Published online Jul 24, 2023. doi: 10.5306/wjco.v14.i7.230
Peer-review started: May 16, 2023
First decision: May 25, 2023
Revised: June 7, 2023
Accepted: June 21, 2023
Article in press: June 21, 2023
Published online: July 24, 2023
Processing time: 64 Days and 0.1 Hours
It is now well established that the biology of cancer is influenced by not only malignant cells but also other components of the tumour microenvironment. Chronic inflammation and fibrosis have long been postulated to be involved in carcinogenesis. Chronic inflammation can promote tumorigenesis via growth factor/cytokine-mediated cellular proliferation, apoptotic resistance, immunosuppression; and free-radical-induced oxidative deoxyribonucleic acid damage. Fibrosis could cause a perturbation in the dynamics of the tumour microenvironment, potentially damaging the genome surveillance machinery of normal epithelial cells. In this review, we will provide an in-depth discussion of various diseases characterised by inflammation and fibrosis that have been associated with an increased risk of malignancy. In particular, we will present a comprehensive overview of the impact of alterations in stromal composition on tumorigenesis, induced as a consequence of inflammation and/or fibrosis. Strategies including the application of various therapeutic agents with stromal manipulation potential and targeted cancer screening for certain inflammatory diseases which can reduce the risk of cancer will also be discussed.
Core Tip: Chronic inflammation and fibrosis have long been postulated to be involved in carcinogenesis via numerous mechanisms including but not limited to growth factor/cytokine-mediated cellular proliferation, apoptotic resistance, immunosuppression; and free-radical-induced oxidative deoxyribonucleic acid damage. In this review, we discuss various inflammatory and/or fibrotic conditions that have been associated with increased cancer risk, with particular emphasis on their pathophysiology. We also review various therapeutic agents and specific cancer screening that could be applicable in reducing the incidence of cancers developing from the corresponding inflammatory and/or fibrotic conditions, thereby reducing morbidity and mortality.
- Citation: Oey O, Sunjaya AF, Khan Y, Redfern A. Stromal inflammation, fibrosis and cancer: An old intuition with promising potential. World J Clin Oncol 2023; 14(7): 230-246
- URL: https://www.wjgnet.com/2218-4333/full/v14/i7/230.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i7.230
In recent years, there is growing consensus that the biology of cancer is not solely defined by malignant cells, but also by the surrounding tumour microenvironment (TME). The TME consists of cellular and non-cellular stroma. The concept that the TME may influence cancer biology was inspired by the observation of immune cells surrounding the tumour by Rudolf Virchow in 1863, and “the seed and soil theory” by Stephen Paget in 1889, in which he hypothesised that the metastatic destination of a certain cancer is dependent on similarities between the TME of primary tumour and the microenvironment at the site of metastases[1,2]. Since then, there have been significant advancements in the understanding of the impact of the TME on the behaviour of malignant cells, from initial tumorigenesis, through progression to therapy resistance[3-5]. This review will focus on the impact of both the physiological and pathological tissue microenvironment, particularly stromal fibrosis and inflammation, on tumorigenesis.
In this context, stroma refers to the component of an organ which provides biomechanical and nutritional support to the corresponding parenchyma. Specifically, it comprises of immune cells, fibroblasts, mesenchymal stromal cells, endothelial cells, pericytes, adipocytes, and the extracellular matrix (ECM). The ECM, consisting of collagen, proteoglycans, glycosaminoglycans and other macromolecules, provides structural and biochemical support for cellular components in the surrounding parenchyma. Of note, some authors do not include immune cells as a component of stroma, however, immune cells such as macrophages, neutrophils and lymphocytes, play an integral role to the function of parenchymal cells and can have far-reaching effects on tumour biology and consequent behaviour, as such they will be classified as a stromal component in this review.
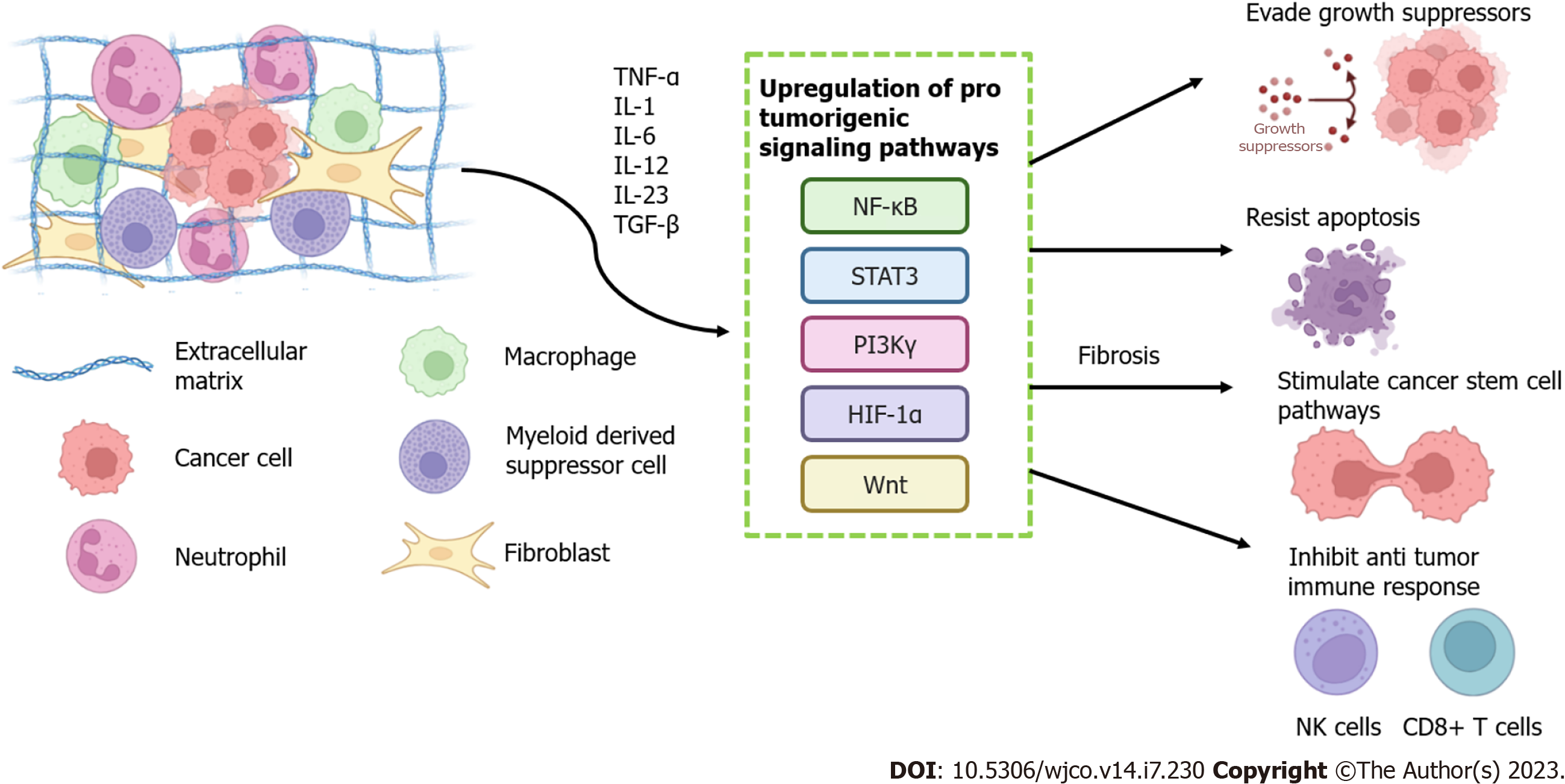
Many stromal components have been shown through various in vitro and animal studies to influence the behaviour and fate of normal cells, including altering the risk of malignant transformation[6-8]. Inflammation and fibrosis are both common processes that significantly alter the cellular and ECM components of normal stroma and so may influence or underlie such behavioural shifts. Both processes have been seen to upregulate the expression of several tumorigenic signalling pathways including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ĸB), signal transducers and activators of transcription (STAT), wingless-related integration site (Wnt) and phosphatidylinositide 3-kinase (PI3K) via the release of pro inflammatory cytokines[9-12]. Hence, several inflammatory and fibrotic conditions have been linked as triggers for tumour development in the organ involved, whether due to autoimmune responses (inflammatory bowel disease and colorectal cancer[13]), bacterial or viral infections (pneumonia or tuberculosis with lung cancer[14]) and environmental factors (silica and lung cancer[15]).
Often, these pathological processes appear to be required for tumorigenesis rather than simply an overrepresentation of certain otherwise normal stromal components. For instance, inflamed adipose mammary tissue in the context of obese mice, increases myofibroblasts number, promoting fibrosis and transformation of normal to malignant breast tissue[6], whereas normal mouse fibroblasts have been shown to prevent clonal proliferation of polyoma virus-transformed cells in vitro[7]. However, there are less frequent precedents where normal stromal components may also contribute to tumorigenesis. Normal fibroblasts have been demonstrated to promote the generation of breast cancer stem cells[8]. Additionally, high mammographic breast density, which results from a higher density of stromal and glandular breast components and a lower proportion of adipocytes, is a potent risk factor for breast cancer development.
In this review we will discuss various medical conditions substantively characterised by inflammation and fibrosis, specifically those known to be linked to increased cancer risk. Furthermore, we will look to whether scenarios exist where physiological variations in stromal composition correlate with differing cancer incidence. In doing so, we will discuss the biological contribution of the various stromal components to tumorigenesis known to date and discuss interventions that may influence these processes to achieve therapeutic advantage.
A range of medical conditions exist that involve one or both of these processes. A common evolution pathogenically is of initial inflammation with subsequent fibrosis. However, each of the processes may occur in isolation. Here we look across a range of scenarios at whether each may affect cancer risk in isolation or whether both appear to be required for tumorigenesis (Table 1).
