Published online Jul 24, 2022. doi: 10.5306/wjco.v13.i7.567
Peer-review started: March 5, 2022
First decision: April 19, 2022
Revised: April 24, 2022
Accepted: June 22, 2022
Article in press: June 22, 2022
Published online: July 24, 2022
Processing time: 139 Days and 3.2 Hours
The classification of central nervous system (CNS) glioma went through a sequence of developments, between 2006 and 2021, started with only histological approach then has been aided with a major emphasis on molecular signatures in the 4th and 5th editions of the World Health Organization (WHO). The recent reformation in the 5th edition of the WHO classification has focused more on the molecularly defined entities with better characterized natural histories as well as new tumor types and subtypes in the adult and pediatric populations. These new subclassified entities have been incorporated in the 5th edition after the continuous exploration of new genomic, epigenomic and transcriptomic discovery. Indeed, the current guidelines of 2021 WHO classification of CNS tumors and European Association of Neuro-Oncology (EANO) exploited the molecular signatures in the diagnostic approach of CNS gliomas. Our current review presents a practical diagnostic approach for diffuse CNS gliomas and circumscribed astrocytomas using histomolecular criteria adopted by the recent WHO classification. We also describe the treatment strategies for these tumors based on EANO guidelines.
Core Tip: Central nervous system (CNS) gliomas went through a sequence of development since 2006. The guidelines of 2021 World Health Organization (WHO) classification of CNS tumors and European Association of Neuro-Oncology (EANO) utilized molecular signatures in the diagnostic approach for CNS gliomas. We herein presents a practical diagnostic approach and the treatment strategies for diffuse CNS gliomas and circumscribed astrocytomas using histomolecular criteria based on the WHO classification.
- Citation: Kurdi M, Moshref RH, Katib Y, Faizo E, Najjar AA, Bahakeem B, Bamaga AK. Simple approach for the histomolecular diagnosis of central nervous system gliomas based on 2021 World Health Organization Classification . World J Clin Oncol 2022; 13(7): 567-576
- URL: https://www.wjgnet.com/2218-4333/full/v13/i7/567.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i7.567
Brain tumors are defined as masses derived from various cells originating from the brain (primary tumors) or distally (secondary tumors, most commonly lung, breast, renal, prostate, and skin cancers) that have undergone metastatic spread[1]. The most prevalent primary intracranial tumors are gliomas, with 80% of the population, and World Health Organization (WHO) grade 4 astrocytoma (previously named glioblastoma) is present in nearly one-half of the patients with astrocytoma, with a 95% mortality rate in a 5-year follow-up period irrespective of age and gender[2,3,4]. Gliomas usually present with headache, nausea and vomiting, blurred vision, focal neurological deficit, alteration in sensation, and other manifestations of high intracranial pressure that warrant investigation by neuroimaging, preferably magnetic resonance imaging (MRI) of the brain[5]. In imaging, cystic change, multicentric enhancement, and hemorrhage are commonly observed[6]. Therefore, histopathological examination is considered the gold standard method for diagnosing and grading the tumors.
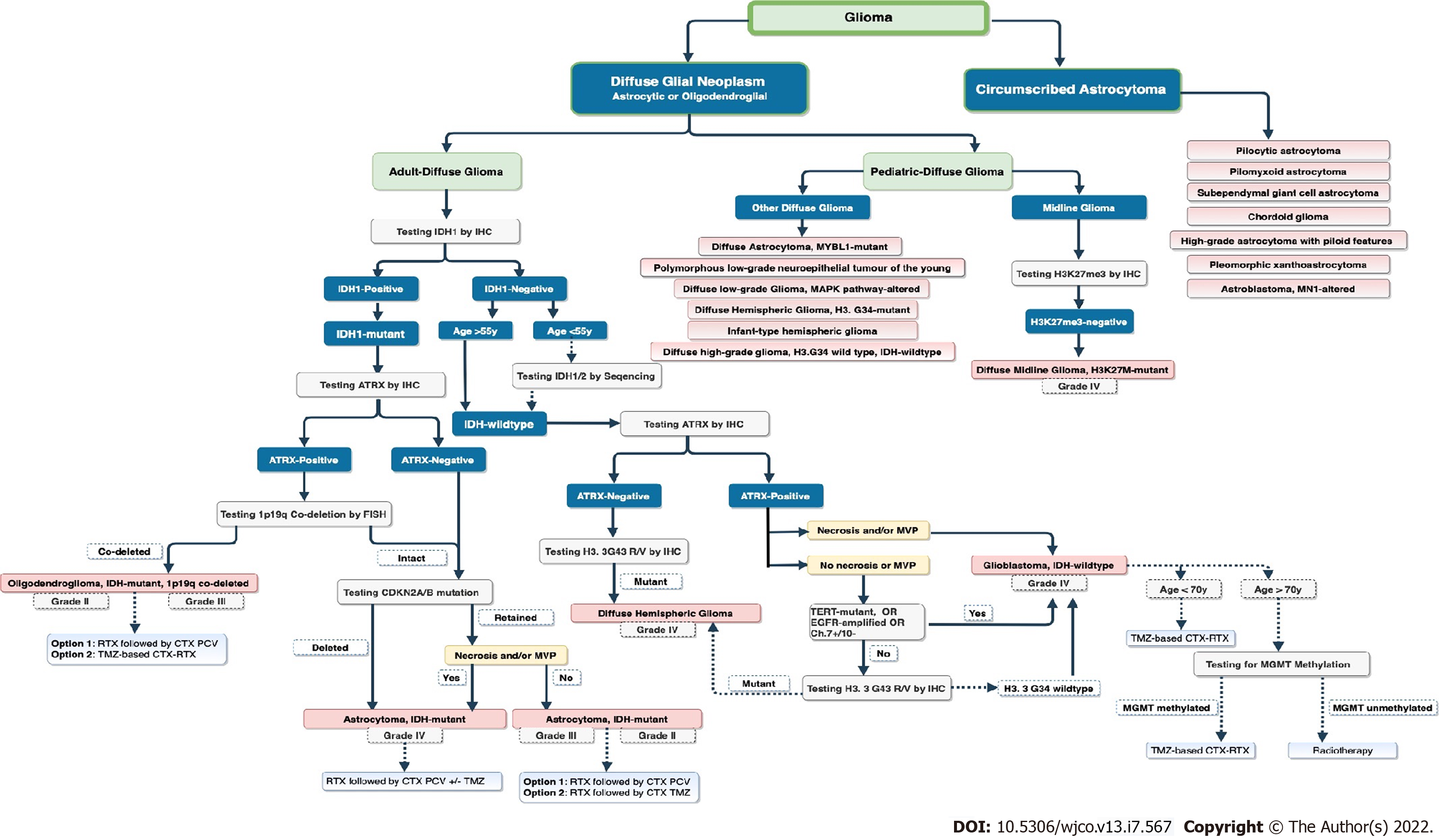
According to the WHO classification, central nervous system (CNS) gliomas can be classified based on their histological and molecular features[1]. The classification initially includes diffuse and non-diffuse gliomas, and it has undergone significant changes since its establishment to the 4th edition in 2016, aided by molecular signatures[2]. The recent 5th edition of the WHO classification has replaced the entity with type and variant with (subtype) group. In addition, the WHO classification has adopted Arabic numerical over the former Roman numerical grading system for grading brain gliomas for easier reading and to avoid confusion when interpreting pathology reports[7]. However, using the Roman numerical grading system is still considered acceptable. Some of the most important changes in the 5th edition involve the classification of gliomas, differentiating gliomas that occur primarily in adults from those that occur mainly in children[8]. Gliomas are traditionally subclassified into three major categories: diffuse gliomas, pediatric diffuse low- and high-grade gliomas, and circumscribed astrocytic gliomas (Figure 1). In addition, glioneuronal tumors, neuronal tumors, and ependymomas were also included in the classification as separate types[7,8].
