Published online Nov 10, 2011. doi: 10.5306/wjco.v2.i11.367
Revised: October 10, 2011
Accepted: October 17, 2011
Published online: November 10, 2011
Lung cancer is currently the leading cause of cancer death in Western nations. Non-small cell lung cancer (NSCLC) represents 80% of all lung cancers, and adenocarcinoma is the predominant histological type. Despite the intensive research carried out on this field and therapeutic advances, the overall prognosis of these patients remains unsatisfactory, with a 5-year overall survival rate of less than 15%. Nowadays, pharmacogenetics and pharmacogenomics represent the key to successful treatment. Recent studies suggest the existence of two distinct molecular pathways in the carcinogenesis of lung adenocarcinoma: one associated with smoking and activation of the K-Ras oncogene and the other not associated with smoking and activation of the epidermal growth factor receptor (EGFR). The K-ras mutation is mainly responsible for primary resistance to new molecules which inhibit tyrosine kinase EGFR (erlotinib and gefitinib) and most of the EGFR mutations are responsible for increased tumor sensitivity to these drugs. This article aims to conduct a systematic review of the literature regarding the molecular pathways involving the EGFR, K-Ras and EGFR targeted therapies in NSCLC tumor behavior.
- Citation: Mello RA, Marques DS, Medeiros R, Araújo AM. Epidermal growth factor receptor and K-Ras in non-small cell lung cancer-molecular pathways involved and targeted therapies. World J Clin Oncol 2011; 2(11): 367-376
- URL: https://www.wjgnet.com/2218-4333/full/v2/i11/367.htm
- DOI: https://dx.doi.org/10.5306/wjco.v2.i11.367
Recently, it was estimated that about 11 million people presently have cancer worldwide[1,2]. In the United States, lung cancer (LC) is the main cause of cancer death, in both genders, and it has a global incidence of about 70 cases per 100 000 inhabitants[3-5]. In Europe, LC incidence is about 52.5 cases per 100 000 inhabitants (82.5/100 000 in males and 23.9/100 000 in females) and mortality is approximately 48.7/100 000 (77/100 000 in males and 23.9/100 000 in females)[3,6]. Smoking status was demonstrated in previous reports to be an important prognostic factor due to its influence on overall survival (OS) regardless of the treatment received[7]. Histology[8,9], co-morbidity using the Charlson score[10] and admission performance status[11] also have an impact on OS and patient outcome.
Non-small cell lung cancer (NSCLC) corresponds to 80%-85% of LC and, although there is progression in the development of new chemotherapeutics, NSCLC prognosis remains unsatisfactory with a 5-year OS of less than 15%[12,13]. Surgery is the best curative therapeutic approach in the early stages (IandII). However, even in these patients, the mean 5-year OS is less than 70%. Thus, most NSCLC patients will be candidates for adjuvant, neoadjuvant or palliative chemotherapy and/or radiotherapy at any time of the disease evolution. Although patients with metastatic disease benefit from standard chemotherapy, its impact on OS is not more than two months. This is why knowledge of the molecular pathways involved in cancer progression is very important[1,6,14-16].
The aim of this study was to conduct a systematic review of the literature regarding the molecular pathways involving the epidermal growth factor receptor (EGFR) and K-Ras in NSCLC behavior and to address some issues on EGFR targeted therapies.
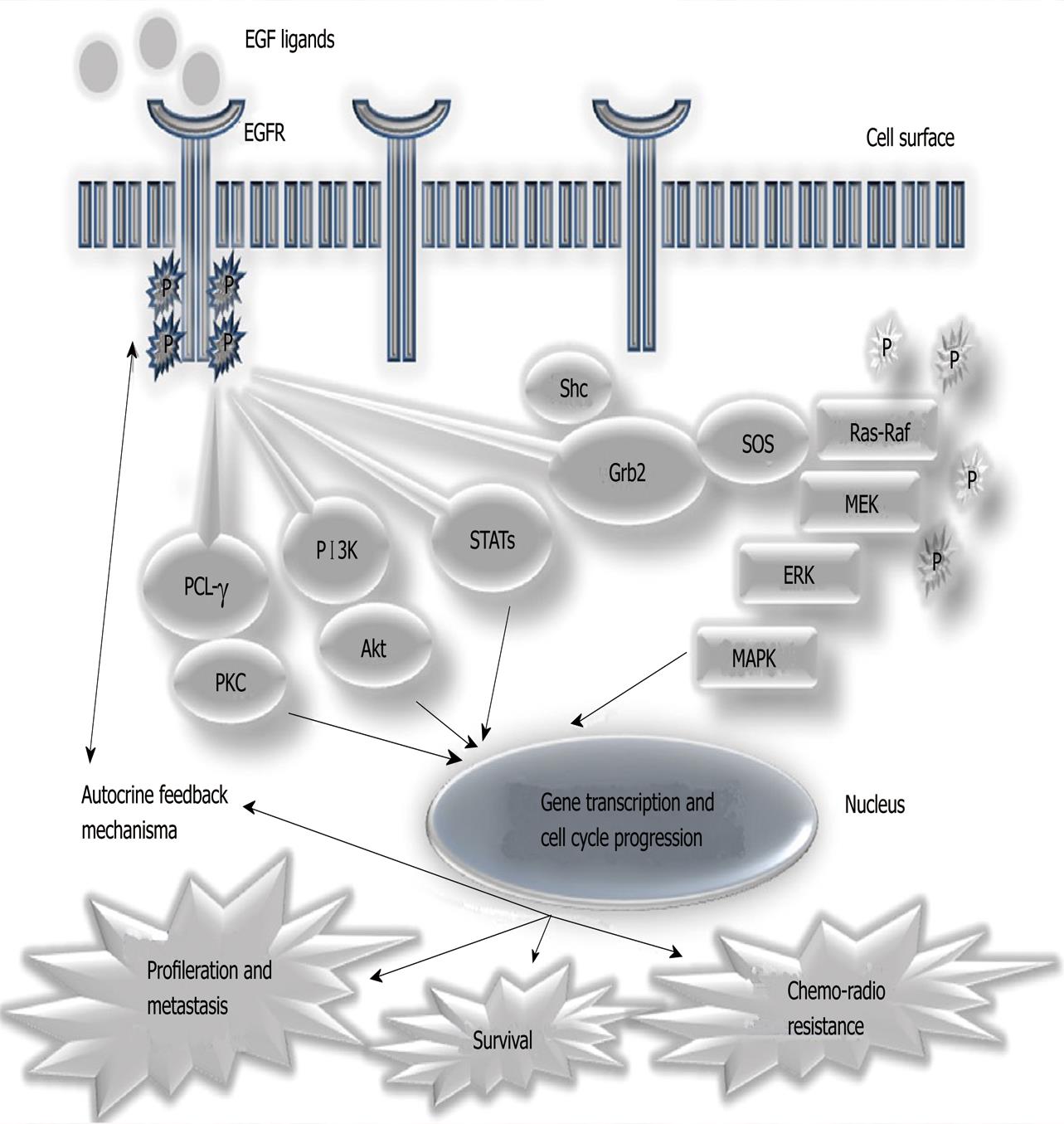
In the last few years, knowledge about molecular mechanisms and cellular transformation in association with cancer behavior has increased[17-19]. More interest has been generated since the development of specific targeted therapies against the processes involved in the carcinogenesis of many types of cancers[20-22]. During the 1990s it was discovered that the EGFR[23] played an important role in tumoral biology and behavior[14]. As summarized in Figure 1, EGFR stimulation activates intracellular signaling and cascades that influence cellular proliferation and mobilization, angiogenesis and other mechanisms. Normal cells are influenced by external factors, in tumor cells it was found that the activation of cell proliferation mediated by this receptor would no longer need external stimuli, but act independently and autonomously[14,24]. In the case of NSCLC, it was shown that the over-expression of this receptor, as well as specific somatic mutations occurred in their intracellular domain with tyrosine kinase activity (between exons 18 and 21), which may influence prognosis, being significantly related to stage, survival and chemotherapy response [14,25,26]. These data led to the development and study of various substances, including monoclonal antibodies directed to the extracellular domain of EGFR (e.g., cetuximab, Erbitux®) and small molecules that inhibit the tyrosine kinase intracellular domain (tyrosine kinase inhibitors, TKIs) of EGFR (e.g., gefitinib and erlotinib)[14,26-30]. Preliminary results of randomized clinical trials conducted with these TKIs have shown that their use in patients with advanced disease is effective, significantly increasing the survival of these patients, especially if they harbor mutations in the EGFR which are more frequently found in a subgroup of non-smoking, female patients, of Asian ethnicity and with adenocarcinoma histological sub-type (especially in the presence of bronchioloalveolar carcinoma). Some of these results were so impressive that this phenomenon was designated, the Lazarus effect, and led to the approval, in the United States and Europe, of erlotinib for the second- and third-line treatment of NSCLC patients; and gefitinib in Europe, for patients harboring the EGFR mutation[25,27-29,31].
