Published online Jun 24, 2025. doi: 10.5306/wjco.v16.i6.105601
Revised: April 8, 2025
Accepted: May 13, 2025
Published online: June 24, 2025
Processing time: 141 Days and 20.3 Hours
Pancreatic ductal adenocarcinoma (PDAC) is characterized by high aggressiveness, poor prognosis, and unsatisfactory survival rates. The incidence of PDAC is increasing annually, and thus, the number of deaths due to PDAC is increasing worldwide. Modern imaging modalities, including multidetector computed to
Core Tip: Pancreatic ductal adenocarcinoma is an aggressive malignancy with a delayed diagnosis and poor prognosis. The newest diagnostic tools and proper management are crucial for determining survival. The current approach must be multidisciplinary and individualized. Surgical resection alone is not enough; it must be accompanied by other novel the
- Citation: Pavlidis ET, Galanis IN, Pavlidis TE. Updates in the diagnosis and management of ductal adenocarcinoma of the pancreas. World J Clin Oncol 2025; 16(6): 105601
- URL: https://www.wjgnet.com/2218-4333/full/v16/i6/105601.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i6.105601
Pancreatic cancer is ductal adenocarcinoma (PDAC) in more than 90% of cases and is characterized by high aggressiveness, poor prognosis, and unsatisfactory survival rates posing considerable management challenges[1-4]. The median survival time is approximately 4 months for untreated patients with locally advanced or metastatic disease, and these patients account for up to 80% of cases. The incidence of PDAC is increasing annually, particularly in men, and, sub
The 5-year overall survival for all patients with PDAC fluctuates between 6% and 10%[9]; however, this rate may increase to 25% after curative surgical resection and adjuvant treatment[7,5]. In inoperable patients, treatment is limited to palliative treatment for symptom relief[8].
Early diagnosis and radical surgical excision offer a unique chance of long-term survival in patients with an otherwise poor prognosis. Unfortunately, at the time of diagnosis, only 10%-20% of patients are amenable to therapeutic operation[2]. Surgical resection is not recommended when distant metastasis is present; only palliative treatment and supportive care are indicated in inoperable cases[2]. In addition, surgery alone is insufficient instead of multimodal treatment to prevent micrometastasis[5,7].
A better understanding of the process of carcinogenesis, the tumor microenvironment, molecular alterations and gene mutations has led to novel treatment modalities. Adjuvant or even neoadjuvant systemic therapy, including che
On the basis of the high-grade malignancy of pancreatic cancer and the disappointing results of its management, this article highlights novel achievements and future research perspective efforts focused on achieving early diagnosis and proper management.
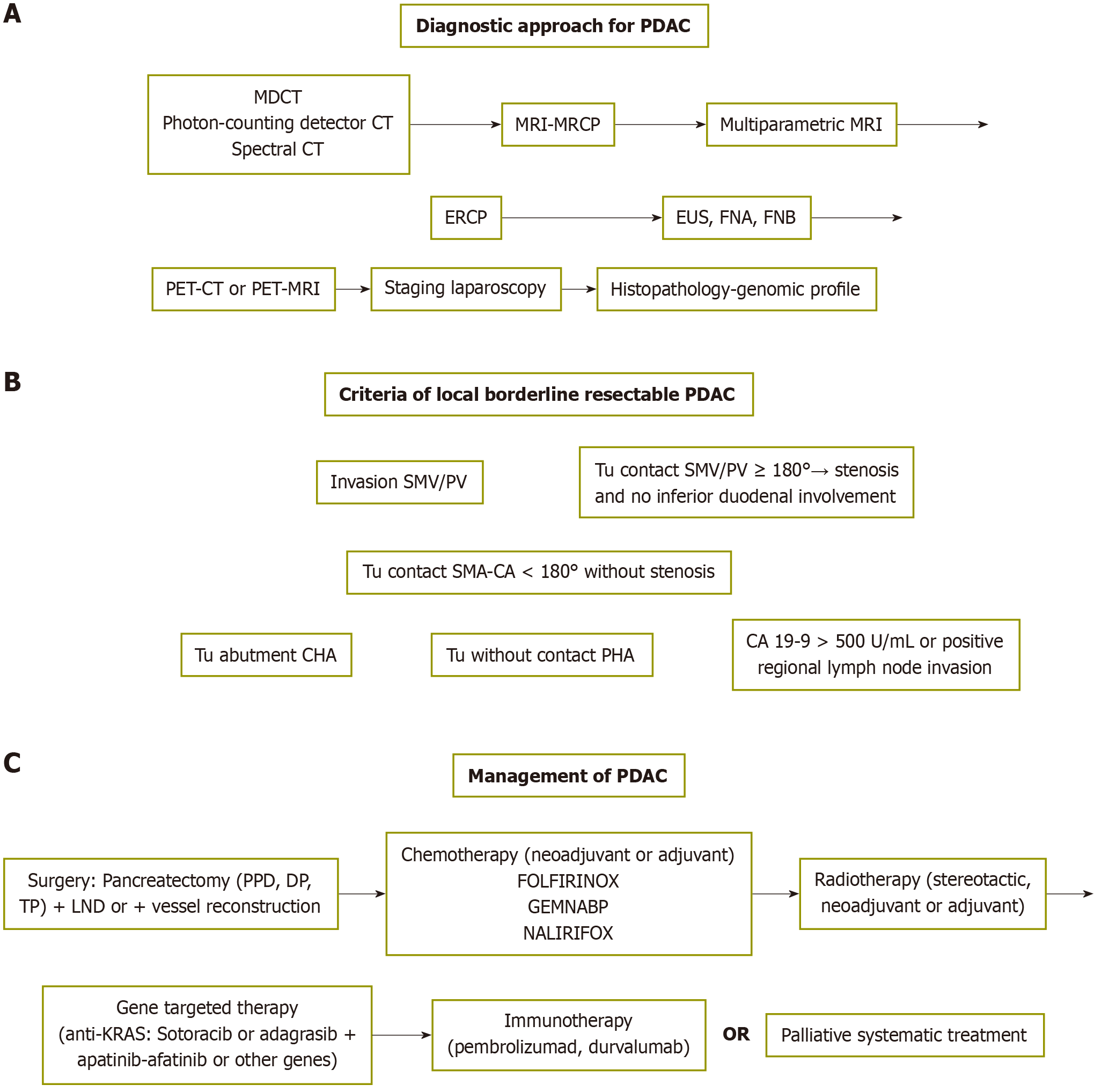
Modern imaging modalities for early accurate diagnosis include (in order of priority): (1) Three-phase contrast-enhanced multidetector computed tomography (CT) or novel photon-counting detector CT[13] and spectral CT for the detection of tumors, local or lymphatic spread, major vessel invasion and distant metastases; (2) Contrast-enhanced magnetic resonance imaging (MRI)-cholangiopancreatography for assessing local tumor conditions or distant metastases, preferably liver metastases and imaging of the common bile duct and pancreatic duct; (3) Endoscopic retrograde cholangiopancreatography (ERCP) for the diagnosis of pancreatic duct or common bile duct stenosis and stenting for palliative treatment in inoperable cases; (4) Positron emission tomography-CT for better staging and detection of small occult or obscure metastases not otherwise visible; and (5) Endoscopic ultrasound (EUS) in ambiguous cases and for obtaining tissue samples for biopsy or gene analysis[8,11].
Integrating recent advancements in artificial intelligence (AI)-assisted imaging and radiomics would provide a more up-to-date perspective[14]. Multiparametric MRI using artificial intelligence is a novel tool for evaluating intratumor characteristic findings that can accurately predict tumor biological behavior and prognosis[15] as well as the response to neoadjuvant treatment[16].
Fine needle aspiration (FNA) or fine-needle tissue biopsy (FNB) during EUS or under imaging guidance and positive cytology of aspirated pancreatic juice with the aid of ERCP safely establish the preoperative diagnosis[8]. The use of novel needles for EUS-FNB ensures that the diagnostic accuracy of core tissue biopsy exceeds 95%, thus replacing mostly traditional EUS-FNA[17].
The most reliable tumor marker that is used worldwide for early preoperative detection, early recurrence assessment, and prognosis prediction is carbohydrate antigen (CA 19-9) when its levels exceed 400 U/mL[3,5,8,18,19]. It is particularly useful for the early diagnosis of pancreatic cancer in patients with chronic pancreatitis and diabetes mellitus[20].
Liquid biopsy detection of tumor DNA, tumor parts or cancer cells can contribute to early diagnosis, therapeutic efficacy monitoring and follow-up[3]. The use of extracellular vesicles (EVs, particularly exosomes) as biomarkers of treatment efficacy increases liquid biopsy accuracy[21]. EVs and circulating tumor DNA (ctDNA) are novel emerging diagnostic tools, and they have the potential to change the management of malignant tumors by possible replacement of tissue biopsy. The diagnostic accuracy of these methods has been further enhanced by novel PCR and next-generation sequencing digital applications[22]. Perspective combination biomarker strategies, such as ctDNA with CA 19-9 or miRNAs, would provide additional value[14].
Histologic confirmation, not only the suspicion of pancreatic adenocarcinoma, is necessary before any surgical intervention or chemotherapy treatment. In cases of negative or unclear preoperative cancer histologic confirmation, it is better to wait for outcomes of liquid biopsy or the other newer molecular tests described herein before proceeding[23,24].
EUS-guided tissue acquisition is a novel valuable modality for the confirmation of PDAC, and its genomic profile evaluation can be used to plan accurate management[23].
In any case, early diagnosis remains challenging despite progress in liquid biopsy, miRNA analysis[25], genomic analysis[26,27], metastasis-associated protein analysis[7] and the identification of circulating cancer-derived exosomes[18]. However, there are difficulties and questions regarding the use of ctDNA liquid biopsy as a biomarker, which can be used to detect primary tumors early in ambiguous cases and determine the strong possibility for a high risk of metastases. Circulating DNA may originate from necrotic or apoptotic cells at primary or metastatic sites. In addition, the circulating cell-free fraction of DNA can be found under normal conditions, including injuries, exercise, inflammation and sepsis[26].
Several genetic and epigenetic changes have been identified that could be used as novel diagnostic biomarkers or promising therapeutic targets[28,29]. The most common gene mutations include those in the KRAS gene and its al
A scheme of the diagnostic approach for PDAC is shown in Figure 1A.
For the staging of PDAC, the American Joint Committee on Cancer 8th edition has been used[34], as shown in Tables 1 and 2. It is important in determining the management plan as well as survival; 5-year overall survival fluctuates between 3% for stage IV patients with distant metastases and 83.7% for early-stage IA patients[35]. In addition, an international consensus has defined the criteria for local borderline resectable cases of PDAD[36], as shown in Figure 1B.
| T | Greatest dimension (cm) |
| Tis | In situ |
| T1 | ≤ 2 |
| T1a | ≤ 0.5 |
| T1b | > 0.5 and < 1 |
| T1c | 1-2 |
| T2 | > 2 and ≤ 4 |
| T3 | > 4 |
| T4 | Involvement of celiac axis, superior mesenteric artery, and/or common hepatic artery |
| Stage | Parameters |
| 0 | Tis, N0, M0 |
| I | |
| Ia | T1, N0, M0 |
| Ib | T2, N0, M0 |
| II | |
| IIa | T3, N0, M0 |
| IIb | T1-T3, N1, M0 |
| III | Any T, N2, M0 or T4, any N, M0 |
| IV | Any T, any N, M1 |
PDAC is inoperable or unresectable in 80%-90% of cases because of locally advanced tumors or distant metastasis, including liver (50%) and peritoneal seeding (30%); in addition, there is overall lymph node involvement of 70%, either local or distant. Cancer is characterized as borderline resectable when it is in close proximity (part of major blood vessels or even invasion), indicating the need for neoadjuvant treatment[36]. The cancer is considered resectable when it is located in the pancreas or just around it[2,5,7,34]. The cornerstone of managing patients with resectable disease is curative surgical resection, followed by adjuvant treatment[5]. However, surgery alone is insufficient, and multimodal treatment is needed. The novel promising therapeutic modalities are immunotherapy and vaccination, gene-targeted therapy and biological agents that inhibit angiogenesis since the biology of pancreatic cancer is the factor that most strongly affects the outcome[7,9,37].
According to the tumor location, the surgical operation includes a. proximal pancreatoduodenectomy with standard lymphadenectomy, including the lymph nodes right to the superior mesenteric artery along with soft tissue around the pancreas, b. retrograde or antegrade distal pancreatectomy with or without spleen preservation or c. even total pancreatectomy[2,38-40]. Among the three types of pancreatectomy, R1 resection (infiltrated resection margins) results in microscopic residual tissue and accounts for 17%-19% of all cases[40]. Radical antegrade modular pancreatosplenectomy is preferable to standard retrograde pancreatosplenectomy for body and tail location, ensuring better lymph node clearance[38]. Vein resection without reconstruction has been proposed for locally advanced disease, increasing the resectability rate[41].
The number of retrieved lymph nodes is a reliable and auspicious prognostic indicator when the number of retrieved nodes is above 12 and without infiltration[42]. Nevertheless, infiltrated resection margins are often found by histopathological assessment, which indicates that systemic therapy is either adjuvant or neoadjuvant[43].
Laparoscopic or robotic pancreatectomy is an evolutionary surgical intervention in the current era of minimally invasive surgery with satisfactory short- and long-term outcomes. Compared with open pancreatectomy, robotic pancreatectomy has a longer duration, results in less blood loss, and is associated with shorter postoperative pain and hospital stays; it has similar rate of pancreatic fistulas but with lower clinical significance, more accurate resection margins (R0 resection) and lymph node clearance, and comparable survival rates[44].
The preferred first-line chemotherapy scheme is FOLFIRINOX, which includes a combination of leucovorin (folinic acid), 5-fluorouracil, irinotecan and oxaliplatin or gemcitabine plus nab-paclitaxel for advanced metastatic disease in well-performing patients. Gemcitabine alone can be used in frail patients[45]. Additionally, modifications of FOLFIRINOX have sometimes been used, i.e., without irinotecan FOLFOX (folinic acid, 5FU and oxaliplatin) or without oxaliplatin FOLFIRI (folinic acid, 5FU and irinotecan)[2,46,47].
As a first-line chemotherapy for inoperable PDAC, FOLFIRINOX has better clinical efficacy than does gemcitabine plus nab-paclitaxel, with median progression-free survival of 6.5 months vs 5.3 months and median overall survival of 12.3 months vs 10.34 months[48].
Cytotoxic chemotherapy is the main option for systemic treatment of metastatic disease[1]. NALIRIFOX (liposomal irinotecan, fluorouracil, leucovorin, oxaliplatin) and GEMNABP (gemcitabine and nab-paclitaxel)[49] are increasingly used for metastatic disease with satisfactory outcomes[1,50]. However, chemoresistance is a major setback in pancreatic cancer, as in other solid tumors, though it can be overcome by precise targeting of malignant cells[43]. Certepetide is a novel antineoplastic drug that regulates the cell microenvironment and may be useful in inoperable advanced cases[51].
Novel stereotactic body radiation therapy and MRI-guided radiotherapy, adjuvant or neoadjuvant, have limited the side effects of radiation, providing accurate targeting and increased effectiveness, thus resulting in tumor shrinkage, restriction and local control; additionally, palliative radiotherapy results in pain relief in advanced cases[2,30,52].
Neoadjuvant treatment, including chemotherapy, radiotherapy[53] or a combination, has been used in borderline operable or locally advanced cases[9,54]. Although neoadjuvant therapy can offer potential benefits, such as reducing tumor size, addressing micrometastases early, and improving surgical outcomes, its universal application in all operable cases remains a topic of debate. Neoadjuvant treatment with gemcitabine plus S-1 (tegafur, a precursor of 5-fluorouracil; gimeracil, an inhibitor of 5-fluorouracil; and oteracil, a limiting agent of 5-fluorouracil toxicity) has beneficial effects[55]. Neoadjuvant chemotherapy can improve the outcome of locally advanced disease by tumor downstaging and gene profile alteration[31], despite the resulting immune suppression[56] and the existing controversy[8]. Additionally, a positive response to neoadjuvant treatment may predict a favorable outcome of potential subsequent surgical in
Gene therapy against the involved genes and novel vaccines have been used with encouraging results[31,60,61]. Vaccines that stimulate the immune system have provided preliminary hopeful outcomes[62]. The combination of herpes virus vaccines with PD-1 antibodies increases therapeutic efficacy[63].
Targeting the KRAS gene mutation, which is found in more than 90% of pancreatic cancers[4,33], may have beneficial effects. High levels of KRAS G12C gene mutations that occur at codon 12 activate the cycle of malignant cells and promote tumor growth, proliferation, invasion and spreading, resulting in a poor prognosis[1,5,64,65]. Similarly, patients with the KRAS G12D and G12V gene mutations had poor progression in contrast to those with the KRAS wild type, whereas those with the KRAS G12R gene mutation had better progression. In these patients, FOLFIRINOX had a better effect than gemcitabine[66].
For anti-KRAS G12C gene mutations, which are found in few cases, the drugs sotoracib or adagrasib have been used in patients with inoperable PDAC. They have modest survival benefits that can be increased by the addition of epidermal growth factor receptor inhibitors, i.e., erlotinib, gefitinib, cetuximab, and necitumuma[1,65,66]. Both drugs were recently approved by the FDA, but resistance to these drugs may occur. However, their combination with son of sevenless homolog 1 inhibitors (lapatinib-afatinib) increases treatment effectiveness[67]. For the Pan-RAS inhibitor RMC-(94)6236, initial results from ongoing studies have been reported[30]. For other gene mutations beyond those of the KRAS gene[68], the following promising therapies have been used: (1) BRAF V600E gene (dablafenib plus trametinib); (2) NTRK gene (larotrectinib or entrectinib); (3) RET gene (selpercatinib); and (4) BRACA1,2 gene (PARP inhibitors, i.e., olaparib, niraparib, rucaparib, and talazoparib, which have the disadvantage of developing resistance frequently limiting their use) and NRG1 gene (zenocutuzumab)[1,30,33]. There are ongoing trials, such as the NCI-MATCH trial, which uses next-generation genetic tests, and the POLO study, which is a phase III clinical trial, evaluating the effectiveness of the PARP inhibitor olaparib in BRCA1/2 metastatic PDAC. Olaparib was associated with increased progression-free survival (7.4 months vs 3.8 months in patients without treatment)[33].
The tumor microenvironment plays a pivotal role in planning management[51,69].
Immunotherapy targeting programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) has been used in many cancers, but pancreatic cancer has a lower response rate[70]. Pembrolizumad against PD-1 is the only drug approved by United States Food and Drug Administration (FDA) for advanced pancreatic cancer, but durvalumab, which targets PD-L1, has been used in clinical trials[9]. The limited success of immune checkpoint inhibitors in treating PDAC may be improved by emerging strategies, such as targeting the tumor microenvironment (extracellular matrix, immune cells, mesenchymal cells, tumor cells and fibroblasts), which can efficiently direct immunotherapy[69].
Recent advancements in immune checkpoint inhibitors, personalized vaccines, and CAR-T-cell therapies have revealed the important role of the continuing safety and efficacy of immunotherapy as a therapeutic choice for adjuvant or even frontline treatment[2,68]. Among its other applications, immunotherapy is a unique alternative for patients in advanced inoperable stages who have minimal or no response to other treatments[62].
The above mentioned management options are shown schematically in Figure 1C.
By incorporating all the relevant information mentioned throughout the manuscript, the predictive factors for favorable prognosis are summarized in Table 3.
| Number | Factor |
| 1 | Stage T1-T2 |
| 2 | Without metastases beyond the pancreas |
| 3 | Curative surgical resection |
| 4 | R0 resection without infiltration of resection margins |
| 5 | G1 (high) cell differentiation or even G2 (moderate); G3 (poor) provides worse outcomes |
| 6 | Absence of lymph node infiltration |
| 7 | Retrieved lymph nodes > 12 |
| 8 | Absence of perineural invasion |
| 9 | Neoadjuvant systemic treatment in borderline resectable cases, at least |
| 10 | Novel adjuvant systemic treatment |
| 11 | Absence of early recurrence |
| 12 | Without malnutrition and sarcopenia |
| 13 | Multimodality treatment |
The best prognosis is related to G1 (high) cell differentiation, followed by G2 (moderate) and G3 (poor) cell differentiation, which results in worse outcomes[35].
Perineural invasion and excruciating pain predict recurrence and a dismal prognosis. Targeting neural invasion with therapeutic biological agents, unless pain is relieved, may restrict tumor progression[71].
A large very recent multicenter trial (GARIBALDI) from Italy including 402 patients with PDAC without metastases who underwent a. surgery (36.6%), including only surgery (3%) and adjuvant chemotherapy (91.8%), b. neoadjuvant chemotherapy and surgery (14.2%) and c. only chemotherapy (49.3%), reported that, during a follow-up period of 57.6 months, 300 deaths (74.6%) occurred[72].
The management options for pancreatic cancer must be multidisciplinary. Modern adjuvant treatment after curative resection has increased the median overall survival from 18.6 months to 38.3 months[2].
In addition to their diagnostic role as PDAC biomarkers, circulating exosomes in liquid biopsy may be used as vehicles for drug delivery, providing targeted treatment against the growth of cancer cells in PDAC and other cancers[18].
Local ablation of the tumor by radiofrequency (RFA), microwaves, irreversible electroporation or electrochemotherapy has been applied in patients who are unfit for surgery or after failed chemotherapy in advanced disease. RFA, along with immunotherapy, constitutes a novel approach[73].
Nanoparticles have been used for the delivery and accurate distribution of chemotherapy, immunotherapy drugs and imaging materials (radiotracers) precisely within tumors[74].
Many studies have focused on novel radiomic methods, including machine learning and artificial intelligence, which could be valuable in early diagnosis, prognosis prediction and management strategic planning[2,75].
The standard adjuvant treatments approved by the FDA include chemotherapy with FOLFIRINOX and its modifications, gemcitabine, nab-paclitaxel, targeting KRAS gene mutation and immunotherapy with pembrolizumad. The other mentioned treatments are novel promising treatments awaiting FDA approval (gene therapies, vaccines and immunotherapy with durvalumab) or further validation through more consistent clinical experience (circulating exosomes and nanoparticles)[1,18,30,33,74].
Future challenging perspectives are anticipated to be based on the evolution of artificial intelligence. This innovation would be capable of analyzing enormous amounts of data regarding precise genomic assessment involved in cancer cell development and accurate selection of responsible gene targeting. The latter could manage the existing gap of resistance to treatment; additionally, it may reduce the high cost of novel oncological drugs, which is a current barrier along with their uncertain effectiveness. AI could be used to validate novel biomarkers for early diagnosis, including genomics and radiomics, or for determining accurate individualized combination immunotherapy, chemotherapy and targeted therapy.
Pancreatic cancer has a poor prognosis. When radical therapeutic surgery is performed whenever possible, multimodal and personalized treatments are necessary for modern management. Novel chemotherapy, radiotherapy, targeted therapy and immunotherapy may improve survival and patient quality of life. Further research efforts at the molecular level are needed to prevent the growth, invasion, and spread of cancer cells. Machine learning and artificial intelligence are promising novel modalities.
| 1. | Buckley CW, O'Reilly EM. Next-generation therapies for pancreatic cancer. Expert Rev Gastroenterol Hepatol. 2024;18:55-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 2. | Del Chiaro M, Sugawara T, Karam SD, Messersmith WA. Advances in the management of pancreatic cancer. BMJ. 2023;383:e073995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 3. | Daamen LA, Molenaar IQ, Groot VP. Recent Advances and Future Challenges in Pancreatic Cancer Care: Early Detection, Liquid Biopsies, Precision Medicine and Artificial Intelligence. J Clin Med. 2023;12:7485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Stoffel EM, Brand RE, Goggins M. Pancreatic Cancer: Changing Epidemiology and New Approaches to Risk Assessment, Early Detection, and Prevention. Gastroenterology. 2023;164:752-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 200] [Reference Citation Analysis (1)] |
| 5. | Olivari A, Agnetti V, Garajovα I. Focus on Therapeutic Options for Surgically Resectable Pancreatic Adenocarcinoma Based on Novel Biomarkers. Curr Oncol. 2023;30:6462-6472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Pavlidis ET, Lambropoulou M, Symeonidis NG, Anagnostopoulos C, Tsaroucha A, Kotini A, Nikolaidou C, Kiziridou A, Simopoulos C. The Immunohistochemical Expression MTA 1 Protein and its Prognostic Value in Pancreatic Cancer. J Invest Surg. 2018;31:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Pavlidis ET, Pavlidis TE. Current Molecular and Genetic Aspects of Pancreatic Cancer, the Role of Metastasis Associated Proteins (MTA): A Review. J Invest Surg. 2018;31:54-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Bugazia D, Al-Najjar E, Esmail A, Abdelrahim S, Abboud K, Abdelrahim A, Umoru G, Rayyan HA, Abudayyeh A, Al Moustafa AE, Abdelrahim M. Pancreatic ductal adenocarcinoma: the latest on diagnosis, molecular profiling, and systemic treatments. Front Oncol. 2024;14:1386699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 9. | Zhang CY, Liu S, Yang M. Clinical diagnosis and management of pancreatic cancer: Markers, molecular mechanisms, and treatment options. World J Gastroenterol. 2022;28:6827-6845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (3)] |
| 10. | Mukund A, Afridi MA, Karolak A, Park MA, Permuth JB, Rasool G. Pancreatic Ductal Adenocarcinoma (PDAC): A Review of Recent Advancements Enabled by Artificial Intelligence. Cancers (Basel). 2024;16:2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 11. | Daher H, Punchayil SA, Ismail AAE, Fernandes RR, Jacob J, Algazzar MH, Mansour M. Advancements in Pancreatic Cancer Detection: Integrating Biomarkers, Imaging Technologies, and Machine Learning for Early Diagnosis. Cureus. 2024;16:e56583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 12. | Takeda T, Sasaki T, Okamoto T, Ishitsuka T, Yamada M, Nakagawa H, Mie T, Furukawa T, Kasuga A, Matsuyama M, Ozaka M, Sasahira N. Prognostic impact of osteosarcopenia in patients with advanced pancreatic cancer receiving gemcitabine plus nab-paclitaxel. Pancreatology. 2023;23:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 13. | Ruff A, Li X, Goldberg JD, Ehrhart M, Ginocchio L, Smereka P, O'Donnell T, Dane B. Optimal virtual monoenergy for the detection of pancreatic adenocarcinoma during the pancreatic parenchymal phase on photon counting CT. Abdom Radiol (NY). 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Yu B, Shao S, Ma W. Frontiers in pancreatic cancer on biomarkers, microenvironment, and immunotherapy. Cancer Lett. 2025;610:217350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 15. | Zhao B, Cao B, Xia T, Zhu L, Yu Y, Lu C, Tang T, Wang Y, Ju S. Multiparametric MRI for Assessment of the Biological Invasiveness and Prognosis of Pancreatic Ductal Adenocarcinoma in the Era of Artificial Intelligence. J Magn Reson Imaging. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Qu C, Zeng P, Hu W, Yang D, Wang H, Yuan H, Cao J, Xiu D. Multiparametric quantitative diffusion weighted magnetic resonance imaging can effectively predict the response to neoadjuvant therapy in borderline resectable pancreatic ductal adenocarcinoma. Eur J Radiol. 2025;183:111893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Marques S, Bispo M, Rio-Tinto R, Fidalgo P, Deviθre J. The Impact of Recent Advances in Endoscopic Ultrasound-Guided Tissue Acquisition on the Management of Pancreatic Cancer. GE Port J Gastroenterol. 2021;28:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Qin C, Li T, Lin C, Zhao B, Li Z, Zhao Y, Wang W. The systematic role of pancreatic cancer exosomes: distant communication, liquid biopsy and future therapy. Cancer Cell Int. 2024;24:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 19. | Fahrmann JF, Yip-Schneider M, Vykoukal J, Spencer R, Dennison JB, Do KA, Long JP, Maitra A, Zhang J, Schmidt CM, Hanash S, Irajizad E. Lead time trajectory of blood-based protein biomarkers for detection of pancreatic cancer based on repeat testing. Cancer Lett. 2025;612:217450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Eze-Odurukwe A, Rehman A, Ayinla L, Anika NN, Shahid R, Ugwuoru AL, Mansoor M, Kamran M. Metabolite Biomarkers for Early Detection of Pancreatic Ductal Adenocarcinoma: A Systematic Review. Cureus. 