CHEMOTHERAPY FOR PANCREATIC CANCER
Gemcitabine
Pancreatic cancer is a devastating disease. The incidence rate is almost identical to the mortality rate highlighting the poor prognosis of this cancer. In addition, the number of patients with pancreatic cancer has been increasing and it is now the fourth or fifth cause of cancer-related death both in Japan and the United States. Little is known about the risks for pancreatic cancer[1]. The majority of patients are identified at unresectable advanced stages such as metastatic or locally advanced disease, and have a median survival of 3 to 6 mo for metastatic disease and 6 to 10 mo for locally advanced disease[2,3].
Systemic chemotherapy with gemcitabine has been the standard therapy for advanced pancreatic cancer after it was confirmed to prolong survival and improve clinical response compared with 5-fluorouracil (5-FU)[4]. In fact, after the introduction of gemcitabine in Japan in 2001, most patients with advanced pancreatic cancer received gemcitabine-based systemic chemotherapy. In our analysis conducted at Tokyo University Hospital, the median survival time and 1-year survival rate were 11.6, 9.3, 6.7, 7.8, 2.4 mo and 47%, 39%, 27%, 22%, 7% in patients treated with gemcitabine, chemoradiotherapy using 5-FU, best supportive care (BSC) for locally advanced disease, and gemcitabine or BSC for metastatic disease, respectively, which showed that both gemcitabine and chemoradiotherapy prolonged overall survival time compared with BSC[5]. Due to the convenience of gemcitabine chemotherapy compared with chemoradiotherapy with 5-FU, and increased toxicities with chemoradiotherapy using gemcitabine, gemcitabine is used in patients with locally advanced disease as well as those with metastases.
S-1
5-FU administered intravenously was the standard chemotherapy for pancreatic cancer before the introduction of gemcitabine. Oral fluoropyrimidines, such as S-1 and capecitabine have recently been developed as new drugs. S-1 is an oral fluoropyrimidine, consisting of tegafur, a prodrug of 5-FU, and two bio-modulators, 5-chloro-2,4-dihydroxypyridine and potassium oxonate, which maintains high serum 5-fluorouracil levels and reduces gastrointestinal toxicity. S-1 has demonstrated efficacy in a variety of solid tumors, especially in Asian patients. This agent has been reported to result in an objective response rate equivalent to that of gemcitabine in patients with advanced pancreatic cancer[6,7]. Therefore, it is now considered to be another important key drug for advanced pancreatic cancer in Japan. In fact, both gemcitabine and S-1 are widely used in our hospital following the introduction of S-1 in February 2005[8].
Combination therapy with gemcitabine
More than 10 years have passed since the systemic administration of gemcitabine became the standard chemotherapy for advanced pancreatic cancer[4]. During this period, numerous attempts have been made to improve survival by combining gemcitabine with other chemotherapeutic agents. However, most of these attempts have failed to prolong overall survival compared with gemcitabine monotherapy. Of the available combinations of cytotoxic drugs, a tendency for better survival was reported for cisplatin, capecitabine, or oxaliplatin, However, significant improvements in survival have not been proved[9-12].
Following the reported efficacy of S-1 as monotherapy, the possibility of improving survival using S-1 in combination with gemcitabine was investigated as a next step[13]. We performed a pilot study using a modified combination chemotherapy regimen with S-1 plus gemcitabine. In our treatment schedule, gemcitabine was administered at a dose of 1000 mg/m2 by a 30-min intravenous injection on days 1 and 15 of each cycle. S-1 was administered orally at a dose of 40 mg/m2 twice daily for the first 14 consecutive days followed by a 14-d rest period. This cycle was repeated every 28 d. The median time to progression and the median survival time were 10 and 20 mo, respectively, without significant toxicity, which suggested better survival using this combination therapy[14]. A larger-scale multicenter prospective study to confirm these results has been conducted at our hospital. A phase III trial was also performed in another multicenter study in Japan.