Other molecular biomarkers have been investigated in NSCLC, such as COX-2, p53 and K-Ras[1,32]. Among these biomarkers, K-Ras was shown to be important in NSCLC carcinogenesis. This biomarker is mutated in about 20% to 40% of these tumors[26,33-35] and over 95% of the mutations described are located at codons 12 and 13, rarely at codons 59 and 61[33]. Several environmental factors are associated with the K-Ras mutation, such as smoking (there is a relationship between the number of cigarettes smoked and the prevalence of mutations) and exposure to asbestos[33,36]. Mutation of K-Ras appears to be an early phenomenon in NSCLC carcinogenesis and it is often associated with other molecular aberrations such as p53 mutation, p16 methylation, Bcl-2, RASSF1 inactivation and increased expression of several growth factors, among them vascular endothelial growth factor, thereby promoting cell proliferation, suppression of apoptosis and angiogenesis[26,33]. Although there is no consensus between the studies, K-Ras mutation seems to be the main poor prognostic factor in LC adenocarcinoma patients in stageI, and possibly stageII, associated with significantly lower survival rates regardless of other involved factors such as the number of treatment regimens[26,33,35]. Similar conclusions can be drawn from the analysis of studies regarding its predictive value in response to currently recommended treatments, targeted chemotherapy and radiation therapy; furthermore, it may be a factor in resistance to therapy in the early stages (I andII), but not in advanced stages[26,33].
Several studies have found an inverse association between K-Ras mutations and mutations in the EGFR tyrosine-kinase domain[20,33]. These data suggest the possible existence of two distinct molecular pathways in lung carcinogenesis: one associated with smoking and activation of K-Ras; and another not associated with smoking and activation of EGFR[28,29,36,37]. When combined, K-Ras mutation[38] is mainly responsible for primary resistance to new molecules which inhibit tyrosine kinase EGFR (e.g. erlotinib and gefitinib)[20,25,26,33,39,40].
EGFR (or ErbB1) is a transmembrane glycoprotein encoded by a gene located on chromosome 7 (7p12.1-12.3). It comprises 1186 amino acids (a.a.) and 26 exons[41]. Exons 1-14 encode the extracellular domain, exon 15 encodes the transmembrane region and exons 16-26 the intracellular domain. This glycoprotein belongs to the ErbB receptor family, which also consists of: ErbB2 (HER2/neu), ErbB3 (HER3) and ErbB4 (HER4). Each of these proteins is structurally composed of an extracellular domain, a hydrophobic transmembrane domain and an intracellular domain with intrinsic tyrosine kinase (TK) activity (except ErbB3). These receptors exist as inactive monomers, being activated by their interaction, through the extracellular domain, with growth factors of the EGF family. The binding of ErbB receptor molecules to one of these ligands leads to its interaction with other monomers of the same family (receptor dimerization). This dimerization can occur between two identical receptors (homodimerization, e.g., ErbB1-ErbB1) or between two different receptors (heterodimerization, e.g., ErbB1-ErbB3). The stimulation caused by a specific ligand triggers a unique pattern of dimerization, which is also specific to the tissue/tumor in which the phenomenon occurs. Dimerization of the receptors leads to their autophosphorylation with activation of TK and activation of a cascade of intracellular biochemical processes that regulate such diverse activities, like proliferation, differentiation, apoptosis and cell migration[14,40] as shown in Figure 1.
Usually, TK activity is regulated by the conformational state of the catalytic domain of the molecule. The conformation of the catalytic domain, either active or inactive, governs the ability of a kinase to transfer phosphate from adenosine triphosphate to a peptic substrate, thereby regulating the intracellular signaling pathways. There are several mechanisms that regulate this balance of active-inactive protein kinase at the atomic level. First, a.a. residues should be properly oriented in order to facilitate the transfer of phosphate and, second, the peptide substrate binding site should not be occluded. There are two important regions of the catalytic domain able to regulate these mechanisms according to their spatial orientation: the activation loop and the helix-C[41].
In the active conformational state, the activation handle extends outside the catalytic cleft of the molecule in order to allow the substrate to bind to it, while the catalytic glutamate residue (C-helix) forms ionic interactions with a lysine residue that coordinate α and β phosphates of ATP. In the inactive conformation, the activation loop changes its conformation drastically and hinders bonding of the peptide substrate to the catalytic domain of the molecule, while the C-helix wheel drags the residue of glutamate clear of the lysine residue[41].
Mutations occurring in these TK molecule catalytic domains lead to conformational changes that promote permanent active status independent of external factors. The most common, accounting for about 85% of the mutations described, include deletions in exon 19 and substitution of a.a. leucine-858 by arginine in exon 21. These mutations increase the sensitivity to TKIs, probably by promoting conformational changes of the molecule so that the fit of the TK catalytic cleft simulates the inactive conformational status[25,41].
On the other hand, the substitution of threonine-790 by methionine in exon 20 is a factor in resistance to TK, probably because the conformational change of the molecule caused by it does not allow the same type of effect observed for other mutations. This mutation may be acquired, having been described in patients with progressive disease after effective treatment with erlotinib and gefitinib, or innate, resulting in increased susceptibility to LC and primary resistance to molecules that inhibit TK[25,39-42].
EGFR amplification and/or over-expression are also predictors of response to TKI treatment[43]. The EGFR over-expression accounts for about 43%-83% of NSCLC, being more common in squamous cell carcinoma (70%), followed by adenocarcinoma (50%) and to a lesser extent, in large cell carcinoma. This phenomenon is very rare in small cell lung cancer patients[14,39,42].
There are four main intracellular signaling pathways involved in the activation of EGFR (Figure 1): Ras/mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K)/Akt, phospholipase Cγ (PLCγ), protein kinase C and signal transducer and activator of transcription (STAT)[14,42]. Activation of PI3K leads to activation of Akt. This is translocated to the cell nucleus and mediates the transcription of many genes while other cytosolic proteins are activated simultaneously (such as mTOR and Bad), resulting in the ultimate expression of several anti-apoptotic proteins. PLCγ hydrolyzes phosphatidylinositol 4, 5-bisphosphate into diacylglycerol and inositol triphosphate with subsequent activation of protein kinase C, resulting in cell cycle progression. STAT proteins are translocated to the active nucleus and regulate transcription of genes essential for survival and proliferation, mediating cellular transformation and progression to carcinoma[33,42]. The Ras/MAPK pathway is described in detail below.
The Ras family (Rat sarcoma viral oncogene), or p21ras (so designated by the molecular weight common to the various elements that constitute the 21Kd), belongs to the super-family of small guanosine triphosphatases (GTPases) and is composed of several members (the most studied are H-Ras, K-Ras and N-ras)[33,44-50].
Unlike the classic G proteins which are heterotrimeric, they remain in the form of monomeric units of connection (similar to the α subunit of classic G proteins), functioning as a small switch that toggles between the inactive monomer bound to guanosine diphosphate (GDP) and actively linked to guanosine triphosphate (GTP)[33,44,47,49,50]. Different stimuli from the cell surface, mediated by several types of transmembrane receptors, activate these proteins, leading to a cascade of intracellular biochemical processes that regulate such diverse activities as proliferation, differentiation, apoptosis and cell migration[33,45-50]. The most studied members of this family share high structural and functional homology (although encoded by different genes); they are expressed in all tissues (there are variations in the level of subtypes expressed); and they are implicated in the carcinogenesis of various types of tumors[33,44-50].
Stimulation of EGFR protein activates both K-Ras 4B and H-Ras in different ways. These are preferentially located in areas of dense cell membrane (e.g., K-Ras 4B), and this is the Ras protein primarily activated[35]. The binding of EGF to its receptor causes dimerization of α and β subunits and subsequent activation of this intrinsic TK receptor, which autophosphorylates the tyrosine residues and thus allows the receptor to bind to proteins such as Shc adaptation or Grb2. These proteins serve as the link between the EGFR and a set of proteins that are able to stimulate the dissociation of guanine (GNEFs - Guanine Nucleotide Exchange Factors). GNEFs promote the dissociation of polymer Ras-GDP, releasing the Ras to bind to GTP and thus causing its activation[33,44,46,48]. In this particular case, the first class of GNEFs to be activated is the Son of sevenless (Sos), which promotes the dissociation and activation of K-Ras 4B at the inner surface of the cell membrane. Secondly, through mechanisms that involve the formation of diacylglycerol , a second class of GNEFs, also called RasGRPs, promote the activation of H-Ras in the Golgi apparatus, by mechanisms not yet completely clarified[44,46].
Generally, the Ras-GTP complex is active only transiently, since each Ras molecule has intrinsic GTPase activity that, when stimulated by GAPs, hydrolyzes rapidly to Ras-GTP binding and promotes its return to inactive baseline status and binds to GDP (GAPs increase the activity of intrinsic Ras-GTPase proteins approximately 10 000 times)[33,44-50].
Once activated, Ras protein promotes the initiation of several distinct signaling cascades, which are reflected at the nuclear transcription of several genes and the production of factors that induce proliferation, differentiation, migration, apoptosis/cell anti-apoptosis and angiogenesis by VEGF secretion[33,44-51]. In the case of K-Ras 4B, the main intracellular signaling pathways activated are the Raf/MEK/ERK (which promotes cell proliferation) and PI3K/Akt (which promotes cell survival by inhibiting apoptosis)[33,44,46,48]. A comparison of the molecular structure of the active form (bound to GTP) and inactive form (bound to GDP) of the Ras protein showed that the transition from an active to inactive form is accompanied by changes in the conformation of the molecule into two regions, designated switchI(residues 30 to 38, the same effectors region in the center) and switch II (residues 60-76). The blockade of residues 63 to 73 using a directed antibody inhibits change of the protein binding of Ras GTP to GDP, proving that this region of the molecule is essential for conformational change of the molecule that allows the passage of the inactive and active state[32,48,52].
As previously mentioned, Ras protein is rapidly inactivated after initial stimulation, remaining as the inactive form most of the time[45]. However, when mutations occur, especially at codons 12, 13, 59, 61, 63, 116, 117, 119 or 146, its structure is altered by binding sites for guanine, affecting its normal function. The effects of these mutations can be translated either in a reduction of the activity of oncoprotein GTPases, blocking it into the active form bound to GTP (especially if they involve the a.a. 12, 13, 59, 61 and 63) or in decreased binding affinity and increasing the change in GDP by GTP attachment (especially if they involve the a.a. 116, 117, 119 and 146)[35]. The inefficient deactivation of oncoprotein is intensified by the fact that GAPs have reduced ability to promote the return to the disabled state (Ras-GDP). All mutations thus facilitate accumulation of the active form (Ras-GTP), contributing to the malignant cell phenotypic change[44,48,53].