2024;16:e74528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Vahabi M, Comandatore A, Centra C, Blandino G, Morelli L, Giovannetti E. Thinking small to win big? A critical review on the potential application of extracellular vesicles for biomarker discovery and new therapeutic approaches in pancreatic cancer. Semin Cancer Biol. 2023;97:50-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Bao Y, Zhang D, Guo H, Ma W. Beyond blood: Advancing the frontiers of liquid biopsy in oncology and personalized medicine. Cancer Sci. 2024;115:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 23. | Ko SW, Jo IH, Yoon SB. Feasibility and clinical utility of endoscopic ultrasound-guided tissue acquisition for comprehensive genomic profiling in pancreatic cancer: A systematic review and meta-analysis. Pancreatology. 2025;25:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Lena J, Alamι M, Italiano A, Soubeyran I, Blouin L, Khalifa E, Cousin S, Pernot S, Palmieri LJ. Extensive molecular profiling of KRAS wild-type as compared to KRAS mutated pancreatic ductal adenocarcinoma on 318 patients. Eur J Cancer. 2025;216:115197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Xia T, Chen XY, Zhang YN. MicroRNAs as biomarkers and perspectives in the therapy of pancreatic cancer. Mol Cell Biochem. 2021;476:4191-4203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Marin AM, Sanchuki HBS, Namur GN, Uno M, Zanette DL, Aoki MN. Circulating Cell-Free Nucleic Acids as Biomarkers for Diagnosis and Prognosis of Pancreatic Cancer. Biomedicines. 2023;11:1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Koustas E, Trifylli EM, Sarantis P, Papadopoulos N, Papanikolopoulos K, Aloizos G, Damaskos C, Garmpis N, Garmpi A, Karamouzis MV. The Emerging Role of MicroRNAs and Autophagy Mechanism in Pancreatic Cancer Progression: Future Therapeutic Approaches. Genes (Basel). 2022;13:1868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Montalvo-Javι EE, Nuρo-Lαmbarri N, Lσpez-Sαnchez GN, Ayala-Moreno EA, Gutierrez-Reyes G, Beane J, Pawlik TM. Pancreatic Cancer: Genetic Conditions and Epigenetic Alterations. J Gastrointest Surg. 2023;27:1001-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 29. | Nwosu ZC, Giza HM, Nassif M, Charlestin V, Menjivar RE, Kim D, Kemp SB, Sajjakulnukit P, Andren A, Zhang L, Lai WK, Loveless I, Steele N, Hu J, Hu B, Wang S, Pasca di Magliano M, Lyssiotis CA. Multidimensional analyses identify genes of high priority for pancreatic cancer research. JCI Insight. 2025;10:e174264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Linehan A, O'Reilly M, McDermott R, O'Kane GM. Targeting KRAS mutations in pancreatic cancer: opportunities for future strategies. Front Med (Lausanne). 2024;11:1369136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 31. | Mahmood U, Carrier E, Khan K. Neoadjuvant management of locally advanced pancreatic ductal adenocarcinoma - Heading towards a promising change in treatment paradigm. Cancer Treat Rev. 2024;127:102750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 32. | Raja Arul GL, Toruner MD, Gatenby RA, Carr RM. Ecoevolutionary biology of pancreatic ductal adenocarcinoma. Pancreatology. 2022;22:730-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Li B, Zhang Q, Castaneda C, Cook S. Targeted Therapies in Pancreatic Cancer: A New Era of Precision Medicine. Biomedicines. 2024;12:2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 34. | Shin DW, Kim J. The American Joint Committee on Cancer 8th edition staging system for the pancreatic ductal adenocarcinoma: is it better than the 7th edition? Hepatobiliary Surg Nutr. 2020;9:98-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Perrotta G, Mohamed G, Larson BK, Osipov A, Ferrone CR, Lo SK, Gaddam S. Accuracy of Clinical Staging in Early-Stage Pancreatic Ductal Adenocarcinoma. JAMA. 2024;332:1108-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 36. | Isaji S, Mizuno S, Windsor JA, Bassi C, Fernαndez-Del Castillo C, Hackert T, Hayasaki A, Katz MHG, Kim SW, Kishiwada M, Kitagawa H, Michalski CW, Wolfgang CL. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 510] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 37. | Ayasun R, Saridogan T, Gaber O, Sahin IH. Systemic Therapy for Patients With Pancreatic Cancer: Current Approaches and Opportunities for Novel Avenues Toward Precision Medicine. Clin Colorectal Cancer. 2023;22:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Li P, Zhang Q, Zhang Q, Liu S, Zhou H, Cui Y, Li H, Wu Q, Song T, Zhang X, Li Q. A LASSO Cox Regression Predictive Model for Patients Undergoing Surgery for Pancreatic Body and Tail Adenocarcinoma Patients: Comparative Long-Term Survival Analysis of Radical Antegrade Modular Pancreatosplenectomy (RAMPS) and Standard Retrograde Pancreatosplenectomy (SPRS). Ann Surg Oncol. 2024;31:8317-8326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 39. | Codjia T, Hobeika C, Platevoet P, Pravisani R, Dokmak S, Aussilhou B, Marique L, Cros J, Cauchy F, Lesurtel M, Sauvanet A. Distal Pancreatectomy for Body Pancreatic Ductal Adenocarcinoma: Is Splenectomy Necessary? A Propensity Score Matched Study. Ann Surg Oncol. 2024;31:4611-4620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Rompen IF, Habib JR, Sereni E, Stoop TF, Musa J, Cohen SM, Berman RS, Kaplan B, Hewitt DB, Sacks GD, Wolfgang CL, Javed AA. What is the optimal surgical approach for ductal adenocarcinoma of the pancreatic neck? - a retrospective cohort study. Langenbecks Arch Surg. 2024;409:224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 41. | Kulkarni RV, Patil V, Bhandare MS, Chaudhari VA, Shrikhande SV. Vein resection without reconstruction (VROR) in pancreatoduodenectomy: expanding the surgical spectrum for locally advanced pancreatic tumours. Langenbecks Arch Surg. 2020;405:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Zheng Y, Li R, Xu J, Shi H, Xing C, Li Z, Cui H, Song J. Prognostic significance of three lymph node staging systems in pancreatic cancer with ≤ 12 and > 12 retrieved lymph nodes. Updates Surg. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Parvaneh S, Miklσs V, Pαhi ZG, Szϋcs D, Monostori T, Pσliska S, Venglovecz V, Pankotai T, Kemιny L, Verιb Z. Chemoresistance in Pancreatic Cancer: The Role of Adipose-Derived Mesenchymal Stem Cells and Key Resistance Genes. Int J Mol Sci. 2025;26:390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 44. | Stefanova I, Vescio F, Nickel F, Merali N, Ammendola M, Lahiri RP, Pencavel TD, Worthington TR, Frampton AE. What are the true benefits of robotic pancreaticoduodenectomy for patients with pancreatic cancer? Expert Rev Gastroenterol Hepatol. 2024;18:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Ettrich TJ, Seufferlein T. Systemic Therapy for Metastatic Pancreatic Cancer. Curr Treat Options Oncol. 2021;22:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 46. | Kamposioras K, Papaxoinis G, Dawood M, Appleyard J, Collinson F, Lamarca A, Ahmad U, Hubner RA, Wright F, Pihlak R, Damyanova I, Razzaq B, Valle JW, McNamara MG, Anthoney A. Markers of tumor inflammation as prognostic factors for overall survival in patients with advanced pancreatic cancer receiving first-line FOLFIRINOX chemotherapy. Acta Oncol. 2022;61:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Ross PJ, Wasan HS, Croagh D, Nikfarjam M, Nguyen N, Aghmesheh M, Nagrial AM, Bartholomeusz D, Hendlisz A, Ajithkumar T, Iwuji C, Wilson NE, Turner DM, James DC, Young E, Harris MT. Results of a single-arm pilot study of (32)P microparticles in unresectable locally advanced pancreatic adenocarcinoma with gemcitabine/nab-paclitaxel or FOLFIRINOX chemotherapy. ESMO Open. 2022;7:100356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 48. | Aseafan M, Alfakeeh AH, Tashkandi E, Mahrous M, Alghamdi M, Alshamsan B, Al-Hajeili M, Bakhsh S, Alshammari K, Almugbel FA, Alfagih AH, Allehebi A, Montaser M, Elsafty MH, Elnaghi KAE, Issa I, Bakshi E, AlSubaie S, AlMutairi B, Mokhtar H, Aboelatta M, Bukhari N, Alzahrani AM, Elhassan T, Alqahtani A, Bazarbashi S. Real-world clinical outcome of unresectable locally advanced & de-novo metastatic pancreatic ductal adenocarcinoma: a multicentre retrospective study. BMC Cancer. 2025;25:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 49. | Xiang Z, Ma L, Li Z, Fu Y, Pan Y. Cost-effectiveness analysis of first-line combination chemotherapy regimens for metastatic pancreatic cancer and evidence-based pricing strategy of liposomal irinotecan in China. Front Pharmacol. 2024;15:1488645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 50. | Αlvarez-Gallego R, Pazo-Cid R, Lσpez de San Vicente B, Macarulla T, Martinez E, Garicano F, Hernαndez I, Granja M, Ghanem I, Martinez J, Ribera P, Diaz R, Martin Valadιs JI, Angeles MC, Cubillo A. Real-world effectiveness and safety of second- or third-line pegylated liposomal irinotecan plus 5-fluorouracil and folinic acid in pancreatic ductal adenocarcinoma in Spain. Ther Adv Med Oncol. 2025;17:17588359241309828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 51. | Winning A, Sietsema WK, Buck KK, Linsmeier A, Wiczling P. Population Pharmacokinetic Modeling of Certepetide in Human Subjects With Metastatic Pancreatic Ductal Adenocarcinoma. Clin Pharmacol Drug Dev. 2025;14:240-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 52. | Zeeshan MS, Ramzan Z. Current controversies and advances in the management of pancreatic adenocarcinoma. World J Gastrointest Oncol. 2021;13:472-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (2)] |
| 53. | Bryant JM, Nakashima J, Khatri VM, Sinnamon AJ, Denbo JW, Hodul P, Malafa M, Hoffe S, Frakes JM. The Evolving Role of Neoadjuvant Radiation Therapy in Pancreatic Adenocarcinoma. J Clin Med. 2024;13:6800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 54. | Ito R, Yoshioka R, Yanagisawa N, Ishii S, Sugitani J, Furuya R, Fujisawa M, Imamura H, Mise Y, Isayama H, Saiura A. ASO Visual Abstract: Survival Analysis of Conversion Surgery in Borderline Resectable and Locally Advanced Unresectable Pancreatic Ductal Adenocarcinoma Addressing Selection and Immortal Time Bias-A Retrospective Single-Center Study. Ann Surg Oncol. 2025;32:2879-2880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Hirashita T, Tada K, Nagasawa Y, Orimoto H, Kawamura M, Fujinaga A, Takayama H, Kawano Y, Masuda T, Endo Y, Inomata M. Benefits of neoadjuvant chemotherapy with gemcitabine plus S-1 for resectable pancreatic ductal adenocarcinoma. Mol Clin Oncol. 2025;22:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 56. | Rupp L, Dietsche I, Kieίler M, Sommer U, Muckenhuber A, Steiger K, van Eijck CWF, Richter L, Istvanffy R, Jδger C, Friess H, van Eijck CHJ, Demir IE, Reyes CM, Schmitz M. Neoadjuvant chemotherapy is associated with suppression of the B cell-centered immune landscape in pancreatic ductal adenocarcinoma. Front Immunol. 2024;15:1378190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 57. | Barnes CA, Tsai S. Novel Considerations in Surgical Management of Individuals with Pancreatic Adenocarcinoma. Hematol Oncol Clin North Am. 2022;36:979-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 58. | Al Masad Q, Sousa A, Pena P, Sammartino CJ, Somasundar P, Abdelfattah T, Espat NJ, Calvino AS, Kwon S. Relationship of Time to First Therapy and Survival Outcomes of Neoadjuvant Chemotherapy Versus Upfront Surgery Approach in Resectable Pancreatic Ductal Adenocarcinoma. J Surg Res. 2025;306:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 59. | Repollet Otero PA, Ibrahim E, Ligato S. Frozen section analysis of pancreatic resection margins during pancreaticoduodenectomy for pancreatic adenocarcinoma is not affected by neoadjuvant therapy. Pancreatology. 2025;25:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 60. | Sharma SK, Mack KN, Piersigilli A, Pourat J, Edwards KJ, Keinδnen O, Jiao MS, Zhao H, White B, Brooks CL, de Stanchina E, Madiyalakan MR, Hollingsworth MA, Radhakrishnan P, Lewis JS, Zeglis BM. ImmunoPET of Ovarian and Pancreatic Cancer with AR9.6, a Novel MUC16-Targeted Therapeutic Antibody. Clin Cancer Res. 2022;28:948-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 61. | Schlick K, Kiem D, Greil R. Recent Advances in Pancreatic Cancer: Novel Prognostic Biomarkers and Targeted Therapy-A Review of the Literature. Biomolecules. 2021;11:1469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Chen SJ, Wang SC, Chen YC. The Immunotherapy for Colorectal Cancer, Lung Cancer and Pancreatic Cancer. Int J Mol Sci. 2021;22:12836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 63. | Gao W, Zhao Z, Bi Y, Li J, Tian N, Zhang C, Pan S, Deng L, Zhang Y. 4-1BBL-Armed Oncolytic Herpes Simplex Virus Exerts Antitumor Effects in Pancreatic Ductal Adenocarcinoma. Vaccines (Basel). 2024;12:1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 64. | Gromisch C, Qadan M, Machado MA, Liu K, Colson Y, Grinstaff MW. Pancreatic Adenocarcinoma: Unconventional Approaches for an Unconventional Disease. Cancer Res. 2020;80:3179-3192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 65. | Yun WG, Kim D, Han Y, Kwon W, Lee SG, Jang JY, Park D. Multiomic quantification of the KRAS mutation dosage improves the preoperative prediction of survival and recurrence in patients with pancreatic ductal adenocarcinoma. Exp Mol Med. 2025;57:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 66. | Norton C, Shaw MS, Rubnitz Z, Smith J, Soares HP, Nevala-Plagemann CD, Garrido-Laguna I, Florou V. KRAS Mutation Status and Treatment Outcomes in Patients With Metastatic Pancreatic Adenocarcinoma. JAMA Netw Open. 2025;8:e2453588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 67. | Isermann T, Sers C, Der CJ, Papke B. KRAS inhibitors: resistance drivers and combinatorial strategies. Trends Cancer. 2025;11:91-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 68. | Hoang T, Tsang ES. Advances in Novel Targeted Therapies for Pancreatic Adenocarcinoma. J Gastrointest Cancer. 2025;56:38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 69. | Szczepanski JM, Rudolf MA, Shi J. Clinical Evaluation of the Pancreatic Cancer Microenvironment: Opportunities and Challenges. Cancers (Basel). 2024;16:794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 70. | Luo W, Zheng L, Zhang T. Do novel treatment strategies enhance T cell-mediated Immunity: Opportunities and challenges in pancreatic cancer immunotherapy. Int Immunopharmacol. 2021;90:107199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Garajovα I, Giovannetti E. Targeting Perineural Invasion in Pancreatic Cancer. Cancers (Basel). 2024;16:4260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 72. | Reni M, Milella M, Bergamo F, Di Marco M, Giommoni E, Cardellino GG, Cavanna L, Bonomi M, Zustovich F, Bozzarelli S, Salmaso F, Spada M, Orsi G, Macchini M, Insolda J, Procaccio L, Santoni A, De Simone I, Caldirola L, Galli F, Pinto C. Survival analysis of the non-metastatic cohort of the Italian Association for Medical Oncology (AIOM) Guideline Application in Real world: multi-Institutional Based survey of Adjuvant and first-Line pancreatic Ductal adenocarcinoma treatment in Italy (GARIBALDI). ESMO Open. 2025;10:104001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 73. | Granata V, Grassi R, Fusco R, Belli A, Palaia R, Carrafiello G, Miele V, Grassi R, Petrillo A, Izzo F. Local ablation of pancreatic tumors: State of the art and future perspectives. World J Gastroenterol. 2021;27:3413-3428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 74. | Liu L, Kshirsagar PG, Gautam SK, Gulati M, Wafa EI, Christiansen JC, White BM, Mallapragada SK, Wannemuehler MJ, Kumar S, Solheim JC, Batra SK, Salem AK, Narasimhan B, Jain M. Nanocarriers for pancreatic cancer imaging, treatments, and immunotherapies. Theranostics. 2022;12:1030-1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 75. | Truntzer C, Ouahbi D, Huppι T, Rageot D, Ilie A, Molimard C, Beltjens F, Bergeron A, Vienot A, Borg C, Monnien F, Bibeau F, Derangθre V, Ghiringhelli F. Deep Multiple Instance Learning Model to Predict Outcome of Pancreatic Cancer Following Surgery. Biomedicines. 2024;12:2754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |