Published online Jun 24, 2025. doi: 10.5306/wjco.v16.i6.108113
Revised: April 22, 2025
Accepted: May 15, 2025
Published online: June 24, 2025
Processing time: 76 Days and 6.8 Hours
Vitamin D deficiency has been associated with prostate cancer, particularly in ethnic minorities. Patients with prostate cancer may still be deficient even in areas of high sun exposure. Although androgen deprivation therapy (ADT) is well documented to affect bone health, its impact on vitamin D levels is still uncertain. This study investigates the subgroups of prostate cancer patients most associated with vitamin D deficiency and ADT’s relation to this.
To examine how prevalent vitamin D deficiency is among prostate cancer patients in a sun-rich environment, with focus on differences by race and disease stage. It also assessed whether ADT is associated with changes in vitamin D levels.
Prostate cancer patients treated at Chao Family Comprehensive Cancer Center between 2014-2024 were retrospectively studied with regards to vitamin D levels across racial groups, disease stages, and ADT exposure. Changes in vitamin D levels pre- and post-ADT over 24 months were assessed by statistical methods including paired t-tests.
Among 120 patients (mean age: 74 years, mean body mass index: 27.6 kg/m²), African American (33.3%) and Hispanic (31.8%) patients had the greatest prevalence of vitamin D deficiency (< 20 ng/mL). With a 28.6% deficit rate, me
Vitamin D deficiency is common in prostate cancer patients, especially racial minorities and those with advanced disease, despite residing in an area with high sun exposure. ADT does not significantly impact vitamin D levels in the short term. Routine screening and supplementation should be considered in these high-risk groups.
Core Tip: This retrospective study investigates the prevalence of vitamin D deficiency in prostate cancer patients who received treatment in Southern California, a region characterized by high annual sun exposure. It underscores the high deficiency rates among advanced-stage disease patients, as well as Hispanic and Black patients. The study also assesses the impact of androgen deprivation therapy on vitamin D and concludes that there is no substantial change in response to treatment. These results provide evidence for routine screening and supplementation of vitamin D, particularly in high-risk subgroups, and challenges the notion that sun exposure alone guarantees adequate vitamin D levels.
- Citation: Hasan N, Rafizadeh D, Gibson S, Kaakour D, Lee B, Khaleghi B, Yazdanpanah O, Rezazadeh Kalebasty A. Vitamin D deficiency in prostate cancer: Prevalence in a sun-rich climate and influence of androgen deprivation therapy. World J Clin Oncol 2025; 16(6): 108113
- URL: https://www.wjgnet.com/2218-4333/full/v16/i6/108113.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i6.108113
Vitamin D is crucial for preserving bone health by facilitating calcium absorption in the intestines and sustaining appropriate serum calcium and phosphate levels, which are vital for bone mineralization[1]. It protects against osteomalacia in adults, and in conjunction with calcium, it aids in safeguarding older populations against osteoporosis[2]. The active form of vitamin D, 1,25-dihydroxyvitamin D (calcitriol), interacts with the vitamin D receptor in the intestines to augment the absorption of dietary calcium and phosphate[3]. Vitamin D also possesses considerable immunomodulatory effects. The vitamin D receptor is present in multiple immune cells, such as B and T lymphocytes, monocytes, and macrophages[3]. Both adaptive and innate immune responses are influenced by vitamin D. It enhances the pathogen-fighting capabilities of monocytes and macrophages, facilitates the differentiation of monocytes into macrophages, and reduces the synthesis of inflammatory cytokines by T helper cells[4]. These properties suggest that adequate vitamin D levels are essential for maintaining a robust immune system and may reduce the likelihood of autoimmune disorders and infections[4].
Numerous studies have demonstrated that vitamin D deficiency correlates with a heightened risk of aggressive prostate cancer. Zhang et al[5] performed an experimental study utilizing both in vivo and in vitro mouse models, revealing that vitamin D deficiency can intensify the growth and metastasis of prostate cancer via mechanisms related to epithelial-mesenchymal transition and β-catenin pathways[5]. This indicates that vitamin D may influence the behavior and growth of cancer cells. Epidemiological data further corroborates this association. Li et al[6] discovered that men exhibiting diminished levels of both 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D faced a markedly elevated risk of aggressive prostate cancer, especially among those with specific vitamin D receptor polymorphisms. This emphasizes the likely genetic links influencing the function of vitamin D on prostate cancer risk. Furthermore, a case-control study by Gilbert et al[7] found that men with low levels of 25-hydroxyvitamin D were more likely than those with adequate levels to have progressed and high-grade prostate cancer. This information supports the theory that more severe forms of prostate cancer could be related with vitamin D deficiency.
Numerous studies have shown that androgen deprivation therapy (ADT) is linked to considerable bone loss, especially during the initial year of treatment. Alibhai et al[8] indicated that men starting ADT, due to a subsequent decrease in androgen levels, encountered substantial reductions in bone mineral density, particularly in the lumbar spine, during the initial year of treatment[8]. Peppone et al[9] similarly discovered that ADT resulted in a significant decrease in bone mineral density at the hip and femoral neck, likely by increasing bone resorption and decreasing bone formation[9].
The research related to the effects of ADT on vitamin D levels is inconclusive. Although numerous studies indicate a reduction in vitamin D levels subsequent to ADT, the evidence remains somewhat inconsistent. Hara et al[10] conducted a prospective cohort study that demonstrated a significant decrease in serum 1,25-dihydroxyvitamin D3 levels in men with prostate cancer after six months of ADT[10]. Khalil et al[11] conducted a prospective cohort study that similarly demonstrated decreased levels of 1,25-dihydroxyvitamin D3 in men who were undergoing ADT. This finding suggests that the therapy may have an initial impact on bone and mineral homeostasis[11]. Nonetheless, alternative investigations have not consistently demonstrated a causal correlation between ADT and vitamin D insufficiency. The review by Datta and Schwartz[12] indicated that whereas ADT is linked to a decrease in bone mineral density, the efficacy of vitamin D supplementation in mitigating this loss is ambiguous. Consequently, although substantial data indicates that ADT may result in reduced vitamin D levels, the results are not uniformly consistent and additional research is needed to reach a stronger conclusion.
Androgens are crucial for maintaining bone density and regulating vitamin D metabolism. Androgens directly stimulate osteoblast activity and inhibit osteoclasts, hence promoting bone growth and reducing resorption[11]. They also enhance renal expression of 1α-hydroxylase, the enzyme responsible for catalyzing the conversion of 25-hydroxyvitamin D into its active form, 1,25-dihydroxyvitamin D[11]. Thus, the inhibition of androgens by ADT not only hastens bone resorption but may also hinder the synthesis and activation of vitamin D. These methods highlight the theory that ADT may affect vitamin D levels, alongside its established impact on bone health.
In spite of the ample sunlight in Southern California, vitamin D deficiency is prevalent among many demographics, including individuals with chronic conditions such as cancer. We hypothesize that deficiency may be more common among prostate cancer patients due to factors such as age, racial differences, and possible treatment-related consequences. A pillar treatment for advanced and metastatic prostate cancer, ADT, is clearly linked to lower bone mineral density and higher risk of osteoporosis and fractures. Nonetheless, its effect on vitamin D levels remains less clear. ADT may also contribute to vitamin D depletion, as it can result in reduced bone metabolism and increased bone resorption[11]. To guide nutritional advice, strategies on supplementation, and general supportive care in this population, it is crucial to investigate whether patients with prostate cancer are prone to vitamin D deficiency and whether ADT contributes to this.
This study assesses whether ADT contributes to a decrease of vitamin D levels and investigates the prevalence of vitamin D deficiency among prostate cancer patients undergoing treatment at a tertiary cancer center situated in Southern California, a region with high annual sunlight exposure (4580 kJ/m²), well above the United States average (3636 kJ/m²)[13]. Considering the unequal impact of deficiency on racial minorities and patients with advanced-stage disease, we also investigate whether particular populations are more adversely affected. We suspect that prostate cancer patients residing in a sun-abundant climate continue to suffer from considerable vitamin D deficiency, especially among ethnic minorities and individuals with advanced-stage disease. We hypothesize that ADT may further deplete vitamin D levels, potentially impacting long-term skeletal and metabolic health in this patient population.
This is a retrospective cohort study that uses electronic medical records from the Chao Family Comprehensive Cancer Center to look at vitamin D levels in men with prostate cancer and investigate whether ADT might have an effect on those levels. The study looked at patient data from January 2014 to January 2024. This allowed for the gathering of clinical and biochemical data on men with prostate cancer who were treated at this hospital.
As this is a retrospective study, all the data came from medical records that were already in place. The study group was selected according to the participation criteria. We collected and analyzed pertinent clinical characteristics, including serum vitamin D concentrations, demographic information, prostate cancer stage, ADT exposure, and duration of treatment.
This study includes patients determined by an established inclusion and exclusion criteria. The aim was to evaluate prostate cancer patients’ vitamin D levels and ascertain whether exposure to ADT influences vitamin D status.
Inclusion criteria: To ensure a clinically relevant cohort, patients were eligible for inclusion if they met the following criteria: Diagnosis of prostate cancer at any disease stage, at least one recorded serum vitamin D level measurement at the time of diagnosis or preceding initial prostate cancer treatment. For secondary analysis, patients receiving ADT with a documented pre-ADT vitamin D level were included to assess changes in vitamin D levels following ADT initiation.
Exclusion criteria: Patients were excluded from the study if they met any of the following conditions: Incomplete medical records particularly those missing baseline vitamin D levels, cancer staging, or treatment details. Patients already on vitamin D supplementation at baseline were excluded as this could confound the assessment of vitamin D status in relation to prostate cancer and ADT. We also excluded patients with conditions known to affect vitamin D levels, such as severe gastrointestinal disorders, parathyroid disease, chronic liver or kidney disease, as well as those taking medications like phenobarbital, carbamazepine, spironolactone, rifampicin, or ritonavir.
Baseline characteristics: Demographic and clinical variables were collected for all eligible patients: Age (years) at prostate cancer diagnosis, race/ethnicity categorized as non-Hispanic White, Black, Hispanic, and Asian, body mass index (BMI), prostate cancer stage at diagnosis with further stratification of stage 4 patients into metastatic castration-sensitive prostate cancer (mCSPC) and metastatic castration-resistant prostate cancer (mCRPC).
Vitamin D levels: Serum vitamin D levels (measured as 25-hydroxyvitamin D) were recorded at two time points: Pre-ADT vitamin D levels defined as the earliest available vitamin D measurement at or near the time of prostate cancer diagnosis. Post-ADT vitamin D levels defined as the most recent available vitamin D measurement within 24 months following ADT initiation. Patients without documented pre-ADT or post-ADT vitamin D levels were excluded from secondary analyses assessing changes in vitamin D levels.
Treatment variables: The median duration of ADT therapy was recorded for all ADT-treated patients. To evaluate potential differences in ADT effects, stratification by cancer stage was performed and ADT duration was analyzed within each stage group (stage 2, 3, and 4) and further categorized into mCSPC and mCRPC subgroups for metastatic disease.
Potential confounders: To improve the reliability of the findings, the following confounders were accounted for during statistical analyses: BMI as obesity is associated with lower vitamin D levels. Seasonal effects were recorded given the known seasonal variation and influence of sunlight exposure in vitamin D synthesis. The month of vitamin D mea
All analyses were conducted using Intercooled Stata, Version 18.0 (StataCorp, College Station, TX, United States). The frequency of vitamin D deficiency in prostate cancer patients was investigated statistically; variations between race and disease stage subgroups were evaluated; and whether ADT was linked with changes in serum vitamin D levels over time. For all analyses, a P value of 0.05 was regarded as statistically significant.
Descriptive statistics: Mean ± SD, median, and interquartile range (IQR) were used to summarize continuous variables including age, BMI, blood vitamin D levels, and ADT duration. Counts (n) and percentages (%), helped to summarize categorical data including race, cancer stage, and vitamin D deficiency status.
Comparative analyses: Differences in vitamin D deficiency prevalence across racial groups (Hispanic, Black, Non-Hispanic White, Asian) and cancer stage categories (stage 2, 3, 4, mCSPC, mCRPC) were assessed using categorical statistical tests. Differences in ADT duration were examined across vitamin D level groups (< 30 ng/mL, 30-49 ng/mL, ≥ 50 ng/mL) and cancer stage categories (stage 4 vs earlier-stage disease).
Correlation analyses: Spearman’s rank correlation coefficient (r) was used to evaluate potential associations between vitamin D levels and cancer stage, vitamin D levels and BMI, and vitamin D levels and age.
Pre-ADT vs post-ADT vitamin D levels: A paired-samples t-test was performed to compare pre-ADT and post-ADT vitamin D levels in patients with available repeat measurements within 24 months of ADT initiation. Subgroup analyses were conducted to assess whether changes in vitamin D levels were influenced by season of diagnosis, patient age (≥ 65 years), or advanced-stage disease.
Our analysis comprised of 120 prostate cancer patients in all. Table 1 shows the mean age at diagnosis was 74 years (SD = 9.5) and the mean BMI was 27.6 (SD = 5.36). While 16.67% had stage 2 and 20.83% had stage 3, most patients had stage 4 disease (62.5%). Of patients in stage 4, 63.4% had mCSPC and 36.6% had mCRPC. The cohort consisted of 57% Non-Hispanic White, 20.7% Asian, 19% Hispanic, and 3.3% Black. At diagnosis, the median blood vitamin D level was 35.4 ng/mL, with levels ranging between 7.8 and 120 ng/mL. Of the overall sample, 12.5% of the patients showed vitamin D deficiency (< 20 ng/mL).
| Characteristic | Value |
| Mean age | 74 ± 9.5 |
| Mean BMI | 27.60 ± 5.36 |
| Stage 2 disease (n = 120) | 20 (16.67) |
| Stage 3 disease (n = 120) | 25 (20.83) |
| Stage 4 disease (n = 120) | 75 (62.5) |
| Percent mCSPC from stage 4 (n = 75) | 47 (63.4) |
| Percent mCRPC from stage 4 (n = 75) | 28 (36.6) |
| Hispanic (n = 120) | 23 (19) |
| Asian (n = 120) | 25 (20.7) |
| Non-Hispanic White (n = 120) | 69 (57) |
| Black (n = 120) | 4 (3.3) |
| Median serum vitamin D level (ng/mL) | 35.4 (7.8-120) |
| Vitamin D deficiency at diagnosis (%) (< 20 ng/mL) | 12.50 |
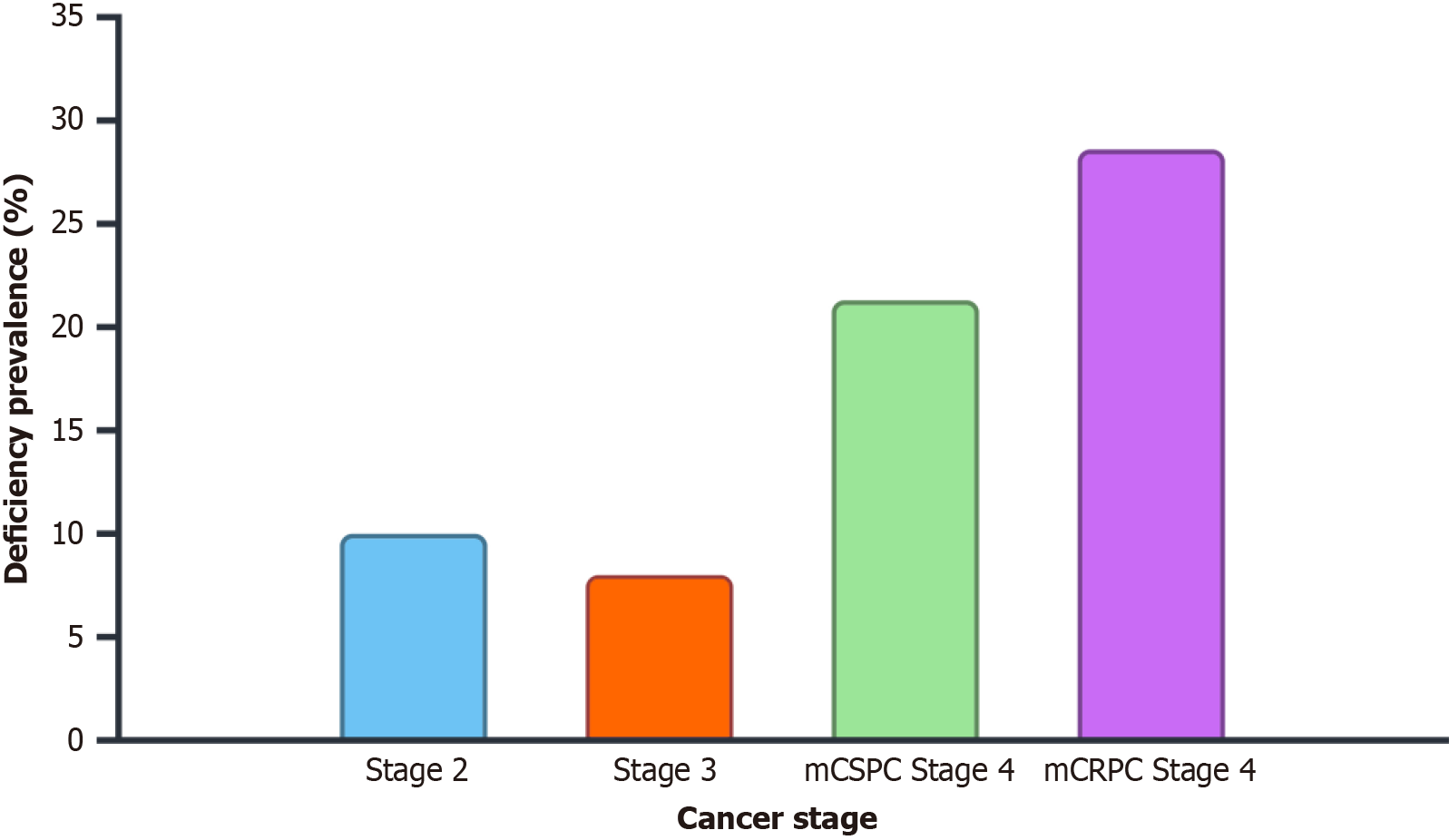
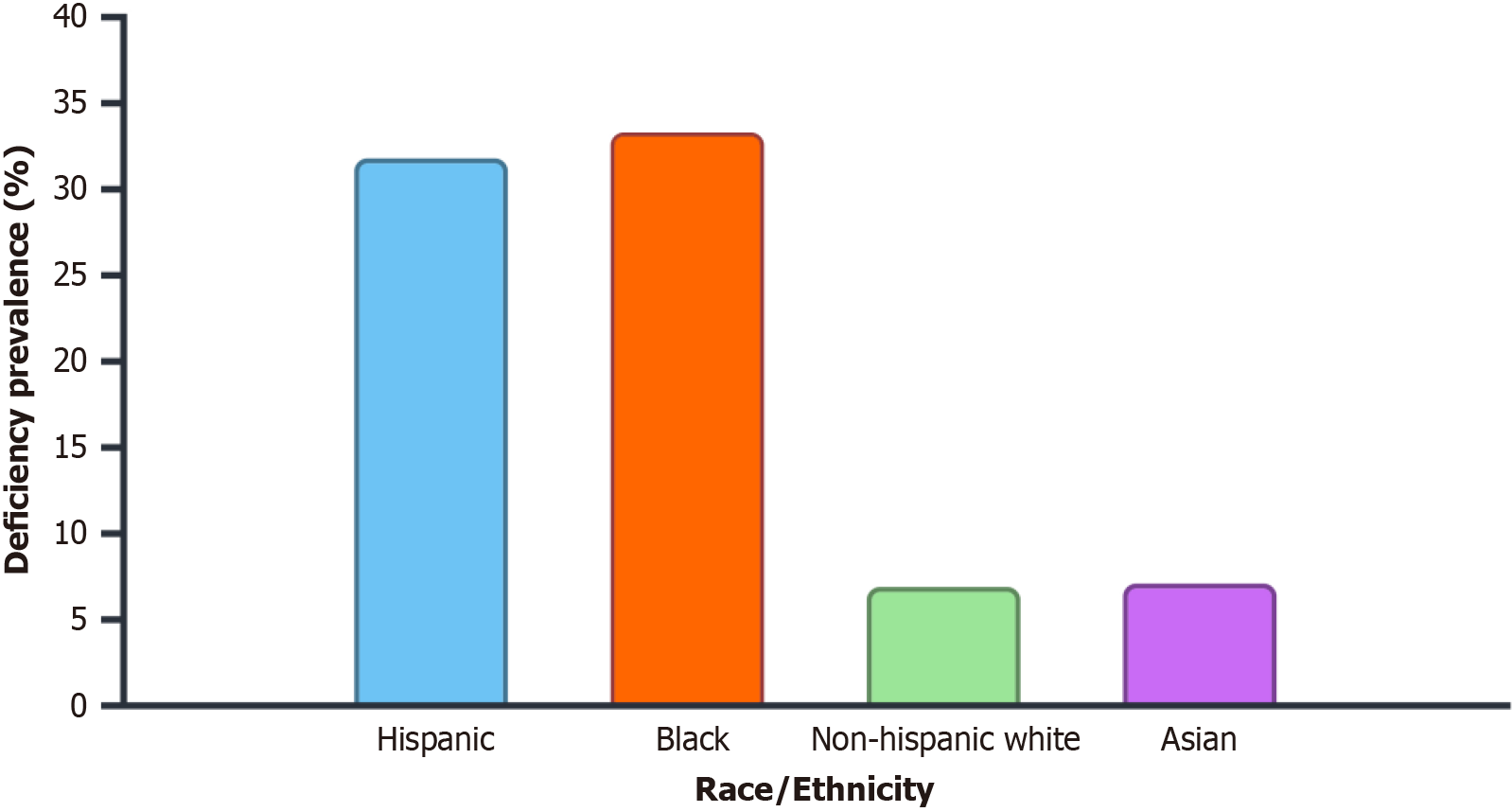
At diagnosis, 12.5% of patients had vitamin D deficiency overall. 10.0% of stage 2 patients, 8.0% of stage 3 patients, and 14.7% of stage 4 patients had a vitamin D deficiency. Among metastatic patients, mCSPC cases had a 21.3% deficiency rate and mCRPC cases had a 28.6% deficiency rate (Table 2 and Figure 1). Whereas deficiency rates were much lower in Non-Hispanic Whites (6.9%) and Asians (7.1%), vitamin D deficiency was most common in Black (33.3%) and Hispanic (31.8%) patients (Table 3 and Figure 2).