| Disease | Associated cancer | Mechanism | Risk ratio | Possible therapeutic targets |
| Inflammatory bowel disease | Colorectal cancer | Increased pro-inflammatory cytokines (TNF-α, IL-6 and TGF-β)[24,30,34]; Increased signalling of pro-tumorigenic molecular pathways, apoptosis resistance, fibrogenesis (NF-ĸB and Wnt/β-catenin)[9,27,30,34] | Ulcerative colitis – 4.8-fold increase[13]; Crohn’s disease – 2-3-fold increase[17] | Thiopurines[173] and anti-inflammatory such as mesalazine[174] and NSAID[149] |
| Chronic pancreatitis | Pancreatic ductal adenocarcinoma | Increased cytokines (TNF-α and TGF-β), growth factors (VEGF, PDGF)[38]; Fibroblast and pancreatic epithelial cell proliferation[40]; Activation of pancreatic stellate cells[39,40]; Increased ECM protein (collagen 1 and 4, laminin, fibronectin) and hyaluronic acid deposition[38] | 20-fold increase[35] | PEGylated Recombinant Human Hyaluronidase[45,46]; NSAID[149] |
| Idiopathic pulmonary fibrosis | Lung cancer | Cellular morphological abnormalities (metaplasia, dysplasia) in fibrotic areas[59]; Reduced immune expression (monocytes, lymphocytes, macrophages) in fibrotic areas[50]; Mutations in tumour-suppressor genes[54]; Upregulated gene expression of ECM components such as collagen and MMP (MMP9 and 11)[57] | 3.5-7.3 fold increase[51] | Anti-fibrotic drugs (pirfenidone and nindetanib)[175] |
| Pneumoconiosis | Lung cancer | Silicosis: Chronic increased release of pro-inflammatory cytokines (IL-12, IL-23 and TNFα) results in DNA damage[66]; Immunosuppression through increased expression of inhibitory immune markers (PD-1, LAG3, FOXP30)[70]. Asbestosis: Increased inflammation (IL-1β, TGF-β and PDGF) and fibrosis through expression of NLRP3[70]; Increased ROS and RNS[64,68]; Increased expression of proliferation signalling pathways (EGFR-ERK)[73] | Silicosis – 3-fold increase[15]; Asbestosis – 1.5-6.8-fold increase[65,65] | Anti-fibrotic drugs (pirfenidone and nindetanib)[152] |
| TB | Lung cancer | Upregulation of anti-apoptotic protein expression via inflammatory cytokines (TNF-α and IL-6)[59,76,78] | Pneumonia – 1.4-fold increase[14]; TB – 1.9-fold increase[14] | NSAID[176] |
| Liver cirrhosis | Hepato-cellular carcinoma | Cellular proliferation, telomere shortening via inflammatory cytokines (TGF-β, TNF-α and interleukins)[83,84]; Genomic instability (p53, Ras, mTOR, Wnt signalling pathways)[11,84]; Reduced expression of CD4+ and CD8+ cytotoxic T cell[85]; Increased regulatory T-cell response[86]; Activation of hepatic stellate cells increase myofibroblast and ECM production[11,87]; Hypoxia in fibrosis leads to genotoxicity (ROS, RNO) and angiogenesis (VEGF)[92] | Hepatitis B related – 1.17-fold increase[81]; Hepatitis C related - 1.15-fold increase[81]; NAFLD-related – 1.6-23.7-fold increase[161] | LOX/LOXL2 inhibitors[161,162]; NSAID, Pentoxifylline[177,178] |
| Primary biliary cholangitis | Cholangiocarcinoma | Increased proliferative signalling via inflammatory cytokines (IL-1β, IL-6 and HGF)[96-98]; IL-6 activates p38-MAPK, increases DNA methyltransferase (DNMT) Mcl-1 and telomerase expression[96]; DNA damage (BRAF, K-ras, cyclin d-1, c-myc, COX-2 and p53) due to dysregulated NO production[98]; Fibroblast proliferation and ECM production (collagen type 1 and 3)[103] | 9-fold increase[94] | Natural anti-inflammatory products (Curcumin)[102] |
| GERD and Barrett’s oesophagus | Oesophageal cancer | Increased inflammatory cell recruitment (macrophages T, B, dendritic cells)[107]; Inflammatory cytokine release (TNF-α, IL-6, IL-1β, IL-8) activates pro tumorigenic signalling pathways (NF-Κb, STAT-3, HIF-1a)[107,108]; Reduced immune response due to immunosuppressive cytokines (IL-10)[112]; Oxidative stress (ROS and RNS) induce mutagenesis of oncogenes and tumor suppressor genes[110] | 30-125-fold increase[106] | NSAID[149] |
| OSF | Oral squamous cell carcinoma | Increased inflammatory cell recruitment[118]; Oxidative stress induces p53 mutation, decreased DMNT and increased HSP70 and MDM2-P2 promoter[120,122]; Increased prostaglandins, cytokines and growth factors (IL-6, TNF-α, PDGF and TGF-β)[118,119]; Fibrogenesis via IL-6 and TGF-β leads to increased ECM protein production (collagen, fibonectin) and inhibit ECM breakdown (PAI-1, TIMP)[124,125]; OSF-associated fibroblast promote dysplastic keratinocyte proliferation via GRO-α release and EGFR/ERK activation[128] | 19-fold increase[114] | Anti-oxidants, steroids and hyaluronidase[178] |
| Physiological breast stromal density, breast conditions – chronic mastitis, sclerosing adenosis | Breast cancer | Mammographically dense breast have higher ECM proportion (collagen, immune cells)[131,133]; Mammographically dense breast have higher proportion of glandular epithelial components and lower proportion of adipocytes[132-134] | Physiological higher MBD: 4-6-fold increase[130]; Chronic mastitis: 3-fold increase[137]; Sclerosing adenosis: 2-fold increase[138] | Anti-estrogens (tamoxifen, raloxifene, exemestane and anastrozole)[154-157]; NSAID[149]; LOX-like inhibitors[159,160,163] |
Inflammatory bowel disease (IBD) is sub-divided into ulcerative colitis (UC), which affects only the large bowel, and Crohn’s disease (CD) which can involve any area of the gut from mouth to rectum[16]. The risk of developing colorectal cancer in UC patients is elevated compared to the disease-free population, with an overall risk of 4.8[13]. Similarly, in CD risk is elevated although to a more moderate degree, by 2-3 times[17]. In keeping with the small bowel involvement in CD, small intestinal tumours are also increased, by a relative risk of 18.75%[17]. The risk in both conditions is associated with duration and extent of inflammation[18]. Beyond inflammation, both conditions can also result in fibrosis although the pattern differs. Fibrosis leading to eventual stricture and potential obstruction is more common in CD than UC, with around 25% of CD sufferers eventually destined to develop a stricture over the course of the illness[19]. On initial consideration this appears at odds with the risk of colorectal cancer, but may be explained by the distribution of fibrotic change. In UC fibrosis is often superficial, affecting only the mucosal and sub-mucosal layers[20] but still, therefore, able to impact the epithelial layer from which neoplasms arise, and generally impacting a longer continuous length of colon. In contrast, Crohn’s disease is characterized by patchy change and skip lesions such that the total area of involved epithelium is often less[16].
Considering these patterns and parallel links in other organs between inflammation, fibrosis and neoplastic transformation, the development of colitis-associated carcinoma (CAC) appears highly likely to be directly attributable to chronic inflammation and consequent fibrosis[21,22]. There is a biological rationale, with previous studies showing that certain inflammatory cytokines prominent in UC, namely TNF-α, IL-6 and TGF-β can promote a pro-tumorigenic microenvironment by stimulating essential cancer stem cell pathways, evading growth suppressors, and resisting apoptosis[23-25]. This occurs via induction of various molecular signalling pathways including NF-ĸB[9], STAT[26] and Wnt pathways[27]. Incidentally, these cytokines can also promote fibrosis. TNF-α has been demonstrated to induce IL-6 production, which is partly responsible for proliferation of fibroblasts[28,29]. In addition, TGF-β, highly expressed in intestinal epithelial cells, inflammatory cells and fibroblasts is known to induce fibrogenesis and ultimately the deposition of ECM such as collagen, via the Wnt/β-catenin pathway, which is also often activated early in dysplastic and surrounding non-dysplastic intestinal epithelial cells, in the setting of CAC carcinogenesis[10,30,31]. This concurs with the upregulation of type 1 collagen, revealed by proteomic analysis in the early stages of colorectal carcinogenesis[32]. Whether or not collagen promotes CAC carcinogenesis remains ambiguous, however increase in collagen may disrupt the polarity of healthy intestinal epithelial cells and stimulate cellular proliferation, thereby promoting malignant transformation.
While it is generally understood that fibrosis occurs as a result of chronic inflammation, it is now understood that fibrosis in IBD may occur without inflammation[33], and further that not all people with IBD develop fibrosis[34]. This prompts the question as to whether either fibrosis or inflammation without the companion process can also trigger carcinogenesis – a question which remains unanswered today due to a lack of cohorts with data that allow the linking degrees of inflammation and fibrosis to cancer risk.
Chronic pancreatitis (CP) is a major risk factor for the development of pancreatic ductal adenocarcinoma (PDAC), increasing the risk of PDAC by 20-fold relative to disease-free population[35]. Both CP and PDAC share a common pathological feature – abundant desmoplastic and inflammatory stroma[36]. Hence, the link between the former and the latter could be attributed to the events occurring in the surrounding inflammatory milieu. This was proven in an animal study involving the insertion of K-ras oncogenes within the endogenous K-ras locus, in which mice without pancreatitis did not develop PDAC, while those with pancreatitis did[37]. Thus, it could be deduced that inflammation is a critical factor in PDAC carcinogenesis, at least in response to this, the commonest of oncogenes implicated in pancreatic cancer. In chronic pancreatitis, the release of inflammatory cytokines such as TNF-α and TGF-β and growth factors such as vascular endothelial growth factor (VEGF) and PDGF trigger the proliferation of fibroblasts and the activation of pancreatic stellate cells (PSC) towards a more myofibroblast-like phenotype[38,39]. Activated PSC have a number of functions, including sustaining proliferative signalling in pancreatic epithelial cells; the release of growth factors; and the synthesis of ECM proteins, notably collagen, fibronectin and laminin[40,41]. The deposition of various ECM proteins could cause a perturbation in the dynamics of the ECM, potentially damaging the genome surveillance machinery of normal epithelial cells. Supportive of a role for certain ECM components in PDAC progression is the finding that collagen 1, 4 and hyaluronic acid which promotes cell survival, proliferation and invasion, with higher levels associated with reduced survival[42-44]. This is further supported by the therapeutic benefit derived from the administration of PEGylated Recombinant Human Hyaluronidase in addition to chemotherapy in PDAC patients[45,46].
However, certain alterations in the ECM can be tumour-inhibitory rather than promoting. Quantitative analysis of stroma density in PDAC samples from patients’ autopsy revealed that tissue stroma density was substantially lower in samples from patients with metastatic PDAC and that higher stromal content was associated with a more favourable outcome[47]. This finding was further supported by Rhim et al[48] who demonstrated that diminished stromal density induced by knocking out sonic hedgehog in an established PDAC mouse model significantly enhanced tumour vascularity and proliferation. Furthermore, another study by Erkan et al[49] in which resected PDAC tumors were analysed for PSC activity and collagen deposition showed that the combination of high collagen deposition and low stromal activity was associated with a better prognosis than low collagen deposition and high stromal activity. While these studies relate to the effect of stroma on tumour progression/regression, considering the similarities between carcinogenesis and organ development, it is likely that these findings apply to PDAC carcinogenesis. Combining findings from these studies, the role of chronic inflammation and fibrosis in influencing PDAC risk remains ambiguous.
Idiopathic pulmonary fibrosis (IPF) is the most common subtype of interstitial lung disease which is characterised by aberrant accumulation of fibrotic tissue in the lung parenchyma[50]. While the pathophysiology of IPF remains to be fully elucidated, the disease is thought to be mainly fibrosis-driven with minimal involvement of inflammation cascade[50]. Over the past decade, many studies have shown that IPF is linked to development of lung cancer, with a relative risk of 3.5-7.3 compared to healthy population[51]. One of the main reasons for this association is that IPF and lung cancer could have similarities in their pathophysiology, in terms of cellular morphological anomalies, dysregulated cytokine signalling and genetic mutations[52]. A study by Kawasaki et al[53] established that morphological aberrations in the lung epithelial layer, ranging from metaplasia and dysplasia to carcinoma, have been identified in fibrotic lung regions of IPF patients. This could be related to microsatellite instability and loss of heterozygosity, including mutations in tumour-suppressor genes such as fragile histidine triad gene, that are present at higher frequency in lung epithelial cells of IPF patients relative to healthy population[54,55]. Genetic alterations like these could be attributed to fibrosis, mainly mediated by TGF-ß released by various immune cells, and other changes in the stroma in IPF patients[56]. Using publicly available datasets, Saito et al[57] confirmed that 10% of the genes upregulated in lung cancer stroma, which include those coding for ECM components, mainly collagen (COL1A2, COL3A1, and COL5A2), and matrix metalloproteinases (MMP9 and 11), are also elevated in IPF. Furthermore, while increased immune cell infiltrates releasing cytokines, which promote epithelial proliferation and resist apoptosis are noted in the early stages of IPF, reduced number of lymphocytes, macrophages and monocytes were reported in fibrotic-predominant areas compared to epithelial-predominant ones in the later stages[57-61]. This implies that lung epithelial cells undergoing malignant transformation in the former are more likely to evade immune surveillance and progress to invasive malignancies in the latter. This observation concurs with the fact that lung cancers associated with IPF tend to develop in the peripheral and lower lobes – the fibrotic-predominant regions[62].
While IPF is mainly driven by fibrosis, other subtypes of ILD such as pneumoconiosis involve an inflammatory-driven condition that has been associated with lung cancer[50,63,64]. Patients with silicosis and asbestosis are about 3 times and 1.5 times more likely to develop lung cancer than the general population[15,65]. Chronic inflammation triggered as a result of the continuous activation of macrophages in an attempt to clear the silica particles is thought to mediate lung carcinogenesis in patients with silicosis[63]. Consequently, there is massive release of cytokines such as IL-12, IL-23, and TNFα which place lung epithelial cells at an increased risk of DNA damage and thus their susceptibility to malignant transformation[66]. This is demonstrated unequivocally by Wang et al[66] in Gprc5a-knockout mice exposed to silica where neoplastic epithelial cells were found in areas of intense lung damage and fibrosis which were thought to be a consequence of chronic inflammation. Furthermore, Freire et al[67] demonstrated increased lung adenocarcinomas in mice treated with the combination of the carcinogen N-nitrosodimethylamine and silica. On histopathological analysis, there was increased expression of various inhibitory immune markers including programmed cell death protein 1, lymphocyte-activation gene 3, and forkhead box P3, as well as the presence of regulatory T cells in mice treated with NMDA and silica compared to silica alone[67]. This produces marked immunosuppression which increases the risk of carcinogenesis, providing another plausible explanation for the link between silicosis and lung cancer.
Similarly, in the case of asbestosis – linked with a 6.8-times and increased incidence of lung cancer respectively compared with the general population – the pathogenesis by which it causes malignancy appears to be a combination of inflammation and the direct genotoxic effect of asbestos fibres on the genome[68,69]. Alveolar macrophages have been known to play a major role in handling asbestosis fibres[68]. The entrapment of asbestos stimulates the activation of NOD-like receptor family, the pyrin domain containing 3 expressed in alveolar macrophages which promotes the activation of IL-1β, along with other cytokines such as TGF-β and PDGF which are responsible for the formation of fibrotic nodules[68,70]. In addition, macrophages increase the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), thereby stimulating genotoxicity, chronic inflammation and thus malignancy transformation[71]. More specifically, numerous studies have demonstrated that chronic inflammation as a result of asbestos exposure affected several cell signalling pathways that are likely responsible for the development of lung cancer including the epidermal growth factor receptor (EGFR)-related extracellular signal–regulated kinase (ERK) signaling that promote lung epithelial cell and fibroblast proliferation[71-73]. While these studies have established the effect of chronic inflammation on development of lung cancer and mesothelioma, there is still a need to ascertain the relevance of fibrosis and lung cancer in vivo.
Infections of the lung have been previously linked with the future development of lung cancer. A meta-analysis by Brenner et al[14] demonstrated that pneumonia and tuberculosis was linked with a 1.4- and 1.9-times increased risk of developing lung cancer in the future. While both pneumonia and tuberculosis constitute as infection of the lung parenchyma, the degree of pulmonary inflammation and subsequent fibrosis likely explains the variation in the risk of developing lung cancer[14]. In regards to the former, pulmonary inflammation occurs for a shorter duration and thus the resulting fibrosis is less if not negligible compared to the latter, where a significant level of inflammation and fibrosis is involved[74,75]. Furthermore, in the setting of further tuberculosis (TB) recurrences which can occur in up to 47% of TB patients, repeated inflammatory response will increase the risk of lung cancer each time, with high cumulative risk associated with more frequent recurrences[76,77]. The mechanism by which inflammation increases cancer risk relates to the action of ROS and RNS produced by immune cells on the genome of lung epithelial cells and the ability of pro-inflammatory cytokines such as TNF-α and IL-6 to upregulate the expression of anti-apoptotic proteins[76,78]. Additionally, recurrent bouts of inflammation results in fibrosis in the surrounding lung parenchyma, which increases the risk of cancer associated with poor lymph drainage[79]. Further supporting the link between inflammation and lung cancer risk is a meta-analysis by Khuder et al[80] which demonstrated that non-steroidal anti-inflammatory drugs (NSAIDs) conferred a protective benefit in reducing lung cancer risk following adjustment for smoking (OR: 0.68; 95%CI: 0.55–0.85). These studies reaffirm the association between inflammation, fibrosis and lung cancer risk.
The link between hepatic cirrhosis and hepatocellular carcinoma (HCC) is well-established, with the 5-year HCC cumulative risk of 17% and 15% respectively for hepatitis B-related and hepatitis C-related cirrhosis respectively[81]. NAFLD-related cirrhosis is also associated with the development of HCC, with multi-centre cohort studies showing 1.6 to 23.7 times increased risk[82]. Chronic inflammation and fibrosis are thought to be the major mechanisms explaining this association. In chronic hepatitis, a multitude of immune cells release various cytokines, most notably, TGF-β, TNF-α and interleukins, which lead to an increase in cellular proliferation, telomere shortening and genomic instability involving signalling pathways such as mechanistic target of rapamycin and Wnt signalling[83,84]. Additionally, previous studies revealed that CD4+ cells – involved in activation of the tumour-killing CD8+ cytotoxic T cells – and regulatory T cells – responsible for suppressing immune response – are diminished and increased respectively in cirrhosis[85,86]. Furthermore, chronic inflammation leads to fibrosis. Specifically, TGF-β released by Kupffer cells (macrophages) promote the activation of quiescent hepatic stellate cells (HSCs), analogous to PSCs in the pancreas, becoming myofibroblasts which are the primary source of ECM proteins including collagen, undulin, fibronectin and elastin[11,87]. More recently, others have identified additional cytokines, growth factors and lipid signals produced by other stromal components including endothelial cells, Kupffer cells and adipocytes are involved in HSC activation[88-90]. Fibrosis impairs the hepatic vasculature and produces a hypoxic environment, triggering the production of reactive oxygen, nitrogen species (ROS and RNO). ROS and RNO in turn can cause oxidative DNA damage among hepatocytes, predisposing them to malignant transformation[91]. Additionally, hypoxia induces the transcription of pro-angiogenic factors such as VEGF which is responsible for angiogenesis[92]. Further exacerbating this tumorigenic environment, neo-angiogenesis promotes the recruitment of immune cells like macrophages which results in further inflammation driving a vicious cycle. Today, the relationship between cirrhosis and HCC is extremely robust, that liver stiffness, a hallmark of hepatic cirrhosis is being studied as a means of assessing HCC risk[93].
Primary biliary cholangitis (PBC) is one of the most common risk factors for cholangiocarcinoma, with ninefold increased risk of developing cholangiocarcinoma[94]. The pathogenesis of cholangiocarcinogenesis in patients with PBC is multifactorial. Apart from the biliary constituent in PBC patients, chronic inflammation involving cytokines and growth factors, notably IL-6, hepatocyte growth factor, and IL-1β, released by various stromal and immune cells have been implicated in sustaining proliferative signalling in biliary cells[95-98]. IL-6 is believed to be a predominant contributor in cholangiocarcinogenesis, with the potential to promote cellular proliferation, survival and immortalisation via different mechanisms – p38MAPK activation[99], increasing DNA methyltransferase[96], Mcl-1 and telomerase expression[100]. In addition, the inflammatory milieu in the surrounding bile duct raises the production of NO which increases the probability of DNA damage, affecting genes such as BRAF, K-ras, cyclin d-1, c-myc, COX-2 and p53[98,101]. Using a hamster model of cholangiocarcinoma, Prakobwong et al[102] demonstrated a decrease in incidence of cholangiocarcinoma, accompanied by decline in pro-inflammatory, growth signalling and anti-apoptotic protein expression including COX-2, cyclin-d1, c-myc, bcl-2 and bcl-xL following administration of curcumin, traditional anti-inflammatory agent derived from turmeric. This highlights the crucial role of inflammation in cholangiocarcinogenesis. Thirdly, fibrosis, instigated by the release of cytokines like IL-6 and TGF-β by immune cells, has also been shown to be involved in the neoplastic transformation of biliary cells. Using a liver cirrhosis mouse model, Farazi et al[103] showed that increased levels of fibroblasts along with type 1 and 3 collagen stimulate intrahepatic cholangiocyte proliferation and subsequent malignant transformation in p53-deficient mice. In another study, Ling et al[104] demonstrated that cholangiocarcinoma was induced in a rat model of thioacetamide (TAA)-induced hepatic fibrosis. The association between inflammation, fibrosis and cholangiocarcinogenesis is sufficiently convincing to stimulate interest in agents such as curcurmin that may diminish the two are being investigated to reduce the risk of cholangiocarcinoma[102,105].
For a long time, chronic gastroesophageal reflux disease (GERD) patients have been known to be at risk of oesophageal cancer (OC), with 10%-20% developing Barrett’s oesophagus (BO), making them 30-125 times more likely than the general population to develop OC[106]. Unlike HCC and cholangiocarcinoma where fibrosis is thought to be crucial to carcinogenesis, the pathophysiology of OC is inflammation-predominant. In GERD patients, chronic inflammation and oesophageal injury initiated by reflux of gastric acid bile and salt, result in BO, which is an intermediate step to progression to OC. More specifically, reflux promotes the recruitment of inflammatory cells, notably macrophages T, B and dendritic cells which release various pro-inflammatory cytokines such as TNF-α, IL-6, IL-1B and IL-8 that are responsible for NF-Κb, STAT-3, and HIF-1a activation[12,107,108]. This in turn leads to cellular proliferation and de-differentiation as part of a metaplastic process, a frequent precursor to neoplastic transformation. Further, immunosuppressive cytokines, notably IL-10 are found at higher levels in BO, and thus, could render healthy squamous epithelial cells undergoing malignant transformation less susceptible to destruction as a result of immune surveillance[109]. Furthermore, chronic inflammation creates a state of oxidative stress, evident by the increased levels of ROS and RNS present in BO[110]. The heightened level of oxidative stress in turn induces mutagenesis of oncogenes and tumour-suppressor genes, including TP53, K-ras, FBXW7 and PI3KCA, thereby contributing to OC carcinogenesis[110]. While chronic inflammation contributes significantly to OC carcinogenesis, the role of other aspects of stroma, including fibrosis on OC carcinogenesis remains unexplored. Interestingly, fibrosis is not apparent in BO, hence providing evidence of an inflammatory condition increasing cancer risk without the need for progression to fibrosis. Considering the reverse situation, we can hypothesise regarding the role of fibrosis on carcinogenesis from studies on eosinophilic oesophagitis, where both inflammation and fibrosis are prominent features but were not found to be associated with increased risk of OC[111]. Several mediators appear to be involved in this fibrosis, namely TGF-β, Th-2 type cytokines and ROS[112,113]. We could hypothesise that fibrosis may suppress neoplastic transformation in this scenario[111]. At this stage, while chronic inflammation substantially elevates OC cancer risk, fibrosis may have differing context specific effects on OC risk.
Apart from tobacco smoking, oral submucosal fibrosis (OSF) is the major risk factor for the development of oral squamous cell carcinoma (OSCC), increasing the likelihood by up to 19-fold compared to a healthy population[114]. The aetiology for OSF has long been established, with increasing incidence attributed to daily consumption of areca nut and betel quid[115,116]. In addition to the carcinogenic potential of constituents of areca nut and betel quid on activating oncogenes and inhibiting tumour-suppressor genes, they are also known to be inflammatory[117]. This promotes the recruitment of immune cells, predominantly, macrophages, T cells and lymphocytes to the oral mucosa, which in turn release ROS, prostaglandins, cytokines and growth factors, notably IL-6, TNF-α, PDGF and TGF-β[118]. These biological mediators, present in the surrounding oral squamous epithelium, promote oral squamous cell proliferation and survival[118]. Additionally, ROS promotes oxidative damage and mutagenesis, resulting in p53 mutations, decreased levels of DNA-methyltransferase repair enzyme and upregulated levels of HSP70 and MDM2-P2 promoter, which ultimately lead to neoplastic transformation in areas of OSF[119-123]. Interestingly, some of the aforementioned biological mediators, namely IL-6 and TGF-ß are significantly involved in fibrogenesis – synthesising ECM proteins like collagen and fibronectin and simultaneously producing plasminogen activator inhibitor-1 (PAI-1) and tissue inhibitor of metalloprotease which inhibit ECM breakdown[124-126]. This produces extensive fibrosis, particularly in the lamina propria, a hallmark feature of OSF. Recently, in an immunohistochemical study involving tissues obtained from patients with normal mucosa and OSF, Gadbail et al[127] demonstrated that Ki67 expression, a marker for cell proliferation, was directly proportional to α-SMA expression, a marker for myofibroblast formation, potentially highlighting that fibrosis may be directly involved in neoplastic transformation. The effect of fibrosis on malignant transformation of oral squamous epithelial cells is further stressed in an in-vitro study by Ye et al[128], who showed that growth-regulated oncogene-α from OSF-associated fibroblasts promote dysplastic keratinocyte cell line proliferation via activation of the EGFR/ERK signalling pathway. The potential of inflammation and fibrosis in OSF to cause neoplastic transformation to OSCC is regarded as high, justifying the ongoing search for anti-inflammatory and anti-fibrotic agents to suppress these processes in OSF[76,129].
Up to this point the breast appears to be a unique case in considering links between stromal composition and cancer risk. The differentiator is the strong established link between mammographic breast density (MBD), as assessed on mammographic images, which ties to the stromal composition of the normal breast, and breast cancer risk. Women with MBD lying in the highest quartile have a 4-6-fold higher risk of developing breast cancer than those in the lowest quartile[130,131]. Dense tissue has been found to correlate with higher proportions of ECM, particularly collagen[132], immune cells[133] and glandular epithelial components, and lower proportions of adipocytes[134]. As well as promoting initial carcinogenesis, higher mammographic density has been found to correlate with a higher risk of local relapse, a lower rate for complete response to chemotherapy[135] and a higher rate of relapse after treatment in locally advanced tumours[136].
This raises the question as to whether higher ‘physiological’ tissue stromal density carries higher risks of cancer in other organs, as well as whether pathological inflammatory and fibrotic processes impact cancer risk in the breast. Considering the latter, inflammatory conditions that result in a sustained inflammatory environment in the breast are relatively rare. Chronic mastitis is a condition whereby there is sustained inflammation usually relating to chronic infection. A retrospective cohort study by Chen et al[137] revealed that patients aged ≥ 40 with a history of mastitis have 3-fold increased risk of developing breast cancer aHR = 3.71, 95%CI = 1.9–7.02) compared to those without a history of mastitis. On the same note, fibrotic condition of the breast such as sclerosing adenosis has also been associated with an approximate doubling of breast cancer risk in a US retrospective cohort[138]. This further highlights the significance of inflammation and fibrosis in influencing cancer risk and emphasises consideration of more rigorous screening for these conditions and therapeutics which could manipulate the stroma and reduce cancer risk.
The abundant evidence for multiple robust links between inflammation, fibrosis and carcinogenesis (Figure 1), as well as the frequently overlapping spectrum of implicated signalling mediators and pathways, suggest that there may be substantial therapeutic benefit to be achieved by detecting and targeting these processes across many cancer types (Table 1).
Knowledge of the links between inflammation and malignancy are widely exploited in the screening of at-risk individuals with a variety of conditions. First there is promise in the assessment of stromal characteristics to predict cancer risk, thereby allowing identification of individuals suitable for screening or for whom screening could be adjusted. For instance, the strong relationship between MBD and breast risk has been described above. Initiatives are already in progress to use MBD levels to tailor screening, both considering the age at which to start screening and the frequency as well as whether other modalities should be considered such as ultrasound or MRI[139,140]. Additionally, robust link between liver cirrhosis and HCC has prompted surveillance quantification of alpha-feto protein and liver as a means to diagnose HCC earlier[141]. Furthermore, there are screening recommendations for patients with BO and IBD to undergo surveillance gastroscopy and colonoscopy to detect the relevant malignancies at early stages[142,143].
Beyond detection, the common mechanisms underlying links between tissue inflammation, fibrosis and malignancy have led to development of a number of strategies to target these underlying processes including the application of therapeutics including anti-proliferatives, anti-inflammatories, anti-estrogens and anti-fibrotics which will be discussed below.
Thiopurines (azathioprine, mercaptopurine and thioguanine) has been a mainstay drug for IBD patients over the last 50 years. Its main drug effect is derived from the production of its metabolites 6-thioguaninenucleotides (6-TGN) and 6-methylmercaptopurine (6-MMP)[144]. These metabolites exert an immunosuppressive and anti-proliferative effect by binding Ras-related C3 botulinum toxin substrate 1 (Rac1) to thioguanosine triphosphate thus mitigating chronic gut inflammation in IBD. This blockade of Rac1 signalling results in decreased anti-apoptotic protein Bcl-xL expression and subsequent promotion of pro-inflammatory T-cell apoptosis[145,146]. A meta-analysis by Zhu et al[147] involving 95397 IBD patients, found that thiopurine use is associated with reduced risk of colorectal neoplasia (case control OR = 0.49, 95%CI: 0.34–0.70; cohort RR = 0.96, 95%CI: 0.94–0.98). While effective as a chemopreventive agent, thiopurine use should be balanced with potential adverse effects such as risk of myelosuppression and in the long term, development of lymphoproliferative disorders[146,148].
NSAID used widely in the treatment of chronic pain syndromes have been studied as a chemopreventive agent in a wide range of cancers. NSAIDs reduce inflammation by reversibly and non-selectively inhibiting cyclooxygenase (COX) enzymes which in turn lead to decreased production of prostaglandins and leukotrienes, mediators which have been implicated in carcinogenesis. A meta-analysis by Qiao et al[149] comprising of 218 studies demonstrated that aspirin use was associated with a significant reduction in risk of gastric, esophageal, colorectal, pancreatic, ovarian, endometrial, breast and prostate cancer with rates ranging from 6%-25%. Another meta-analysis investigating the link between NSAID and skin cancer risk has also shown positive results, with significant reduction in risk of developing basal cell carcinoma, squamous cell carcinoma and non-melanoma skin cancer, but not melanoma. Interestingly, no significant chemopreventive effect is observed for COX-2 selective-NSAIDs and NSAID use among European populations[150].
5-aminosalicylates (5-ASA) is a drug class with anti-inflammatory and immunosuppressive properties, generally utilized in treatment of IBD and various rheumatologic conditions which has recently been found to possess chemopreventive properties. It works via multifactorial mechanisms but two well-understood mechanisms are the inhibition of prostaglandins and leukotrienes synthesis and scavenging of reactive oxygen species[151]. Previous systematic review of 31 independent observational studies in IBD has demonstrated that 5-ASA use is associated with a 43% reduction in risk of colorectal malignancy among patients with IBD. Of note, the reduction in risk of colorectal malignancy of 50% was more prominent in UC as compared to CD, where the risk reduction was non-significant. Furthermore, the incidence of IBD-related colorectal cancer have significantly declined in recent years and whilst numerous factors could cause this, the role of 5-ASA and other immunomodulatory agents are likely to have contributed to the decrease in cancer incidence[13].
Nintedanib and pirfenidone are two anti-fibrotic agents which have been approved for the management of IPF. Both work via modulation of fibrogenic growth factors, thereby decreasing fibroblast proliferation, myofibroblast differentiation, collagen and fibronectin synthesis, and extracellular matrix deposition[152]. Recent retrospective study by Naoi et al[153] demonstrated that the cumulative incidence of lung cancer in patients with IPF treated with antifibrotic agent was significantly lower than those who were not (2.2% vs 4.4% at 1 year, 2.2% vs 6.7% at 3 years, and 3.3% vs 9.7% at 5 years, respectively; P = 0.004)[153]. Interestingly, the use of anti-fibrotic agent was also associated with lower lung-cancer related mortality (1.6% vs 15.2%, respectively; P = 0.0001)[153]. With established benefits in terms of slowing progression, possibly improving survival in IPF and more recently, preventing lung cancer development, the use of anti-fibrotic agents should be strongly considered in all IPF patients provided that there are no contraindications.
Anti-estrogens inhibit the synthesis or antagonise action of estrogen in target organs. Anti-estrogens encompass selective estrogen receptor modulators (SERMs), selective estrogen receptor degrader, aromatase inhibitors, gonadotrophin release hormone agonists and antagonists. Previous studies have shown that tamoxifen, raloxifene, exemestane and anastrozole have significantly reduced the incidence of breast cancer in high-risk women by 49%[154], 76%[155], 65%[156], 49%[157] respectively. Currently, two SERMs, tamoxifen and raloxifene, are approved by the FDA for breast cancer chemoprevention, with anastrozole and exemestane pending approval. The mechanism of action by which antiestrogens prevent breast cancer remains unclear, however, the reduction of breast stromal density brought about by antiestrogen use is thought to confer a less pro-tumorigenic environment and hence lowering breast cancer risk.
Lysyl oxidase (LOX) and LOX-like inhibitors are another drug class targeting the stroma of immense chemopreventive potential. LOXL is amine oxidase which catalyse the cross-linking of collagen and elastin in normal tissue and extracellular matrix, facilitating carcinogenesis, cell proliferation, migration and metastases[158]. Whilst previous studies have mainly investigated LOXL inhibitors as an anti-cancer agent, the preliminary results have been promising and LOX role in carcinogenesis make it a particularly interesting target to prevent carcinogenesis. Anti-GS341, antibody targeting LOXL-2 has been shown to significantly reduce tumour volume and lung metastases in a breast cancer xenograft model using MDA-MB-231 cells into immunocompromised SCID mice[159]. Additionally, an orally bioavailable LOX/LOXL2 inhibitor, CCT365623, developed by Leung et al[160] produced significant diminution in tumor growth and metastases in an in vivo model of transgenic LOX-dependent breast tumor mice[160]. These promising preclinical findings have translated to clinical trials exploring LOX/LOXL inhibitor in numerous diseases including myelofibrosis, cirrhosis, and breast cancer[161-164].
Another potential stromal disruption agent targets the extracellular matrix, particularly degradation of hyaluronic acid (HA), an important component of the ECM known to participate in carcinogenesis, tumor progression and metastasis in various cancers[165]. PEGPH20 is a PEGylated human hyaluronidase that showed promise both as single agent or in combination, in numerous preclinical studies[165-167]. Thompson et al[168] showed that repetitive PEGPH20 administration significantly inhibited tumor growth by 70% in high-HA prostate PC3 tumors and improved both docetaxel and liposomal doxorubicin activity in PC3 tumors. Additionally, using HA synthase 3-overexpressing and wild-type SKOV3 ovarian cancer model and in the BxPC3 pancreas xenograft tumour model, Morosi et al[166] showed that PEGPH20 enhanced the antitumor activity of paclitaxel by modifying the tumour tissue architecture. Despite the promising potential of PEGPH20 in preclinical studies, clinical trials of PEGPH20 in various advanced solid tumours have been disappointing with PEGPH20 failing to meet its primary end point of improvement in overall survival[169]. However, it is crucial to note that PEGPH20 has not been explored in preventing carcinogenesis such as in the context of IBD, cirrhosis and IPF. Considering the significance of the ECM in carcinogenesis, future studies should study the effect of ECM-degrading agents such as PEGPH20 in carcinogenesis.
In addition to targeting the ECM, agents targeting other components of the ECM have been studied. Most notably, agents targeting myofibroblasts which produce pathological fibrosis and thus a pro-carcinogenic environment have shown promising results in previous studies. Depletion of myofibroblasts by targeting its marker, fibroblast activation protein-α, has been shown to inhibit tumor growth by augmenting anti-tumor immunity[170,171]. Additionally, agents targeting TGF-β, an important cytokine in myofibroblast activation have also been studied as TGF-β inhibition has been demonstrated to prevent myofibroblast activation and prevent immunosuppression and thus cancer progression[172]. Again while these agents are studied as anti-cancer therapies, these drugs have immense potential to be utilised as chemopreventive agents in disorders of chronic inflammation and fibrosis to prevent carcinogenesis.
In conclusion, the correlation between chronic inflammation, fibrosis and cancer risk is complex, with the former being more straightforward. Chronic inflammation in the stroma of different body tissues promotes carcinogenesis via different mechanisms – growth factor/cytokine-mediated cellular proliferation, apoptotic resistance and immunosuppression; and free-radical-induced oxidative DNA damage. However, certain immune cells, involved in tumour-surveillance may be depleted, as seen in IPF and hepatic cirrhosis, thereby raising cancer risk by compromising immune surveillance of tumours. The relationship between stromal fibrosis and cancer risk varies in different organs, implying that the effects of fibrosis could be tissue-specific. Increased stromal fibrosis is associated with an increased cancer risk in organs like the lung, liver, biliary tract and colorectal region. Conversely, in other organs such as pancreas and potentially, oesophagus, increased stromal fibrosis may confer a lower cancer risk.
At this current time, the mechanism by which fibrosis influences cancer risk is still ambiguous. We propose two hypotheses. Firstly, a fibrotic environment contributes to an aberration in ECM dynamics which affects normal cellular behaviour and ultimately neoplastic transformation. Secondly, we hypothesise that fibrosis may present as a safe alternative to cellular regeneration which has the potential to produce aberrant DNA mutations, resulting in tumour formation. What determines the former or the latter are a multitude of factors which could include fibroblast heterogeneity and plasticity; extent of fibrosis; inflammation; and the predominance of certain mediators over others. Therefore, future studies, especially in-vitro and animal studies, should investigate the mechanisms by which fibrosis contributes to carcinogenesis in various organs in further depth and determine if fibrosis, alone or only in conjunction with inflammation would promote carcinogenesis. Furthermore, the role of surveillance screening and therapeutic agents with stroma manipulation potential in patients with diseases which involve chronic inflammation and fibrosis should be further studied to reduce the incidence of relevant cancers.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Agostino SD, Italy; Gutiérrez-Cuevas J, Mexico S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98-101. [PubMed] |
| 2. | Virchow R. Cellular pathology. As based upon physiological and pathological histology. Lecture XVI--Atheromatous affection of arteries. 1858. Nutr Rev. 1989;47:23-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 220] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Franco M, Bustuoabad OD, di Gianni PD, Goldman A, Pasqualini CD, Ruggiero RA. A serum-mediated mechanism for concomitant resistance shared by immunogenic and non-immunogenic murine tumours. Br J Cancer. 1996;74:178-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022-7029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 854] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 5. | Zaynagetdinov R, Sherrill TP, Polosukhin VV, Han W, Ausborn JA, McLoed AG, McMahon FB, Gleaves LA, Degryse AL, Stathopoulos GT, Yull FE, Blackwell TS. A critical role for macrophages in promotion of urethane-induced lung carcinogenesis. J Immunol. 2011;187:5703-5711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Seo, BR, Bhardwaj P, Choi S, Gonzalez J, Eguiluz RCA, Wang K, Mohanan S, Morris PG, Du B, Zhou XK, Vahdat LT, Verma A, Elemento O, Hudis CA, Williams RM, Gourdon D, Dannenberg AJ, Fischbach C. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 249] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 7. | Stoker MG, Shearer M, O'Neill C. Growth inhibition of polyoma-transformed cells by contact with static normal fibroblasts. J Cell Sci. 1966;1:297-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 142] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 8. | Wang, B, Xi C, Liu M, Sun H, Liu S, Song L, Kang H. Breast fibroblasts in both cancer and normal tissues induce phenotypic transformation of breast cancer stem cells: a preliminary study. Peer J. 2018;e4805. [DOI] [Full Text] |
| 9. | Burkitt MD, Hanedi AF, Duckworth CA, Williams JM, Tang JM, O'Reilly LA, Putoczki TL, Gerondakis S, Dimaline R, Caamano JH, Pritchard DM. NF-κB1, NF-κB2 and c-Rel differentially regulate susceptibility to colitis-associated adenoma development in C57BL/6 mice. J Pathol. 2015;236:326-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007;26:227-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 300] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 11. | Cai CX, Buddha H, Castelino-Prabhu S, Zhang Z, Britton RS, Bacon BR, Neuschwander-Tetri BA. Activation of Insulin-PI3K/Akt-p70S6K Pathway in Hepatic Stellate Cells Contributes to Fibrosis in Nonalcoholic Steatohepatitis. Dig Dis Sci. 2017;62:968-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Dvorak K, Chavarria M, Payne CM, Ramsey L, Crowley-Weber C, Dvorakova B, Dvorak B, Bernstein H, Holubec H, Sampliner RE, Bernstein C, Prasad A, Green SB, Garewal H. Activation of the interleukin-6/STAT3 antiapoptotic pathway in esophageal cells by bile acids and low pH: relevance to barrett's esophagus. Clin Cancer Res. 2007;13:5305-5313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 658] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 14. | Brenner DR, McLaughlin JR, Hung RJ. Previous lung diseases and lung cancer risk: a systematic review and meta-analysis. PLoS One. 2011;6:e17479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 15. | Partanen T, Pukkala E, Vainio H, Kurppa K, Koskinen H. Increased incidence of lung and skin cancer in Finnish silicotic patients. J Occup Med. 1994;36:616-622. [PubMed] |
| 16. | Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. 2014;7:113-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 336] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 17. | Laukoetter MG, Mennigen R, Hannig CM, Osada N, Rijcken E, Vowinkel T, Krieglstein CF, Senninger N, Anthoni C, Bruewer M. Intestinal cancer risk in Crohn's disease: a meta-analysis. J Gastrointest Surg. 2011;15:576-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis. 2013;19:789-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 375] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 19. | Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 705] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 20. | LUMB G, PROTHEROE RH. Ulcerative colitis; a pathologic study of 152 surgical specimens. Gastroenterology. 1958;34:381-407. [PubMed] [DOI] [Full Text] |
| 21. | Gordon IO, Agrawal N, Willis E, Goldblum JR, Lopez R, Allende D, Liu X, Patil DY, Yerian L, El-Khider F, Fiocchi C, Rieder F. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment Pharmacol Ther. 2018;47:922-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 22. | Boldeanu MV, Siloşi I, Ghiluşi M, Cojocaru M, Biciuşcă V, Avrămescu CS, Cojocaru IM, Ciurea T, Albu DF, Siloşi CA. Investigation of inflammatory activity in ulcerative colitis. Rom J Morphol Embryol. 2014;55:1345-1351. [PubMed] |
| 23. | Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C, Strand D, Czaja J, Schlaak JF, Lehr HA, Autschbach F, Schürmann G, Nishimoto N, Yoshizaki K, Ito H, Kishimoto T, Galle PR, Rose-John S, Neurath MF. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 1026] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 24. | Francescone R, Hou V, Grivennikov SI. Cytokines, IBD, and colitis-associated cancer. Inflamm Bowel Dis. 2015;21:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 219] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 25. | Korkaya H, Liu S, Wicha MS. Regulation of cancer stem cells by cytokine networks: attacking cancer's inflammatory roots. Clin Cancer Res. 2011;17:6125-6129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 260] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 26. | Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1747] [Cited by in RCA: 1768] [Article Influence: 110.5] [Reference Citation Analysis (0)] |
| 27. | Xing Y, Chen X, Cao Y, Huang J, Xie X, Wei Y. Expression of Wnt and Notch signaling pathways in inflammatory bowel disease treated with mesenchymal stem cell transplantation: evaluation in a rat model. Stem Cell Res Ther. 2015;6:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Li C, Iness A, Yoon J, Grider JR, Murthy KS, Kellum JM, Kuemmerle JF. Noncanonical STAT3 activation regulates excess TGF-β1 and collagen I expression in muscle of stricturing Crohn's disease. J Immunol. 2015;194:3422-3431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 29. | Okuno T, Andoh A, Bamba S, Araki Y, Fujiyama Y, Fujiyama M, Bamba T. Interleukin-1beta and tumor necrosis factor-alpha induce chemokine and matrix metalloproteinase gene expression in human colonic subepithelial myofibroblasts. Scand J Gastroenterol. 2002;37:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Claessen, MM, Schipper MEI, Oldenburg B, Siersema PD, Offerhaus GJA, Vleggaar fp. WNT-pathway activation in IBD-associated colorectal carcinogenesis: potential biomarkers for colonic surveillance. Cell Oncol. 2010;32:303-310. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Babyatsky MW, Rossiter G, Podolsky DK. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996;110:975-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 285] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 32. | Zou X, Feng B, Dong T, Yan G, Tan B, Shen H, Huang A, Zhang X, Zhang M, Yang P, Zheng M, Zhang Y. Up-regulation of type I collagen during tumorigenesis of colorectal cancer revealed by quantitative proteomic analysis. J Proteomics. 2013;94:473-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Hünerwadel A, Fagagnini S, Rogler G, Lutz C, Jaeger SU, Mamie C, Weder B, Ruiz PA, Hausmann M. Severity of local inflammation does not impact development of fibrosis in mouse models of intestinal fibrosis. Sci Rep. 2018;8:15182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Latella G, Di Gregorio J, Flati V, Rieder F, Lawrance IC. Mechanisms of initiation and progression of intestinal fibrosis in IBD. Scand J Gastroenterol. 2015;50:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 35. | Krejs GJ. Pancreatic cancer: epidemiology and risk factors. Dig Dis. 2010;28:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Haeberle L, Steiger K, Schlitter AM, Safi SA, Knoefel WT, Erkan M, Esposito I. Stromal heterogeneity in pancreatic cancer and chronic pancreatitis. Pancreatology. 2018;18:536-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Guerra C, Schuhmacher AJ, Cañamero M, Grippo PJ, Verdaguer L, Pérez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 953] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 38. | Luttenberger T, Schmid-Kotsas A, Menke A, Siech M, Beger H, Adler G, Grünert A, Bachem MG. Platelet-derived growth factors stimulate proliferation and extracellular matrix synthesis of pancreatic stellate cells: implications in pathogenesis of pancreas fibrosis. Lab Invest. 2000;80:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 151] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Mews P, Phillips P, Fahmy R, Korsten M, Pirola R, Wilson J, Apte M. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 2002;50:535-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 290] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 40. | Apte MV, Haber PS, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut. 1999;44:534-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 482] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 41. | Marzoq AJ, Mustafa SA, Heidrich L, Hoheisel JD, Alhamdani MSS. Impact of the secretome of activated pancreatic stellate cells on growth and differentiation of pancreatic tumour cells. Sci Rep. 2019;9:5303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 42. | Cheng XB, Kohi S, Koga A, Hirata K, Sato N. Hyaluronan stimulates pancreatic cancer cell motility. Oncotarget. 2016;7:4829-4840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 43. | Linder S, Castaños-Velez E, von Rosen A, Biberfeld P. Immunohistochemical expression of extracellular matrix proteins and adhesion molecules in pancreatic carcinoma. Hepatogastroenterology. 2001;48:1321-1327. [PubMed] |
