Published online Jun 24, 2023. doi: 10.5306/wjco.v14.i6.215
Peer-review started: April 27, 2023
First decision: May 12, 2023
Revised: May 18, 2023
Accepted: June 6, 2023
Article in press: June 6, 2023
Published online: June 24, 2023
Processing time: 54 Days and 15 Hours
Several studies report the useful therapeutic results of regional hyperthermia in association with chemotherapy (CHT) and radiotherapy for the treatment of pancreatic cancer. Modulated electro-hyperthermia (mEHT) is a new hyperthermia technique that induces immunogenic death or apoptosis of pancreatic cancer cells in laboratory experiments and increases tumor response rate and survival in pancreatic cancer patients, offering beneficial therapeutic effects against this severe type of cancer.
To assess survival, tumor response and toxicity of mEHT alone or combined with CHT compared with CHT for the treatment of locally advanced or metastatic pancreatic cancer.
This was a retrospective data collection on patients affected by locally advanced or metastatic pancreatic cancer (stage III and IV) performed in 9 Italian centers, members of International Clinical Hyperthermia Society-Italian Network. This study included 217 patients, 128 (59%) of them were treated with CHT (no-mEHT) and 89 (41%) patients received mEHT alone or in association with CHT. mEHT treatments were performed applying a power of 60-150 watts for 40-90 min, simultaneously or within 72 h of administration of CHT.
Median patients’ age was 67 years (range 31-92 years). mEHT group had a median overall survival greater than non-mEHT group (20 mo, range 1.6-24, vs 9 mo, range 0.4-56.25, P < 0.001). mEHT group showed a higher number of partial responses (45% vs 24%, P = 0.0018) and a lower number of progressions (4% vs 31%, P < 0.001) than the no-mEHT group, at the three months follow-up. Adverse events were observed as mild skin burns in 2.6% of mEHT sessions.
mEHT seems safe and has beneficial effects on survival and tumor response of stage III-IV pancreatic tumor treatment. Further randomized studies are warranted to confirm or not these results.
Core Tip: Pancreatic cancer has very poor prognosis with a 5-year overall survival of 5% and a median overall survival (OS) time of 8-12 mo. The concomitant use of modulated electro-hyperthermia (mEHT) in addition to chemotherapy has been introduced. mEHT has specific antitumor effects, increasing survival and tumor response. This was a retrospective data collection on 217 patients affected by locally advanced pancreatic cancer performed in 9 Italian centres, aiming to assess survival, tumour response and toxicity. The mEHT group had a greater OS (20 mo vs 9 mo, P < 0.001), higher number of partial responses (45% vs 24%, P = 0.0018) and a lower number of progressions (4% vs 31%, P < 0.001) than no-mEHT group. Adverse events were observed in 2.6% of mEHT sessions. mEHT have beneficial effects on survival and tumor response of stage III-IV pancreatic tumor patients, without adding toxicity.
- Citation: Fiorentini G, Sarti D, Mambrini A, Hammarberg Ferri I, Bonucci M, Sciacca PG, Ballerini M, Bonanno S, Milandri C, Nani R, Guadagni S, Dentico P, Fiorentini C. Hyperthermia combined with chemotherapy vs chemotherapy in patients with advanced pancreatic cancer: A multicenter retrospective observational comparative study. World J Clin Oncol 2023; 14(6): 215-226
- URL: https://www.wjgnet.com/2218-4333/full/v14/i6/215.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i6.215
Pancreatic cancer (PC) is a particularly serious disease with poor prognosis[1,2]. Main risk factors of pancreatic cancer are non-hereditary and environmental factors, such as increased age, diabetes, obesity, smoking, alcohol intake and diet high in fat and low in vegetables. Only 10% of PC are familial or hereditary. Numerous molecular biology studies are underway the genes and pathological conditions involved in PC onset, in particular, the most studied are: Lynch syndrome, hereditary pancreatitis, Peutz-Jeghers syndrome, cystic fibrosis and breast cancer gene (BRCA)[2].
The Lynch syndrome is an inherited condition that is associated with 5% of colon cancer cases. Patients with this syndrome have about 10-fold increased risk of developing PC[3].
Hereditary pancreatitis is another rare inherited condition that is usually diagnosed in young individuals (< 20 years); its main symptoms are frequent episodes of severe inflammation of the pancreatic gland, leading to chronic pancreatitis. This pathology increases of about a 50% the risk of developing PC; this risk is increased when associated to smoke[4].
Peutz-Jeghers Syndrome is characterized by polyps in the small intestine and pigmented spots on nose and lips. Patients with this syndrome have a 10%-35% risk of developing PC[5]. Cystic fibrosis induces pancreatic insufficiency and chronic pancreatitis and increases of 5 to 6 times the risk of developing pancreatic cancer[6].
BRCA 1 and 2 mutations are often related to inherited ovarian and breast cancer. It is well known that the BRCA1 mutation can also induce an increased risk (4-9 times) of developing PC[7].
Over the past twenty years, PC prognosis has been improved by early diagnosis, the use of neoadjuvant, adjuvant and palliative therapies and the increased number of centres expert in hepatic-pancreatic surgery.
Today PC is the seventh cause of cancer-related death worldwide with a median overall survival < 1 year[1,2]. Incidence and mortality of this cancer have increased significantly in the last two decades[2]. The most common PC histology is adenocarcinoma that accounts for about 90% of cases. Radical surgery followed by adjuvant radiotherapy (RT) and/or chemotherapy (CHT) is considered the gold standard treatment for this tumor; however, surgery is indicated only in 10% of patients at the first diagnosis, whereas, 90% are non-resectable because they are locally advanced or metastatic. Neoadjuvant CHT or a combination of RT and chemo-therapy can be performed in locally advanced PC without evidence of distant metastases, in order to allow surgical approach[8,9].
FOLFIRINOX (leucovorin, fluorouracil, irinotecan, oxaliplatin) and the combination of nab-paclitaxel and gemcitabine are the more suitable upfront CHT for PC patients with good performance status (PS). These protocols have partial activity, resulting in similar overall survival (32-54 mo), but they have frequently severe side effects[8-10].
Recent studies on cisplatin therapeutic use in PC patients with BRCA 1 and 2 mutations report clinical benefit, although superiority over other chemotherapeutic treatments and optimal dosing and combination therapies are still ongoing. PARP inhibitors such as niraparib and olaparib have also promising results in this setting. They prevent single-strand DNA break repair, resulting in double-stranded DNA breaks that cannot to be repaired by homologous recombination deficiency tumors, causing cell cycle arrest and apoptosis. Olaparib increases progression-free survival, when used as a maintenance therapy for PC patients responding to first-line platinum-based therapy. Although these drugs show interesting results, however their efficacy is undermined by drug resistance and high cost[7].
PC is generally refractory to CHT and RT because of its low perfused and hypoxic microenvironment that is mainly due to large accumulation of non-tumor cells (stroma), obstructing vascularization and the delivery systemic chemotherapeutics. This also reduces oxygen delivery and hence the sensitivity to CHT and RT, impacting negatively on prognosis. In vitro experiments have shown that hyperthermia can increase the effectiveness of conventional treatments[15-19].
Regional hyperthermia (RHT) optimize the distribution of drugs in cancer cells, improves blood circulation, increases district oxygen level, reduces DNA repair and promotes cancer cell death[16]. RHT increases temperature inside the tumor up to 39.5/43 °C, using an external radiofrequency equipment. RHT is often associated to CHT and RT, in order to increase responses and overall survival (OS) in PC[20-24]. A systematic review shows the benefits of hyperthermia added to CHT and/or RT for PC patients from 14 clinical trials, resulting in median OS of 6-18.6 mo that is longer than that observed in the control cohorts and improved tumor response with 31.3% overall response rate that is greater than the control cohort. These data suggest that this combination positively affect tumor response and OS for PC patients[24-28].
Modulated Electro- Hyperthermia (mEHT) is obtained with a 13.56 MHz capacitive equipment and is a relatively new RHT technique (introduced in clinical practice in the last decade)[25,27,28-30]. This type of RHT is more selective in killing tumor cells and overcoming the limited tissue permeation of radio-frequency. There is no direct way to measure the temperature inside the tissues in vivo, however it can be predicted from input power applied, because of the high efficacy of the electric field[29-34]. mEHT increases the temperature of the targeted malignant cells over 3 °C higher than surrounding environment[29].
The high heterogeneity of PC cells is counteracted by mEHT, which selectively damages the external membrane of cancer cells that have different energy uptake on the membrane rafts from healthy tissue and different electron impedance compared to normal cells[17-19].
Data from the literature show that mEHT is safe and feasible and has not only palliative but also therapeutic effects in several advanced cancer and also in pancreatic adenocarcinoma in monotherapy or in combination with CHT and/or RT[25,27,28,32-35]. This method seems to re-sensitize patients who are refractory to CHT and RT and improve palliation, increasing survival time, tumor response and quality of life (QoL), prolonging OS and improving QoL[25,27,28,32-34,36-39].
The purpose of this multicenter observational comparative study was to assess the advantages of mEHT in association to CHT compared to CHT alone in locally advanced or metastatic pancreatic tumors. This study was carried out by members of International Clinical Hyperthermia Society-Italian Network and created the platform for a large randomized phase III trial to compare outcomes with or without mEHT application.
This study was multicenter, retrospective, observational, comparative and case-control, aiming to assess survival and tumor response of CHT or mEHT plus CHT for the treatment of locally advanced or metastatic pancreatic tumors. Inclusion criteria were: Older than 18 years, informed consent signed, histological diagnosis of locally advanced or metastatic pancreatic tumor, PS of 0-2, treatment with CHT, mEHT or mEHT plus CHT, data on tumor response and survival. From 2003 to 2021, 628 patients with locally advanced or metastatic pancreatic tumor were treated in nine Italian centers, 217 of them were included in this study, 89 (41%) of them were treated with mEHT plus CHT (mETH group) and 128 (59%) with only CHT (no-mEHT group). CHT regimen was mostly gemcitabine-based in both study groups (Table1). The majority (95%) of gemcitabine-based treatments were administered on the same day of mEHT treatment. In a minority of patients (5%), it was administered the following day or within the following 72 h because of precarious clinical conditions and geographic accessibility. Even if gemcitabine had a half-life of 42-94 min and was eliminated within 5-11 h after infusion, the pharmacokinetic elimination half-life for dFdU varies between 2 and 24 h, and it is still present systemically in concentrations greater than 1 μmol/L up to 1 wk after infusion.
This was a retrospective data collection, CHT type was chosen by singular physician, we collected the data when the treatments (CHT and mEHT) were already done.
mEHT was performed with EHY-2000 plus device (CE0123 Oncotherm, Torisdorf, Germany) and a radiofrequency current of 13.56 MHz as previously described[28-31]. mEHT treatments were performed 1-3 times/week for a total of 4-6 wk, starting at 60 W/40 min and increasing up to 150 W/ 90 min in 2 wk[28]. Power increase was performed in the initial mEHT sessions, in order to assess patient treatment tolerance. Starting with the increased potency could have caused a feeling of heat and pain on the scars, as indicated in previous protocols that we have set up[32,36]. Power increase is performed in the initial mEHT sessions and was not used for efficacy purpose, the efficacy was measured after reaching the final power of 150 W/90 min. The computer connected to the hyperthermia machine has a program that calculates and converts the Kilojoules dispensed by the machine into degrees of temperature at the treatment site. This was represented on the screen and printed on graphs. Temperature on the target was 41-42.5°C during mEHT sessions as they were assessed in previous publications from the manufacturer of the device[38]. As concerning metastases, most patients had disease located in the upper abdomen and for this reason the treatment was suitable because we used an applicator-antenna of 30 cm diameter that allowed to treat the entire upper abdomen and multiple liver or nodal metastases at the same time.
The primary objective was OS that was measured from date of diagnosis to date of death or last available follow up date. The analysis of OS was made for the whole mEHT and no-mEHT group or by age ≥ 70 years or < 70 years. Secondary objectives were tumor response and treatment tolerability. Progression free survival (PFS) was computed from date of treatment start to progression.
Tumor response was measured with the Response Evaluation Criteria in Solid Tumors version 1.4 at three months follow up imaging. In case of multiple tumors, the target lesion was considered that with the largest diameter in outcome measures. Complete response was obtained if every target lesion disappeared; partial response (PR) if tumor diameter decreased by > 30%; progressive disease (PD) was observed if tumor size increased by > 20%, or one or more new lesions appeared. Stable disease (SD) was considered in all the other cases.
Since no data were available on cardiac toxicity due to radiofrequency disturbance from mEHT applied on upper abdomen; twenty-one patients out 89 treated with mEHT were monitored with electrocardiogram and echocardiogram before and after treatment in order to evaluate any alterations in cardiac rhythm and morphology.
As concerning the other adverse events were all monitored according to clinical practice and classified using the Common Terminology Criteria for Adverse Events version 5.0.
Survival and patients’ age were indicated as median and range values, whereas frequencies were indicated as percentages. Kaplan-Meier method and log-rank test were used or OS analysis, with survival probability on the Y axis and time (months) on the X axis. Mann-Whitney test and Student’s test for proportions were used to assess statistical significance (P ≤ 0.05) among differences of patient characteristics. χ2 test was used to assess statistical significance (P ≤ 0.05) among differences of tumor response.
The sample included 217 pancreatic patients: 122 (56%) were males and 95 (44%) were females, 89 (41%) of these were treated with the combination of CHT and mEHT (mETH group) and 128 (59%) with only CHT (no-mEHT group). Their median age was 67 years (37-89 years, range). Most patients were metastatic (65%). In the 217 patients, 235 metastatic sites were observed, with liver being the most frequent metastatic site (132/235, 57%). Previous CHT was administered to 136 (63%) patients, previous RT to 10 (5%) and surgery to 51 (24%). Most frequent CHT regimen was gemcitabine-based regimens: Gemcitabine-oxaliplatin (35%), gemcitabine (29%) and gemcitabine-abraxane (9%). Inclusion criteria indicated both radio and CHT in concomitant used with mEHT, however, none of the patients included in the study received RT in association to mEHT. The two groups had similar characteristics (Table 1).
| Whole sample, n = 217 | mEHT, n = 89 | No-mEHT, n = 128 | P value1 | ||||
| Median | Range | Median | Range | Median | Range | ||
| Age (yr) | 67 | 34-89 | 64 | 38-82 | 69 | 34-89 | NS |
| Student’s test for proportions | |||||||
| M | 122 | 56 | 58 | 65 | 64 | 50 | NS |
| F | 95 | 44 | 31 | 35 | 64 | 50 | NS |
| Metastatic | 142 | 65 | 70 | 79 | 72 | 56 | 0.004 |
| Previous chemotherapy | 136 | 63 | 68 | 76 | 68 | 53 | 0.005 |
| Previous Radiotherapy | 10 | 5 | 1 | 1 | 9 | 7 | NS |
| Current chemotherapy type | |||||||
| Gemcitabine/oxaliplatin | 76 | 35 | 34 | 38 | 42 | 33 | NS |
| Gemcitabine | 62 | 29 | 26 | 29 | 36 | 28 | NS |
| Gemcitabine/abraxane | 39 | 18 | 9 | 10 | 30 | 23 | NS |
| Gemcitabine/5-fluorouracil | 5 | 2 | 4 | 4 | 1 | 1 | NS |
| Gemcitabine/cisplatin | 10 | 5 | 2 | 2 | 8 | 6 | NS |
| Gemcitabine/Nab-paclitaxel | 2 | 1 | 0 | 0 | 2 | 2 | NS |
| FOLFIRINOX | 5 | 2 | 1 | 1 | 4 | 3 | NS |
| Other | 10 | 5 | 5 | 6 | 5 | 4 | NS |
| None | 8 | 4 | 8 | 9 | 0 | 0 | NS |
| Site of metastases | (N = 235) | (N = 132) | (N = 103) | ||||
| Liver | 132 | 57 | 70 | 53 | 63 | 61 | NS |
| Peritoneum | 55 | 23 | 35 | 27 | 20 | 19 | NS |
| Lymph nodes | 37 | 16 | 22 | 17 | 15 | 15 | NS |
| Other | 10 | 4 | 5 | 4 | 5 | 5 | NS |
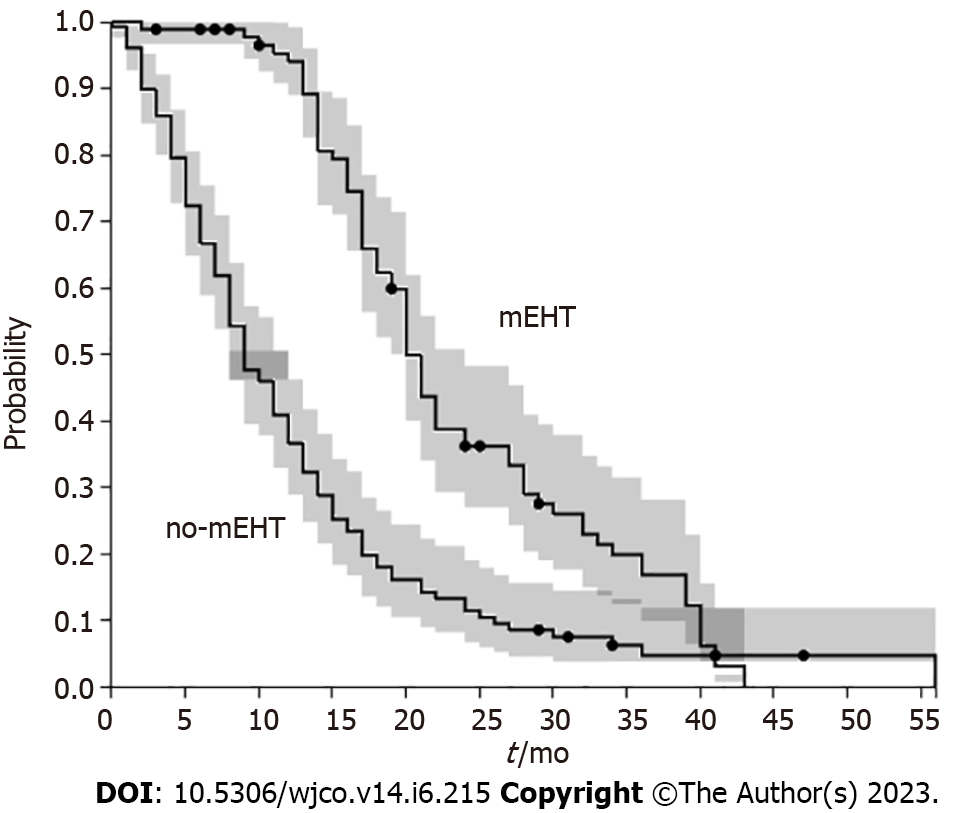
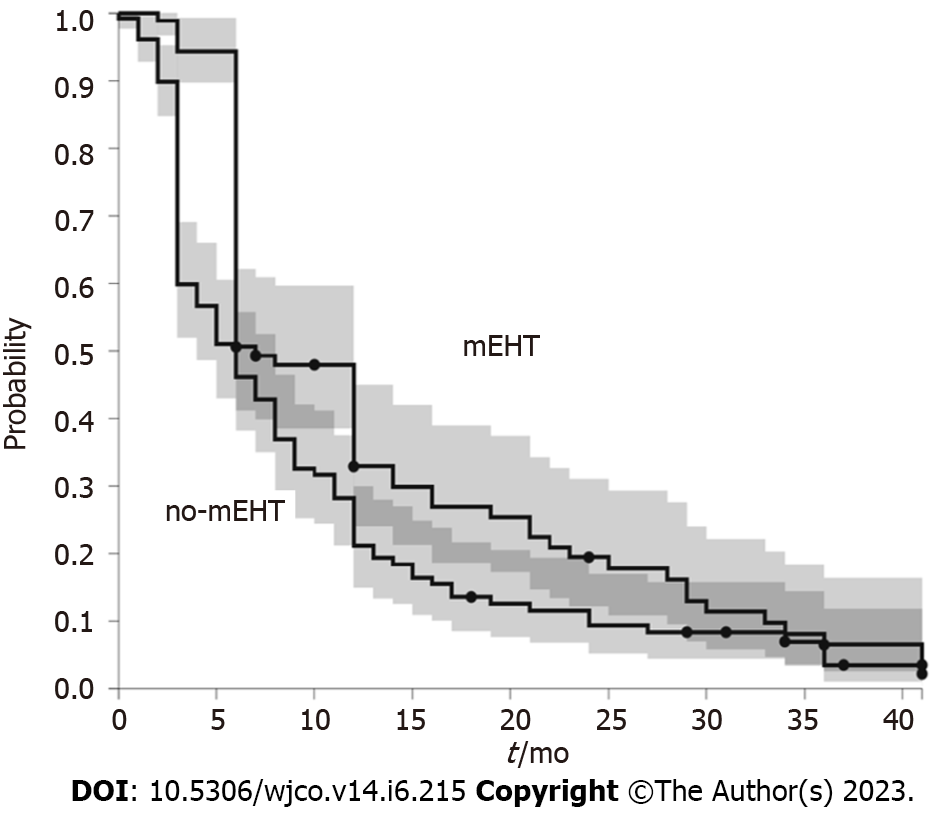
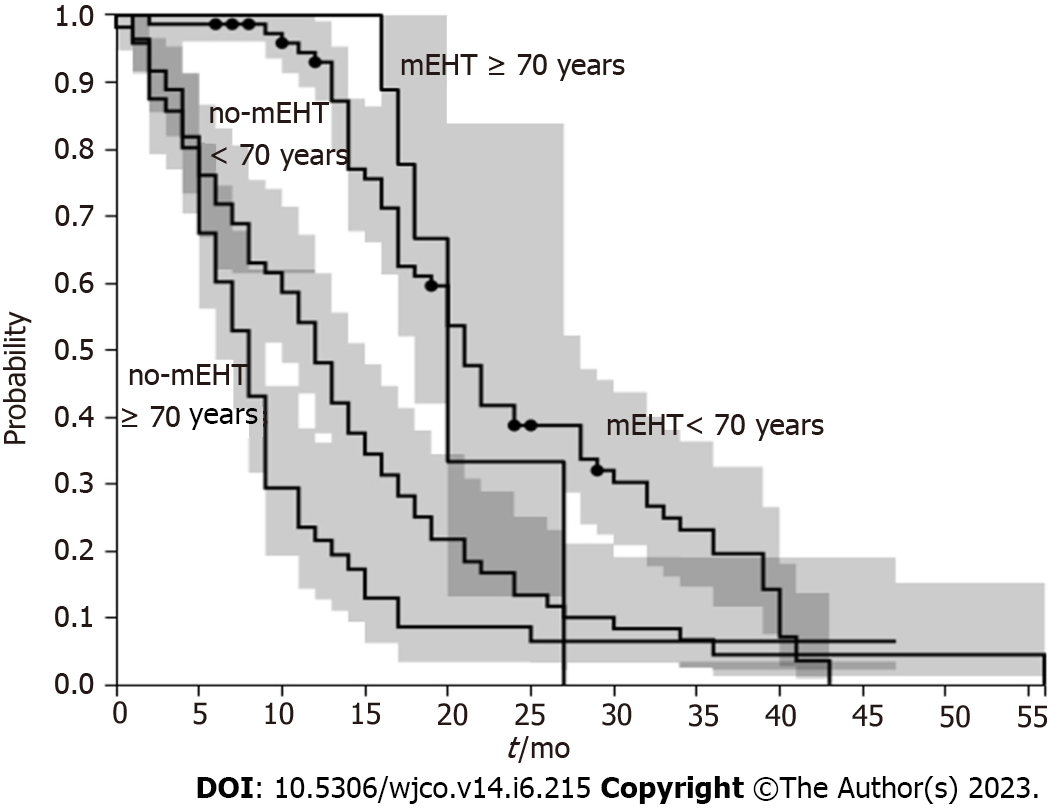
mEHT group had a median OS greater than non-mEHT group (20 mo, range 1.6-24, vs 9 mo, range 0.4-56.25, P < 0.001) (Figure 1). mEHT group had a median PFS greater than non-mEHT group (7 mo, range 2-24, vs 5 mo, range 0.4-41, P < 0.05) (Figure 2). The analysis of OS by age ≥ 70 years or < 70 years showed that there was no difference in OS between mEHT ≥ 70 years (20 mo, 2-43 mo range) and < 70 years (20 mo 3-27 mo range) P = 0.235, whereas no-mEHT < 70 years had a higher OS than no-mEHT ≥ 70 years group (12 mo range 1-56 vs 8 mo range 1-47, P = 0.01) (Figure 3). mEHT had a longer OS than no-mEHT group both among ≥ 70 years (20 mo range 3-27 vs 8 mo range 1-47, P < 0.01) and < 70 years (20 mo range 2-43 vs 12 mo range 1-56, P < 0.01).
Tumor response at three months follow-up was available for 87 (98%) of mEHT and 111 (88%) patients for non-mEHT group. mEHT patients showed a higher number of PR (45% vs 24%, P = 0.0018) and a lower number of progressions (PD) (4% vs 31%, P < 0.01) than no-mEHT group. SD had similar value in both groups: 51% for mEHT and 45% for no-mEHT (Table 2).
| mEHT | n = 87 | no-mEHT | n = 111 | P value | |
| PR | 39 | 45 | 27 | 24 | 0.0018 |
| SD | 44 | 51 | 50 | 45 | 0.8430 |
| PD | 4 | 4 | 34 | 31 | < 0.001 |
Median mEHT sessions was 16.8 (range 6-25), resulting 1495 mEHT delivered sessions. Adverse events were observed in 2.6% of cases and included: Low grade (G1) skin pain in 22 (1.5%) sessions and low-mild grade (G1-2) burns in 16 (1.1%) cases that resolved in a few days. Hyperthermia did not increase haematological, hepatic, pulmonary and metabolic toxicity due to CHT.
In particular no increased blood pressure or any other cardiac changes were observed for mEHT sessions in patients who received adequate cardiological monitoring including clinical examination, electrocardiogram and echocardiogram.
Hyperthermia involves the application of heat-generating energy to tumors, increasing their temperature to 39-43.5 ◦C and improving the response to systemic therapies. Hyperthermia has been studied for cancer treatment since the 80s, especially for the benefits observed in laboratory experiments, clinical case series and phase II studies. The results of these studies show that hyperthermia enhances CHT, RT and immunotherapy efficacy (tumor response and survival) for several cancers and also in pancreatic adenocarcinoma[16-20,24-28,32-36].
Hyperthermia can be performed with different electromagnetic devices/techniques that can be classified as loco-regional or whole-body methods, according to the size of the body treated[26,31,40-44]. First studies on hyperthermia effects in PC were made mainly using whole-body hyperthermia, whereas, more recent clinical studies apply mainly loco-RHT, such as mEHT that is non-invasive technique that balances low-power thermal effects and non-thermal electric processes, operating with capacitive coupled impedance on a radiofrequency of 13.56 MHz[25-31,37-38]. Current mEHT protocols show that optimal treatment is obtained when the mEHT is performed two or three-times a week, resulting in improvements of OS, disease control and QoL and PFS[25-28,33,39,40]. mEHT has also benefits in tumor control when used as neoadjuvant therapy in combination with CHT and RT[42].
mEHT can counteract heterogenicity of PC and its resistance to systemic therapy, because it targets selectively tumor cells (heating homogeneously the target tissues), exploiting their several biophysical differences from normal cells, such as energy absorption and damage-associated molecular patterns[16-19,37,38]. mEHT induces programmed or immunogenic apoptosis of tumor cells, increasing DNA fragmentation, MAPK/ERK signaling pathways and pro-apoptotic Bcl-2 activation, low mitochondrial membrane potential, the concentration of intracellular Ca2+, Fas and c-Jun N-terminal kinases and the expression of pro-apoptotic genes (EGR1, JUN, and CDKN1A), while silencing other genes that are associated with cytoprotective functions[16-19,30].
In our study, mEHT group had a longer OS than the no-mEHT group (20 mo vs 9 mo, P < 0.001). This effect was present also among ≥ 70 years patients (20 mo, range 3-27 vs 8 mo, range 1-47, P < 0.01). mEHT group had also a median PFS greater than non-mEHT group (7 mo, range 2-24, vs 5 mo, range 0.4-41, P < 0.05). OS improvement was observed in other studies when mEHT was associated with CHT, resulting in an OS of 12.9 mo (95%CI: 9.9-15.9) and disease control rate of 50% in pancreatic cancer treatment[35-38]. Other studies on mEHT reported an OS of 8.9-19 mo and a PFS of 3.9-12.9 mo in advanced pancreatic adenocarcinoma[25-28,32,33]. The results of our study are in agreement with the above data, even if they used different types of deep hyperthermia devices but similar CHT regimens.
OS improvement was of particular interest for elderly patients who suffer most from side effects of CHT. The analysis of OS by age ≥ 70 years or < 70 years showed that there was no difference in OS between mEHT ≥ 70 years and < 70 years, whereas no-mEHT < 70 years had a higher OS than no-mEHT ≥ 70 years group (12 mo range 1-56 vs 8 mo range 1-47, P = 0.01). These data would recommend to use mEHT in elderly patients instead of a second or third line of CHT with heavy side effects.
The combination mEHT with CHT also improved tumor response, disease control rate (DCR = 96%), as it was reported in other studies (DCR = 71%-96%)[21,23,24,29]. mEHT group had a higher number of PR (45% vs 24%, P = 0.0018) and a lower number of progressions (PD) (4% vs 31%, P < 0.001) than the no-mEHT group. This was also reported in other studies, showing higher DCR of mEHT group than that of no-mEHT group (96% vs 77%, P < 0.05)[16-19].
Adverse events that were related to hyperthermia were observed in 2.6% of the sample, showing G1-2 pain and a skin burns. These data agree with toxicity reported in other studies (5%) and suggest that mEHT-related toxicity is low[25,28,33]. Little has been published on the possible cardiotoxicity of radiofrequencies produced by mEHT when applied to the upper abdomen, in locations close to the heart. Twenty-one patients out 89 treated with mEHT were evaluated by electrocardiogram, echocardiogram before and after treatment with mEHT. We observed that upper abdominal mEHT does not generate rhythm disturbance, changes in electrocardiogram, cardiac morphology and blood pressure elevation.
The novelty of this study is the accurate reporting of a significant number of patients treated with mEHT compared with an equally large number of patients who received second and third-line CHT. The data on such a large total number of patients (217) and on the therapeutic effect of mEHT against PC has not been published before in the literature, of particular interest is also the improvement the of older patients’ prognosis of older patients that reaches values comparable to that observed in younger patients.
The observational and retrospective nature of the study is the main limitation together with the long period of observation and inclusion from 2003 to 2021 during which many diagnostic and therapeutic approaches were modified and the still low number of patients treated with mEHT due the limited number of centers using mEHT in Italy. Future immunological research for PC treatment should be directed on circulating tumor cells (CTCs). It should be of interest characterize the transcriptomes of human pancreatic ductal adenocarcinoma CTCs, primary, and metastatic lesions at single-cell scale. Cell-interaction analysis and functional studies in vitro and in vivo reveal that CTCs and natural killer (NK) cells interact via the immune checkpoint molecule pair HLA-E:CD94-NKG2A. The breakdown of this interaction by blockade of NKG2A or knockdown of HLA-E expression could enhance NK-mediated tumor cell killing in vitro and prevents tumor metastasis in vivo[43].
Other methods of hyperthermia when the tumor is metastatic are currently being studied such as whole body hyperthermia but still seem to be at an early stage of application in clinical practice or burdened with excessive complexity[44].
Our study would like to open a new avenue in the treatment of locally advanced pancreatic cancer including mEHT in therapeutic options, since the results of CHT alone are not satisfactory. It is also necessary to know that the centers that practice hyperthermia at a good level are few both in Europe and in the World. Therefore, it is not easy to reach case studies with numerous patients to implement randomized trials. Future studies with a greater number of patients and randomized protocols are needed to confirm these results.
Skilled doctors who use RHT on a daily basis argue that the reasons for the progress hampered in this field are not the lack of effectiveness of technique but the paucity of qualified hyperthermia centres, lack of quality assurance processes, poor temperature monitoring and heterogeneous practices, lack of funding, poorer tolerance of older technology, limited access for the patients.
To overcome these limitations, we performed this study on a disease with high lethality where current therapeutic options in advanced stages are still unsatisfactory.
Therefore, our observations do not have the value of a prospective randomized study with defined and adequate times and methods of unfolding but, this study, despite many limitations, showed that mEHT group had improved OS and disease control rate in stage III-IV pancreatic cancer. These data are also reported for elderly patients. These data suggest that the association of mEHT to CHT is effective for the treatment of pancreatic tumors. mEHT was safe and had only mild toxicity. Notably not adding any toxicity compared to CHT. We hope that the results of our study will orient the scientific community to perform prospective, randomized, clinical study to further define the mEHT safety and efficacy in pancreatic cancer patients.
Modulated Electro- Hyperthermia (mEHT) optimize the distribution of drugs in cancer cells, improves blood circulation, increases district oxygen level, reduces DNA repair and promotes cancer cell death. mEHT is effective for different types of tumors and also in pancreatic adenocarcinoma resulting in better tumor response and longer survival, as reported in clinical case series and phase II studies.
Pancreatic cancer is generally refractory to chemotherapy (CHT) and radiotherapy because of its low perfused and hypoxic microenvironment that reduces the efficacy and sensitivity to these treatments. The high heterogeneity of pancreatic cancer cells is counteracted by mEHT, which selectively damages the external membrane of cancer cells that have different energy uptake on the membrane rafts from healthy tissue, inducing immunogenic death of cancer cells or apoptosis.
The aim of this study was to assess the advantages of mEHT in association to CHT compared to CHT alone in locally advanced or metastatic pancreatic tumors.
This was a retrospective data collection on patients affected by metastatic or locally advanced pancreatic cancer performed in 9 Italian centres. This study included 217 patients, 128 (59%) of them were treated with CHT (no-mEHT) and 89 (41%) patients received mEHT alone or in association with CHT. mEHT treatments were performed applying a power of 60-150 watts for 40-90 min two or three times a week for 4-6 wk.
mEHT group had a median overall survival (OS) greater than non-mEHT group (20 mo, range 1.6-24, vs 9 mo, range 0.4-56.25, P < 0.001). mEHT group showed a higher number of partial responses (45% vs 24%, P = 0.0018) and a lower number of progressions (PD) (4% vs 31%, P < 0.001) than the no-mEHT group, at the three months follow-up. Adverse events were observed in 2.6% of mEHT sessions.
The results obtained in this study provided new evidence that mEHT is safe and has beneficial effects on survival and tumor response of stage III-IV pancreatic tumor treatment. Further studies are warranted.
This multicentre retrospective observational comparative study on 217 patients provides further evidence that mEHT improved OS and disease control rate in stage III-IV pancreatic cancer. This study would like to open a new avenue in the treatment of locally advanced pancreatic cancer including mEHT in therapeutic options, since the results of CHT alone are not satisfactory.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lee L, South Korea; Ma X S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15305] [Article Influence: 3061.0] [Reference Citation Analysis (4)] |
| 2. | International Agency for Research on Cancer. World Health Organization. Global Cancer Observatory 2018. Available from: https://gco.iarc.fr/. |
| 3. | Zalevskaja K, Mecklin JP, Seppälä TT. Clinical characteristics of pancreatic and biliary tract cancers in Lynch syndrome: A retrospective analysis from the Finnish National Lynch Syndrome Research Registry. Front Oncol. 2023;13:1123901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 4. | Panchoo AV, VanNess GH, Rivera-Rivera E, Laborda TJ. Hereditary pancreatitis: An updated review in pediatrics. World J Clin Pediatr. 2022;11:27-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Tacheci I, Kopacova M, Bures J. Peutz-Jeghers syndrome. Curr Opin Gastroenterol. 2021;37:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Archangelidi O, Cullinan P, Simmonds NJ, Mentzakis E, Peckham D, Bilton D, Carr SB. Incidence and risk factors of cancer in individuals with cystic fibrosis in the UK; a case-control study. J Cyst Fibros. 2022;21:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Kindler HL, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, Bordia S, McGuinness D, Cui K, Locker GY, Golan T. Overall Survival Results From the POLO Trial: A Phase III Study of Active Maintenance Olaparib Versus Placebo for Germline BRCA-Mutated Metastatic Pancreatic Cancer. J Clin Oncol. 2022;40:3929-3939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 119] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 8. | Chin V, Nagrial A, Sjoquist K, O'Connor CA, Chantrill L, Biankin AV, Scholten RJ, Yip D. Chemotherapy and radiotherapy for advanced pancreatic cancer. Cochrane Database Syst Rev. 2018;3:CD011044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Martin-Perez E, Domínguez-Muñoz JE, Botella-Romero F, Cerezo L, Matute Teresa F, Serrano T, Vera R. Multidisciplinary consensus statement on the clinical management of patients with pancreatic cancer. Clin Transl Oncol. 2020;22:1963-1975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 10. | Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Boige V, Bérille J, Conroy T. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol. 2013;31:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 340] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 11. | Ye LF, Ren C, Bai L, Liang JY, Hu MT, Yang H, Wang ZQ, Wang FH, Xu RH, Li YH, Wang DS. Efficacy and safety of modified FOLFIRINOX as salvage therapy for patients with refractory advanced biliary tract cancer: a retrospective study. Invest New Drugs. 2021;39:836-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4882] [Article Influence: 406.8] [Reference Citation Analysis (0)] |
| 13. | Feliu J, Jorge Fernández M, Macarulla T, Massuti B, Albero A, González González JF, Quintero-Aldana G, Delgado-Mingorance JI, Fernández Montes A, García Piernavieja C, Valladares-Ayerbes M, López Muñoz AM, Mondéjar Solís R, Vicente P, Casado Gonzalez E, González Cebrián I, López-Vivanco G. Phase II clinical trial of nab-paclitaxel plus gemcitabine in elderly patients with previously untreated locally advanced or metastatic pancreatic adenocarcinoma: the BIBABRAX study. Cancer Chemother Pharmacol. 2021;87:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Oba A, Ho F, Bao QR, Al-Musawi MH, Schulick RD, Del Chiaro M. Neoadjuvant Treatment in Pancreatic Cancer. Front Oncol. 2020;10:245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (1)] |
| 15. | Weniger M, Honselmann KC, Liss AS. The Extracellular Matrix and Pancreatic Cancer: A Complex Relationship. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 198] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 16. | Maurici CE, Colenbier R, Wylleman B, Brancato L, van Zwol E, Van den Bossche J, Timmermans JP, Giovannetti E, Mori da Cunha MGMC, Bogers J. Hyperthermia Enhances Efficacy of Chemotherapeutic Agents in Pancreatic Cancer Cell Lines. Biomolecules. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Forika G, Balogh A, Vancsik T, Zalatnai A, Petovari G, Benyo Z, Krenacs T. Modulated Electro-Hyperthermia Resolves Radioresistance of Panc1 Pancreas Adenocarcinoma and Promotes DNA Damage and Apoptosis In Vitro. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Forika G, Kiss E, Petovari G, Danko T, Gellert AB, Krenacs T. Modulated Electro-Hyperthermia Supports the Effect of Gemcitabine Both in Sensitive and Resistant Pancreas Adenocarcinoma Cell Lines. Pathol Oncol Res. 2021;27:1610048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Krenacs T, Meggyeshazi N, Forika G, Kiss E, Hamar P, Szekely T, Vancsik T. Modulated Electro-Hyperthermia-Induced Tumor Damage Mechanisms Revealed in Cancer Models. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Ohguri T, Imada H, Yahara K, Narisada H, Morioka T, Nakano K, Korogi Y. Concurrent chemoradiotherapy with gemcitabine plus regional hyperthermia for locally advanced pancreatic carcinoma: initial experience. Radiat Med. 2008;26:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Ishikawa T, Kokura S, Sakamoto N, Ando T, Imamoto E, Hattori T, Oyamada H, Yoshinami N, Sakamoto M, Kitagawa K, Okumura Y, Yoshida N, Kamada K, Katada K, Uchiyama K, Handa O, Takagi T, Yasuda H, Sakagami J, Konishi H, Yagi N, Naito Y, Yoshikawa T. Phase II trial of combined regional hyperthermia and gemcitabine for locally advanced or metastatic pancreatic cancer. Int J Hyperthermia. 2012;28:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Maebayashi T, Ishibashi N, Aizawa T, Sakaguchi M, Sato T, Kawamori J, Tanaka Y. Treatment outcomes of concurrent hyperthermia and chemoradiotherapy for pancreatic cancer: Insights into the significance of hyperthermia treatment. Oncol Lett. 2017;13:4959-4964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Datta NR, Pestalozzi B, Clavien PA, Siebenhüner A, Puric E, Khan S, Mamot C, Riesterer O, Knuchel J, Reiner CS, Bodis S; members of the HEATPAC Trial Group. "HEATPAC" - a phase II randomized study of concurrent thermochemoradiotherapy versus chemoradiotherapy alone in locally advanced pancreatic cancer. Radiat Oncol. 2017;12:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | van der Horst A, Versteijne E, Besselink MGH, Daams JG, Bulle EB, Bijlsma MF, Wilmink JW, van Delden OM, van Hooft JE, Franken NAP, van Laarhoven HWM, Crezee J, van Tienhoven G. The clinical benefit of hyperthermia in pancreatic cancer: a systematic review. Int J Hyperthermia. 2018;34:969-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Fiorentini G, Sarti D, Casadei V, Milandri C, Dentico P, Mambrini A, Nani R, Fiorentini C, Guadagni S. Modulated Electro-Hyperthermia as Palliative Treatment for Pancreatic Cancer: A Retrospective Observational Study on 106 Patients. Integr Cancer Ther. 2019;18:1534735419878505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Rogers SJ, Datta NR, Puric E, Timm O, Marder D, Khan S, Mamot C, Knuchel J, Siebenhüner A, Pestalozzi B, Guckenberger M, Bodis S, Riesterer O. The addition of deep hyperthermia to gemcitabine-based chemoradiation may achieve enhanced survival in unresectable locally advanced adenocarcinoma of the pancreas. Clin Transl Radiat Oncol. 2021;27:109-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Sarti D, Milandri C, Fiorentini C, Mambrini A, Fiorentini G. Modulated electro-hyperthermia for the treatment of elderly pancreatic cancer patients. Argum Geriatr Oncol. 2020;5:1-7. |
| 28. | Fiorentini G, Sarti D, Ranieri G, Gadaleta CD, Fiorentini C, Milandri C, Mambrini A, Guadagni S. Modulated electro-hyperthermia in stage III and IV pancreatic cancer: Results of an observational study on 158 patients. World J Clin Oncol. 2021;12:1064-1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 29. | Szasz O. Bioelectromagnetic Paradigm of Cancer Treatment—Modulated Electro-Hyperthermia (mEHT). Open J Biophys. 2019;9:98-109. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Szasz A. Thermal and nonthermal effects of radiofrequency on living state and applications as an adjuvant with radiation therapy. J Radiat Cancer Res. 2019;10:1-17.. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Lee SY, Szigeti GP, Szasz AM. Oncological hyperthermia: The correct dosing in clinical applications. Int J Oncol. 2019;54:627-643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Alshaibi HF, Al-Shehri B, Hassan B, Al-Zahrani R, Assiss T. Modulated Electrohyperthermia: A New Hope for Cancer Patients. Biomed Res Int. 2020;2020:8814878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Petenyi FG, Garay T, Muhl D, Izso B, Karaszi A, Borbenyi E, Herold M, Herold Z, Szasz AM, Dank M. Modulated Electro-Hyperthermic (mEHT) Treatment in the Therapy of Inoperable Pancreatic Cancer Patients-A Single-Center Case-Control Study. Diseases. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 34. | Szasz AM, Minnaar CA, Szentmártoni G, Szigeti GP, Dank M. Review of the Clinical Evidences of Modulated Electro-Hyperthermia (mEHT) Method: An Update for the Practicing Oncologist. Front Oncol. 2019;9:1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Peters GJ, Clavel M, Noordhuis P, Geyssen GJ, Laan AC, Guastalla J, Edzes HT, Vermorken JB. Clinical phase I and pharmacology study of gemcitabine (2', 2'-difluorodeoxycytidine) administered in a two-weekly schedule. J Chemother. 2007;19:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Fiorentini G, Sarti D, Casadei V, Minnaar C, Szász MA. Modulated Electro-Hyperthermia (mEHT)[oncothermia®] Protocols as Complementary Treatment. Oncothermia J. 2019;25:85-115. |
| 37. | Szasz A. Challenges and Solutions of Oncological Hyperthermia. Thermal Medicine. 2013;29:1-23. [DOI] [Full Text] |
| 38. | Szasz O, Szasz A. Heating, efficacy and dose of local hyperthermia. Open J Bioph. 2016;6:10-18. [DOI] [Full Text] |
| 39. | Tschoep-Lechner KE, Milani V, Berger F, Dieterle N, Abdel-Rahman S, Salat C, Issels RD. Gemcitabine and cisplatin combined with regional hyperthermia as second-line treatment in patients with gemcitabine-refractory advanced pancreatic cancer. Int J Hyperthermia. 2013;29:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 40. | Maluta S, Schaffer M, Pioli F, Dall'oglio S, Pasetto S, Schaffer PM, Weber B, Giri MG. Regional hyperthermia combined with chemoradiotherapy in primary or recurrent locally advanced pancreatic cancer : an open-label comparative cohort trial. Strahlenther Onkol. 2011;187:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Fiorentini G, Sarti D, Gadaleta CD, Ballerini M, Fiorentini C, Garfagno T, Ranieri G, Guadagni S. A Narrative Review of Regional Hyperthermia: Updates From 2010 to 2019. Integr Cancer Ther. 2020;19:1534735420932648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Kim S, Lee JH, Cha J, You SH. Beneficial effects of modulated electro-hyperthermia during neoadjuvant treatment for locally advanced rectal cancer. Int J Hyperthermia. 2021;38:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Liu X, Song J, Zhang H, Liu X, Zuo F, Zhao Y, Yin X, Guo X, Wu X, Xu J, Hu J, Jing J, Ma X, Shi H. Immune checkpoint HLA-E:CD94-NKG2A mediates evasion of circulating tumor cells from NK cell surveillance. Cancer Cell. 2023;41:272-287.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 178] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 44. | Dbouk M, Katona BW, Brand RE, Chak A, Syngal S, Farrell JJ, Kastrinos F, Stoffel EM, Blackford AL, Rustgi AK, Dudley B, Lee LS, Chhoda A, Kwon R, Ginsberg GG, Klein AP, Kamel I, Hruban RH, He J, Shin EJ, Lennon AM, Canto MI, Goggins M. The Multicenter Cancer of Pancreas Screening Study: Impact on Stage and Survival. J Clin Oncol. 2022;40:3257-3266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 129] [Article Influence: 43.0] [Reference Citation Analysis (0)] |