| Stage | Deficiency rate (%) |
| Stage 2 (n = 20) | 2 (10) |
| Stage 3 (n = 25) | 2 (8) |
| Overall stage 4 (n = 75) | 11 (14.7) |
| mCSPC stage 4 (n = 47) | 10 (21.3) |
| mCRPC stage 4 (n = 28) | 8 (28.6) |
| Stage | Deficiency rate (%) |
| Hispanic (n = 22) | 7 (31.8) |
| Black (n = 6) | 2 (33.3) |
| Non-Hispanic White (n = 58) | 4 (6.9) |
| Asian (n = 28) | 8 (7.1) |
With r = 0.07, P = 0.46, Spearman’s correlation revealed no appreciable relationship between vitamin D levels and cancer stage. Though it did not approach statistical significance, a modest negative connection was noted between vitamin D levels and BMI (r = -0.14, P = 0.13). In contrast, statistically significant by weak positive correlation was found between vitamin D levels and age (r = 0.23, P = 0.013) (Table 4).
| Variable | Correlation (r) | P value |
| Vitamin D vs stage | 0.07 | 0.46 |
| Vitamin D vs BMI | -0.14 | 0.13 |
| Vitamin D vs age | 0.23 | 0.013 |
The median duration of ADT therapy was 18 months (IQR: 10-33.5) (Table 5). ADT duration was similar across vitamin D level groups, with patients in the < 30 ng/mL group receiving a median of 18 months (IQR: 10-41), those in the 30-49 ng/mL group receiving 18 months (IQR: 8-30), and those in the ≥ 50 ng/mL group receiving 20 months (IQR: 14-28). However, patients with stage 4 disease had a significantly longer ADT duration of 21 months (IQR: 12-39), compared to 14 months (IQR: 8-20) in earlier-stage patients (P = 0.05).
| Vitamin D level group | Duration of ADT therapy (months), median (IQR) |
| Overall | 18 (10-33.5) |
| < 30 ng/mL | 18 (10-41) |
| 30-49 ng/mL | 18 (8-30) |
| ≥ 50 ng/mL | 20 (14-28) |
| Stage 2 and 3 | 14 (8-20) |
| Stage 4 | 21 (12-39) |
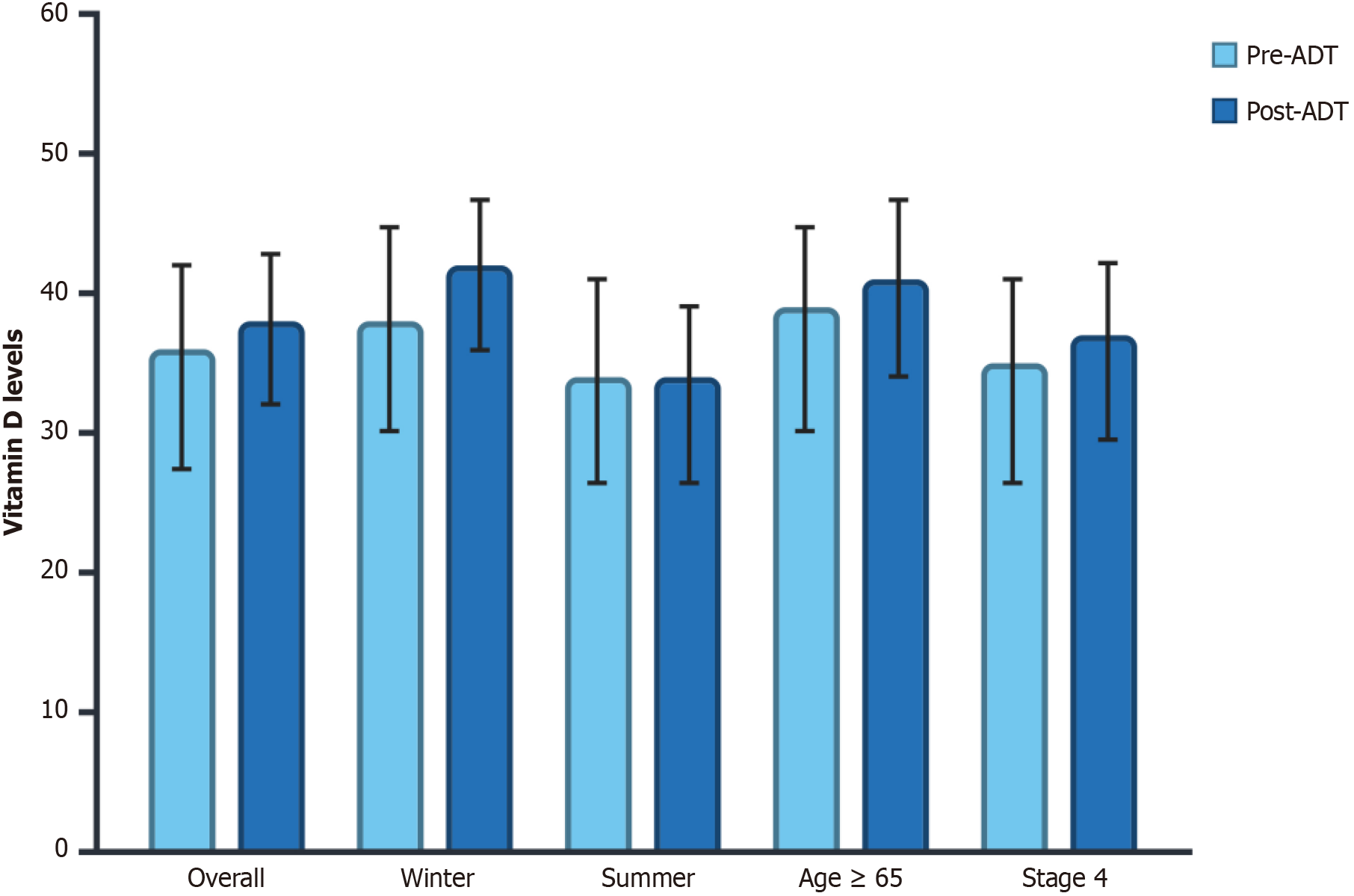
Twenty-six of the included patients (21.7%) had repeat serum vitamin D levels within 24 months of ADT initiation. Among these patients, a paired-samples t-test showed no significant difference between pre-ADT (36 ± 20 ng/mL) and post-ADT (38 ± 19 ng/mL) vitamin D levels (P = 0.45) (Table 6). Analysis of subgroups showed no significant variations in vitamin D levels among different clinical factors. Post-ADT vitamin D levels did not show any appreciable variation between measurements conducted in the winter (38 ± 20 vs 42 ± 18; P = 0.30) or summer (34 ± 20 vs 34 ± 21; P = 0.95). Similarly, patients aged ≥ 65 years showed no significant difference in pre- vs post-ADT vitamin D levels (39 ± 20 vs 41 ± 21 ng/mL, P = 0.70). A comparable lack of difference was observed in patients with advanced-stage disease (35 ± 20 vs 37 ± 20 ng/mL, P = 0.53) (Figure 3).
| Category | Pre-ADT | Post-ADT | P value |
| Overall pre-ADT vs post-ADT | 36 ± 20 | 38 ± 19 | 0.45 |
| Winter diagnosis | 38 ± 20 | 42 ± 18 | 0.3 |
| Summer diagnosis | 34 ± 20 | 34 ± 21 | 0.95 |
| Older adults (≥ 65 years) | 39 ± 20 | 41 ± 21 | 0.7 |
| Stage 4 | 35 ± 20 | 37 ± 20 | 0.53 |
Stage: Our results revealed that vitamin D deficiency overall was present in 12.5% of prostate cancer patients at diagnosis. Rates were consistently higher in metastatic disease. mCRPC patients had the highest deficiency rate (28.6%), which was higher than those observed in mCSPC patients (21.3%) and those with earlier-stage disease (8.0%-10.0%). These results imply that vitamin D level could be influenced by disease severity. In the cross-sectional study by Trump et al[14], 18% of men with localized prostate cancer were found to be vitamin D deficient (< 20 ng/mL) compared to the higher rate of 40% in men with recurrent prostate cancer which included those with metastatic disease. There may be clinical disparities in prostate cancer related to this. One study found, for instance, that African American men with vitamin D deficiency had an odds ratio of 3.1 for aggressive prostate cancer when their serum vitamin D levels fell below 20 ng/mL. Another study found that African American men with notable vitamin D inadequacy (< 12 ng/mL) had advanced tumor stages and higher Gleason scores. Vitamin D deficiency is more prevalent in patients with more aggressive and advanced prostate cancer due to several mechanisms. Zhang et al[5] demonstrated that vitamin D deficiency can exacerbate the growth and metastasis of prostate cancer in mouse models by promoting epithelial-mesenchymal transition through β-catenin-related mechanisms, which are critical for cancer cell invasion and metastasis. There is also a possibility that inflammation is involved. High levels of inflammatory markers like C-reactive protein and interleukin-8 were found in those with prostate cancer who also had low levels of vitamin D and these markers are linked to more severe disease[15].
The rates of deficiency found in our study, regardless of prostate cancer stage, are in contrast to the general population, where 5% of United States citizens had deficient vitamin D levels, according to data from the National Health and Nutrition Examination Survey 2011-2014[16]. These differences highlight the likelihood that patients with prostate cancer may have greater vitamin D deficiency compared to the general population. One key component is that prostate cancer cells have decreased activity of the enzyme 1α-hydroxylase, which converts 25-hydroxyvitamin D into its active form, 1,25-dihydroxyvitamin D. This reduced enzymatic activity limits the local synthesis of active vitamin D inside the prostate, therefore lowering overall vitamin D levels in patients with prostate cancer[17].
Race: Patients with advanced disease and racial minority groups had the highest deficit rates. Deficiency rates were greater among Black (33.3%) and Hispanic (31.8%) patients than among Non-Hispanic White (6.9%) and Asian (7.1%) patients. There is historical evidence of racial differences in vitamin D insufficiency among patients with prostate cancer, with African American men being disproportionately affected. Several studies found that African American men suffer disproportionately from prostate cancer and vitamin D deficiency[18]. Globally, the incidence is high in Australia/New Zealand, North America, and Europe, and the highest incidence is in Black people. African Americans are more likely to have vitamin D deficiency due to disparities in dietary consumption and supplementing habits as well as lower cutaneous vitamin D production[19,20].
ADT: In contrast to the hypothesis that ADT could reduce vitamin D levels, there was no significant difference between pre-ADT and post-ADT vitamin D levels among patients who had repeat levels within 24 months of the initiation of ADT (P = 0.45). Subgroup analysis also indicated that post-ADT vitamin D levels were not significantly influenced by cancer stage, older age (≥ 65 years), or seasonal changes. Our results imply that the main driver of vitamin D depletion in this patient population may not primarily be ADT. The lack of a significant decline in vitamin D levels following ADT initiation contrasts with some prior studies that have suggested a potential relationship between ADT and impaired vitamin D metabolism. Hara et al[10] reported that serum 1,25-dihydroxyvitamin D3 levels decreased significantly within six months of ADT initiation, suggesting that androgen deprivation may alter vitamin D homeostasis via its effects on calcium metabolism and bone turnover. Khalil et al[11] also discovered that patients who were treated with ADT had lower levels of 1,25-dihydroxyvitamin D than controls who were not treated with ADT[11]. Not all studies, however, have shown a direct link between ADT and vitamin D levels. In their review of the existing literature, Datta and Schwartz[12] determined that, despite the significant association between ADT and bone loss, there is inconclusive evidence linking ADT to vitamin D depletion. This broader uncertainty is consistent with the results of our investigation, which did not identify a substantial decline in serum vitamin D levels following ADT.
Climate: Although we conducted our study in Southern California, an area with strong year-round sun exposure, our results show that a sizable proportion of prostate cancer patients, 12.5%, still have untreated vitamin D deficiency. Centers for Disease Control and Prevention data on annual average solar irradiation shows Southern California gets roughly 4580 kJ/m² of sunlight annually, compared to the national average of 3636 kJ/m², an almost 26% difference[13]. The expectation that residing in a sun-rich climate would protect against deficiency in this population was not supported by our data. The fact that seasonal variation (winter vs summer diagnosis) did not significantly impact post-ADT vitamin D levels (P = 0.30, P = 0.95, respectively) further suggests that individual sun exposure behaviors and endogenous factors play a greater role than simple geographic sun availability. One such factor is the altered expression of vitamin D metabolizing enzymes in prostate cancer cells. Prostate cancer tissues have revealed, for example, higher expression of CYP24A1, which deactivates vitamin D, and lower expression of CYP27B1, which activates vitamin D. This imbalance reduces the bioavailability of active vitamin D, regardless of sun exposure[21-23]. Prostate diseases and aging might further disrupt vitamin D metabolism. In men, CYP27B1 expression drops as they become older whereas CYP24A1 rises, which lowers local synthesis of active vitamin D in the prostate. This is exacerbated by a lower expression of the vitamin D receptor, therefore limiting the protective role of vitamin D signaling[24]. Finally, even in sunny climates, many people might not get enough sun exposure because of lifestyle choices including indoor activities, sunscreen application, or protective clothing.
Several strengths of this study help to explain its relevance. First, it is among the few studies looking at a prostate cancer group living in a sun-rich environment for vitamin D deficiency. Although vitamin D deficiency is usually linked to low sun exposure, our results show that deficiency is rather common even in Southern California. Second, this study provides a detailed analysis of vitamin D status in metastatic prostate cancer, specifically distinguishing between mCSPC and mCRPC patients. Prior studies have generally focused on localized prostate cancer or grouped metastatic patients together, making it difficult to discern whether vitamin D deficiency correlates with disease progression. Our results suggest that mCRPC patients have the highest rates of deficiency, which warrants more research on the possible relation of vitamin D metabolism in disease aggressiveness. Third, this study incorporates a racial subgroup analysis, therefore adding to the expanding body of data on variations in vitamin D levels among prostate cancer patients.
This study has some constraints inherent in the retroactive approach despite the advantages. The study’s retrospective nature limits causal conclusions. Although patients with metastatic prostate cancer showed more vitamin D insufficiency, we are not certain if deficiency caused the disease to proceed or resulted from advanced disease. Establishing a temporal link would require a prospective study design including serial vitamin D assessments at several times periods both before and after illness onset. Finally, longer follow-up times and a bigger sample size might help determine whether ADT causes a gradual drop in vitamin D levels over time.
This study’s findings have significant clinical implications for managing vitamin D deficiency in prostate cancer patients, especially among ethnic minorities and individuals with advanced-stage disease. Despite occurring in a sun-abundant environment, vitamin D deficiency persisted in a considerable percentage of these patients, underscoring the necessity for regular screening and supplementation, particularly for high-risk populations such as African American and Hispanic individuals. Particularly for patients with mCRPC, who had the highest deficiency rates in our study, clinicians ought to include regular vitamin D level evaluations into thorough prostate cancer management considering the correlation between vitamin D deficiency and advanced prostate cancer. Although ADT had no appreciable effect on vitamin D levels in the short run, more research is needed on the long term metabolic effects of androgen deprivation on bone health and vitamin D metabolism.
Future studies should look at whether prolonged ADT causes cumulative vitamin D deficiency and if so, whether early vitamin D treatment might mitigate harmful skeletal effects. Moreover, future studies with larger cohorts and longer follow-up periods are crucial to clarify the temporal connection between vitamin D insufficiency and the progression of prostate cancer.
This study emphasizes that even in places with high sun exposure there is a need for vitamin D monitoring in prostate cancer patients. Important directions for future studies are addressing racial differences in vitamin D deficiency and looking at long-term consequences of ADT on vitamin D metabolism. Advancing our knowledge on the role of vitamin D in prostate cancer biology and treatment outcomes will help us to create more tailored plans for management and prevention of vitamin D deficiency in this patient population.
| 1. | Gil Á, Plaza-Diaz J, Mesa MD. Vitamin D: Classic and Novel Actions. Ann Nutr Metab. 2018;72:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 325] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 2. | Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, Lips P, Munns CF, Lazaretti-Castro M, Giustina A, Bilezikian J. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr Rev. 2019;40:1109-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 670] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 3. | Khammissa RAG, Fourie J, Motswaledi MH, Ballyram R, Lemmer J, Feller L. The Biological Activities of Vitamin D and Its Receptor in Relation to Calcium and Bone Homeostasis, Cancer, Immune and Cardiovascular Systems, Skin Biology, and Oral Health. Biomed Res Int. 2018;2018:9276380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 4. | Charoenngam N, Holick MF. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 560] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 5. | Zhang ZH, Liu MD, Yao K, Xu S, Yu DX, Xie DD, Xu DX. Vitamin D deficiency aggravates growth and metastasis of prostate cancer through promoting EMT in two β-catenin-related mechanisms. J Nutr Biochem. 2023;111:109177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 6. | Li H, Stampfer MJ, Hollis JB, Mucci LA, Gaziano JM, Hunter D, Giovannucci EL, Ma J. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med. 2007;4:e103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 203] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Gilbert R, Metcalfe C, Fraser WD, Donovan J, Hamdy F, Neal DE, Lane JA, Martin RM. Associations of circulating 25-hydroxyvitamin D with prostate cancer diagnosis, stage and grade. Int J Cancer. 2012;131:1187-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Alibhai SM, Mohamedali HZ, Gulamhusein H, Panju AH, Breunis H, Timilshina N, Fleshner N, Krahn MD, Naglie G, Tannock IF, Tomlinson G, Warde P, Duff Canning S, Cheung AM. Changes in bone mineral density in men starting androgen deprivation therapy and the protective role of vitamin D. Osteoporos Int. 2013;24:2571-2579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Peppone LJ, Kleckner AS, Fung C, Puzas JE, Reschke JE, Culakova E, Inglis J, Kamen C, Friedberg JW, Janelsins M, Mustian K, Heckler CE, Mohile S. High-dose vitamin D to attenuate bone loss in patients with prostate cancer on androgen deprivation therapy: A phase 2 RCT. Cancer. 2024;130:2538-2551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Hara N, Ishizaki F, Saito T, Nishiyama T, Kawasaki T, Takahashi K. Decrease in lean body mass in men with prostate cancer receiving androgen deprivation therapy: mechanism and biomarkers. Urology. 2013;81:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Khalil R, Antonio L, Laurent MR, David K, Kim NR, Evenepoel P, Eisenhauer A, Heuser A, Cavalier E, Khosla S, Claessens F, Vanderschueren D, Decallonne B. Early effects of androgen deprivation on bone and mineral homeostasis in adult men: a prospective cohort study. Eur J Endocrinol. 2020;183:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Datta M, Schwartz GG. Calcium and vitamin D supplementation during androgen deprivation therapy for prostate cancer: a critical review. Oncologist. 2012;17:1171-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Amos HM, Skaff NK, Uz SS, Policelli FS, Slayback D, Macorps E, Jo MJ, Patel K, Keller CA, Abue P, Buchard V, Werner AK. Public Health Data Applications Using the CDC Tracking Network: Augmenting Environmental Hazard Information With Lower-Latency NASA Data. Geohealth. 2023;7:e2023GH000971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Trump DL, Chadha MK, Sunga AY, Fakih MG, Ashraf U, Silliman CG, Hollis BW, Nesline MK, Tian L, Tan W, Johnson CS. Vitamin D deficiency and insufficiency among patients with prostate cancer. BJU Int. 2009;104:909-914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Xie DD, Chen YH, Xu S, Zhang C, Wang DM, Wang H, Chen L, Zhang ZH, Xia MZ, Xu DX, Yu DX. Low vitamin D status is associated with inflammation in patients with prostate cancer. Oncotarget. 2017;8:22076-22085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Herrick KA, Storandt RJ, Afful J, Pfeiffer CM, Schleicher RL, Gahche JJ, Potischman N. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 211] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 17. | Ma JF, Nonn L, Campbell MJ, Hewison M, Feldman D, Peehl DM. Mechanisms of decreased Vitamin D 1alpha-hydroxylase activity in prostate cancer cells. Mol Cell Endocrinol. 2004;221:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Duraki A, Krieger KD, Nonn L. The double disparity: Vitamin D deficiency and lethal prostate cancer in black men. J Steroid Biochem Mol Biol. 2025;247:106675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Ames BN, Grant WB, Willett WC. Does the High Prevalence of Vitamin D Deficiency in African Americans Contribute to Health Disparities? Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 20. | Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr. 2005;135:2478-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Nelson SM, Batai K, Ahaghotu C, Agurs-Collins T, Kittles RA. Association between Serum 25-Hydroxy-Vitamin D and Aggressive Prostate Cancer in African American Men. Nutrients. 2016;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Murphy AB, Nyame Y, Martin IK, Catalona WJ, Hollowell CM, Nadler RB, Kozlowski JM, Perry KT, Kajdacsy-Balla A, Kittles R. Vitamin D deficiency predicts prostate biopsy outcomes. Clin Cancer Res. 2014;20:2289-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Campolina-Silva GH, Barata MC, Werneck-Gomes H, Maria BT, Mahecha GAB, Belleannée C, Oliveira CA. Altered expression of the vitamin D metabolizing enzymes CYP27B1 and CYP24A1 under the context of prostate aging and pathologies. J Steroid Biochem Mol Biol. 2021;209:105832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Schafer EJ, Laversanne M, Sung H, Soerjomataram I, Briganti A, Dahut W, Bray F, Jemal A. Recent Patterns and Trends in Global Prostate Cancer Incidence and Mortality: An Update. Eur Urol. 2025;87:302-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 15.0] [Reference Citation Analysis (0)] |