Published online Aug 10, 2015. doi: 10.5306/wjco.v6.i4.57
Peer-review started: December 22, 2014
First decision: March 6, 2015
Revised: April 22, 2015
Accepted: June 15, 2015
Article in press: June 16, 2015
Published online: August 10, 2015
Processing time: 240 Days and 19.1 Hours
AIM: To better define the efficacy and the safety of intra-arterial infusion performed with or without hemofiltration for recurrent limb melanoma.
METHODS: Patients with the following characteristics were included in the study: recurrent limb melanoma not indicated for surgical resection, measurable disease in the extremity, > 18 years, performances status (Eastern Cooperative Oncology Group ) was 0-1 and life expectancy of at least 6 mo. Twenty nine consecutive patients were enrolled in the study. Patients underwent fluoroscopic placement of angiographic arterial and venous catheters to infuse the drug in the artery [isolated limb infusion (ILI)], and to stop the out flow (venous). Melphalan was rapidly infused into the isolated limb via the arterial catheter after the inflation of venous balloon catheter. Then the circulation of the limb was completely blocked with a pneumatic cuff at the root of the limb. Haemofiltration (HF) was available only in the main center, and was performed with an extracorporeal perfusion system, in order to reduce high systemic toxic peaks of drug.
RESULTS: Thirty seven ILI were done in 29 cases (31 ILI-HF and 6 ILI) between 2001 and 2014 at Ancona and Pesaro Hospitals, Italy. Clinical outcomes were monitored 30 d after treatment. Eleven patients (38%) received infusion of melphalan alone, 7 (24%) melphalan associated to mitomicin C and 7 (24%) melphalan associated to cisplatin, the remaining 4 were treated with cisplatin, melphalan and epirubicin or cisplatin and mitomicin C. The overall response rate was 66%, in particular, 3 patients (10%) were complete responders and 16 (56%) were partial responders; whereas 7 patients (24%) had stable disease, and 3 (10%) showed progressive disease. Limb toxicity was assessed adopting Wieberdink scale, with evidence of 90% of low grade (I and II) toxicity.
CONCLUSION: ILI-HF and ILI are effective and safe treatments for recurrent non-resectable limb melanoma. They present evidence of favorable clinical benefit and is effective in delaying progression.
Core tip: Isolated limb infusion (ILI) is a regional treatment of limb melanoma, allowing selective delivery of toxic agents to the arm or leg with the tumor, with limited leakage. Hemofiltration can reduce the high toxic peaks of drug in the blood, hence, limiting post-procedural side effects. In this paper we report results of an Italian Registry applying the ILI technique with or without Hemofiltration to recurrent non-resectable limb melanoma. The overall response rate was 66%. Low grade toxicity was observed in 90% of patients. ILI with/without hemofiltration is an efficacious and safe treatment for recurrent non-resectable limb melanoma.
- Citation: Cecchini S, Sarti D, Ricci S, Vergini LD, Sallei M, Serresi S, Ricotti G, Mulazzani L, Lattanzio F, Fiorentini G. Isolated limb infusion chemotherapy with or without hemofiltration for recurrent limb melanoma. World J Clin Oncol 2015; 6(4): 57-63
- URL: https://www.wjgnet.com/2218-4333/full/v6/i4/57.htm
- DOI: https://dx.doi.org/10.5306/wjco.v6.i4.57
The treatments for recurrent limb melanoma are several, including, repeated surgical excision, local injection of drugs, chemotherapy and immuotherapy. Systemic chemotherapy, radiotherapy or regional chemotherapy are the most used techniques when the metastases are several and large[1-3]. Doses of general chemotherapy are restrictive because of adverse events; for this reason isolated limb perfusion (ILP, surgical method) and isolated limb infusion (ILI) (radiological method) are the preferred approaches[4-6].
Melphalan is used as referring drug for melanoma and sarcoma therapy (primary tumor and recurrences at the limbs)[7]. In-transit metastases are observed in 3%-8% of melanoma cases[8]. In this circumstance, treatment of these metastases with ILP or ILI can be very indicated, since they allow using high doses of toxic agents directly in the affected limb with limited outflow[9,10].
ILI is a less invasive procedure than ILP, moreover, it is safe and can be repeatable also in the old and frail patients[11,12]. ILI avoids the use of complex surgical procedures, is shorter in execution, generate lower temperatures, but more hypoxic and acidotic environment, improving in this way the clinical outcome[11-16].
ILI has, moreover, comparable tumor response to ILP, for this reason it may be the future first choice treatment of isolated limb melanoma, alone or in association with systemic targeted therapies[17].
ILI can also be associated to hemofiltration (ILI-HF), for the reduction of unbound drug that can be present in the plasma after ILI procedure, which can cause side effects. This method can be particularly useful for elderly and frail patients[18,19]. The interruption of limb blood circulation can be obtained placing external tourniquets or vascular catheters with terminal inflating balloon, in the main blood vessel of the interested arm or leg[19,20].
The selective exposition of the tumor area allows the administration of higher drug doses (also 10 ×) with an acceptable general toxicity[13,16].
Our angiographic method permits an almost complete vascular isolation of the tumor-affected limb, and is combined with an extra-corporeal circuit that maintains the limb perfusion for 30 min. In this way, the perfusion is performed under hypoxic conditions and with normal thermal conditions.
We have been performing ILI for our patients for several years, and decided to collect data on our procedures, in order to verify if this procedure can be comparable to ILP, which normally is performed by surgeons, and is a more complex and expensive procedure.
The results of our study confirm that the new research avenue ILI and ILI-HF, which are non invasive loco-regional treatments, can be as effective as more aggressive treatments, showing similar responses to ILP.
ILI is a simple method allowing the treatment even of patients with distant metastases which would be excluded from ILP. ILI permits also a good palliation of painful lesions and control of disease. Literature reports in this field are still limited and require further confirmations.
Our purpose is to monitor effectiveness and tolerability of ILI and ILI-HF in patients with limb relapsed melanoma, data are collected from a regional registry of Marche Region (situated in the central part of Italy with 1.4 million of inhabitants).
This was a retrospective study; data were collected from 29 consecutive patients that were treated with ILI or ILI-HF from January 2001 to September 2014 at INRCA Hospital of Ancona, and at Department of Oncology-Hematology of Pesaro General Hospital. Patients were included in the study if they were older that 18 years, and had histological proven recurrent, arm or leg metastatic melanoma that was not suitable for surgical resection, performances status was 0-1 (ECOG scale) and life expectancy of at least 6 mo.
Upon admittance to the radiology room, hydration, antibiotic and analgesic therapy were administered as previously reported by Thompson et al[6]. It included the use tropisetron before the beginning of procedure; and 1 vial of 10 mg morphine hydrochloride during melphalan infusion. Intra-arterial premedication was performed with verapamil 5 mg, and lidocaine 2%, 20 mg.
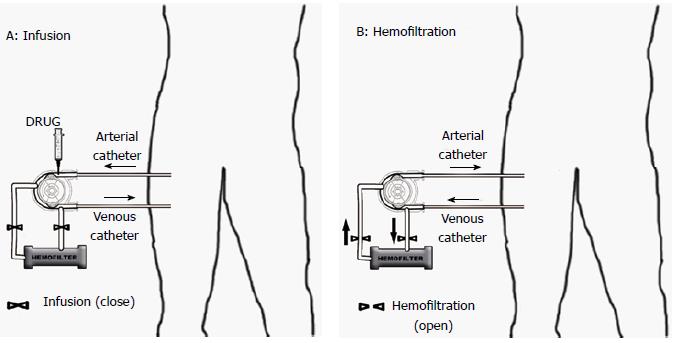
The catheters were introduced into the vessels by percutaneous vascular puncture according to Seldinger’s technique, after loco-regional anesthesia and systemic heparin treatment (100 UI/kg). The catheters with terminal balloon (Boston Scientific Occlusion Balloon Catheters 6F) were placed in the main vessels of the affected limb under fluoroscopic guidance. The balloons were then cuffed, in order to occlude the vascular lumen and to start drug administration. The circulation of the limb was completely interrupted applying a pneumatic tourniquet at the root of the affected limb. The simultaneous blockage of both blood inflow and outflow allowed the complete vascular isolation and the following infusion of the affected limb. Vascular isolation created a local pH decrease and hypoxia, which enhanced the cytotoxic activity of melphalan, doxorubicin and mitomycin. The arterial and the venous catheters were connected with an extra-corporeal circuit sustained by a roller pump that allowed the perfusion of the affected limb at a flow rate range 100-150 mL/min (Figure 1A). Melphalan 1 mg/kg was administered rapidly for the first 4 min and then was recirculated for 30 min. Longer drugs exposure could create severe tissue acidosis and compromise the pre-cellular drug concentration. Haemofilter (two capillary filter system, model FH88, Gambro Dialysontaen, KG, Hechingen, FRG) was included in the extracorporeal perfusion system, in order to eliminate the excess of drug at the end of the procedure. Limb blockade was performed for 30 min, during the last five minutes the hemofiltration was started, and it was prolonged after the opening of limb circulation, by clearing the blood for 45 min or till the production of at least five liters of ultrafiltrate (Figure 1B). Our ILI procedure respected the standard Thomson protocol[6,9,16], with the only exception that we did not use hyperthermia, because our hospital did not have the required equipment. We believed that hyperthermia could increase the therapeutic results, but unfortunately we were not able to perform it due to technical issues.
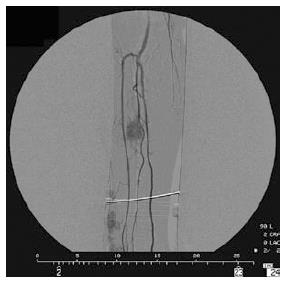
Sequential arteriograms were obtained before intra-arterial infusion of chemotherapeutic agents, in order to detect and localize the lesions (Figure 2). The rationale for the use of this angiograms was that in responders we observed the disappearance and/or reduction of tumor blush in the lesions.
Patients with little or no response after 4 wk from first ILI were indicated to receive up to 2 additional treatments (after 30 and 90 d). Patients were hospitalized for 24-48 h, keeping the limb elevated.
ILI was performed with the most used drugs for loco-regional treatments, such as Melphalan, the standard drug, which was associated to Mitomicin C (bio-reductive agent), because of its property of being more active under hypoxic conditions[10,21-23].
Complete blood counts were obtained daily for the first week following therapy. The Wieberdink Toxicity Grading scale was used for the assessment of limb toxicity (I-V)[24].
Tumor response was assessed using clinical evaluation, according to the Recist 1.1 version; CT scans were performed only in case of profound lesions. First assessments were made at 30 d after the procedure, because in our experience the response was promptly observed, and was maintained for several months, as it could be noticed from the progression free survival, and time to progression (TTP).
We used the validated Italian version of the Edmonton Symptom Assessment Scale (ESAS), which monitored nine of the most frequent cancer symptoms[25]. Patients were asked to assess the severity of each item giving a score from 0 (not at all serious) to 10 (the worst possible severity) on a visual numeric scale (VNS).
Final scores were summed to obtain the total score (overall distress). Overall distress > 5 indicated a poor quality of life (QoL). QoL was monitored at 30 d after treatment. Time to walking recover was another monitored parameter as index of quality of life. Fifteen to twenty days were normally needed for walking recovery after surgical ILP[4,8]. ILI was less invasive procedure and allowed to recover the walking ability in a shorter time range (1-3 d).
Data of the whole sample (n = 29) were analyzed, and quantitative variables were reported as the mean and ± SD or median. Proportions were expressed in percentage. There was no statistical test except to demonstrate the variability and trends of the response to treatment. The statistical review of the study was performed by a biomedical statistician. IBM SPSS Statistic was used for all calculations.
Thirty-seven ILI procedures were performed in 29 patients between 2001 and 2014 in Ancona and Pesaro Hospitals. Main patients’ characteristics were reported in Table 1. They all had distant metastases, the suggested reason for which ILI was done. All patients had been excluded from ILP by surgeon opinion. Palpable nodes were observed in 50% of patients, however no lymph-node dissection was performed. Presenting symptoms were painful nodes, ulcerated lesions, and functional impairment.
| n = 29 | ||
| Average age (yr) | 74,17 (std 12.90) | |
| Range (yr) | 34-93 | |
| Sex | n | % |
| Females | 13 | 45 |
| Males | 16 | 55 |
| Tumor localization | ||
| Upper limb | 5 | 17 |
| Lower limb | 24 | 83 |
| Previous therapy | ||
| SURG + INF | 10 | 34 |
| SURG | 9 | 31 |
| SURG + CHT + INF | 7 | 24 |
| SURG + CHT | 2 | 7 |
| SURG + PERF + CHT + INF | 1 | 3 |
| Treatment type | ||
| ILI + HF | 30 | 76 |
| ILI | 4 | 14 |
| ILI + (ILI + HF) | 3 | 10 |
| Drug type | ||
| Melphalan | 11 | 38 |
| Melphalan + cisplatin | 7 | 24 |
| Melphalan + mitomicin C | 7 | 24 |
| Cisplatin | 2 | 7 |
| Melphalan + epirubicin | 1 | 3 |
| Cisplatin + mitomicin C | 1 | 3 |
The majority of patients, 23 (79%), received only one infusion, whereas 4 (14%) and 2 (7%) patients received two and three infusions respectively. Twenty-three patients received ILI + HF and six patients ILI, of these three underwent HF following progression after the first ILI.
The median follow-up duration after the first procedure was 14.4 mo (range 3-65 mo). The melanoma nodules were monitored with clinical observation and computed tomography scans if in case of deep lesions.
The median infusion liquid was 1.5 L in the treated leg, whereas it was of 1 L for the upper limb infusion. Eleven patients (38%) received infusion of melphalan alone, 7 (24%) melphalan associated to mitomicin C and 7 (24%) melphalan associated to cisplatin, the remaining 4 were treated with cisplatin, melphalan and epirubicin or cisplatin and mitomicin C. The average melphalan dose administered was 50.63 (± 18.46) mg per liter of infused solution, average Mitomicin C dose was 14.4 (± 10.05) mg per liter, average cisplatin dose was 47.69 (± 31.90) mg per liter of tissue, and epirubicin dose was 50 mg per liter.
Therapies performed before ILI infusion were: 10 (34%) surgery followed by interferon (INF) therapy; 9 (31%) surgery alone; 2 (7%) surgery followed by chemotherapy, 7 (24%) surgery followed by chemotherapy and interferon (INF) or interleukin-2 (IL-2). There was also one patient that 4 years after surgery received a hypertermic isolated limb perfusion, with complete response for 10 years, and after progression received ILI, reporting a further complete remission lasting 24 mo. The patient was included in the study.
The overall response rate (ORR) was 66% one month after treatment (Table 2). In particular, 3 (10%) were complete responders, 16 (56%) were partial responders and 7 (24%) stable disease, whereas 3 (10%) showed progression disease.
| n = 29 | ||
| Response | n | % |
| PR | 16 | 56 |
| SD | 7 | 22 |
| CR | 3 | 11 |
| PD | 3 | 11 |
Six (21%) patients showed an early progression (3.5 mo median), whereas median overall TTP was 14.0 ± 4.01 mo. Seventeen (59%) patients were lost to follow up at 36 mo after ILI. Overall survival was 36 mo (range 3-65).
The toxicity (n = 37) was grade I in 30 cases (82%), grade II in 3 cases (8%), and grade III in 4 cases (10%); and there weren’t any grade IV and V limb toxicity. The average hospitalization was 2 d after both ILI and ILI + HF.
The majority of sample, 26 patients (90%), had an improvement of QoL according Edmonton’ scale 30 d after the procedure, and came back to their daily activity. Fifteen (52%) patients, moreover, interrupted analgesic or symptomatic therapy two weeks after the local infusion procedure, for more than one year.
Walking ability and rotary motion were resumed 24 and 48 h after treatment in the lower limbs and in the upper limbs respectively.
Regional chemotherapy is the most indicated therapy for metastatic melanoma[2-9,18]. Isolated limb perfusion is the first extensively internationally procedure for regional treatment of metastatic melanoma, lately, ILI has become widely used because of its advantages: procedural good tolerability in patients (especially old and frail patients), and relative easy repetition of the procedure[15,21]. ILI has also the important advantage of reducing the complication rate (limb toxicity and morbidity), because it is a less invasive and non surgical procedure[12].
Our data confirm the above findings, showing an overall low toxicity with mainly grade 1 or 2 (Wieberdink scale). The median follow-up was 14.4 (range 3-65) mo, and the ORR was 66% one month after treatment. In particular, 3 (10%) were complete responders, 16 (56%) were partial responders and 7 (24%) stable disease, whereas 3 (10%) showed progression disease. Our response rates were comparable to those of previously published ILI studies that reported overall response ranging from 64% to 84%[6-9].
Hemofiltration resulted in a clear reduction of expected drug related systemic toxicity. Patients treated with ILI-HF, indeed, had reduced fall-down of withe blood cells and platelets, than ILI without HF. The repetition of the procedure was performed only in cases of none or low response after the first ILI. The best cytotoxic agent for ILI is Melphalan in our opinion; we believe that the addition of other drug for the loco-regional treatment did not add anything in terms of response.
The measurement of HF leakage has already been performed in our previous studies that showed that there is a reduction of unbounded drugs of more than 30%[10,13,23].
The main limitation of our study is the number of patients included in the analysis, in respect to the time of enrollment and the number of patients lost to follow up. Our data, however, showed similar results to those presented in the literature, suggesting the low risk and good efficacy of ILI for metastatic limb melanoma therapy.
ILI is a simple method, less expensive and with lower morbidity than the surgical approach. Long-term survivors are reported after ILI also in our experience. Scientific advances in understanding oncogenic signalling and the immunobiology of melanoma lead to the most recent therapies in the treatment of advanced melanoma, such as the targeted therapy with BRaf inhibitor, Ipilimumab and anti PD-1[26,27]. These new drugs could be successfully integrated with ILI in the next future.
In conclusion, our study suggested that isolated limb infusion with or without HF is an efficacious procedure for the palliation of melanoma metastastases confined to a limb, showing evidence of responses and clinical benefit. For this reason we suggest ILI as an effective treatment option for patients with advanced limb melanoma, in which surgical resection is not possible.
The treatments for recurrent limb melanoma are several, including, repeated surgical excision, local injection of drugs, chemotherapy and vaccine therapy. Doses of general chemotherapy are restrictive because of adverse events; for this reason isolated limb infusion (ILI) is the preferred method in cases of limb melanoma, allowing the selective delivery of cytotoxic agents to tumor, with limited general diffusion.
ILI is a simpler procedure than isolated limb perfusion (ILP), avoiding complex surgical procedures and shortening operating duration. It is safe and can be repeatable also in the old and frail patients.
ILI has, moreover, comparable tumor response to ILP, for this reason it may be the future first choice treatment of isolated limb melanoma, alone or in association with systemic targeted therapies, due to lack of side effects, and could be combined with other systemic target therapies.
The results of this study suggested that isolated limb infusion with or without HF is an efficacious procedure for the palliation of melanoma metastases confined to a limb, showing. For this reason the authors suggest ILI as an effective treatment option for patients with advanced limb melanoma, in which surgical resection is not possible.
ILI is a regional treatment of limb melanoma, allowing selective delivery of toxic agents to the target arm or leg, with limited leakage. Hemofiltration (HF) can reduce the high toxic peaks of drug in the blood, hence, limiting post-procedural side effects. Hemofiltration can be associated to ILI (ILI-HF) in order to further reduce drug leakage and systemic diffusion.
This is a retrospective review of the experience of ILI as a regional treatment of melanoma localized to a limb. The sample is short but the article is well written.
P- Reviewer: Araujo AMF, Kupeli S, Lasithiotakis K, Reim D S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Garbe C, Peris K, Hauschild A, Saiag P, Middleton M, Spatz A, Grob JJ, Malvehy J, Newton-Bishop J, Stratigos A. Diagnosis and treatment of melanoma: European consensus-based interdisciplinary guideline. Eur J Cancer. 2010;46:270-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 2. | Krathen M. Malignant melanoma: advances in diagnosis, prognosis, and treatment. Semin Cutan Med Surg. 2012;31:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Hoekstra HJ. The European approach to in-transit melanoma lesions. Int J Hyperthermia. 2008;24:227-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Vrouenraets BC, Nieweg OE, Kroon BB. Thirty-five years of isolated limb perfusion for melanoma: indications and results. Br J Surg. 1996;83:1319-1328. [PubMed] |
| 5. | Thompson JF, Hunt JA, Shannon KF, Kam PC. Frequency and duration of remission after isolated limb perfusion for melanoma. Arch Surg. 1997;132:903-907. [PubMed] |
| 6. | Thompson JF, Kam PC, Waugh RC, Harman CR. Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Semin Surg Oncol. 1998;14:238-247. [PubMed] |
| 7. | Defty CL, Marsden JR. Melphalan in regional chemotherapy for locally recurrent metastatic melanoma. Curr Top Med Chem. 2012;12:53-60. [PubMed] |
| 8. | Takkenberg RB, Vrouenraets BC, van Geel AN, Nieweg OE, Noorda EM, Eggermont AM, Kroon BB. Palliative isolated limb perfusion for advanced limb disease in stage IV melanoma patients. J Surg Oncol. 2005;91:107-111. [PubMed] |
| 9. | Hayes AJ, Neuhaus SJ, Clark MA, Thomas JM. Isolated limb perfusion with melphalan and tumor necrosis factor alpha for advanced melanoma and soft-tissue sarcoma. Ann Surg Oncol. 2007;14:230-238. [PubMed] |
| 10. | Guadagni S, Aigner KR, Palumbo G, Cantore M, Fiorentini G, Pozone T, Deraco M, Clerico M, Chaudhuri PK. Pharmacokinetics of mitomycin C in pelvic stopflow infusion and hypoxic pelvic perfusion with and without hemofiltration: a pilot study of patients with recurrent unresectable rectal cancer. J Clin Pharmacol. 1998;38:936-944. [PubMed] |
| 11. | Coventry BJ, Kroon HM, Giles MH, Henderson M, Speakman D, Wall M, Barbour A, Serpell J, Paddle P, Coventry AG. Australian multi-center experience outside of the Sydney Melanoma Unit of isolated limb infusion chemotherapy for melanoma. J Surg Oncol. 2014;109:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Giles MH, Coventry BJ. Isolated limb infusion chemotherapy for melanoma: an overview of early experience at the Adelaide Melanoma Unit. Cancer Manag Res. 2013;5:243-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Guadagni S, Santinami M, Patuzzo R, Pilati PL, Miotto D, Deraco M, Rossi CR, Fiorentini G, Di Filippo F, Valenti M. Hypoxic pelvic and limb perfusion with melphalan and mitomycin C for recurrent limb melanoma: a pilot study. Melanoma Res. 2003;13:51-58. [PubMed] |
| 14. | Lindnér P, Doubrovsky A, Kam PC, Thompson JF. Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann Surg Oncol. 2002;9:127-136. [PubMed] |
| 15. | Kroon HM, Moncrieff M, Kam PC, Thompson JF. Outcomes following isolated limb infusion for melanoma. A 14-year experience. Ann Surg Oncol. 2008;15:3003-3013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Guadagni S, Russo F, Rossi CR, Pilati PL, Miotto D, Fiorentini G, Deraco M, Santinami M, Palumbo G, Valenti M. Deliberate hypoxic pelvic and limb chemoperfusion in the treatment of recurrent melanoma. Am J Surg. 2002;183:28-36. [PubMed] |
| 17. | Hu-Lieskovan S, Robert L, Homet Moreno B, Ribas A. Combining targeted therapy with immunotherapy in BRAF-mutant melanoma: promise and challenges. J Clin Oncol. 2014;32:2248-2254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 18. | Kroon HM, Thompson JF. Isolated limb infusion: a review. J Surg Oncol. 2009;100:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Lucchi L, Fiore GB, Guadagni G, Perrone S, Malaguti V, Caruso F, Fumero R, Albertazzi A. Clinical evaluation of internal hemodiafiltration (iHDF): a diffusive-convective technique performed with internal filtration enhanced high-flux dialyzers. Int J Artif Organs. 2004;27:414-419. [PubMed] |
| 20. | Thompson JF, Siebert GA, Anissimov YG, Smithers BM, Doubrovsky A, Anderson CD, Roberts MS. Microdialysis and response during regional chemotherapy by isolated limb infusion of melphalan for limb malignancies. Br J Cancer. 2001;85:157-165. [PubMed] |
| 21. | Beasley GM, Caudle A, Petersen RP, McMahon NS, Padussis J, Mosca PJ, Zager JS, Hochwald SN, Grobmyer SR, Delman KA. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg. 2009;208:706-715; discussion 715-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Kroon HM, Lin DY, Kam PC, Thompson JF. Efficacy of repeat isolated limb infusion with melphalan and actinomycin D for recurrent melanoma. Cancer. 2009;115:1932-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Guadagni S, Kanavos E, Schietroma M, Fiorentini G, Amicucci G. Selected hypoxic stop-flow perfusions: indication and limits. Tumori. 2006;92:402-406. [PubMed] |
| 24. | Wieberdink J, Benckhuysen C, Braat RP, van Slooten EA, Olthuis GA. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol. 1982;18:905-910. [PubMed] |
| 25. | Moro C, Brunelli C, Miccinesi G, Fallai M, Morino P, Piazza M, Labianca R, Ripamonti C. Edmonton symptom assessment scale: Italian validation in two palliative care settings. Support Care Cancer. 2006;14:30-37. [PubMed] |
| 26. | Noorda EM, Vrouenraets BC, Nieweg OE, van Coevorden F, van Slooten GW, Kroon BB. Isolated limb perfusion with tumor necrosis factor-alpha and melphalan for patients with unresectable soft tissue sarcoma of the extremities. Cancer. 2003;98:1483-1490. [PubMed] |
| 27. | Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368:1365-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 550] [Article Influence: 45.8] [Reference Citation Analysis (0)] |