The modified molecule is independent of stimulation by the activation of cell membrane receptors. Thus, it is understandable that patients with NSCLC who have a mutated K-Ras do not respond to treatment with TKI[25]. Brugger et al[54] demonstrated that K-Ras works as a prognostic factor for reduced progression-free survival (PFS) regardless of the treatment for advanced NSCLC.
The search for new targeted therapies able to inactivate K-Ras has led to the discovery of farnesyltransferase inhibitors[42]. These inhibitors act at a protein level by blocking K-Ras farnesylation and preventing their anchor to the cell membrane. The K-Ras molecule, thus trapped in the cytoplasm, is not able to activate effectors of intracellular signaling pathways. Several farnesyltransferase inhibitors have been investigated in LC with unsatisfactory results. Tipifarnib, although capable of effectively blocking this enzyme, did not result in clinical response. Moreover, preliminary clinical trials conducted with lonafarbin (in combination with paclitaxel in patients with NSCLC resistant to taxanes) showed that this combination therapy was effective in controlling some 50% of patients and is currently under further Phase III clinical trials[42].
Over the few last decades, the platinum-based treatment of NSCLC has remained unsatisfactory[55]. Many researchers have tried to identify biomarkers of response to chemotherapy such as ERCC1 (excision repair cross complementing 1) and platinum response[56], ribonucleotide reductase subunit M1 (RRM1) and gemcitabine resistance[57], K-Ras mutation and EGFR status[38,58]. At the moment, some studies such as those shown in Table 1, have demonstrated that personalized therapy through the EGFR pathway have the potential to improve the survival of advanced NSCLC patients[22,27,59-65]. However, the results are not linear. Many factors influence OS, PFS and response rate, such as EGFR mutation status, clinical TNM stage, gender and ethnicity[66].
| Study | Molecule | Place of study | EGFR positive selectedmutations | No. ofpatients | Clinical stage | Responserate (%) | Median OS(mo) | Median PFS(mo) |
| Kris et al, 2003 | Gefitinib | United States | No | 221 | IIIB and IV | 22 | 6-7 | - |
| Perez-Soler et al, 2004 | Gefitinib | United States | No | 57 | IIIB and IV | 12.3 | 8.4 | - |
| Maemondo et al, 2010 | Gefitinib | Asia | Yes | 230 | IIIB and IV | 73.7 | 30.5 | 10.8 |
| Mok et al, 2009 | Gefitinib | Asia | No | 609 | III and IV | 71.2 | 18.6 | 5.7 |
| Mitsudomi et al, 2010 | Gefitinib | Japan | yes | 177 | IIIB and IV | 62.1 | 30.9 | 9.2 |
| Shepherd et al, 2005 | Erlotinib | America, Europe and Asia | No | 731 | IIIB and IV | 8.9 | 6.7 | 2.2 |
| Herbst et al, 2005 | Erlotinib | United States | No | 526 | IIIB and IV | 30 | 10.6 | 6 |
| Capuzzo et al, 2010 | Erlotinib | Italy | yes | 437 | IIIB and IV | 11.9 | 12.3 | 12.3 |
Since 2005, based on the study by Shepherd et al[27], erlotinib has been used for second/third-line therapy[63] to prolong survival in refractory NSCLC IIIb and IV patients irrespective of EGFR status. This drug showed improvements in response rate, OS and 1-year-survival when added to carboplatin and paclitaxel in a recent evaluation[67]. Furthermore, as showed in the TRIBUNE phase III trial, it was first studied in United States, Asia and Europe, but did not have consistent results following approval by the US Food and Drug Administration (FDA) and European Medicine Agency (EMEA) in first-line chemotherapy patients[27,30,61,62,68]. The genetic mutation of EGFR in exons 19 and 21 of chromosome 7 demonstrated an association with response to erlotinib [40,68]. Therefore, erlotinib is approved for the treatment of patients with refractory disease. In April 2010, based on the SATURN phase III trial conducted by Capuzzo et al[63], erlotinib was also approved for maintenance treatment in patients with locally advanced or metastatic NSCLC without progression after four chemotherapy cycles in the first-line setting. In 2011, an American Society of Clinical Oncology provisional opinion panel started to consider erlotinib as first-line therapy for advanced NSCLC[69]. A recent Chinese phase 3 trial (OPTIMAL, CTONG 0802), enrolled 83 patients with advanced NSCLC and mutations of the EGFR gene (exon 19 and 21) from 22 centers in China[70]. This study was conducted in order to compare the efficacy and tolerability of the TKI erlotinib versus standard chemotherapy, in this case gemcitabine plus carboplatin. They showed significantly higher PFS in patients treated with erlotinib compared with those treated with standard chemotherapy: 13.1 mo (95% CI 10.58-16.53) vs 4.6 months (95% CI 4.21-5.42). Furthermore, grade III and IV toxicities, mainly neutropenia and thrombocytopenia, were more frequent in the chemotherapy arm. Thus, these findings suggested that erlotinib might be an important agent in the first-line treatment of advanced NSCLC patients with positive EGFR mutations (mainly in exon 21). Based on these results, erlotinib was approved in Europe in September 2011, as first-line therapy in patients with locally advanced or metastatic NSCLC harboring EGFR activating mutations. Currently, erlotinib is also recommended as second- and third-line therapy in a subset of advanced NSCLC patients irrespective of their EGFR status, due to its impressive results described in the above studies[27,63,70].
Gefitinib did not initially show significant clinical benefit on OS, PSF and tumor response in Western patients with NSCLC IIIB and IV stages[59,61]. The iressa survival evaluation in lung cancer (ISEL) trial showed disappointing results when no improvement in OS was observed in patients treated with gefitinib in either the overall or adenocarcinoma population[60]. However, others studies (IPASS) reported the superiority of gefitinib when compared with platinum-taxane-based therapy protocols mainly in patients with EGFR mutations, adenocarcinoma histology, nonsmokers or former light smokers in East Asia[64,65]. Subsequently, it was also confirmed that EGFR mutations had a predictive role in the response of lung adenocarcinoma to gefitinib as compared with carboplatin-paclitaxel treatment[43,64,70,71]. These findings resulted in the approval of gefitinib by EMEA for use in the first-line treatment of patients with advanced metastatic NSCLC EGFR mutation positive tumors[65].
Recently, the MET proto-oncogene was discovered which encodes for the high affinity cell surface receptor for hepatocyte growth factor (HGF) and also control the main steps of carcinogenesis: cell growth, invasion, proliferation and apoptosis[72,74]. Thus, MET inhibitors emerged as a promising new class of targeted drugs in patients with MET-mediated resistance to EGFR inhibitors[66,74]. Nowadays, dual MET-EGFR multi-target TKI therapies may be considered a good approach for MET-mediated resistance to EGFR inhibitors to improve NSCLC patient outcome. In 2010, a recent multi-target of MET, VEGFR2 and RET, called XL 184, in association with erlotinib in NSCLC EGFR T790M and MET amplified patients was presented at the American Society of Clinical Oncology annual meeting as a promising choice in these patients[75]. Another drug, ARQ197, which is a selective non ATP competitive inhibitor of c-MET, when combined with erlotinib in the second/third-line treatment of EGFR inhibition naïve NSCLC patients showed increased PSF, mainly among patients with non-squamous histology, K-Ras mutations, and EGFR wild-type status[73]. Other drugs targeting MET pathways, such as AMG102[74], a monoclonal antibody against HGF, and MetMab (Genentech)[77], a human recombinant agonist of the HGF-Met signaling pathway, are still in phaseIstudies and show promise in the treatment of NSCLC patients[76-78].
In the last few years, the echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK) fusion gene has been identified by Fluorecence in Situ Hybridization (FISH) Assay as an oncogene in about 11.3% of patients with NSCLC[79]. These patients are resistant to EGFR TKI therapies and should be directed to ALK-targeted agents[80]. FISH and real time polymerase chain reaction represent two primary methods to assess ALK fusions[81]. Recently, crizotinib, was identified as a potent inhibitor of ALK and MET tyrosine kinases[82,83]. It was demonstrated in previous studies that this drug was well tolerated and resulted in important tumor shrinkage in NSCLC EML-4ALK positive patients [84,85]. Crizotinib has been shown to significantly control disease when used in NSCLC patients with EML4-ALK mutation fusion who were refractory to EGFR TKI treatment[82,86]. However, some patients have resistance to crizotinib and other EML4-ALK inhibitors are in development[87]. Other studies[88] showed that EML4-ALK mutation prolonged PSF in patients treated with premetrexed. Thus, this should be considered in trials involving patients treated with this drug. Recently, it was reported that crizotinib does not cross the blood brain barrier and thus its cerebrospinal fluid levels are insufficient to control brain metastases[89]. Further studies are warranted to assess this situation.
In recent decades, therapeutic advances in LC studies, with the use of combined platinum-based chemotherapy strategies, radiotherapy or surgery, have not been completely satisfactory in terms of overall survival; and thus the prognosis associated with this disease remains very poor[54,90-95]. The need to find new targeted agents has renewed interest in the study and understanding of the molecular pathways involved in lung carcinogenesis[3,9,17,18,58,91,92], and several targeted therapeutic molecules have been synthesized[22,96-99]. However, it has also became evident that there are multiple pathogenic mechanisms in lung cancer working in parallel or with several loops of activation/inhibition, thus therapeutic exploration with the goal of disease control can not be based on the study of a single mechanism[22,97-99]. Through experience with molecules such as gefitinib and erlotinib, it is now understood that the benefit of EGFR TKIs depend on several biological characteristics in individual patients[30,59,100-103]. The study of new targeted agents and their combination in order to optimize therapy should therefore take into account the individual characteristics of each patient[55,58]. This is currently a promising field of cancer research in which genetics, tumor molecular biology and clinical experience interact to achieve more effective combination therapies adjusted to the patient profile.
We would like to thank Dr. Miguel Nunes from Department of Informatics of Faculty of Medicine of the University of Porto, Porto, Portugal and Professor. Daniel H. Pozza, Department of Experimental Biology, Faculty of Medicine of the University of Porto, for his help in technical support.
Peer reviewer: Yong-Song Guan, Professor, Oncology and Radiology, State Key Laboratory of Biotherapy, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China
S-Editor Yang XC L-Editor Webster JR E-Editor Yang XC
| 1. | Schrump D, Giaccone G, Kelsey K, Marks L. Non Small Cell Lung Cancer. DeVita, Hellman, and Rosenberg's Cancer: principles & practice of oncology. 8 ed. 2008;896-939. |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25536] [Article Influence: 1824.0] [Reference Citation Analysis (7)] |
| 3. | Alberg AJ, Brock MV, Samet JM. Epidemiology of lung cancer: looking to the future. J Clin Oncol. 2005;23:3175-3185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 297] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 4. | Herbst RS, Bajorin DF, Bleiberg H, Blum D, Hao D, Johnson BE, Ozols RF, Demetri GD, Ganz PA, Kris MG. Clinical Cancer Advances 2005: major research advances in cancer treatment, prevention, and screening--a report from the American Society of Clinical Oncology. J Clin Oncol. 2006;24:190-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8283] [Cited by in RCA: 8224] [Article Influence: 483.8] [Reference Citation Analysis (0)] |
| 6. | Felip E, Stahel RA, Pavlidis N. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of non-small-cell lung cancer (NSCLC). Ann Oncol. 2005;16 Suppl 1:i28-i29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Hanagiri T, Sugio K, Mizukami M, Ichiki Y, Sugaya M, Yasuda M, Takenoyama M, Yasumoto K. Significance of Smoking as a Postoperative Prognostic Factor in Patients with Non-small Cell Lung Cancer. J Thorac Oncol. 2008;3:1127-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543-3551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2484] [Cited by in RCA: 2487] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 9. | Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M, Muller T. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1940] [Cited by in RCA: 1896] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 10. | Asmis TR, Ding K, Seymour L, Shepherd FA, Leighl NB, Winton TL, Whitehead M, Spaans JN, Graham BC, Goss GD. Age and comorbidity as independent prognostic factors in the treatment of non small-cell lung cancer: a review of National Cancer Institute of Canada Clinical Trials Group trials. J Clin Oncol. 2008;26:54-59. [PubMed] |
| 11. | Dajczman E, Kasymjanova G, Kreisman H, Swinton N, Pepe C, Small D. Should patient-rated performance status affect treatment decisions in advanced lung cancer. J Thorac Oncol. 2008;3:1133-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Sculier JP, Chansky K, Crowley JJ, Van Meerbeeck J, Goldstraw P. The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th Edition of the TNM Classification of Malignant Tumors and the proposals for the 7th Edition. J Thorac Oncol. 2008;3:457-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 252] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 13. | Carvalho L, Cardoso E, Nunes H, Baptista V, Gomes A, Couceiro P. [The IASLC lung cancer staging project. Comparing the current 6(th) TNM edition with the proposed 7(th) edition]. Rev Port Pneumol. 2009;15:67-76. [PubMed] |
| 14. | Araújo A, Ribeiro R, Azevedo I, Coelho A, Soares M, Sousa B, Pinto D, Lopes C, Medeiros R, Scagliotti GV. Genetic polymorphisms of the epidermal growth factor and related receptor in non-small cell lung cancer--a review of the literature. Oncologist. 2007;12:201-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Lopez A, Collishaw N, Piha T. A descriptive model of the cigarette epidemic in developed countries. Tob Control. 1994;3:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 674] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 16. | Anderson GP, Bozinovski S. Acquired somatic mutations in the molecular pathogenesis of COPD. Trends Pharmacol Sci. 2003;24:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Chiang AC, Massagué J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 751] [Cited by in RCA: 760] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 18. | Nikolinakos PG, Altorki N, Yankelevitz D, Tran HT, Yan S, Rajagopalan D, Bordogna W, Ottesen LH, Heymach JV. Plasma cytokine and angiogenic factor profiling identifies markers associated with tumor shrinkage in early-stage non-small cell lung cancer patients treated with pazopanib. Cancer Res. 2010;70:2171-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Fong KM, Minna JD. Molecular biology of lung cancer: clinical implications. Clin Chest Med. 2002;23:83-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Oliveira PA, Colaço A, Chaves R, Guedes-Pinto H, De-La-Cruz P LF, Lopes C. Chemical carcinogenesis. An Acad Bras Cienc. 2007;79:593-616. [PubMed] |
| 21. | Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3965] [Cited by in RCA: 4049] [Article Influence: 176.0] [Reference Citation Analysis (0)] |
| 22. | Dy GK, Adjei AA. Emerging therapeutic targets in non-small cell lung cancer. Proc Am Thorac Soc. 2009;6:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003;21:2237-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2363] [Cited by in RCA: 2255] [Article Influence: 102.5] [Reference Citation Analysis (0)] |
| 24. | Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919-8923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 955] [Cited by in RCA: 1005] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 25. | Toschi L, Cappuzzo F. Understanding the new genetics of responsiveness to epidermal growth factor receptor tyrosine kinase inhibitors. Oncologist. 2007;12:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Rosell R, Cuello M, Cecere F, Santarpia M, Reguart N, Felip E, Taron M. Usefulness of predictive tests for cancer treatment. Bull Cancer. 2006;93:E101-E108. [PubMed] |
| 27. | Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4223] [Cited by in RCA: 4215] [Article Influence: 210.8] [Reference Citation Analysis (0)] |
| 28. | Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8739] [Cited by in RCA: 8776] [Article Influence: 417.9] [Reference Citation Analysis (0)] |
| 29. | Ichihara S, Toyooka S, Fujiwara Y, Hotta K, Shigematsu H, Tokumo M, Soh J, Asano H, Ichimura K, Aoe K. The impact of epidermal growth factor receptor gene status on gefitinib-treated Japanese patients with non-small-cell lung cancer. Int J Cancer. 2007;120:1239-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Lind JS, Dingemans AM, Groen HJ, Thunnissen FB, Bekers O, Heideman DA, Honeywell RJ, Giovannetti E, Peters GJ, Postmus PE. A multicenter phase II study of erlotinib and sorafenib in chemotherapy-naive patients with advanced non-small cell lung cancer. Clin Cancer Res. 2010;16:3078-3087. [PubMed] |
| 31. | Hirsch FR, Varella-Garcia M, McCoy J, West H, Xavier AC, Gumerlock P, Bunn PA, Franklin WA, Crowley J, Gandara DR. Increased epidermal growth factor receptor gene copy number detected by fluorescence in situ hybridization associates with increased sensitivity to gefitinib in patients with bronchioloalveolar carcinoma subtypes: a Southwest Oncology Group Study. J Clin Oncol. 2005;23:6838-6845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 469] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 32. | Scheffzek K, Ahmadian MR, Kabsch W, Wiesmüller L, Lautwein A, Schmitz F, Wittinghofer A. The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science. 1997;277:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1173] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 33. | Aviel-Ronen S, Blackhall FH, Shepherd FA, Tsao MS. K-ras mutations in non-small-cell lung carcinoma: a review. Clin Lung Cancer. 2006;8:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 34. | Gautschi O, Bigosch C, Huegli B, Jermann M, Marx A, Chasse E, Ratschiller D, Weder W, Joerger M, Betticher DC. Circulating deoxyribonucleic acid as prognostic marker in non-small-cell lung cancer patients undergoing chemotherapy. J Clin Oncol. 2004;22:4157-4164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 205] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 35. | Mascaux C, Iannino N, Martin B, Paesmans M, Berghmans T, Dusart M, Haller A, Lothaire P, Meert AP, Noel S. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2005;92:131-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 455] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 36. | Westra WH, Baas IO, Hruban RH, Askin FB, Wilson K, Offerhaus GJ, Slebos RJ. K-ras oncogene activation in atypical alveolar hyperplasias of the human lung. Cancer Res. 1996;56:2224-2228. [PubMed] |
| 37. | Wistuba II, Gazdar AF. Lung cancer preneoplasia. Annu Rev Pathol. 2006;1:331-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 162] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 38. | Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, Ince WL, Jänne PA, Januario T, Johnson DH. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900-5909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1164] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 39. | Zhu CQ, da Cunha Santos G, Ding K, Sakurada A, Cutz JC, Liu N, Zhang T, Marrano P, Whitehead M, Squire JA. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2008;26:4268-4275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 533] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 40. | Miller VA, Riely GJ, Zakowski MF, Li AR, Patel JD, Heelan RT, Kris MG, Sandler AB, Carbone DP, Tsao A. Molecular characteristics of bronchioloalveolar carcinoma and adenocarcinoma, bronchioloalveolar carcinoma subtype, predict response to erlotinib. J Clin Oncol. 2008;26:1472-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 219] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 41. | Kumar A, Petri ET, Halmos B, Boggon TJ. Structure and clinical relevance of the epidermal growth factor receptor in human cancer. J Clin Oncol. 2008;26:1742-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 223] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 42. | Choong NW, Salgia R, Vokes EE. Key signaling pathways and targets in lung cancer therapy. Clin Lung Cancer. 2007;8 Suppl 2:S52-S60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Sholl LM, Xiao Y, Joshi V, Yeap BY, Cioffredi LA, Jackman DM, Lee C, Jänne PA, Lindeman NI. EGFR mutation is a better predictor of response to tyrosine kinase inhibitors in non-small cell lung carcinoma than FISH, CISH, and immunohistochemistry. Am J Clin Pathol. 2010;133:922-934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 44. | Omerovic J, Laude AJ, Prior IA. Ras proteins: paradigms for compartmentalised and isoform-specific signalling. Cell Mol Life Sci. 2007;64:2575-2589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 45. | Singh A, Sowjanya AP, Ramakrishna G. The wild-type Ras: road ahead. FASEB J. 2005;19:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Ehrhardt A, David MD, Ehrhardt GR, Schrader JW. Distinct mechanisms determine the patterns of differential activation of H-Ras, N-Ras, K-Ras 4B, and M-Ras by receptors for growth factors or antigen. Mol Cell Biol. 2004;24:6311-6323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Walker SA, Lockyer PJ. Visualizing Ras signalling in real-time. J Cell Sci. 2004;117:2879-2886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Macaluso M, Russo G, Cinti C, Bazan V, Gebbia N, Russo A. Ras family genes: an interesting link between cell cycle and cancer. J Cell Physiol. 2002;192:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 49. | Adjei AA. Blocking oncogenic Ras signaling for cancer therapy. J Natl Cancer Inst. 2001;93:1062-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 618] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 50. | Denhardt D. Signal-transducing protein phosphorylation cascades mediated by Ras/Rho proteins in the mammalian cell: the potential for multiplex signalling. Biochem J. 1996;318:729-747. [PubMed] |
| 51. | Konishi T, Huang CL, Adachi M, Taki T, Inufusa H, Kodama K, Kohno N, Miyake M. The K-ras gene regulates vascular endothelial growth factor gene expression in non-small cell lung cancers. Int J Oncol. 2000;16:501-511. [PubMed] |
| 52. | Milburn MV, Tong L, deVos AM, Brünger A, Yamaizumi Z, Nishimura S, Kim SH. Molecular switch for signal transduction: structural differences between active and inactive forms of protooncogenic ras proteins. Science. 1990;247:939-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 863] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 53. | Barbacid M. ras oncogenes: their role in neoplasia. Eur J Clin Invest. 1990;20:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 174] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | Brugger W, Triller N, Blasinska-Morawiec M, Curescu S, Sakalauskas R, Manikhas GM, Mazieres J, Whittom R, Ward C, Mayne K. Prospective Molecular Marker Analyses of EGFR and KRAS From a Randomized, Placebo-Controlled Study of Erlotinib Maintenance Therapy in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2011;Oct 3 [Epub ahead of print]. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 214] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 55. | Mahalingam D, Mita A, Mita MM, Nawrocki ST, Giles FJ. Targeted therapy for advanced non-small cell lung cancers: historical perspective, current practices, and future development. Curr Probl Cancer. 2009;33:73-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Olaussen KA, Dunant A, Fouret P, Brambilla E, André F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983-991. [PubMed] |
| 57. | Shepherd FA, Rosell R. Weighing tumor biology in treatment decisions for patients with non-small cell lung cancer. J Thorac Oncol. 2007;2 Suppl 2:S68-S76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353:172-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 975] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 59. | Kris MG, Natale RB, Herbst RS, Lynch TJ, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2064] [Cited by in RCA: 2004] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 60. | Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, Thongprasert S, Tan EH, Pemberton K, Archer V. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005;366:1527-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1624] [Cited by in RCA: 1621] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 61. | Pérez-Soler R, Chachoua A, Hammond LA, Rowinsky EK, Huberman M, Karp D, Rigas J, Clark GM, Santabárbara P, Bonomi P. Determinants of tumor response and survival with erlotinib in patients with non--small-cell lung cancer. J Clin Oncol. 2004;22:3238-3247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 826] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 62. | Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, Kris MG, Tran HT, Klein P, Li X. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892-5899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1120] [Cited by in RCA: 1113] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 63. | Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczésna A, Juhász E, Esteban E, Molinier O, Brugger W, Melezínek I. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 905] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 64. | Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5906] [Cited by in RCA: 6468] [Article Influence: 404.3] [Reference Citation Analysis (0)] |
| 65. | Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380-2388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3961] [Cited by in RCA: 4387] [Article Influence: 292.5] [Reference Citation Analysis (0)] |
| 66. | Roberts PJ, Stinchcombe TE, Der CJ, Socinski MA. Personalized medicine in non-small-cell lung cancer: is KRAS a useful marker in selecting patients for epidermal growth factor receptor-targeted therapy. J Clin Oncol. 2010;28:4769-4777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 67. | Riely GJ, Rizvi NA, Kris MG, Milton DT, Solit DB, Rosen N, Senturk E, Azzoli CG, Brahmer JR, Sirotnak FM. Randomized phase II study of pulse erlotinib before or after carboplatin and paclitaxel in current or former smokers with advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 68. | Tan E, Ramlau R, Pluzanska A, Kuo HP, Reck M, Milanowski J, Au JS, Felip E, Yang PC, Damyanov D. A multicentre phase II gene expression profiling study of putative relationships between tumour biomarkers and clinical response with erlotinib in non-small-cell lung cancer. Ann Oncol. 2010;21:217-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Keedy VL, Temin S, Somerfield MR, Beasley MB, Johnson DH, McShane LM, Milton DT, Strawn JR, Wakelee HA, Giaccone G. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) Mutation testing for patients with advanced non-small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol. 2011;29:2121-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 396] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 70. | Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2700] [Cited by in RCA: 3258] [Article Influence: 232.7] [Reference Citation Analysis (0)] |
| 71. | Takano T, Ohe Y, Sakamoto H, Tsuta K, Matsuno Y, Tateishi U, Yamamoto S, Nokihara H, Yamamoto N, Sekine I. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23:6829-6837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 590] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 72. | Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2837] [Cited by in RCA: 3282] [Article Influence: 218.8] [Reference Citation Analysis (0)] |
| 73. | Toschi L, Cappuzzo F. Clinical implications of MET gene copy number in lung cancer. Future Oncol. 2010;6:239-247. [PubMed] |
| 74. | Sequist LV, von Pawel J, Garmey EG, Akerley WL, Brugger W, Ferrari D, Chen Y, Costa DB, Gerber DE, Orlov S. Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. J Clin Oncol. 2011;29:3307-3315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 330] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 75. | Liu X, Newton RC, Scherle PA. Developing c-MET pathway inhibitors for cancer therapy: progress and challenges. Trends Mol Med. 2010;16:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 76. | Wakelee H, Gettinger S, Engelman J, Janne P, West H, Subramaniam D, Leach J. W., Wax M. B., Yaron Y. and Lara P. A phase Ib/II study of XL184 (BMS 907351) with and without erlotinib (E) in patients (pts) with non-small cell lung cancer (NSCLC). J Clin Oncol. 2010;28 suppl 15:3017. |
| 77. | Rosen PJ, Sweeney CJ, Park DJ, Beaupre DM, Deng H, Leitch IM, Shubhakar P, Zhu M, Oliner KS, Anderson A. A phase Ib study of AMG 102 in combination with bevacizumab or motesanib in patients with advanced solid tumors. Clin Cancer Res. 2010;16:2677-2687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 78. | Moss R, Bothos J, Filvaroff E, Merchant M, Eppler S, Yu W, Harris K. Scott P, Mehnert J M, Patel P H. Phase Ib dose-escalation study of MetMAb, a monovalent antagonist antibody to the receptor MET, in combination with bevacizumab in patients with locally advanced or metastatic solid tumors. J Clin Oncol. 2010;28 suppl 15:e13050. |
| 79. | Laino C. NSCLC: MetMAb erlotinib extends survival in subset of lung cancer patients. Oncology Times. 2010;32:38-39. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 80. | Lin E, Li L, Guan Y, Soriano R, Rivers CS, Mohan S, Pandita A, Tang J, Modrusan Z. Exon array profiling detects EML4-ALK fusion in breast, colorectal, and non-small cell lung cancers. Mol Cancer Res. 2009;7:1466-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 245] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 81. | Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, Solomon B, Stubbs H, Admane S, McDermott U. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247-4253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1345] [Cited by in RCA: 1511] [Article Influence: 94.4] [Reference Citation Analysis (0)] |
| 82. | Zhang X, Zhang S, Yang X, Yang J, Zhou Q, Yin L, An S, Lin J, Chen S, Xie Z. Fusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expression. Mol Cancer. 2010;9:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 246] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 83. | Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693-1703. [PubMed] |
| 84. | Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, Yatabe Y, Takeuchi K, Hamada T, Haruta H. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 891] [Cited by in RCA: 933] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 85. | Rodig SJ, Shapiro GI. Crizotinib, a small-molecule dual inhibitor of the c-Met and ALK receptor tyrosine kinases. Curr Opin Investig Drugs. 2010;11:1477-1490. [PubMed] |
| 86. | Shen L, Ji HF. More on crizotinib. N Engl J Med. 2011;364:777-778. [PubMed] |
| 87. | Chihara D, Suzuki R. More on crizotinib. N Engl J Med. 2011;364:776-77; author reply 778. [PubMed] |
| 88. | Cheng M, Ott GR. Anaplastic lymphoma kinase as a therapeutic target in anaplastic large cell lymphoma, non-small cell lung cancer and neuroblastoma. Anticancer Agents Med Chem. 2010;10:236-249. [PubMed] |
| 89. | Camidge DR, Kono SA, Lu X, Okuyama S, Barón AE, Oton AB, Davies AM, Varella-Garcia M, Franklin W, Doebele RC. Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol. 2011;6:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 90. | Costa DB, Kobayashi S, Pandya SS, Yeo WL, Shen Z, Tan W, Wilner KD. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29:e443-e445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 500] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 91. | de Mello RA, Costa BM, Reis RM, Hespanhol V. Insights into Angiogenesis in Non-Small Cell Lung Cancer: Molecular Mechanisms, Polymorphic Genes, and Targeted Therapies. Recent Pat Anticancer Drug Discov. 2011;Aug 22 [Epub ahead of print]. [PubMed] |
| 92. | Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: a decade of progress. Chest. 2002;122:1037-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 432] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 93. | Scappaticci FA. Mechanisms and future directions for angiogenesis-based cancer therapies. J Clin Oncol. 2002;20:3906-3927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 156] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 94. | Haura EB. Treatment of advanced non-small-cell lung cancer: a review of current randomized clinical trials and an examination of emerging therapies. Cancer Control. 2001;8:326-336. [PubMed] |
| 95. | Dong J, Dai J, Shu Y, Pan S, Xu L, Chen W, Wang Y, Jin G, Ma H, Zhang M. Polymorphisms in EGFR and VEGF contribute to non-small-cell lung cancer survival in a Chinese population. Carcinogenesis. 2010;31:1080-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 96. | Duda DG, Jain RK, Willett CG. Antiangiogenics: the potential role of integrating this novel treatment modality with chemoradiation for solid cancers. J Clin Oncol. 2007;25:4033-4042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 97. | Blumenschein G, Reckamp K, Stephenson GJ, O'Rourke T, Gladish G, McGreivy J, Sun YN, Ye Y, Parson M, Sandler A. Phase 1b study of motesanib, an oral angiogenesis inhibitor, in combination with carboplatin/paclitaxel and/or panitumumab for the treatment of advanced non–small cell lung cancer. Clin Cancer Res. 2010;16:279-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 98. | Dy GK, Adjei AA. Novel targets for lung cancer therapy: part I. J Clin Oncol. 2002;20:2881-2894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 99. | Hanrahan EO, Lin HY, Kim ES, Yan S, Du DZ, McKee KS, Tran HT, Lee JJ, Ryan AJ, Langmuir P. Distinct patterns of cytokine and angiogenic factor modulation and markers of benefit for vandetanib and/or chemotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2010;28:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 100. | Singhal S, Vachani A, Antin-Ozerkis D, Kaiser LR, Albelda SM. Prognostic implications of cell cycle, apoptosis, and angiogenesis biomarkers in non-small cell lung cancer: a review. Clin Cancer Res. 2005;11:3974-3986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 225] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 101. | Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1876] [Cited by in RCA: 1998] [Article Influence: 117.5] [Reference Citation Analysis (0)] |
| 102. | Jain RK, Duda DG, Willett CG, Sahani DV, Zhu AX, Loeffler JS, Batchelor TT, Sorensen AG. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6:327-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 474] [Cited by in RCA: 450] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 103. | Inoue A, Kobayashi K, Usui K, Maemondo M, Okinaga S, Mikami I, Ando M, Yamazaki K, Saijo Y, Gemma A. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol. 2009;27:1394-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 368] [Article Influence: 23.0] [Reference Citation Analysis (0)] |