Further propositions of the 2021 WHO classification of CNS tumors was adapted by the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy - Not Officially WHO (cIMPACT-NOW), published in 2020/2021. Moreover, they included various molecular genomic studies, including methylation profiling, isocitrate dehydrogenase (IDH1-2) codon mutation, and alpha-thalassemia-mental retardation X-chromosome (ATRX) mutation. This genomic profiling has affected such tumors' management and treatment modalities[9]. Moreover, three major subtypes of diffuse gliomas have been emphasized, based on molecular genomic signatures, including IDH-mutant oligodendroglioma with 1p/19q codeletion, IDH-mutant astrocytoma, and IDH-wild-type glioblastoma[8].
In this review, we presented a simple and practical diagnostic approach using histomolecular characteristics to define diffuse CNS gliomas accurately. We also associated the newly published 5th edition of the 2021 WHO classification with the current and updated European Association of Neuro-Oncology (EANO) treatment guidelines.
Before 2016, the classification of CNS tumors was established based on histological findings and immunohistochemical tests. Between 2016 and 2021, molecular biomarkers were incorporated into the diagnostic criteria to differentiate CNS tumors into clustered groups[7,9]. The current 5th edition of the 2021 WHO classification does not recommend a specific assessment method to identify molecular alterations unless a distinct tumor subtype is in the differential diagnosis. The cIMPACT-NOW has emphasized the confirmatory diagnosis of astrocytoma with the presence of IDH mutation or wild type, ATRX loss, TP53 mutation, and lack of 1p/q19 codeletion[7,10]. In comparison, diffuse gliomas (classified as WHO grades 2 and 3) are either IDH wild type or IDH1- mutant[11,12]. As a key feature of oligodendroglioma, it, by definition, must harbor an IDH mutation and 1p/19q codeletion.
If IDH1R132H is immunonegative in astrocytic or oligodendroglial tumors of WHO grades 2 and 3 or in patients aged less than 55 years, IDH1 (132 codon) and/or IDH2 (172 codon) Deoxyribonucleic acid (DNA) sequencing should be performed using the Sanger method or polymerase chain reaction (PCR)[13,14]. Otherwise, IDH1 immunonegative in patients aged greater than 55 years is likely to be IDH-wild type with an incidence rate of < 1% and acts like a high-grade glioma. Meanwhile, IDH1/2 DNA sequencing is preferable for detecting non-canonical mutations[15]. A non-canonical IDH mutation and a loss of ATRX mutation and O(6)-methylguanine-DNA methyltransferase (MGMT) promoter methylation[7] have been associated with a family history of cancer and astrocytoma of the infratentorial region being identified at 80% of the time in multicentric astrocytoma[16].
ATRX mutation should also be tested in all gliomas, whether IDH1/2 is a mutant or wild type. ATRX can be tested by immunohistochemistry (IHC). In oligodendroglioma, positive ATRX, negative TP53, and TERT mutations in IDH-mutant tumors are prevalent; nonetheless, 1p19q codeletion using fluorescence in situ hybridization (FISH) should be tested. However, false-positive results are expected to be less than 4% in most cases[17,18]. In both astrocytomas and oligodendrogliomas, the presence of homozygous deletion of CDKN2A/B at 9p21 is associated with poor prognosis in such groups, leading to decreased overall survival (OS)[11]. CDKN2A/B gene mutation can be tested through next-generation sequencing or the Sanger method if the tumor is IDH-mutant and 1p19q is not codeleted[19] (Figure 1). Loss of p16 expression (a marker of CDKNA/B) is associated with poor prognosis in IDH-mutant tumors and those with 1p/19q codeletion[20]. CDKN2A/B deletion is detected in both the wild-type and mutant IDH; however, it has been related to a reduction in OS in wild-type tumors[21-23].
Glioblastomas are routinely diagnosed based on histological findings of microvascular proliferation (MVP) and/or necrosis, and are molecularly defined as either IDH-mutant (10%) or IDH-wild-type (90%) tumors with significantly different biology and prognoses[8]. The terminology of IDH-mutant glioblastoma was omitted from the cIMPACT as they are biologically different from IDH-wild-type astrocytomas and were defined as IDH-mutant WHO grade 4 astrocytomas. Glioblastoma is referred to as IDH-wild-type glioblastoma or astrocytoma. There are no longer IDH-mutant glioblastomas. Another new terminology is an astrocytic glioma with IDH wild type, but with the presence of a histone 3 (H3) mutation, classified as a WHO grade 4 astrocytoma[9,24] (Figure 1). In glioblastoma, Telomerase reverse transcriptase (TERT) promoter mutations, Epidermal growth factor receptor (EGFR) mutations, and/or loss of chromosome 10 with gain of chromosome 7 are all prevalent[25]. If one of these features is detected, glioblastoma should be immediately diagnosed regardless of the presence of necrosis and/or MVP[26]. The presence of the H3F3A G34 histone mutation with chromosomal 1q gain distinguishes pediatric diffuse hemispheric glioma from adult diffuse hemispheric glioma. EGFR, TERT, CDKNB2A/B, and Ch10-Ch7+ are usually identified in adults; however, they should be tested for H3F3A gene mutation[27,28]. In comparison, the 2021 WHO classification has included IDH wild type and H3F3A in diffuse high-grade pediatric astrocytoma (most commonly from the pons in more than two-thirds of the cases, followed by the spinal cord and thalamus)[29].
H3-K27 is another commonly reported histone mutation in midline gliomas, ependymomas, and gangliogliomas[30-32]. Altered mitogen activated protein kinase (MAPK) pathway and H3-K27-mutant tumors are associated with diffuse low-grade midline gliomas and are associated with prolonged OS of more than 10 years[33]. The astroblastoma tumor is a new entity, MN1-altered, with better OS compared to C11Orf95-RELA and BRAF-positive astrocytic tumors[34]. Four other categories of diffuse astrocytomas were described in the 5th edition of the WHO classification, which included angiocentric gliomas, polymorphous low-grade neuroepithelial tumors of the young, and diffuse low-grade gliomas (MAP altered) (Figure 1). Infantile gliomas have a special genetic signature with fusion genes of MET, ROS1, ALK, or NTRK1/2/3[35,36].
A positive prognostic factors in diffuse gliomas include young patients, good Karnofsky performance status (KPS), total resection, and MGMT promoter methylation[37]. Surgical resection is considered the cornerstone of therapy, with a 5-year survival rate (80%) followed by watchful waiting in low-grade gliomas. However, total resection may result in neurological deficits; thus, awake craniotomy and imaging modalities, such as tractography, may be utilized on a case-by-case basis[38,39]. Postoperatively, MRI can detect residuals, and perfusion studies may detect progression[40]. Therefore, the care plan must be through a multidisciplinary approach with neurooncologists, neuropathologists, and neurosurgeons on board to discuss management modalities.
For low-grade gliomas, such as IDH-mutant and 1p/19q codeleted oligodendroglioma (WHO grade 2), careful, a watchful waiting strategy is an option, particularly for totally resected tumors or younger patients (< 40 years) with incomplete tumor resection. However, this would come at a cost: the patient’s life, neurological impairments development, and a substantial increase in histological grading over time[41,42]. As a result, disease progression must be monitored with neuroimaging every 2–3 months. According to the National Comprehensive Cancer Network guidelines, patients should be followed up every 2 months with an MRI brain scan, then every 3 months if they have been off therapy for a year[40]. Progression may occur after 4–8 weeks, necessitating a brain MRI. Perfusion studies and spectroscopy can help to differentiate between progression and pseudoprogression, and, in doubt, a multidisciplinary team should be counseled.
Post-surgical resection radiotherapy is the best therapeutic effect to prevent recurrence or delay the progression of diffuse gliomas (WHO grades 2-4)[43]. The role of radiotherapy is to maintain the control of tumor progression, but it leads to neurotoxicity if used in high doses. Thus, the most used doses are 50-60 Gy administered 3-5 times postoperatively[44]. Choi et al[45] found that patients with WHO grade 4 astrocytoma who received 50-60 Gy lived an average of 9 months longer than those who received 45 Gy for 3 months.
Radiotherapy is recommended for all WHO grade 2 gliomas with incomplete resection or for patients aged > 40 years and all WHO grade 3–4 gliomas. Early radiotherapy has been shown to prolong progression-free survival but not OS[46]. Whole-brain radiation therapy is usually not preferable in clinical practice because it is associated with cognitive effects[45]. A follow-up MRI 3-4 weeks after radiotherapy completion is typically performed to monitor disease progression[40]. The use of chemotherapy without radiation remains under investigation but might be an option if radiotherapy is not possible, for example, in patients with large tumors or elderly patients who might not be candidates for radiation.
Another adjunct treatment modality for diffuse gliomas is pharmacological treatment, mainly for patients with high-grade gliomas. The most used drug is the alkylating agent temozolomide (TMZ) for its immunomodulation and contribution to tumor-acquiring cell death. TMZ was first discovered in 1987 and has been widely applied as an effective first-line chemotherapeutic agent for treating patients with glioblastoma since the Food and Drug Administration has approved its efficacy in 2005[47,48]. Other drugs used as adjuvants in treating gliomas include nitrosourea, which includes lomustine, carmustine, nimustine, and fotemustine. However, this class of drugs may cause pronounced low platelet count when administered long-term. As a result, patients with oligodendroglioma frequently receive a procarbazine, vincristine, and lomustine (PCV) regimen, as dose-related side effects are more evident in other nitrosourea classes, such as gastrointestinal disorders, decreased cell count, and ototoxicity[48]. Anti-vascular endothelial growth factor antibody (bevacizumab) has also been used as an adjuvant treatment, but it’s clear benefit is uncertain[49].
In the new 2021 WHO, cIMPACT-NOW, and EANO guidelines, therapeutic options are targeted at the genomic types of tumors[7,9]. As total surgical resection remains the standard treatment for all CNS gliomas, radiotherapy is still considered the first-line targeted therapy after surgical resection for all WHO grade 2 and 3 oligodendrogliomas or astrocytomas, as mentioned previously[50-52].
In patients with IDH-mutant oligodendroglioma (WHO grade 2 or 3) with 1p19q codeletion, aged > 40 years or with no totally resected tumor, and associated with comorbidities, residuals, or recurrence > 15 cm[3], radiotherapy followed by PCV chemotherapy regimen is recommended[51]. According to the two trials (EORTC 26951 and RTOG 9402), the combination of radiotherapy and PCV regimen showed a considerable benefit[52,53].
Radiotherapy followed by chemotherapy is recommended for all patients with IDH-mutant WHO grade 2, 3, or 4 astrocytomas, particularly in patients aged > 40 years, with incompletely resected tumors, and associated with neurological deficits[51]. TMZ is often preferred over PCV owing to its safety and ease of administration. However, radiotherapy followed by PCV constitutes the current standard of care for patients with IDH-mutant astrocytomas (WHO grade 2)[51]. The RTOG 9802 trial reported a major prolongation of OS with the addition of PCV to radiotherapy, from 7 to 13 years in patients with WHO grade 2 gliomas who had undergone a subtotal resection or in those aged ≥ 40 years[54]. To prevent functional deficits, diffusion tensor imaging and functional MRI can be utilized[55]. In recurrence, repeat surgery with radiation and chemotherapy (TMZ and nitrosourea) can be considered equally efficient in treatment[9]. For IDH-mutant WHO grade 3 astrocytoma, the EORTC 26053 trial of radiotherapy alone, with concomitant or maintenance TMZ, showed a significant prolongation of OS in patients receiving radiotherapy followed by maintenance TMZ[56]. Therefore, TMZ chemotherapy is considered as the standard treatment for tumour progression after surgery and radiotherapy for most patients with IDH-mutant gliomas (WHO grade 2 or 3).
Glioblastoma (IDH-wild-type grade 4 astrocytoma) is best managed by gross total resection followed by radiotherapy[51]. In non-feasible or nearly total resection cases with age ≥ 70 years, radiotherapy (60 Gy in 30 fractions) or over fractionated radiotherapy (40 Gy in 15 fractions) is preferable to increase OS[9]. Higher survival rates are recorded in younger age groups < 65 years at diagnosis, with a median of up to 40 weeks. However, TERT mutation, gain of chromosome 7, and loss of chromosome 10 are associated with poor prognosis[57]. Neurocognitive outcomes can be affected by overfractionated radiotherapy. In patients with good KPS and aged < 70 years, a combination of radiotherapy and chemotherapy (TMZ) is the standard therapy[58]. Combined TMZ with lomustine in early diagnosis may increase OS, particularly in MGMT-methylated glioblastomas[59-61]. Hypofractionated radiotherapy is preferable for patients aged ≥ 70 years. The standard-of-care treatment for patients with recurrent glioblastoma has not yet been clarified; treatment is selected based on the prior therapy, patient’s age, KPS score, MGMT promoter methylation status, and disease progression. Therefore, surgery and radiotherapy should be considered. Nitrosourea regimens, TMZ, with consideration of bevacizumab are options for pharmacotherapy but have an unconfirmed effect on OS. In patients who did not benefit from adjuvant radiotherapy or had an early symptomatic progression, a second surgery could be considered 6 months after the initial surgery aiming to increase OS[62].
There is a limitation of surgical management in H3-K27M-mutant diffuse midline glioma (WHO grade 4) because of its eloquent structures, including the pituitary, thalamus, midbrain, pons, and medulla, and it has a 5-year survival period of < 1%. Radiotherapy is often used but is associated with a poor prognosis. However, in hemispheric glioma, chemoradiotherapy drugs can be used because most of these tumors are MGMT-methylated[63].
In this review, we presented a simple diagnostic approach to differentiate diffuse CNS gliomas into molecularly defined subtypes using histomolecular features, based on the 5th edition of 2021 WHO classification of CNS tumors. This is to emphasize that molecular profiling is important in the diagnostic classification and grading of diffuse CNS gliomas. We also defined the role of different treatment modalities of surgery, radiotherapy, and pharmacotherapy in the treatment of these molecular defined gliomas. In fact, our review intends to serve as a simple reference for the diagnosis of CNS gliomas for healthcare providers.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Saudi Arabia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kotelevets SM, Russia; Liu Z, China; Mijwil MM, Iraq S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Butowski NA. Epidemiology and diagnosis of brain tumors. Continuum (Minneap Minn). 2015;21:301-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh KM, Wrensch MR, Barnholtz-Sloan JS. The epidemiology of glioma in adults: a "state of the science" review. Neuro Oncol. 2014;16:896-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1515] [Cited by in RCA: 1554] [Article Influence: 141.3] [Reference Citation Analysis (0)] |
| 3. | Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro Oncol. 2019;21:v1-v100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1781] [Article Influence: 356.2] [Reference Citation Analysis (0)] |
| 4. | Jung E, Alfonso J, Osswald M, Monyer H, Wick W, Winkler F. Emerging intersections between neuroscience and glioma biology. Nat Neurosci. 2019;22:1951-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 5. | Wesseling P, Capper D. WHO 2016 Classification of gliomas. Neuropathol Appl Neurobiol. 2018;44:139-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 551] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 6. | Ly KI, Wen PY, Huang RY. Imaging of Central Nervous System Tumors Based on the 2016 World Health Organization Classification. Neurol Clin. 2020;38:95-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23:1231-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4523] [Cited by in RCA: 6537] [Article Influence: 1634.3] [Reference Citation Analysis (1)] |
| 8. | Wen PY, Packer RJ. The 2021 WHO Classification of Tumors of the Central Nervous System: clinical implications. Neuro Oncol. 2021;23:1215-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 9. | Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, French P, Hegi ME, Jakola AS, Platten M, Roth P, Rudà R, Short S, Smits M, Taphoorn MJB, von Deimling A, Westphal M, Soffietti R, Reifenberger G, Wick W. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18:170-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 1094] [Article Influence: 218.8] [Reference Citation Analysis (0)] |
| 10. | Tanboon J, Williams EA, Louis DN. The Diagnostic Use of Immunohistochemical Surrogates for Signature Molecular Genetic Alterations in Gliomas. J Neuropathol Exp Neurol. 2016;75:4-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Reinhardt A, Stichel D, Schrimpf D, Sahm F, Korshunov A, Reuss DE, Koelsche C, Huang K, Wefers AK, Hovestadt V, Sill M, Gramatzki D, Felsberg J, Reifenberger G, Koch A, Thomale UW, Becker A, Hans VH, Prinz M, Staszewski O, Acker T, Dohmen H, Hartmann C, Mueller W, Tuffaha MSA, Paulus W, Heß K, Brokinkel B, Schittenhelm J, Monoranu CM, Kessler AF, Loehr M, Buslei R, Deckert M, Mawrin C, Kohlhof P, Hewer E, Olar A, Rodriguez FJ, Giannini C, NageswaraRao AA, Tabori U, Nunes NM, Weller M, Pohl U, Jaunmuktane Z, Brandner S, Unterberg A, Hänggi D, Platten M, Pfister SM, Wick W, Herold-Mende C, Jones DTW, von Deimling A, Capper D. Anaplastic astrocytoma with piloid features, a novel molecular class of IDH wildtype glioma with recurrent MAPK pathway, CDKN2A/B and ATRX alterations. Acta Neuropathol. 2018;136:273-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 210] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 12. | Jones DTW, Kieran MW, Bouffet E, Alexandrescu S, Bandopadhayay P, Bornhorst M, Ellison D, Fangusaro J, Fisher MJ, Foreman N, Fouladi M, Hargrave D, Hawkins C, Jabado N, Massimino M, Mueller S, Perilongo G, Schouten van Meeteren AYN, Tabori U, Warren K, Waanders AJ, Walker D, Weiss W, Witt O, Wright K, Zhu Y, Bowers DC, Pfister SM, Packer RJ. Pediatric low-grade gliomas: next biologically driven steps. Neuro Oncol. 2018;20:160-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 13. | van den Bent MJ, Chang SM. Grade II and III Oligodendroglioma and Astrocytoma. Neurol Clin. 2018;36:467-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Kim M, Jung SY, Park JE, Jo Y, Park SY, Nam SJ, Kim JH, Kim HS. Diffusion- and perfusion-weighted MRI radiomics model may predict isocitrate dehydrogenase (IDH) mutation and tumor aggressiveness in diffuse lower grade glioma. Eur Radiol. 2020;30:2142-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 15. | Barresi V, Eccher A, Simbolo M, Cappellini R, Ricciardi GK, Calabria F, Cancedda M, Mazzarotto R, Bonetti B, Pinna G, Sala F, Ghimenton C, Scarpa A. Diffuse gliomas in patients aged 55 years or over: A suggestion for IDH mutation testing. Neuropathology. 2020;40:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Poetsch L, Bronnimann C, Loiseau H, Frénel JS, Siegfried A, Seizeur R, Gauchotte G, Cappellen D, Carpentier C, Figarella-Branger D, Eimer S, Meyronet D, Ducray F; POLA network. Characteristics of IDH-mutant gliomas with non-canonical IDH mutation. J Neurooncol. 2021;151:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Ohba S, Kuwahara K, Yamada S, Abe M, Hirose Y. Correlation between IDH, ATRX, and TERT promoter mutations in glioma. Brain Tumor Pathol. 2020;37:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Ball MK, Kollmeyer TM, Praska CE, McKenna ML, Giannini C, Raghunathan A, Jentoft ME, Lachance DH, Kipp BR, Jenkins RB, Ida CM. Frequency of false-positive FISH 1p/19q codeletion in adult diffuse astrocytic gliomas. Neurooncol Adv. 2020;2:vdaa109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Kong Y, Sharma RB, Ly S, Stamateris RE, Jesdale WM, Alonso LC. CDKN2A/B T2D Genome-Wide Association Study Risk SNPs Impact Locus Gene Expression and Proliferation in Human Islets. Diabetes. 2018;67:872-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Park JW, Kang J, Lim KY, Kim H, Kim SI, Won JK, Park CK, Park SH. The prognostic significance of p16 expression pattern in diffuse gliomas. J Pathol Transl Med. 2021;55:102-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Ma S, Rudra S, Campian JL, Dahiya S, Dunn GP, Johanns T, Goldstein M, Kim AH, Huang J. Prognostic impact of CDKN2A/B deletion, TERT mutation, and EGFR amplification on histological and molecular IDH-wildtype glioblastoma. Neurooncol Adv. 2020;2:vdaa126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Okajima K, Ohta Y. [Diagnostic imaging of high-grade astrocytoma: heterogeneity of clinical manifestation, image characteristics, and histopathological findings]. Brain Nerve. 2012;64:1151-1157. [PubMed] |
| 23. | López GY, Perry A, Harding B, Li M, Santi M. CDKN2A/B Loss Is Associated with Anaplastic Transformation in a Case of NTRK2 Fusion-positive Pilocytic Astrocytoma. Neuropathol Appl Neurobiol. 2019;45:174-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Brat DJ, Aldape K, Colman H, Holland EC, Louis DN, Jenkins RB, Kleinschmidt-DeMasters BK, Perry A, Reifenberger G, Stupp R, von Deimling A, Weller M. cIMPACT-NOW update 3: recommended diagnostic criteria for "Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV". Acta Neuropathol. 2018;136:805-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 613] [Article Influence: 87.6] [Reference Citation Analysis (0)] |
| 25. | Stichel D, Ebrahimi A, Reuss D, Schrimpf D, Ono T, Shirahata M, Reifenberger G, Weller M, Hänggi D, Wick W, Herold-Mende C, Westphal M, Brandner S, Pfister SM, Capper D, Sahm F, von Deimling A. Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclassification of IDHwt astrocytoma to glioblastoma. Acta Neuropathol. 2018;136:793-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 219] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 26. | Campos B, Olsen LR, Urup T, Poulsen HS. A comprehensive profile of recurrent glioblastoma. Oncogene. 2016;35:5819-5825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 27. | Sturm D, Bender S, Jones DT, Lichter P, Grill J, Becher O, Hawkins C, Majewski J, Jones C, Costello JF, Iavarone A, Aldape K, Brennan CW, Jabado N, Pfister SM. Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat Rev Cancer. 2014;14:92-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 423] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 28. | Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D, Koelsche C, Sahm F, Chavez L, Reuss DE, Kratz A, Wefers AK, Huang K, Pajtler KW, Schweizer L, Stichel D, Olar A, Engel NW, Lindenberg K, Harter PN, Braczynski AK, Plate KH, Dohmen H, Garvalov BK, Coras R, Hölsken A, Hewer E, Bewerunge-Hudler M, Schick M, Fischer R, Beschorner R, Schittenhelm J, Staszewski O, Wani K, Varlet P, Pages M, Temming P, Lohmann D, Selt F, Witt H, Milde T, Witt O, Aronica E, Giangaspero F, Rushing E, Scheurlen W, Geisenberger C, Rodriguez FJ, Becker A, Preusser M, Haberler C, Bjerkvig R, Cryan J, Farrell M, Deckert M, Hench J, Frank S, Serrano J, Kannan K, Tsirigos A, Brück W, Hofer S, Brehmer S, Seiz-Rosenhagen M, Hänggi D, Hans V, Rozsnoki S, Hansford JR, Kohlhof P, Kristensen BW, Lechner M, Lopes B, Mawrin C, Ketter R, Kulozik A, Khatib Z, Heppner F, Koch A, Jouvet A, Keohane C, Mühleisen H, Mueller W, Pohl U, Prinz M, Benner A, Zapatka M, Gottardo NG, Driever PH, Kramm CM, Müller HL, Rutkowski S, von Hoff K, Frühwald MC, Gnekow A, Fleischhack G, Tippelt S, Calaminus G, Monoranu CM, Perry A, Jones C, Jacques TS, Radlwimmer B, Gessi M, Pietsch T, Schramm J, Schackert G, Westphal M, Reifenberger G, Wesseling P, Weller M, Collins VP, Blümcke I, Bendszus M, Debus J, Huang A, Jabado N, Northcott PA, Paulus W, Gajjar A, Robinson GW, Taylor MD, Jaunmuktane Z, Ryzhova M, Platten M, Unterberg A, Wick W, Karajannis MA, Mittelbronn M, Acker T, Hartmann C, Aldape K, Schüller U, Buslei R, Lichter P, Kool M, Herold-Mende C, Ellison DW, Hasselblatt M, Snuderl M, Brandner S, Korshunov A, von Deimling A, Pfister SM. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555:469-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1746] [Cited by in RCA: 2006] [Article Influence: 286.6] [Reference Citation Analysis (0)] |
| 29. | Louis DN, Giannini C, Capper D, Paulus W, Figarella-Branger D, Lopes MB, Batchelor TT, Cairncross JG, van den Bent M, Wick W, Wesseling P. cIMPACT-NOW update 2: diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol. 2018;135:639-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 271] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 30. | Hochart A, Escande F, Rocourt N, Grill J, Koubi-Pick V, Beaujot J, Meignan S, Vinchon M, Maurage CA, Leblond P. Long survival in a child with a mutated K27M-H3.3 pilocytic astrocytoma. Ann Clin Transl Neurol. 2015;2:439-443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Johnson A, Severson E, Gay L, Vergilio JA, Elvin J, Suh J, Daniel S, Covert M, Frampton GM, Hsu S, Lesser GJ, Stogner-Underwood K, Mott RT, Rush SZ, Stanke JJ, Dahiya S, Sun J, Reddy P, Chalmers ZR, Erlich R, Chudnovsky Y, Fabrizio D, Schrock AB, Ali S, Miller V, Stephens PJ, Ross J, Crawford JR, Ramkissoon SH. Comprehensive Genomic Profiling of 282 Pediatric Low- and High-Grade Gliomas Reveals Genomic Drivers, Tumor Mutational Burden, and Hypermutation Signatures. Oncologist. 2017;22:1478-1490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 183] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 32. | Kleinschmidt-DeMasters BK, Donson A, Foreman NK, Dorris K. H3 K27M Mutation in Gangliogliomas can be Associated with Poor Prognosis. Brain Pathol. 2017;27:846-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, Orisme W, Punchihewa C, Parker M, Qaddoumi I, Boop FA, Lu C, Kandoth C, Ding L, Lee R, Huether R, Chen X, Hedlund E, Nagahawatte P, Rusch M, Boggs K, Cheng J, Becksfort J, Ma J, Song G, Li Y, Wei L, Wang J, Shurtleff S, Easton J, Zhao D, Fulton RS, Fulton LL, Dooling DJ, Vadodaria B, Mulder HL, Tang C, Ochoa K, Mullighan CG, Gajjar A, Kriwacki R, Sheer D, Gilbertson RJ, Mardis ER, Wilson RK, Downing JR, Baker SJ, Ellison DW; St. Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45:602-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 526] [Cited by in RCA: 626] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 34. | Lehman NL, Usubalieva A, Lin T, Allen SJ, Tran QT, Mobley BC, McLendon RE, Schniederjan MJ, Georgescu MM, Couce M, Dulai MS, Raisanen JM, Al Abbadi M, Palmer CA, Hattab EM, Orr BA. Genomic analysis demonstrates that histologically-defined astroblastomas are molecularly heterogeneous and that tumors with MN1 rearrangement exhibit the most favorable prognosis. Acta Neuropathol Commun. 2019;7:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 35. | Guerreiro Stucklin AS, Ryall S, Fukuoka K, Zapotocky M, Lassaletta A, Li C, Bridge T, Kim B, Arnoldo A, Kowalski PE, Zhong Y, Johnson M, Ramani AK, Siddaway R, Nobre LF, de Antonellis P, Dunham C, Cheng S, Boué DR, Finlay JL, Coven SL, de Prada I, Perez-Somarriba M, Faria CC, Grotzer MA, Rushing E, Sumerauer D, Zamecnik J, Krskova L, Garcia Ariza M, Cruz O, Morales La Madrid A, Solano P, Terashima K, Nakano Y, Ichimura K, Nagane M, Sakamoto H, Gil-da-Costa MJ, Silva R, Johnston DL, Michaud J, Wilson B, van Landeghem FKH, Oviedo A, McNeely PD, Crooks B, Fried I, Zhukova N, Hansford JR, Nageswararao A, Garzia L, Shago M, Brudno M, Irwin MS, Bartels U, Ramaswamy V, Bouffet E, Taylor MD, Tabori U, Hawkins C. Alterations in ALK/ROS1/NTRK/MET drive a group of infantile hemispheric gliomas. Nat Commun. 2019;10:4343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 228] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 36. | Pathania M, De Jay N, Maestro N, Harutyunyan AS, Nitarska J, Pahlavan P, Henderson S, Mikael LG, Richard-Londt A, Zhang Y, Costa JR, Hébert S, Khazaei S, Ibrahim NS, Herrero J, Riccio A, Albrecht S, Ketteler R, Brandner S, Kleinman CL, Jabado N, Salomoni P. H3.3K27M Cooperates with Trp53 Loss and PDGFRA Gain in Mouse Embryonic Neural Progenitor Cells to Induce Invasive High-Grade Gliomas. Cancer Cell. 2017;32:684-700.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 37. | Settle SH, Sulman EP. Tumor profiling: development of prognostic and predictive factors to guide brain tumor treatment. Curr Oncol Rep. 2011;13:26-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Brown T, Shah AH, Bregy A, Shah NH, Thambuswamy M, Barbarite E, Fuhrman T, Komotar RJ. Awake craniotomy for brain tumor resection: the rule rather than the exception? J Neurosurg Anesthesiol. 2013;25:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 39. | Zhang JJY, Lee KS, Voisin MR, Hervey-Jumper SL, Berger MS, Zadeh G. Awake craniotomy for resection of supratentorial glioblastoma: a systematic review and meta-analysis. Neurooncol Adv. 2020;2:vdaa111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 40. | White ML, Zhang Y, Yu F, Shonka N, Aizenberg MR, Adapa P, Kazmi SAJ. Post-operative perfusion and diffusion MR imaging and tumor progression in high-grade gliomas. PLoS One. 2019;14:e0213905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Jakola AS, Skjulsvik AJ, Myrmel KS, Sjåvik K, Unsgård G, Torp SH, Aaberg K, Berg T, Dai HY, Johnsen K, Kloster R, Solheim O. Surgical resection versus watchful waiting in low-grade gliomas. Ann Oncol. 2017;28:1942-1948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 212] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 42. | Zikou A, Sioka C, Alexiou GA, Fotopoulos A, Voulgaris S, Argyropoulou MI. Radiation Necrosis, Pseudoprogression, Pseudoresponse, and Tumor Recurrence: Imaging Challenges for the Evaluation of Treated Gliomas. Contrast Media Mol Imaging. 2018;2018:6828396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 43. | Hau E, Shen H, Clark C, Graham PH, Koh ES, L McDonald K. The evolving roles and controversies of radiotherapy in the treatment of glioblastoma. J Med Radiat Sci. 2016;63:114-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Keime-Guibert F, Chinot O, Taillandier L, Cartalat-Carel S, Frenay M, Kantor G, Guillamo JS, Jadaud E, Colin P, Bondiau PY, Meneï P, Loiseau H, Bernier V, Honnorat J, Barrié M, Mokhtari K, Mazeron JJ, Bissery A, Delattre JY; Association of French-Speaking Neuro-Oncologists. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356:1527-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 545] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 45. | Choi J, Kim G, Cho SB, Im HJ. Radiosensitizing high-Z metal nanoparticles for enhanced radiotherapy of glioblastoma multiforme. J Nanobiotechnology. 2020;18:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 46. | van den Bent MJ, Afra D, de Witte O, Ben Hassel M, Schraub S, Hoang-Xuan K, Malmström PO, Collette L, Piérart M, Mirimanoff R, Karim AB; EORTC Radiotherapy and Brain Tumor Groups and the UK Medical Research Council. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 680] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 47. | Karachi A, Dastmalchi F, Mitchell DA, Rahman M. Temozolomide for immunomodulation in the treatment of glioblastoma. Neuro Oncol. 2018;20:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 194] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 48. | Levin VA, Uhm JH, Jaeckle KA, Choucair A, Flynn PJ, Yung WKA, Prados MD, Bruner JM, Chang SM, Kyritsis AP, Gleason MJ, Hess KR. Phase III randomized study of postradiotherapy chemotherapy with alpha-difluoromethylornithine-procarbazine, N-(2-chloroethyl)-N'-cyclohexyl-N-nitrosurea, vincristine (DFMO-PCV) versus PCV for glioblastoma multiforme. Clin Cancer Res. 2000;6:3878-3884. [PubMed] |
| 49. | An Z, Aksoy O, Zheng T, Fan QW, Weiss WA. Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies. Oncogene. 2018;37:1561-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 401] [Cited by in RCA: 440] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 50. | Schiff D, Van den Bent M, Vogelbaum MA, Wick W, Miller CR, Taphoorn M, Pope W, Brown PD, Platten M, Jalali R, Armstrong T, Wen PY. Recent developments and future directions in adult lower-grade gliomas: Society for Neuro-Oncology (SNO) and European Association of Neuro-Oncology (EANO) consensus. Neuro Oncol. 2019;21:837-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 51. | Weller M, van den Bent M, Tonn JC, Stupp R, Preusser M, Cohen-Jonathan-Moyal E, Henriksson R, Le Rhun E, Balana C, Chinot O, Bendszus M, Reijneveld JC, Dhermain F, French P, Marosi C, Watts C, Oberg I, Pilkington G, Baumert BG, Taphoorn MJB, Hegi M, Westphal M, Reifenberger G, Soffietti R, Wick W; European Association for Neuro-Oncology (EANO) Task Force on Gliomas. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18:e315-e329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 776] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 52. | Wick W, Roth P, Hartmann C, Hau P, Nakamura M, Stockhammer F, Sabel MC, Wick A, Koeppen S, Ketter R, Vajkoczy P, Eyupoglu I, Kalff R, Pietsch T, Happold C, Galldiks N, Schmidt-Graf F, Bamberg M, Reifenberger G, Platten M, von Deimling A, Meisner C, Wiestler B, Weller M; Neurooncology Working Group (NOA) of the German Cancer Society. Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol. 2016;18:1529-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 53. | Dono A, Ballester LY, Primdahl D, Esquenazi Y, Bhatia A. IDH-Mutant Low-grade Glioma: Advances in Molecular Diagnosis, Management, and Future Directions. Curr Oncol Rep. 2021;23:20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR, Coons S, Ricci P, Bullard D, Brown PD, Stelzer K, Brachman D, Suh JH, Schultz CJ, Bahary JP, Fisher BJ, Kim H, Murtha AD, Bell EH, Won M, Mehta MP, Curran WJ Jr. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N Engl J Med. 2016;374:1344-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 742] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 55. | Krivosheya D, Prabhu SS, Weinberg JS, Sawaya R. Technical principles in glioma surgery and preoperative considerations. J Neurooncol. 2016;130:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 56. | van den Bent M, Erridge S, Vogelbaum M, Nowak A, Sanson M, Brandes A, Wick W, Clement P, Baurain JF, Mason W, Wheeler H, Weller M, aldape K, Wesseling P, Kros J, Tesileanu M, Golfinopoulos V, Gorlia T, Baumert B, French P. ACTR-11. Second interim and 1st molecular analysis of the eortc randomized phase iii intergroup catnon trial on concurrent and adjuvant temozolomide in anaplastic glioma without 1p/19q codeletion. Neuro Oncol. 2019;21:vi14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Galbraith K, Kumar A, Abdullah KG, Walker JM, Adams SH, Prior T, Dimentberg R, Henderson FC, Mirchia K, Sathe AA, Viapiano MS, Chin LS, Corona RJ, Hatanpaa KJ, Snuderl M, Xing C, Brem S, Richardson TE. Molecular Correlates of Long Survival in IDH-Wildtype Glioblastoma Cohorts. J Neuropathol Exp Neurol. 2020;79:843-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 58. | Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M. Management of glioblastoma: State of the art and future directions. CA Cancer J Clin. 2020;70:299-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 1226] [Article Influence: 245.2] [Reference Citation Analysis (0)] |
| 59. | Kosti A, de Araujo PR, Li WQ, Guardia GDA, Chiou J, Yi C, Ray D, Meliso F, Li YM, Delambre T, Qiao M, Burns SS, Lorbeer FK, Georgi F, Flosbach M, Klinnert S, Jenseit A, Lei X, Sandoval CR, Ha K, Zheng H, Pandey R, Gruslova A, Gupta YK, Brenner A, Kokovay E, Hughes TR, Morris QD, Galante PAF, Tiziani S, Penalva LOF. The RNA-binding protein SERBP1 functions as a novel oncogenic factor in glioblastoma by bridging cancer metabolism and epigenetic regulation. Genome Biol. 2020;21:195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 60. | Erel-Akbaba G, Carvalho LA, Tian T, Zinter M, Akbaba H, Obeid PJ, Chiocca EA, Weissleder R, Kantarci AG, Tannous BA. Radiation-Induced Targeted Nanoparticle-Based Gene Delivery for Brain Tumor Therapy. ACS Nano. 2019;13:4028-4040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 156] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 61. | Byron SA, Tran NL, Halperin RF, Phillips JJ, Kuhn JG, de Groot JF, Colman H, Ligon KL, Wen PY, Cloughesy TF, Mellinghoff IK, Butowski NA, Taylor JW, Clarke JL, Chang SM, Berger MS, Molinaro AM, Maggiora GM, Peng S, Nasser S, Liang WS, Trent JM, Berens ME, Carpten JD, Craig DW, Prados MD. Prospective Feasibility Trial for Genomics-Informed Treatment in Recurrent and Progressive Glioblastoma. Clin Cancer Res. 2018;24:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 62. | Suchorska B, Weller M, Tabatabai G, Senft C, Hau P, Sabel MC, Herrlinger U, Ketter R, Schlegel U, Marosi C, Reifenberger G, Wick W, Tonn JC, Wirsching HG. Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the DIRECTOR trial. Neuro Oncol. 2016;18:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 63. | Nikolaev A, Fiveash JB, Yang ES. Combined Targeting of Mutant p53 and Jumonji Family Histone Demethylase Augments Therapeutic Efficacy of Radiation in H3K27M DIPG. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |