supports the activation of apoptosis (Figure 4). Taken together, our study suggests that the FLX-induced UPR and autophagy in rapidly dividing SUM149PT and Late MCF10A promotes apoptosis, thus showing the intricate crosstalk between ER and mitochondria. Despite the network complexity, observations reported by us and others provide further evidence how individual components of UPR, autophagy, and apoptosis coordinately regulate cell fate (Figure 5). In regards to FLX-induced UPR in SUM149PT after 48 h, we hypothesized that activation of NFκB may promote transcription of apoptotic genes (i.e., Bax) over cell survival genes (i.e., Bcl-2), thereby favoring cell death through the mitochondrial pathway.
There are several limitations in the present study that precluded us from making general conclusions about the effects of FLX in molecular subtypes of breast cancer. First, each cell type has innate adaptive response to ER and metabolic stress. The extent to which cells will either adapt to stress or die will depend on (1) the genetic/biochemical makeup that dictates proper cellular response, (2) time of exposure to external and/or internal stress stimuli, and (3) the delicate balance between cell survival or death promoting genes. Second, the extent of cytotoxic response in basal-like SUM149PT may not be similar to other subtypes of TNBC that have been recently described[47]. Third, comparison of cytotoxic profiles between FLX and classical inducers of ER stress was not performed in the present study. Xenograft models of SUM149PT and another TNBC subtype will be an important follow-up in vivo study to obtain important insights to the mechanism of action of FLX and potent ER stress inducer, thapsigargin.
In summary, our study demonstrated the complex actions of FDA-approved drug in malignant mammary epithelial TNBC cells that go beyond the inhibition of selective serotonin re-uptake. In addition to its utility for treating clinical depression, FLX has been used to improve quality of life in cancer patients[48]. Recently, FLX has been shown to reverse the multidrug resistance in cancer cells, enhancing the apoptotic effects of chemotherapeutics[23]. Here, we employed high-throughput RPPM to monitor FLX-induced changes in the expression of proteins encompassing the RTK/Akt/mTOR, MEK/ERK, and apoptotic pathways to complement our functional data. Our data analysis pointed to few proteins that play a role in cellular homeostasis and stress response. These proteins are components of the highly integrated autophagy, UPR, and apoptosis in response to ER and metabolic stress. The apparent sensitivity of TNBC SUM149PT to stress-mediated apoptosis has important clinical implications, given the aggressive biology of the inflammatory breast tumor that the cell line was originally derived and frequency of therapeutic resistance. Currently, a multi-modal approach (systemic chemotherapy, surgery, and radiation) is used to treat inflammatory breast cancer. Given the safety profile[49], potential as a chemosensitizer[23], and induction of ER stress-mediated apoptosis (Figure 5), FLX may provide additional benefit to current treatment modality for inflammatory TNBC. The anti-proliferative effect of FLX alone and in combination with chemotherapeutic will have to be tested in an in vivo model of TNBC in the near future.
We thank Dr. Stephen Ethier (Karmanos Cancer Institute, Detroit, MI), Dr. Neil Spector (Duke University Medical Center, Durham, NC), and Dr. David Beebe, (University of Wisconsin, Madison, WI) for cell lines.
The ability of widely prescribed antidepressant fluoxetine to induce cell death or chemosensitize cancer cells has been described previously, but the mechanism of action is not well understood and appears to be cell type-dependent. While the inhibition of extracellular signal regulated kinase pathway and cell cycle progression has been proposed in two different breast cancer cell lines, the comparative studies did not employ non-transformed breast epithelial cells as additional control. Thus, information regarding the selectivity of fluoxetine-induced growth inhibition in molecular subtypes of breast cancer and normal breast cells is lacking.
Given the heterogeneity of breast cancers, including the aggressive triple negative breast cancer subtypes, efforts to identify targets of therapeutic intervention are greatly needed to improve clinical outcome and survival of women diagnosed with such phenotype.
The authors’ study is the first to describe the selective cytotoxicity of fluoxetine for basal-like inflammatory triple negative breast cancer (TNBC) cells over non-transformed mammary epithelial cells that involves the unfolded protein response and autophagy pathways.
Inflammatory breast cancers have a high likelihood of residual disease and recurrence. The ability of fluoxetine to promote unfolded protein response, autophagy, and subsequent death in our preclinical model of inflammatory breast cancer may not only alleviate psychological stress but also potentially reverse therapeutic resistance. The utility of FLX as a potential adjuvant to treatment regimen of inflammatory breast cancer will have to be evaluated in zenograft models of TNBC.
Unfolded protein response (UPR) and autophagy are both physiological responses to oxidative and metabolic stress with the goal of restoring balance in correctly folded proteins and energy sources, respectively. However, prolonged cellular stress can lead to apoptosis. Because UPR and autophagy promote survival and death outcomes, mechanistic insights to their inter-dependent functions may lead to development of new treatment strategies against many diseases where both processes have been implicated.
The paper is good, it has accomplished with many different cell lines.
P- Reviewer: Constantinos D, Hosseini N
S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Prat A, Adamo B, Cheang MC, Anders CK, Carey LA, Perou CM. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist. 2013;18:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 408] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 2. | Yang ZJ, Chee CE, Huang S, Sinicrope FA. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther. 2011;10:1533-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 982] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 3. | Cloonan SM, Williams DC. The antidepressants maprotiline and fluoxetine induce Type II autophagic cell death in drug-resistant Burkitt’s lymphoma. Int J Cancer. 2011;128:1712-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Shao Y, Gao Z, Marks PA, Jiang X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci USA. 2004;101:18030-18035. [PubMed] |
| 5. | Kanzawa T, Zhang L, Xiao L, Germano IM, Kondo Y, Kondo S. Arsenic trioxide induces autophagic cell death in malignant glioma cells by upregulation of mitochondrial cell death protein BNIP3. Oncogene. 2005;24:980-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 309] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 6. | Turcotte S, Chan DA, Sutphin PD, Hay MP, Denny WA, Giaccia AJ. A molecule targeting VHL-deficient renal cell carcinoma that induces autophagy. Cancer Cell. 2008;14:90-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 7. | Clarke R, Cook KL, Hu R, Facey CO, Tavassoly I, Schwartz JL, Baumann WT, Tyson JJ, Xuan J, Wang Y. Endoplasmic reticulum stress, the unfolded protein response, autophagy, and the integrated regulation of breast cancer cell fate. Cancer Res. 2012;72:1321-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 8. | Tam AB, Mercado EL, Hoffmann A, Niwa M. ER stress activates NF-κB by integrating functions of basal IKK activity, IRE1 and PERK. PLoS One. 2012;7:e45078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 276] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 9. | Benbrook DM, Long A. Integration of autophagy, proteasomal degradation, unfolded protein response and apoptosis. Exp Oncol. 2012;34:286-297. [PubMed] |
| 10. | Li J, Ni M, Lee B, Barron E, Hinton DR, Lee AS. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008;15:1460-1471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 374] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 11. | Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1987] [Cited by in RCA: 2173] [Article Influence: 86.9] [Reference Citation Analysis (0)] |
| 12. | Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220-9231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 1480] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 13. | Isbister GK, Bowe SJ, Dawson A, Whyte IM. Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose. J Toxicol Clin Toxicol. 2004;42:277-285. [PubMed] |
| 14. | Wong DT, Bymaster FP, Engleman EA. Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci. 1995;57:411-441. [PubMed] |
| 15. | Brandes LJ, Arron RJ, Bogdanovic RP, Tong J, Zaborniak CL, Hogg GR, Warrington RC, Fang W, LaBella FS. Stimulation of malignant growth in rodents by antidepressant drugs at clinically relevant doses. Cancer Res. 1992;52:3796-3800. [PubMed] |
| 16. | Coogan PF, Palmer JR, Strom BL, Rosenberg L. Use of selective serotonin reuptake inhibitors and the risk of breast cancer. Am J Epidemiol. 2005;162:835-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Wernli KJ, Hampton JM, Trentham-Dietz A, Newcomb PA. Antidepressant medication use and breast cancer risk. Pharmacoepidemiol Drug Saf. 2009;18:284-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Abdul M, Logothetis CJ, Hoosein NM. Growth-inhibitory effects of serotonin uptake inhibitors on human prostate carcinoma cell lines. J Urol. 1995;154:247-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Arimochi H, Morita K. Characterization of cytotoxic actions of tricyclic antidepressants on human HT29 colon carcinoma cells. Eur J Pharmacol. 2006;541:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Stepulak A, Rzeski W, Sifringer M, Brocke K, Gratopp A, Kupisz K, Turski L, Ikonomidou C. Fluoxetine inhibits the extracellular signal regulated kinase pathway and suppresses growth of cancer cells. Cancer Biol Ther. 2008;7:1685-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Lee CS, Kim YJ, Jang ER, Kim W, Myung SC. Fluoxetine induces apoptosis in ovarian carcinoma cell line OVCAR-3 through reactive oxygen species-dependent activation of nuclear factor-kappaB. Basic Clin Pharmacol Toxicol. 2010;106:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Krishnan A, Hariharan R, Nair SA, Pillai MR. Fluoxetine mediates G0/G1 arrest by inducing functional inhibition of cyclin dependent kinase subunit (CKS)1. Biochem Pharmacol. 2008;75:1924-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Zhou T, Duan J, Wang Y, Chen X, Zhou G, Wang R, Fu L, Xu F. Fluoxetine synergys with anticancer drugs to overcome multidrug resistance in breast cancer cells. Tumour Biol. 2012;33:1299-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Levkovitz Y, Gil-Ad I, Zeldich E, Dayag M, Weizman A. Differential induction of apoptosis by antidepressants in glioma and neuroblastoma cell lines: evidence for p-c-Jun, cytochrome c, and caspase-3 involvement. J Mol Neurosci. 2005;27:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Seewaldt VL, Johnson BS, Parker MB, Collins SJ, Swisshelm K. Expression of retinoic acid receptor beta mediates retinoic acid-induced growth arrest and apoptosis in breast cancer cells. Cell Growth Differ. 1995;6:1077-1088. [PubMed] |
| 26. | D’Amato NC, Ostrander JH, Bowie ML, Sistrunk C, Borowsky A, Cardiff RD, Bell K, Young LJ, Simin K, Bachelder RE. Evidence for phenotypic plasticity in aggressive triple-negative breast cancer: human biology is recapitulated by a novel model system. PLoS One. 2012;7:e45684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Espina V, Liotta LA, Petricoin EF. Reverse-phase protein microarrays for theranostics and patient tailored therapy. Methods Mol Biol. 2009;520:89-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Harlow E, Lane D. Antibodies: a laboratory manual. : Cold Spring Harbor Laboratory 1988; 449. |
| 29. | Charafe-Jauffret E, Ginestier C, Monville F, Finetti P, Adélaïde J, Cervera N, Fekairi S, Xerri L, Jacquemier J, Birnbaum D. Gene expression profiling of breast cell lines identifies potential new basal markers. Oncogene. 2006;2273-2284 [. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 431] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 30. | Boyd ZS, Wu QJ, O’Brien C, Spoerke J, Savage H, Fielder PJ, Amler L, Yan Y, Lackner MR. Proteomic analysis of breast cancer molecular subtypes and biomarkers of response to targeted kinase inhibitors using reverse-phase protein microarrays. Mol Cancer Ther. 2008;7:3695-3706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Fumagalli F, Molteni R, Calabrese F, Frasca A, Racagni G, Riva MA. Chronic fluoxetine administration inhibits extracellular signal-regulated kinase 1/2 phosphorylation in rat brain. J Neurochem. 2005;93:1551-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Tait L, Soule HD, Russo J. Ultrastructural and immunocytochemical characterization of an immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6087-6094. [PubMed] |
| 33. | Kondo Y, Kondo S. Autophagy and cancer therapy. Autophagy. 2006;2:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 240] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 34. | Gething MJ. Role and regulation of the ER chaperone BiP. Semin Cell Dev Biol. 1999;10:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 386] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 35. | Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161-10168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 524] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 36. | Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1484] [Cited by in RCA: 1573] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 37. | Copetti T, Bertoli C, Dalla E, Demarchi F, Schneider C. p65/RelA modulates BECN1 transcription and autophagy. Mol Cell Biol. 2009;29:2594-2608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 211] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 38. | Szegezdi E, Macdonald DC, Ní Chonghaile T, Gupta S, Samali A. Bcl-2 family on guard at the ER. Am J Physiol Cell Physiol. 2009;296:C941-C953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 39. | Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887-894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 902] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 40. | Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 751] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 41. | Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 579] [Cited by in RCA: 656] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 42. | Brewer JW, Diehl JA. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc Natl Acad Sci USA. 2000;97:12625-12630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 363] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 43. | Sivaprasad U, Basu A. Inhibition of ERK attenuates autophagy and potentiates tumour necrosis factor-alpha-induced cell death in MCF-7 cells. J Cell Mol Med. 2008;12:1265-1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 44. | Oh SH, Lim SC. Endoplasmic reticulum stress-mediated autophagy/apoptosis induced by capsaicin (8-methyl-N-vanillyl-6-nonenamide) and dihydrocapsaicin is regulated by the extent of c-Jun NH2-terminal kinase/extracellular signal-regulated kinase activation in WI38 lung epithelial fibroblast cells. J Pharmacol Exp Ther. 2009;329:112-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 45. | Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J. 2010;277:2-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 1040] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 46. | Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2141] [Cited by in RCA: 2145] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 47. | Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750-2767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3591] [Cited by in RCA: 3995] [Article Influence: 285.4] [Reference Citation Analysis (0)] |
| 48. | Navari RM, Brenner MC, Wilson MN. Treatment of depressive symptoms in patients with early stage breast cancer undergoing adjuvant therapy. Breast Cancer Res Treat. 2008;112:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Barbey JT, Roose SP. SSRI safety in overdose. J Clin Psychiatry. 1998;59 Suppl 15:42-48. [PubMed] |