Molecular-targeted drugs
Erlotinib: A number of new molecular-targeted drugs have recently been developed for the treatment of malignant tumors. Many of these drugs were investigated for the treatment of pancreatic cancer[15,16]. However, most of these agents failed to prolong overall survival compared with gemcitabine alone. To date, erlotinib in combination with gemcitabine has been the only drug to show prolonged survival in advanced pancreatic cancer[17]. However, the survival benefit was only two weeks despite high costs and greater toxicity than gemcitabine monotherapy.
One of the reasons for little or no improvement in survival benefit with molecular-targeted drugs may be attributed to the characteristic molecular abnormalities in pancreatic cancer. Genetic abnormalities in K-ras, p16, p53 and DPC4 have been identified in pancreatic cancer. Among these, a peculiar feature is that most pancreatic adenocarcinomas contain K-ras gene mutations[18]. Ras gene mutations are prevalent in all human cancers, but the incidence varies among these cancers. Pancreatic cancer has the highest frequency of ras gene mutations of all human cancers. Erlotinib is a low molecular weight drug that inhibits signal transmission by binding to the epidermal growth factor receptor (EGFR), an important cell-surface receptor. As over-expression of the EGFR is associated with tumor progression, inhibition of EGFR leads to an anti-cancer effect. Consequently, it is one of the important molecular targets for anticancer therapies.
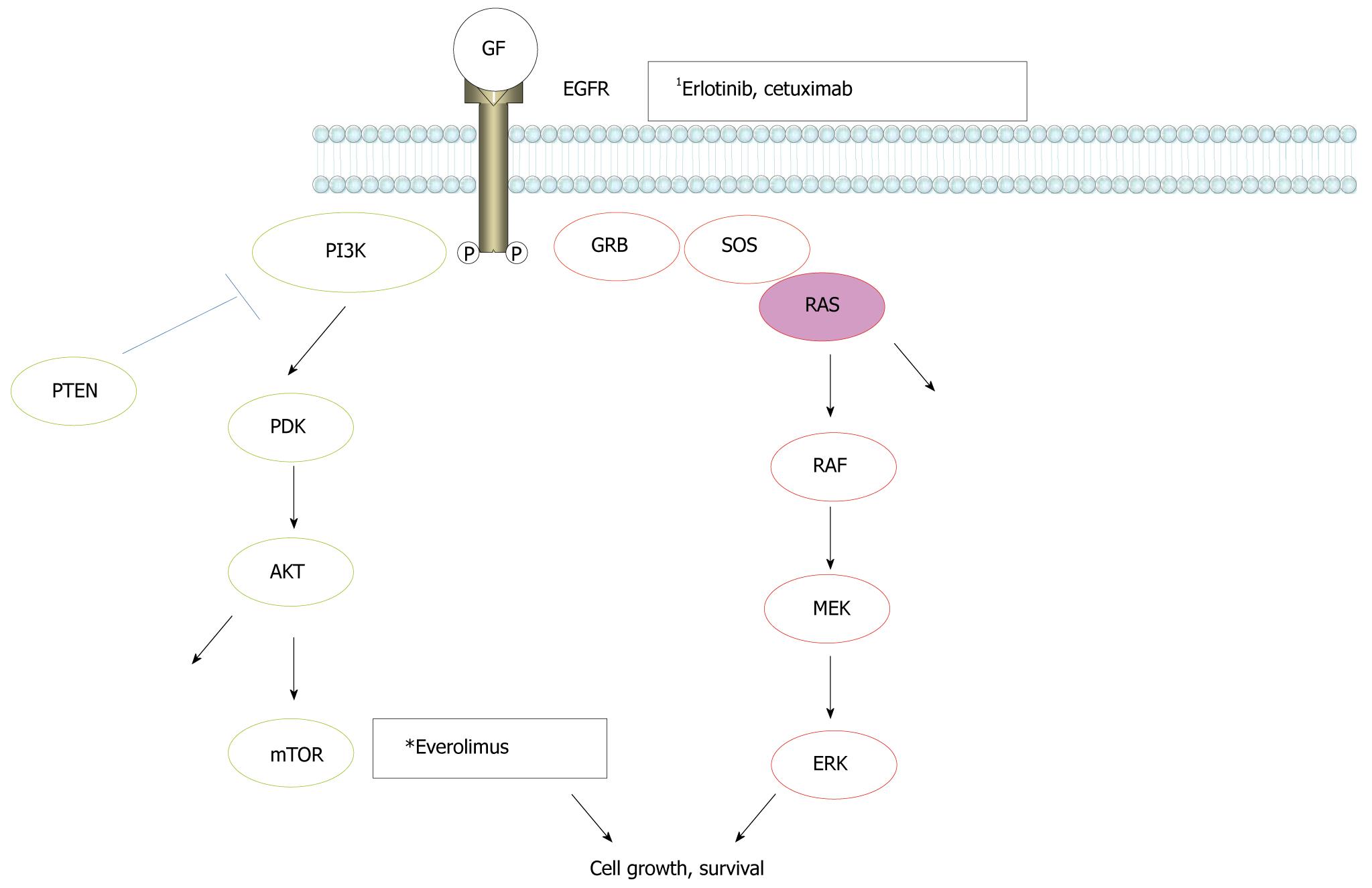
Recently, it was shown that cetuximab, a monoclonal antibody against the EGFR, was effective in patients with metastatic colorectal cancer. However, it was not effective in cases of colon cancer harboring K-ras gene mutations[19]. There are two major signaling pathways downstream of the EGFR, the RAS/RAF/MAP kinase (MAPK) and PI3K/AKT/mTOR pathways, respectively (Figure 1). K-ras is a small G-protein downstream of the EGFR and an essential component of the EGFR signaling cascade of the RAS/RAF/MAPK pathway. Mutation of the K-ras gene leads to the activation of these intracellular pathways downstream of the EGFR. The fact that K-ras mutation is associated with the inefficacy of cetuximab in colon cancer probably reflects the very strong influence of the K-ras mutational effect on signal transduction of the EGFR related signaling cascade, regardless of inhibition of the EGFR. This characteristic strong influence of K-ras mutation on the EGFR signaling system may explain why erlotinib, another inhibitor of the EGFR, showed minimal survival benefit in pancreatic cancer. To date, no potent inhibitor of ras activation has been developed. If a molecular-targeted drug could be developed that suppresses the RAS/RAF/MAPK signaling cascade, a dramatic survival benefit in patients with pancreatic cancer may be achieved in the future.
Figure 1 Signal transmission in the epidermal growth factor receptor pathways.
RAS/RAF/MAPK and PI3K/AKT/mTOR cascades are major pathways downstream of the epidermal growth factor receptor (EGFR). 1Inhibition sites by molecular-targeted drugs.
Everolimus: Everolimus is a molecular-targeted drug that inhibits the PI3K/AKT/mTOR pathway, which is another major signaling cascade downstream of the EGFR (Figure 1). Following the benefit of everolimus in patients with pancreatic neuroendocrine tumors shown in a phase II trial which also had lower toxicity and the convenience of oral dosing[20], the latest results show that everolimus increased progression-free survival to 11 mo, compared with 4.6 mo for placebo and BSC in a phase III clinical trial. Pancreatic endocrine tumors are rare compared with adenocarcinoma of the pancreas. Different from pancreatic adenocarcinoma, these tumors rarely contain K-ras mutations. In this sense, it is not surprising that inhibition of one of the major signaling cascades downstream of the EGFR could be effective in this tumor type.
New promising regimens
Angiotensin converting enzyme inhibitors: Angiotensin I converting enzyme inhibitors (ACEIs) and angiotensin II type-1 receptor blockers (ARBs) are widely used as anti-hypertensive drugs, and have organ protective effects. Long-term use of these drugs has been reported to reduce the incidence of various types of cancer. Therefore, these drugs could be effective as anti-cancer therapies, although they are neither cytotoxic nor molecular-targeted drugs. Our retrospective analysis suggested that ACEIs or ARBs in combination with gemcitabine might improve clinical outcomes in patients with advanced pancreatic cancer[21]. Following these results, we have initiated a phase I trial of candesartan, an ARB, in combination with gemcitabine.
FOLFIRINOX: FOLFIRINOX (5-FU, leucovorin, irinotecan and oxaliplatin) was the first non-gemcitabine-based regimen to show a significantly longer overall survival compared with gemcitabine alone in recent a phase III randomized study of metastatic pancreatic cancer[22]. This therapy not only improved progression-free survival and response rate but also improved overall survival from 6.8 to 11.1 mo. Good performance status patients are thought to be good candidates for FOLFIRINOX due to its high toxicity, which is characteristic of multiple combinations of cytotoxic drugs.
CHEMOTHERAPY FOR BILIARY TRACT CANCER
Gemcitabine, S-1
Biliary tract cancer is the sixth leading cause of cancer death in Japan, although it is relatively rare worldwide compared with pancreatic cancer. The majority of patients are identified at unresectable advanced stages with a poor prognosis that is comparable to, but slightly better than that of pancreatic cancer. Because of the small number of patients with biliary tract cancer, the efficacy of chemotherapy for biliary cancer is often analyzed by including multiple subtypes, such as extrahepatic bile duct cancer, gallbladder cancer, intrahepatic cholangiocarcinoma, and periampullary carcinoma, although these tumors have different biologic behaviors and are associated with different factors and conditions. In addition, analysis can sometimes include both unresectable advanced cases and recurrent cases following surgery. Management of biliary drainage is critical in patients with biliary tract cancer. Chemotherapy is often interrupted by recurrent biliary obstruction accompanying cholangitis, even in cases with appropriate biliary drainage by stenting, especially when tumor obstruction includes hilar bile ducts of the liver.
Gemcitabine is widely used for the treatment of advanced biliary tract cancer, as several clinical studies including phase II trials showed that gemcitabine was moderately effective, although survival did not improve satisfactorily[23,24]. More recently, S-1 was introduced after the results of a few phase II studies showed a median overall survival of 8-9 mo, equivalent to that of gemcitabine[25-27].
Recently, as described previously, combination chemotherapy using gemcitabine plus S-1 has shown good anti-tumor effects and tolerability in patients with advanced pancreatic cancer. We also conducted a multicenter, phase II study to evaluate the efficacy and safety of gemcitabine plus S-1 combination chemotherapy in patients with advanced bile duct cancer[28]. The median overall survival time was 11.6 mo and the median time to progression was 5.9 mo, which were equivalent to those of gemcitabine plus cisplatin combination chemotherapy[29]. These results suggested that this regimen has a promising tumor response without an increased risk of severe drug-related adverse events. A phase III study of gemcitabine plus S-1 combination chemotherapy vs gemcitabine plus cisplatin may be required to identify the most effective regimen.
Gemcitabine plus cisplatin
A large phase III study of biliary tract cancer was recently reported which showed for the first time the superiority of gemcitabine plus cisplatin combination chemotherapy compared to gemcitabine monotherapy[29]. The median overall survival and progression-free survival were 11.7 and 8.0 mo in the gemcitabine plus cispatin group and were 8.1 and 5.0 mo in the gemcitabine alone group, respectively. Although this 3-mo extension in survival was modest, the results indicated that the combination of gemcitabine plus cisplatin should be considered a new standard treatment for patients with advanced biliary tract cancer. Because equivalent results were also obtained in Japanese patients included in a randomized phase II study[30,31], this regimen will be approved for clinical practice in Japan in the near future.
New promising regimen
Cetuximab plus GEMOX (gemcitabine and oxalipatin): The finding that the addition of cetuximab to gemcitabine plus oxaliplatin combination therapy was associated with increased antitumor activity in patients with advanced biliary tract cancer was published very recently[32]. In this phase II study, the response rate was very high at 63% (19 of 30 patients). In addition, 9 patients (30%) were able to undergo secondary curative resection due to down-staging following this regimen. Different from pancreatic cancer, K-ras mutation is uncommon in biliary tract cancers[33] with the exception of intrahepatic cholangiocarcinoma[34]. Therefore, cetuximab, a monoclonal antibody against the EGFR, could be effective in the majority of biliary tract cancers.