| 44. | Whatcott CJ, Diep CH, Jiang P, Watanabe A, LoBello J, Sima C, Hostetter G, Shepard HM, Von Hoff DD, Han H. Desmoplasia in Primary Tumors and Metastatic Lesions of Pancreatic Cancer. Clin Cancer Res. 2015;21:3561-3568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 470] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 45. | Hingorani SR, Harris WP, Beck JT, Berdov BA, Wagner SA, Pshevlotsky EM, Tjulandin SA, Gladkov OA, Holcombe RF, Korn R, Raghunand N, Dychter S, Jiang P, Shepard HM, Devoe CE. Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin Cancer Res. 2016;22:2848-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 260] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 46. | Hingorani SR, Zheng L, Bullock AJ, Seery TE, Harris WP, Sigal DS, Braiteh F, Ritch PS, Zalupski MM, Bahary N, Oberstein PE, Wang-Gillam A, Wu W, Chondros D, Jiang P, Khelifa S, Pu J, Aldrich C, Hendifar AE. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J Clin Oncol. 2018;36:359-366. [DOI] [Full Text] |
| 47. | Torphy RJ, Wang Z, True-Yasaki A, Volmar KE, Rashid N, Yeh B, Anderson JM, Johansen JS, Hollingsworth MA, Yeh JJ, Collisson EA. Stromal Content Is Correlated With Tissue Site, Contrast Retention, and Survival in Pancreatic Adenocarcinoma. JCO Precis Oncol. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 48. | Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, Westphalen CB, Kitajewski J, Fernandez-Barrena MG, Fernandez-Zapico ME, Iacobuzio-Donahue C, Olive, KP, Stanger BZ. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014; 25: 735-747. [DOI] [Full Text] |
| 49. | Erkan M, Michalski CW, Rieder S, Reiser-Erkan C, Abiatari I, Kolb A, Giese NA, Esposito I, Friess H, Kleeff J. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2008;6:1155-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 353] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 50. | Barratt SL, Creamer A, Hayton C, Chaudhuri N. Idiopathic Pulmonary Fibrosis (IPF): An Overview. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 243] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 51. | Naccache JM, Gibiot Q, Monnet I, Antoine M, Wislez M, Chouaid C, Cadranel J. Lung cancer and interstitial lung disease: a literature review. J Thorac Dis. 2018;10:3829-3844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 52. | Debes JD, van Tilborg M, Groothuismink ZMA, Hansen BE, Schulze Zur Wiesch J, von Felden J, de Knegt RJ, Boonstra A. Levels of Cytokines in Serum Associate With Development of Hepatocellular Carcinoma in Patients With HCV Infection Treated With Direct-Acting Antivirals. Gastroenterology. 2018;154:515-517.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 53. | Kawasaki H, Ogura T, Yokose T, Nagai K, Nishiwaki Y, Esumi H. p53 gene alteration in atypical epithelial lesions and carcinoma in patients with idiopathic pulmonary fibrosis. Hum Pathol. 2001;32:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Uematsu K, Yoshimura A, Genma A, Mochimaru H, Hosoya Y, Kunugi S, Matsuda K, Seike M, Kurimoto F, Takenaka K, Koizumi K, Fukuda Y, Tanaka S, Chin K, Jablons DM, Kudoh S. Aberrations in the fragile histidine triad (FHIT) gene in idiopathic pulmonary fibrosis. Cancer Res. 2001;61:8527-8533. [DOI] [Full Text] |
| 55. | Vassilakis DA, Sourvinos G, Spandidos DA, Siafakas NM, Bouros D. Frequent genetic alterations at the microsatellite level in cytologic sputum samples of patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2000;162:1115-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Bergeron A, Soler P, Kambouchner M, Loiseau P, Milleron B, Valeyre D, Hance AJ, Tazi A. Cytokine profiles in idiopathic pulmonary fibrosis suggest an important role for TGF-beta and IL-10. Eur Respir J. 2003;22:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 57. | Saito A, Horie M, Micke P, Nagase T. The Role of TGF-β Signaling in Lung Cancer Associated with Idiopathic Pulmonary Fibrosis. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 58. | Car BD, Meloni F, Luisetti M, Semenzato G, Gialdroni-Grassi G, Walz A. Elevated IL-8 and MCP-1 in the bronchoalveolar lavage fluid of patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am J Respir Crit Care Med. 1994;149:655-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 177] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 59. | Yoshida M, Sakuma J, Hayashi S, Abe K, Saito I, Harada S, Sakatani M, Yamamoto S, Matsumoto N, Kaneda Y. A histologically distinctive interstitial pneumonia induced by overexpression of the interleukin 6, transforming growth factor beta 1, or platelet-derived growth factor B gene. Proc Natl Acad Sci U S A. 1995;92:9570-9574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 60. | Furuie H, Yamasaki H, Suga M, Ando M. Altered accessory cell function of alveolar macrophages: a possible mechanism for induction of Th2 secretory profile in idiopathic pulmonary fibrosis. Eur Respir J. 1997;10:787-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 61. | Flavia Greiffo IF, Marion Frankenberger, Jürgen Behr, Oliver Eickelberg. Circulating monocytes from interstitial lung disease patients show an activated phenotype. European Respiratory Journal. 2016;48. [DOI] [Full Text] |
| 62. | Aburto M, Herráez I, Iturbe D, Jiménez-Romero A. Diagnosis of Idiopathic Pulmonary Fibrosis: Differential Diagnosis. Med Sci (Basel). 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Davis GS. The pathogenesis of silicosis. State of the art. Chest. 1986;89:166S-169S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 64. | Kamp DW, Weitzman SA. Asbestosis: clinical spectrum and pathogenic mechanisms. Proc Soc Exp Biol Med. 1997;214:12-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | Kwak K, Kang D, Paek D. Environmental exposure to asbestos and the risk of lung cancer: a systematic review and meta-analysis. Occup Environ Med. 2022;79:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Wang X, Xu D, Liao Y, Zhong S, Song H, Sun B, Zhou BP, Deng J, Han B. Epithelial neoplasia coincides with exacerbated injury and fibrotic response in the lungs of Gprc5a-knockout mice following silica exposure. Oncotarget. 2015;6:39578-39593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Freire J, Ajona D, de Biurrun G, Agorreta J, Segura V, Guruceaga E, Bleau AM, Pio R, Blanco D, Montuenga LM. Silica-induced chronic inflammation promotes lung carcinogenesis in the context of an immunosuppressive microenvironment. Neoplasia. 2013;15:913-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 68. | Liu G, Cheresh P, Kamp DW. Molecular basis of asbestos-induced lung disease. Annu Rev Pathol. 2013;8:161-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 69. | Selikoff IJ, Churg J, Hammond EC. Classics in Oncology: Asbestos exposure and neoplasia. CA Cancer J Clin. 1984;34:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 70. | Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674-677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2196] [Cited by in RCA: 2084] [Article Influence: 122.6] [Reference Citation Analysis (0)] |
| 71. | Heintz NH, Janssen-Heininger YM, Mossman BT. Asbestos, lung cancers, and mesotheliomas: from molecular approaches to targeting tumor survival pathways. Am J Respir Cell Mol Biol. 2010;42:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 72. | Mossman BT, Lippmann M, Hesterberg TW, Kelsey KT, Barchowsky A, Bonner JC. Pulmonary endpoints (lung carcinomas and asbestosis) following inhalation exposure to asbestos. J Toxicol Environ Health B Crit Rev. 2011;14:76-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 73. | Shukla A, Hillegass JM, MacPherson MB, Beuschel SL, Vacek PM, Butnor KJ, Pass HI, Carbone M, Testa JR, Heintz NH, Mossman BT. ERK2 is essential for the growth of human epithelioid malignant mesotheliomas. Int J Cancer. 2011;129:1075-1086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Grief SN, Loza JK. Guidelines for the Evaluation and Treatment of Pneumonia. Prim Care. 2018;45:485-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 75. | Hunter RL. The Pathogenesis of Tuberculosis: The Early Infiltrate of Post-primary (Adult Pulmonary) Tuberculosis: A Distinct Disease Entity. Front Immunol. 2018;9:2108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 76. | Lin CY, Hsieh PL, Liao YW, Peng CY, Yu CC, Lu MY. Arctigenin Reduces Myofibroblast Activities in Oral Submucous Fibrosis by LINC00974 Inhibition. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 77. | Mirsaeidi M, Sadikot RT. Patients at high risk of tuberculosis recurrence. Int J Mycobacteriol. 2018;7:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 78. | Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 919] [Cited by in RCA: 962] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 79. | Ardies CM. Inflammation as cause for scar cancers of the lung. Integr Cancer Ther. 2003;2:238-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 80. | Khuder SA, Herial NA, Mutgi AB, Federman DJ. Nonsteroidal antiinflammatory drug use and lung cancer: a metaanalysis. Chest. 2005;127:748-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 81. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1791] [Article Influence: 85.3] [Reference Citation Analysis (2)] |
| 82. | Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, Li L, Desiderio R, Thrift AP, Asch SM, Chu J, El-Serag HB. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology. 2018;155:1828-1837.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 566] [Article Influence: 80.9] [Reference Citation Analysis (0)] |
| 83. | Estevez J, Chen VL, Podlaha O, Li B, Le A, Vutien P, Chang ET, Rosenberg-Hasson Y, Jiang Z, Pflanz S, Ge D, Gaggar A, Nguyen MH. Differential Serum Cytokine Profiles in Patients with Chronic Hepatitis B, C, and Hepatocellular Carcinoma. Sci Rep. 2017;7:11867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 84. | Plentz RR, Caselitz M, Bleck JS, Gebel M, Flemming P, Kubicka S, Manns MP, Rudolph KL. Hepatocellular telomere shortening correlates with chromosomal instability and the development of human hepatoma. Hepatology. 2004;40:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 85. | McGovern BH, Golan Y, Lopez M, Pratt D, Lawton A, Moore G, Epstein M, Knox TA. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 86. | Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457-2464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 486] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 87. | Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. J Hepatol. 1999;30:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 317] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 88. | Meng F, Wang K, Aoyoma T, Grivennikov SI, Paik YH, Scholten D, Cong M, Iwaisako K, Liu X, Zhang M, Osterreicher CH, Stickel F, Ley K, Brenner DA, Kisesleva T. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143:765-776 e763. [DOI] [Full Text] |
| 89. | Shafiei MS, Shetty S, Scherer PE, Rockey DC. Adiponectin regulation of stellate cell activation via PPARγ-dependent and -independent mechanisms. Am J Pathol. 2011;178:2690-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 90. | Xie G, Wang X, Wang L, Atkinson RD, Kanel GC, Gaarde WA, Deleve LD. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology. 2012;142:918-927.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 297] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 91. | Breimer LH. Molecular mechanisms of oxygen radical carcinogenesis and mutagenesis: the role of DNA base damage. Mol Carcinog. 1990;3:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 250] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 92. | Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, Poupon R, Housset C, Rosmorduc O. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35:1010-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 369] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 93. | Adler M, Larocca L, Trovato FM, Marcinkowski H, Pasha Y, Taylor-Robinson SD. Evaluating the risk of hepatocellular carcinoma in patients with prominently elevated liver stiffness measurements by FibroScan: a multicentre study. HPB (Oxford). 2016;18:678-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 94. | Boonstra K, Bokelaar R, Stadhouders PH, Tuynman HA, Poen AC, van Nieuwkerk KM, Witteman EM, Hamann D, Witteman BJ, Beuers U, Ponsioen CY. Increased cancer risk in a large population-based cohort of patients with primary biliary cirrhosis: follow-up for up to 36 years. Hepatol Int. 2014;8:266-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 95. | Yoon JH, Canbay AE, Werneburg NW, Lee SP, Gores GJ. Oxysterols induce cyclooxygenase-2 expression in cholangiocytes: implications for biliary tract carcinogenesis. Hepatology. 2004;39:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 96. | Sugawara H, Yasoshima M, Katayanagi K, Kono N, Watanabe Y, Harada K, Nakanuma Y. Relationship between interleukin-6 and proliferation and differentiation in cholangiocarcinoma. Histopathology. 1998;33:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 97. | Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 112] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 98. | Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 169] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 99. | Wehbe H, Henson R, Meng F, Mize-Berge J, Patel T. Interleukin-6 contributes to growth in cholangiocarcinoma cells by aberrant promoter methylation and gene expression. Cancer Res. 2006;66:10517-10524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 100. | Yamagiwa Y, Meng F, Patel T. Interleukin-6 decreases senescence and increases telomerase activity in malignant human cholangiocytes. Life Sci. 2006;78:2494-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 101. | Haswell-Elkins MR, Satarug S, Tsuda M, Mairiang E, Esumi H, Sithithaworn P, Mairiang P, Saitoh M, Yongvanit P, Elkins DB. Liver fluke infection and cholangiocarcinoma: model of endogenous nitric oxide and extragastric nitrosation in human carcinogenesis. Mutat Res. 1994;305:241-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 102. | Prakobwong S, Khoontawad J, Yongvanit P, Pairojkul C, Hiraku Y, Sithithaworn P, Pinlaor P, Aggarwal BB, Pinlaor S. Curcumin decreases cholangiocarcinogenesis in hamsters by suppressing inflammation-mediated molecular events related to multistep carcinogenesis. Int J Cancer. 2011;129:88-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 103. | Farazi PA, Zeisberg M, Glickman J, Zhang Y, Kalluri R, DePinho RA. Chronic bile duct injury associated with fibrotic matrix microenvironment provokes cholangiocarcinoma in p53-deficient mice. Cancer Res. 2006;66:6622-6627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 104. | Ling H, Roux E, Hempel D, Tao J, Smith M, Lonning S, Zuk A, Arbeeny C, Ledbetter S. Transforming growth factor β neutralization ameliorates pre-existing hepatic fibrosis and reduces cholangiocarcinoma in thioacetamide-treated rats. PLoS One. 2013;8:e54499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 105. | Strack I, Schulte S, Varnholt H, Schievenbusch S, Töx U, Wendland K, Steffen HM, Drebber U, Dienes HP, Odenthal M. β-Adrenoceptor blockade in sclerosing cholangitis of Mdr2 knockout mice: antifibrotic effects in a model of nonsinusoidal fibrosis. Lab Invest. 2011;91:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 106. | Wild CP, Hardie LJ. Reflux, Barrett's oesophagus and adenocarcinoma: burning questions. Nat Rev Cancer. 2003;3:676-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 193] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 107. | O'Riordan JM, Abdel-latif MM, Ravi N, McNamara D, Byrne PJ, McDonald GS, Keeling PW, Kelleher D, Reynolds JV. Proinflammatory cytokine and nuclear factor kappa-B expression along the inflammation-metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Gastroenterol. 2005;100:1257-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 108. | Kimura S, Kitadai Y, Tanaka S, Kuwai T, Hihara J, Yoshida K, Toge T, Chayama K. Expression of hypoxia-inducible factor (HIF)-1alpha is associated with vascular endothelial growth factor expression and tumour angiogenesis in human oesophageal squamous cell carcinoma. Eur J Cancer. 2004;40:1904-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 109. | Fitzgerald RC, Abdalla S, Onwuegbusi BA, Sirieix P, Saeed IT, Burnham WR, Farthing MJ. Inflammatory gradient in Barrett's oesophagus: implications for disease complications. Gut. 2002;51:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 110. | Sihvo EI, Salminen JT, Rantanen TK, Rämö OJ, Ahotupa M, Färkkilä M, Auvinen MI, Salo JA. Oxidative stress has a role in malignant transformation in Barrett's oesophagus. Int J Cancer. 2002;102:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 111. | Syed A, Maradey-Romero C, Fass R. The relationship between eosinophilic esophagitis and esophageal cancer. Dis Esophagus. 2017;30:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 112. | Rieder F, Nonevski I, Ma J, Ouyang Z, West G, Protheroe C, DePetris G, Schirbel A, Lapinski J, Goldblum J, Bonfield T, Lopez R, Harnett K, Lee J, Hirano I, Falk G, Biancani P, Fiocchi C. T-helper 2 cytokines, transforming growth factor β1, and eosinophil products induce fibrogenesis and alter muscle motility in patients with eosinophilic esophagitis. Gastroenterology. 2014;146:1266-77.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 113. | Yisireyili M, Wulamu W, Aili A, Li Y, Alimujiang A, Aipire A, Aizezi M, Zhang W, Cao Z, Mijiti A, Abudureyimu K. Chronic restraint stress induces esophageal fibrosis with enhanced oxidative stress in a murine model. Exp Ther Med. 2019;18:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 114. | Merchant A, Husain SS, Hosain M, Fikree FF, Pitiphat W, Siddiqui AR, Hayder SJ, Haider SM, Ikram M, Chuang SK, Saeed SA. Paan without tobacco: an independent risk factor for oral cancer. Int J Cancer. 2000;86:128-131. [PubMed] [DOI] [Full Text] |
| 115. | Adel M, Liao CT, Lee LY, Hsueh C, Lin CY, Fan KH, Wang HM, Ng SH, Lin CH, Tsao CK, Huang SF, Kang CJ, Fang KH, Wang YC, Chang KP, Fang TJ, Yang LY, Yen TC. Incidence and Outcomes of Patients With Oral Cavity Squamous Cell Carcinoma and Fourth Primary Tumors: A Long-term Follow-up Study in a Betel Quid Chewing Endemic Area. Medicine (Baltimore). 2016;95:e2950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 116. | van Wyk CW, Stander I, Padayachee A, Grobler-Rabie AF. The areca nut chewing habit and oral squamous cell carcinoma in South African Indians. A retrospective study. S Afr Med J. 1993;83:425-429. [PubMed] |
| 117. | Shafique K, Mirza SS, Vart P, Memon AR, Arain MI, Tareen MF, Haq ZU. Areca nut chewing and systemic inflammation: evidence of a common pathway for systemic diseases. J Inflamm (Lond). 2012;9:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 118. | Jeng JH, Wang YJ, Chiang BL, Lee PH, Chan CP, Ho YS, Wang TM, Lee JJ, Hahn LJ, Chang MC. Roles of keratinocyte inflammation in oral cancer: regulating the prostaglandin E2, interleukin-6 and TNF-alpha production of oral epithelial cells by areca nut extract and arecoline. Carcinogenesis. 2003;24:1301-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 119. | Baral R, Patnaik S, Das BR. Co-overexpression of p53 and c-myc proteins linked with advanced stages of betel- and tobacco-related oral squamous cell carcinomas from eastern India. Eur J Oral Sci. 1998;106:907-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 120. | Lee HC, Yin PH, Yu TN, Chang YD, Hsu WC, Kao SY, Chi CW, Liu TY, Wei YH. Accumulation of mitochondrial DNA deletions in human oral tissues -- effects of betel quid chewing and oral cancer. Mutat Res. 2001;493:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 121. | Lee SS, Tsai CH, Ho YC, Yu CC, Chang YC. Heat shock protein 27 expression in areca quid chewing-associated oral squamous cell carcinomas. Oral Dis. 2012;18:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 122. | Lee SS, Tsai CH, Yu CC, Ho YC, Hsu HI, Chang YC. The expression of O(6) -methylguanine-DNA methyltransferase in human oral keratinocytes stimulated with arecoline. J Oral Pathol Med. 2013;42:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 123. | Liu TY, Chen CL, Chi CW. Oxidative damage to DNA induced by areca nut extract. Mutat Res. 1996;367:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 124. | Haque MF, Meghji S, Khitab U, Harris M. Oral submucous fibrosis patients have altered levels of cytokine production. J Oral Pathol Med. 2000;29:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 125. | Leivonen SK, Lazaridis K, Decock J, Chantry A, Edwards DR, Kähäri VM. TGF-β-elicited induction of tissue inhibitor of metalloproteinases (TIMP)-3 expression in fibroblasts involves complex interplay between Smad3, p38α, and ERK1/2. PLoS One. 2013;8:e57474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 126. | Yang SF, Hsieh YS, Tsai CH, Chou MY, Chang YC. The upregulation of type I plasminogen activator inhibitor in oral submucous fibrosis. Oral Oncol. 2003;39:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 127. | Gadbail AR, Chaudhary M, Sarode SC, Gondivkar S, Tekade SA, Zade P, Hande A, Sarode GS, Patil S. Ki67, CD105, and α-SMA expression supports the transformation relevant dysplastic features in the atrophic epithelium of oral submucous fibrosis. PLoS One. 2018;13:e0200171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 128. | Ye MY, Chen MY, Chang YH, Huang JS, Huang TT, Wong TY, Hong TM, Chen YL. Growth-regulated oncogene-α from oral submucous fibrosis fibroblasts promotes malignant transformation of oral precancerous cells. J Oral Pathol Med. 2018;47:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 129. | Daga D, Singh RK, Pal US, Gurung T, Gangwar S. Efficacy of oral colchicine with intralesional hyaluronidase or triamcinolone acetonide in the Grade II oral submucous fibrosis. Natl J Maxillofac Surg. 2017;8:50-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 130. | Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, Lockwood GA, Tritchler DL, Yaffe MJ. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87:670-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 829] [Cited by in RCA: 753] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 131. | Green AK, Hankinson SE, Bertone-Johnson ER, Tamimi RM. Mammographic density, plasma vitamin D levels and risk of breast cancer in postmenopausal women. Int J Cancer. 2010;127:667-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 132. | Bodelon C, Mullooly M, Pfeiffer RM, Fan S, Abubakar M, Lenz P, Vacek PM, Weaver DL, Herschorn SD, Johnson JM, Sprague BL, Hewitt S, Shepherd J, Malkov S, Keely PJ, Eliceiri KW, Sherman ME, Conklin MW, Gierach GL. Mammary collagen architecture and its association with mammographic density and lesion severity among women undergoing image-guided breast biopsy. Breast Cancer Res. 2021;23:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 133. | Huo CW, Chew G, Hill P, Huang D, Ingman W, Hodson L, Brown KA, Magenau A, Allam AH, McGhee E, Timpson P, Henderson MA, Thompson EW, Britt K. High mammographic density is associated with an increase in stromal collagen and immune cells within the mammary epithelium. Breast Cancer Res. 2015;17:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 134. | Engin AB, Engin A, Gonul II. The effect of adipocyte-macrophage crosstalk in obesity-related breast cancer. J Mol Endocrinol. 2019;62:R201-R222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 135. | Skarping I, Förnvik D, Sartor H, Heide-Jørgensen U, Zackrisson S, Borgquist S. Mammographic density is a potential predictive marker of pathological response after neoadjuvant chemotherapy in breast cancer. BMC Cancer. 2019;19:1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 136. | Hwang KT, Chu AJ, Kim J, Lee JY, Chang JH, Oh S, Kim YA, Jung J, Oh B. Prognostic Influence of Preoperative Mammographic Breast Density in Operable Invasive Female Breast Cancer. Sci Rep. 2018;8:16075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 137. | Chen YC, Chan CH, Lim YB, Yang SF, Yeh LT, Wang YH, Chou MC, Yeh CB. Risk of Breast Cancer in Women with Mastitis: A Retrospective Population-Based Cohort Study. Medicina (Kaunas). 2020;56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 138. | Visscher DW, Nassar A, Degnim AC, Frost MH, Vierkant RA, Frank RD, Tarabishy Y, Radisky DC, Hartmann LC. Sclerosing adenosis and risk of breast cancer. Breast Cancer Res Treat. 2014;144:205-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 139. | Pinsky RW, Helvie MA. Mammographic breast density: effect on imaging and breast cancer risk. J Natl Compr Canc Netw. 2010;8:1157-64; quiz 1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 140. | Mann RM, Athanasiou A, Baltzer PAT, Camps-Herrero J, Clauser P, Fallenberg EM, Forrai G, Fuchsjäger MH, Helbich TH, Killburn-Toppin F, Lesaru M, Panizza P, Pediconi F, Pijnappel RM, Pinker K, Sardanelli F, Sella T, Thomassin-Naggara I, Zackrisson S, Gilbert FJ, Kuhl CK; European Society of Breast Imaging (EUSOBI). Breast cancer screening in women with extremely dense breasts recommendations of the European Society of Breast Imaging (EUSOBI). Eur Radiol. 2022;32:4036-4045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 217] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 141. | Choi DT, Kum HC, Park S, Ohsfeldt RL, Shen Y, Parikh ND, Singal AG. Hepatocellular Carcinoma Screening Is Associated With Increased Survival of Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2019;17:976-987.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 142. | Huguet JM, Ferrer-Barceló L, Suárez P, Sanchez E, Prieto JD, Garcia V, Sempere J. Colorectal cancer screening and surveillance in patients with inflammatory bowel disease in 2021. World J Gastroenterol. 2022;28:502-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 143. | Spechler SJ. Barrett esophagus and risk of esophageal cancer: a clinical review. JAMA. 2013;310:627-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 187] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 144. | Van Asseldonk DP, de Boer NK, Peters GJ, Veldkamp AI, Mulder CJ, Van Bodegraven AA. On therapeutic drug monitoring of thiopurines in inflammatory bowel disease; pharmacology, pharmacogenomics, drug intolerance and clinical relevance. Curr Drug Metab. 2009;10:981-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 145. | Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, Lehr HA, Wirtz S, Becker C, Atreya R, Mudter J, Hildner K, Bartsch B, Holtmann M, Blumberg R, Walczak H, Iven H, Galle PR, Ahmadian MR, Neurath MF. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003;111:1133-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 572] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 146. | de Boer NKH, Peyrin-Biroulet L, Jharap B, Sanderson JD, Meijer B, Atreya I, Barclay ML, Colombel JF, Lopez A, Beaugerie L, Marinaki AM, van Bodegraven AA, Neurath MF. Thiopurines in Inflammatory Bowel Disease: New Findings and Perspectives. J Crohns Colitis. 2018;12:610-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 147. | Zhu Z, Mei Z, Guo Y, Wang G, Wu T, Cui X, Huang Z, Zhu Y, Wen D, Song J, He H, Xu W, Cui L, Liu C. Reduced Risk of Inflammatory Bowel Disease-associated Colorectal Neoplasia with Use of Thiopurines: a Systematic Review and Meta-analysis. J Crohns Colitis. 2018;12:546-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 148. | Gargallo-Puyuelo CJ, Laredo V, Gomollón F. Thiopurines in Inflammatory Bowel Disease. How to Optimize Thiopurines in the Biologic Era? Front Med (Lausanne). 2021;8:681907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 149. | Qiao Y, Yang T, Gan Y, Li W, Wang C, Gong Y, Lu Z. Associations between aspirin use and the risk of cancers: a meta-analysis of observational studies. BMC Cancer. 2018;18:288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 150. | Ma Y, Yu P, Lin S, Li Q, Fang Z, Huang Z. The association between nonsteroidal anti-inflammatory drugs and skin cancer: Different responses in American and European populations. Pharmacol Res. 2020;152:104499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 151. | Ehrenpreis ED, Kruchko DH. Rapid Review: Nonsteroidal Anti-inflammatory Agents and Aminosalicylates in COVID-19 Infections. J Clin Gastroenterol. 2020;54:602-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 152. | Llanos-González AB, Martínez JB, Peloche GB, Suárez-Cuartín G, Zygmunt VV, Planas-Cerezales L, López JMP, Martín LP, Masanes RJ, Zamora NP, Gil SG, Sargatal JD, Gonzálvez AM, Fernández OA, Molina MM. Antifibrotic treatment in progressive non-IPF fibrotic interstitial lung diseases. Eur Respir J. 2019;54:1731. [DOI] [Full Text] |
| 153. | Naoi H, Suzuki Y, Mori K, Aono Y, Kono M, Hasegawa H, Yokomura K, Inoue Y, Hozumi H, Karayama M, Furuhashi K, Enomoto N, Fujisawa T, Nakamura Y, Inui N, Nakamura H, Suda T. Impact of antifibrotic therapy on lung cancer development in idiopathic pulmonary fibrosis. Thorax. 2022;77:727-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 154. | Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4008] [Cited by in RCA: 3604] [Article Influence: 133.5] [Reference Citation Analysis (0)] |
| 155. | Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, Norton L, Nickelsen T, Bjarnason NH, Morrow M, Lippman ME, Black D, Glusman JE, Costa A, Jordan VC. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1363] [Cited by in RCA: 1191] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 156. | Zhang Y, Simondsen K, Kolesar JM. Exemestane for primary prevention of breast cancer in postmenopausal women. Am J Health Syst Pharm. 2012;69:1384-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 157. | Cuzick J, Sestak I, Forbes JF, Dowsett M, Cawthorn S, Mansel RE, Loibl S, Bonanni B, Evans DG, Howell A; IBIS-II investigators. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395:117-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 158. | Ferreira S, Saraiva N, Rijo P, Fernandes AS. LOXL2 Inhibitors and Breast Cancer Progression. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 159. | Grossman M, Ben-Chetrit N, Zhuravlev A, Afik R, Bassat E, Solomonov I, Yarden Y, Sagi I. Tumor Cell Invasion Can Be Blocked by Modulators of Collagen Fibril Alignment That Control Assembly of the Extracellular Matrix. Cancer Res. 2016;76:4249-4258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 160. | Leung L, Niculescu-Duvaz D, Smitten D, Lopes F, Calens C, McLeary R, Saturno G, Davies L, Aljarah M, Bown M, Johnson L, Zambon A, Chambers T, Menard D, Bayliss N, Knight R, Fish L, Lawrence R, Challinor M, Tang HR, Marais R, Springer C. Anti-metastatic Inhibitors of Lysyl Oxidase (LOX): Design and Structure-Activity Relationships. Journal of Medicinal Chemistry. 2019;62: 5863-5884. [DOI] [Full Text] |
| 161. | Meissner EG, McLaughlin M, Matthews L, Gharib AM, Wood BJ, Levy E, Sinkus R, Virtaneva K, Sturdevant D, Martens C, Porcella SF, Goodman ZD, Kanwar B, Myers RP, Subramanian M, Hadigan C, Masur H, Kleiner DE, Heller T, Kottilil S, Kovacs JA, Morse CG. Simtuzumab treatment of advanced liver fibrosis in HIV and HCV-infected adults: results of a 6-month open-label safety trial. Liver Int. 2016;36:1783-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 162. | Sciences G. Simtuzumab (GS-6624) in the Prevention of Progression of Liver Fibrosis in Adults With Primary Sclerosing Cholangitis (PSC). Vol. 2022 (ClinicalTrials.gov, 2012).. [DOI] [Full Text] |
| 163. | Center MSKC. Phase II Study of Tetrathiomolybdate (TM) in Patients With Breast Cancer. Vol. 2022 (clinicaltrials.gov, 2005). [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 164. | Pharmacist. Study to Evaluate Safety, Pharmacokinetic and Pharmacodynamic Dose Escalation and Expansion Study of PXS-5505 in Patients With Primary, Post-polycythemia Vera or Post-essential Thrombocythemia Myelofibrosis. Vol. 2022 (ClinicaTrials.gov, 2020).. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 165. | Caon I, Bartolini B, Parnigoni A, Caravà E, Moretto P, Viola M, Karousou E, Vigetti D, Passi A. Revisiting the hallmarks of cancer: The role of hyaluronan. Semin Cancer Biol. 2020;62:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 166. | Morosi L, Meroni M, Ubezio P, Fuso Nerini I, Minoli L, Porcu L, Panini N, Colombo M, Blouw B, Kang DW, Davoli E, Zucchetti M, D'Incalci M, Frapolli R. PEGylated recombinant human hyaluronidase (PEGPH20) pre-treatment improves intra-tumour distribution and efficacy of paclitaxel in preclinical models. J Exp Clin Cancer Res. 2021;40:286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 167. | Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, Lolkema MP, Jiang P, Kultti A, Thompson CB, Maneval DC, Jodrell DI, Frost GI, Shepard HM, Skepper JN, Tuveson DA. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 775] [Cited by in RCA: 863] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 168. | Thompson CB, Shepard HM, O'Connor PM, Kadhim S, Jiang P, Osgood RJ, Bookbinder LH, Li X, Sugarman BJ, Connor RJ, Nadjsombati S, Frost GI. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol Cancer Ther. 2010;9:3052-3064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 229] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 169. | Doherty GJ, Tempero M, Corrie PG. HALO-109-301: a Phase III trial of PEGPH20 (with gemcitabine and nab-paclitaxel) in hyaluronic acid-high stage IV pancreatic cancer. Future Oncol. 2018;14:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 170. | Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 956] [Cited by in RCA: 948] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 171. | Arnold JN, Magiera L, Kraman M, Fearon DT. Tumoral immune suppression by macrophages expressing fibroblast activation protein-α and heme oxygenase-1. Cancer Immunol Res. 2014;2:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 172. | Belhabib I, Zaghdoudi S, Lac C, Bousquet C, Jean C. Extracellular Matrices and Cancer-Associated Fibroblasts: Targets for Cancer Diagnosis and Therapy? Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 173. | Jess T, Simonsen J, Jørgensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143:375-81.e1; quiz e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 384] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 174. | Miura Y, Saito T, Tanaka T, Takoi H, Yatagai Y, Inomata M, Nei T, Saito Y, Gemma A, Azuma A. Reduced incidence of lung cancer in patients with idiopathic pulmonary fibrosis treated with pirfenidone. Respir Investig. 2018;56:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 175. | Bittoni MA, Carbone DP, Harris RE. Ibuprofen and fatal lung cancer: A brief report of the prospective results from the Third National Health and Nutrition Examination Survey (NHANES III). Mol Clin Oncol. 2017;6:917-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 176. | Tao Y, Li Y, Liu X, Deng Q, Yu Y, Yang Z. Nonsteroidal anti-inflammatory drugs, especially aspirin, are linked to lower risk and better survival of hepatocellular carcinoma: a meta-analysis. Cancer Manag Res. 2018;10:2695-2709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 177. | Gutiérrez-Cuevas J, Lucano-Landeros S, López-Cifuentes D, Santos A, Armendariz-Borunda J. Epidemiologic, Genetic, Pathogenic, Metabolic, Epigenetic Aspects Involved in NASH-HCC: Current Therapeutic Strategies. Cancers (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 178. | Rai A, Shrivastava PK, Kumar A, Prasad K, Shakeel S, Ul Haque Z. Comparative effectiveness of medicinal interventions for oral submucous fibrosis: A network meta-analysis. J Stomatol Oral Maxillofac Surg. 2023;124:101423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |