Published online Aug 24, 2025. doi: 10.5306/wjco.v16.i8.108865
Revised: May 22, 2025
Accepted: July 1, 2025
Published online: August 24, 2025
Processing time: 110 Days and 23.5 Hours
Familial adenomatous polyposis (FAP) is an autosomal dominant syndrome that results from a germline mutation in the adenomatous polyposis coli gene. It is characterized by the early development of hundreds of adenomas in the colon during the second decade of life. If prophylactic colectomy is not performed, most patients eventually develop colorectal cancer (CRC).
We present the mutational profile of a case of FAP that progressed to CRC. A 45-year-old Saudi man presented with intestinal obstruction and underwent a total colectomy. The colon showed hundreds of polyps and two infiltrative ulcerative lesions, which proved to be adenocarcinoma according to histopathology. We performed next-generation sequencing and found mutations in the TP53, NRAS, EGFR PDGFR, MET, KIT, ERBB2, and GUSP genes.
To the best of our knowledge, this case report is the first to sheds the light on the mutation profile of FAP that progressed to CRC in Saudi Arabia.
Core Tip: This case report is the first to highlight the mutation profile of a patient with familial adenomatous polyposis that progressed to colon cancer using next-generation sequencing in Saudi Arabia. The findings provide valuable insight into the genetic landscape of familial adenomatous polyposis-related colorectal cancer in the region. This information may have important prognostic significance and could guide future therapeutic strategies, potentially improving personalized treatment approaches for patients with similar genetic backgrounds.
- Citation: Esheba GE, Kamel HF, Youssef HM, Badawood HF, Alshamrani AA, Alharbi RJ, Nassir R. Mutational profile of a Saudi patient with Familial adenomatous polyposis that progressed to colon cancer: A case report. World J Clin Oncol 2025; 16(8): 108865
- URL: https://www.wjgnet.com/2218-4333/full/v16/i8/108865.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i8.108865
Familial adenomatous polyposis (FAP) (MIM# 175100) is an autosomal dominant inherited disease which is characterized by multiple adenomatous polyps arising from the mucosa of gastrointestinal tract. The incidence of FAP is uncommon with an average of 1% of all colorectal cancer cases globally[1,2]. Adenomatous polyps start to develop during childhood and mainly in the rectosigmoid colon. By the time of adolescence, these polyps are present throughout the colon and increase in size and number thereafter. Patients with classic FAP have an almost 100% risk of developing CRC by the fourth decade of life, which is significantly earlier than sporadic CRC[1].
Since FAP is a complex genetic disorder with multiple genotype variations, the tumor development, clinical symptoms, and treatment plan are quite challenging. Most patients with FAP have a family history of colorectal polyps and cancer. However, about a third of patients do not have clinical or genetic evidence of FAP among family members. This could be explained by either de-novo mutations or germline mosaicism[1,3].
FAP results from a germline mutation in the adenomatous polyposis coli (APC) gene. Most of these mutations are nonsense mutations. APC is a tumor suppressor gene that is located on the q-arm of chromosome 5. This gene inhibits the Wnt/β-catenin pathway by regulating the degradation of β-catenin. Binding of APC to β-catenin leads to activation of ubiquitin-mediated β-catenin degradation. Therefore, loss of APC function in FAP results in accumulation of β-catenin in the nucleus, activation of the Wnt signaling pathway, and activation of β-catenin’s tumorigenic effects. Up-regulation of the Wnt signaling pathway in colonic epithelial cells has been associated with epithelial hyperplasia[4]. Which explains the development of hundreds to thousands of intestinal adenomas in FAP[5]. Patients with FAP develop new mutations through enhanced cellular proliferation and survival, which occurs at a higher rate compared to healthy individuals. Currently, FAP is rarely seen in countries with efficient screening programs for CRC[1].
The principal targets of FAP management are prevention of cancer and maintaining good quality of life. Regular follow-up should be offered to all patients, and prophylactic surgery should be performed by the patient’s late teens or early twenties. In patients with FAP, screening colonoscopy combined with prophylactic surgery has led to a 55% reduction in the incidence of colorectal cancer as the initial clinical manifestation[6]. We present a case of patient who underwent next-generation sequencing as a molecular genetic study to define new mutations that may have led to the development of CRC in the context of FAP. This study highlights the importance of recognizing familial colorectal cancer cases.
Vomiting, constipation and abdominal pain.
A 45-year-old Saudi man presented with intestinal obstruction. The patient had suffered from vomiting, constipation, and abdominal pain for 2 weeks.
Unremarkable.
The patient’s personal, medical, and surgical histories were unremarkable, and his family history was negative for FAP. The patient had not been admitted to hospital before and had not undergone any investigation or physical examination.
On admission, the general physical examination was unremarkable.
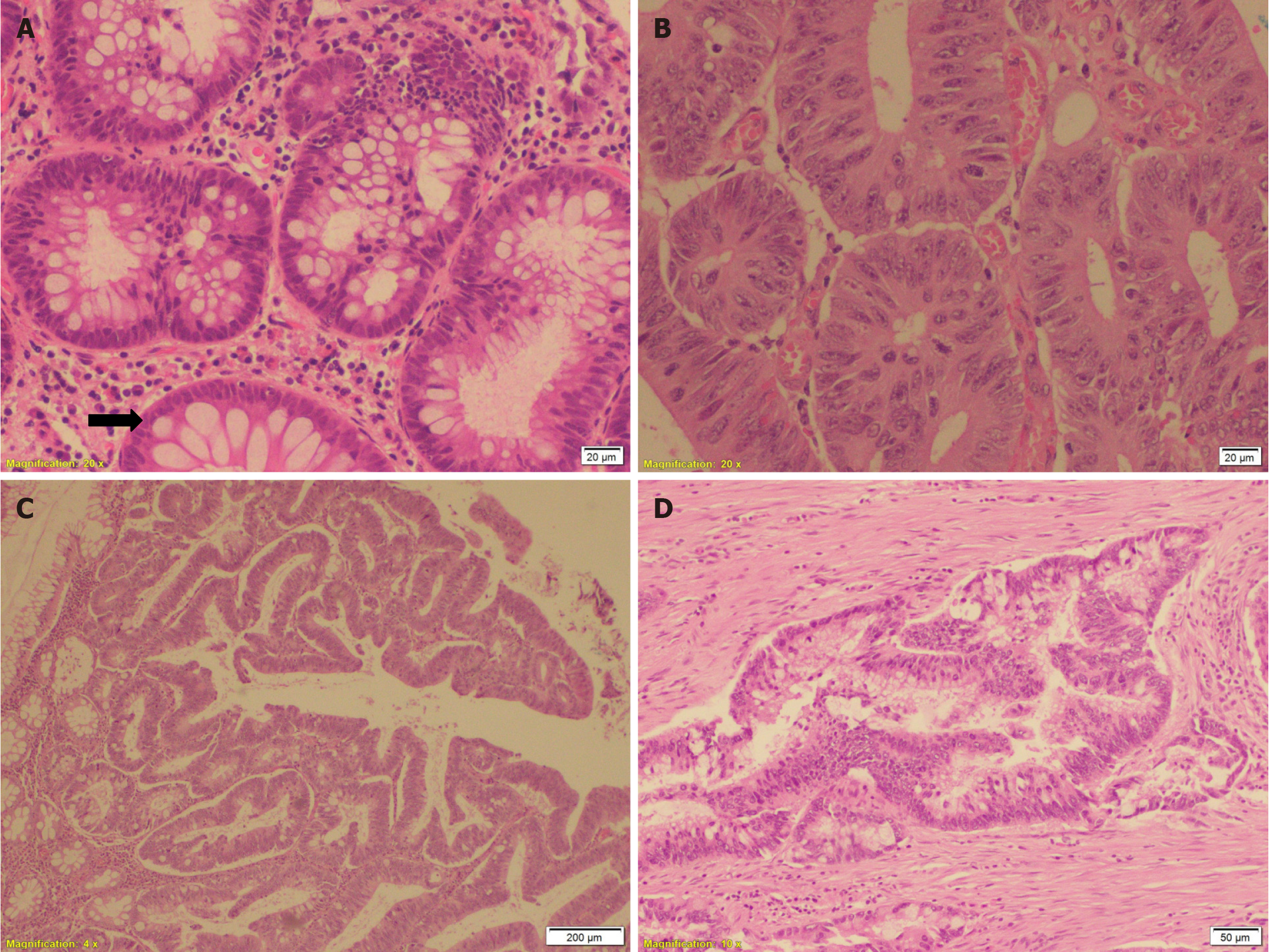
All routine laboratory tests showed results within normal ranges. Multiple biopsies were taken from different polyps and the ulcerative lesions. Histopathology revealed that most polyps were tubular adenomas with low-grade dysplasia, which showed polypoid colonic mucosa lined by dysplastic pseudostratified epithelium with elongated and hy
A few polyps were tubulovillous adenomas showing an admixture of tubular and villous components in the form of finger-like projections containing fibrovascular cores, which were covered with dysplastic crowded epithelium with elongated hyperchromatic nuclei (Figure 1C). Biopsies from the ulcerative lesions revealed moderately differentiated infiltrating adenocarcinoma (grade II) (Figure 1D).
The patient underwent proctocolectomy with ileal pouch–anal anastomosis. On grossing, the colon was studied and showed hundreds of variable sizes and shapes of polyps in addition to two infiltrative firm grayish white ulcerative growths in the mid-portion of the colon, each measured 3 cm × 2 cm. Both tumors infiltrated deeply into the muscularis propria up to the pericolic fat. Dissection of the pericolic adipose tissue revealed 6 lymph nodes, and the largest measured 1 cm × 0.5 cm.
Histopathology of the polyps confirmed the diagnosis of the previous biopsies. Sections from both infiltrative ulcerative lesions revealed infiltration of the wall of the colon by malignant epithelial cells exhibiting cribriform and tubular configuration. They infiltrated the entire wall thickness in a desmoplastic stroma with extension into the pericolic fibroadipose tissue. Perineural and lymphovascular invasion was detected. Out of 6 lymph nodes, 4 nodes were found to be infiltrated by malignant cells in nests, tubules, and individually, along with capsular invasion and extension to perinodular tissue. The resection margins, terminal ileum, and appendix were free of malignancy.
After obtaining tumor tissue, genomic DNA was extracted using a Qiagen kit. Qubit and Nano-drop were used to measure the concentration of DNA and verify its purity. Illumina second-generation sequencing equipment (Illumina, San Diego, CA, United States) was utilized with TruSight Tumor 15. MiSeq reporter software was used for assessment and comparing the reads with the hg19/GRch37 reference sequence, TruSight Tumor 15, which is approved by the Saudi national health for diagnostic purposes. BaseSpcae Variant Interpreter was used to identify the pathogenic variances precisely. To reduce false positives, we raised the threshold values for read depth, InDel repeat length, and genotyping quality. Based on the results of sequencing, we found mutations in the following genes: NRAS, PDGFRA, KIT, EGFR, MET, GUSB, AKT1, TP53, and ERBB2 (Table 1). No mutations have been detected in APC gene.
| Gene | Chromosome number | SNV | Variant | Consequence |
| NRAS | 1 | 115256530 | C>A | Missense |
| PDGFRA | 4 | 55141055 | A>G | Synonymous |
| PDGFRA | 4 | 55141085 | G>A | Synonymous |
| KIT | 4 | 55593709 | G>T | Splice region |
| EGFR | 7 | 55249003 | G>A | Synonymous |
| EGFR | 7 | 55249063 | C>T | Synonymous |
| EGFR | 7 | 55241726 | C>T | Missense |
| MET | 7 | 116312986 | T>C | Missense |
| GUSB | 7 | 65425894 | T>C | Missense |
| AKT1 | 14 | 105246606 | C>T | Synonymous |
| TP53 | 17 | 7577094 | C>T | Missense |
| TP53 | 17 | 7577121 | C>T | Missense |
| TP53 | 17 | 7578457 | G>A | Missense |
| TP53 | 17 | 7579835 | A>G | Splice region |
| TP53 | 17 | 7579855 | 58delT | Frameshift indels |
| ERBB2 | 17 | 37880988 | G>A | Missense |
Colonoscopy revealed multiple polyps of different sizes affecting the whole colon. The largest polyp measured 2.3 mm in diameter, and there were also 2 ulcerative lesions each measuring 2 cm × 2 cm.
FAP complicated with invasive adenocarcinoma.
The patient underwent proctocolectomy with ileal pouch-anal anastomosis.
The patient was followed after surgery for 6 months. Serum carcinoembryonic antigen level was done and it was within normal level. In addition, computed tomography of the chest, abdomen, and pelvis was also performed and it was free. Unfortunately, 6 months later, the patient was lost to follow up.
Approximately 85% of colorectal cancers are sporadic, while 15% are familial. Most CRC results from the conventional adenoma–carcinoma sequence, in which adenomas with either low-grade or high-grade dysplasia progress to infiltrating adenocarcinoma. This occurs through a stepwise accumulation of genetic and epigenetic changes[1,4]. Symptoms of FAP are relatively uncommon at a young age until adenomas enlarge in size, increase in number, and cause rectal bleeding. Other manifestations of FAP include constipation, diarrhea, abdominal pain, and weight loss. It is possible for patients with FAP to remain asymptomatic, as in the present case[1].
Other gastrointestinal manifestations of FAP include fundic gland polyps and adenomatous polyps in the duodenum and small intestine. Patients with FAP may also present with extraintestinal manifestations such as osteomas, fibromas, adrenal adenomas, lipomas, dental abnormalities, and extracolonic malignancies in the pancreas, thyroid, liver, bile ducts, and central nervous system. Hence, patients with APC mutations should be screened for other FAP-associated tumors[2,3].
Our patient was asymptomatic, and physical examination, colonoscopy, and imaging studies revealed no other gastrointestinal or extraintestinal manifestations. Additionally, the patient had a no family history of FAP. More than 700 mutations in the APC gene have been identified in FAP, and frameshift and nonsense mutations are the most common (more than 90% of cases). The rate of germline mutations causing the evolution of a new APC allele was found to be 5 to 9 mutations/million gametes. Therefore, FAP may develop in people with a negative family history. In addition, these new APC mutations arise during embryogenesis; consequently, the patient’s siblings have no risk for FAP[5].
Diagnosis of FAP is based on a family history, clinical findings, and colonoscopy. The clinical diagnosis should be confirmed by genetic testing in addition to screening of all first-degree relatives whenever possible[4,5]. Genetic testing helps to avoid unnecessary cost and risks related to repeated colonoscopies in individuals who prove to be unaffected[6]. Unfortunately, there are insufficient data from Saudi Arabia on the prevalence, clinical features, genetic profiles, and management of FAP. The reported rates of genetic testing among patients with FAP and their relatives are 40%-60%. However, in Saudi Arabia, this rate is 36%[6]. This could be explained by a lack of referral to geneticists and reservations toward genetic testing due to fear of stigmatization among affected families, in spite of the easy accessibility to highly equipped genetic counseling centers in Saudi Arabia. Therefore, patient awareness and genetic testing among FAP family members should be increased in Saudi Arabia[7].
In the current case, we identified 18 pathogenic variants that are associated with the development of colon cancer. The genetic testing using TruSight Tumor 15 targeted coding and splicing regions and gene-targeted deletion/duplication analyses. However, due to the limitation of TruSight Tumor 15, we could not detect more genetic variants. This finding provides motivational to perform further molecular analyses to obtain more information.
We could not detect any mutations in the APC gene in our patient and unfortunately, we did not have the chance to look at other alternative mechanisms, such as mosaic mutations or epigenetic changes, which could potentially explain the observed phenotype in the absence of detectable germline APC mutations in this case. Assessing for mosaicism or epigenetic alterations would require fresh tumor samples for deep sequencing or methylation analysis, respectively which was not feasible within the scope of this study as this was a retrospective case, and the patient underwent surgery prior to the study with subsequent loss to follow-up. Therefore, we were unable to obtain additional samples beyond those initially collected.
Direct sequencing showed variable APC gene mutation rate in FAP patients across the world ranging from 48.39% to 69.7%. This divergence might be the consequence of different detection methods and small sample sizes[8]. Younis et al[9] conducted an extensive literature review on the genetics of colorectal cancer to provide insights into gene mutations associated with CRC in Saudi patients. The authors found APC gene mutation rate in Saudi patients ranged from 36.4% to 96% in different studies.
In conclusion, we have described the results of sequencing in a case of colorectal adenoma and carcinoma in a Saudi patient with FAP, which may have prognostic significance and therapeutic implications. However, validation studies on a larger scale are required to confirm these findings. Furthermore, genomic analyses should be considered by applying conventional genetic testing regardless of the family history of the cancer syndrome.
| 1. | Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis. 2009;4:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 376] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 2. | Ali A, Ahmad A, Taj S, Qaudeer SA, Ahmed SE. Familial Adenomatous Polyposis (FAP) Presenting as Iron Deficiency Anemia in a 33-Year-Old Female: A Case Report. Cureus. 2022;14:e24603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Xu M, Zheng Y, Zuo Z, Zhou Q, Deng Q, Wang J, Wang D. De novo familial adenomatous polyposis associated thyroid cancer with a c.2929delG frameshift deletion mutation in APC: a case report and literature review. World J Surg Oncol. 2023;21:73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Nieuwenhuis MH, Vasen HF. Correlations between mutation site in APC and phenotype of familial adenomatous polyposis (FAP): a review of the literature. Crit Rev Oncol Hematol. 2007;61:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 246] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 5. | Ma H, Brosens LAA, Offerhaus GJA, Giardiello FM, de Leng WWJ, Montgomery EA. Pathology and genetics of hereditary colorectal cancer. Pathology. 2018;50:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 6. | Alhassan N, Helmi H, Alzamil A, Alshammari A, Altamimi A, Alshammari S, Bin Traiki T, Albanyan S, AlKhayal K, Zubaidi A, Al-Obeed O. Surveillance Compliance and Quality of Life Assessment Among Surgical Patients with Familial Adenomatous Polyposis Syndrome. J Epidemiol Glob Health. 2024;14:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Balobaid A, Qari A, Al-Zaidan H. Genetic counselors' scope of practice and challenges in genetic counseling services in Saudi Arabia. Int J Pediatr Adolesc Med. 2016;3:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Kim B, Won D, Jang M, Kim H, Choi JR, Kim TI, Lee ST. Next-generation sequencing with comprehensive bioinformatics analysis facilitates somatic mosaic APC gene mutation detection in patients with familial adenomatous polyposis. BMC Med Genomics. 2019;12:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Younis NS, AlMasoud ES, Al Khawajah F, Alghazal FJ, AlMofarfesh HM, Al-Khalaf LH, Al Otaibi MS, Alkhamis SM, Al Naser ZA, Al Mousa ZH, Alabdulaziz ZI, Mohamed ME. Potential genetic biomarker of Saudi Arabian patients with colorectal cancer. Eur Rev Med Pharmacol Sci. 2022;26:3109-3126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |