Published online Dec 24, 2022. doi: 10.5306/wjco.v13.i12.943
Peer-review started: September 2, 2022
First decision: September 27, 2022
Revised: October 10, 2022
Accepted: December 8, 2022
Article in press: December 8, 2022
Published online: December 24, 2022
Processing time: 107 Days and 17.8 Hours
Approximately 7%-29% of patients with colorectal cancer present with colonic obstruction. The concept of self-expandable metal stent (SEMS) insertion as a bridge to surgery (BTS) is appealing. However, concerns on colonic stenting possibly impairing oncologic outcomes have been raised. This study aimed to review current evidence on the short- and long-term oncologic outcomes of SEMS insertion as BTS for left-sided malignant colonic obstruction. For short-term outcomes, colonic stenting facilitates a laparoscopic approach, increases the likelihood of primary anastomosis without a stoma, and may decrease postoperative morbidity. However, SEMS-related perforation also increases local recurrence and impairs overall survival. Moreover, colonic stenting may cause negative oncologic outcomes even without perforation. SEMS can induce shear forces on the tumor, leading to increased circulating cancer cells and aggressive pathological characteristics, including perineural and lymphovascular invasion. The conflicting evidence has led to discordant guidelines. Well-designed collaborative studies that integrate both oncologic outcomes and data on basic research (e.g., alteration of circulating tumors) are needed to clarify the actual benefit of colonic stenting as BTS.
Core Tip: Although the concept of self-expandable metal stent (SEMS) insertion as a bridge to surgery in patients with left-sided malignant colonic obstruction is promising, there remain concerns of adverse oncologic outcomes. Nowadays, three possible mechanisms of tumor dissemination from SEMS have been proposed: (1) SEMS-related perforation; (2) increased circulating tumor cells; and (3) aggressive pathological features after SEMS placement. However, among these, only SEMS-related perforation clearly influences adverse oncologic outcomes. The other two mechanisms lack consistent clinical evidence for their association with decreased survival. Therefore, further collaborating studies are needed to validate the clinical impact of these hypotheses.
- Citation: Pattarajierapan S, Sukphol N, Junmitsakul K, Khomvilai S. Oncologic safety of colonic stenting as a bridge to surgery in left-sided malignant colonic obstruction: Current evidence and prospects. World J Clin Oncol 2022; 13(12): 943-956
- URL: https://www.wjgnet.com/2218-4333/full/v13/i12/943.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i12.943
Colorectal cancer (CRC) is the fourth most common malignant disease worldwide, with more than 1.9 million new cases recorded in 2020[1]. Patients with CRC are presented with bowel obstruction for 7%-29%[2]. The outcomes of emergency surgery (ES) for patients with obstructed CRC are worse than those of elective surgery for patients without obstruction. Patients with obstructed CRC also have a higher mortality rate than those without obstruction (17% vs 6%, respectively)[3]. The causes of the high morbidity and mortality of ES are advanced-stage cancer, malnutrition, electrolyte abnormalities, colonic mucosa injury from distention, and fecal loading of the obstructed colon[4]. The self-expandable metal stent (SEMS) insertion as a bridge to surgery (BTS) concept, which converts an emergency condition to an elective one, is fascinating. Colonic decompression by SEMS gives time to stabilize medical conditions that distinctly benefit high-risk patients.
The benefits of SEMS as BTS for right-sided malignant colonic obstruction (RMCO, defined as an obstructed tumor located between the cecum and distal transverse colon) are limited. Currently, right colectomy with primary anastomosis is the recommended treatment for RMCO[5]. Ileocolic anastomosis is associated with the lowest incidence of leaks, ranging from 1% to 3%, and can be performed in cases with obstructive situation[6,7]. Therefore, the World Society of ES (WSES) guideline does not reco
However, the long-term oncologic outcomes are a matter of concern. SEMS induces shear force to the tumor and may lead to cancer cell dissemination into the peritoneal cavity, lymphatic fluid, and bloodstream[9,10]. A few studies suggested that SEMS insertion was associated with worse oncologic outcomes than ES, especially in patients with SEMS-related perforation[11,12]. Nevertheless, recent studies with low complication rates reported good oncologic outcomes with SEMS placement as BTS[13-19]. As a result, current guidelines are dynamic and discordant because of the conflicting evidence[20-24].
As such, this study aimed to perform a comprehensive review of the current evidence on the short- and long-term oncologic outcomes of SEMS insertion as BTS for LMCO.
Emergent procedures for LMCO include various procedures such as Hartmann’s procedure, segmental colectomy with/without on-table lavage, and subtotal/total colectomy. These procedures result in high morbidity and mortality because of the limited time to stabilize the patient’s condition before surgery[4]. Among these, Hartmann’s procedure remains one of the most common emergency procedures for the left colon because of the short operative time and avoidance of anastomotic leakage[25]. However, the rate of reversal of Hartmann’s procedure is less than 50% because this operation is associated with high morbidity and possible mortality[26,27]. As a result, most of them turn to permanent stomas that severely affect the patient’s quality of life. Laparoscopic approach for ES of LMCO has limited application because of technical difficulties during surgery. The WSES guideline does not recommend its use except in selected cases in specialist centers[5].
Dohmoto et al[28] was the first to report the idea of using plastic tubes as colonic stenting for palliation of obstructed rectal cancer in 1991. One year later, Spinelli et al[29] started using SEMS insertion as a palliative modality with good results. After the success of palliative SEMS placement, the BTS concept was introduced by Tejero et al[30] in 1994. They described 3 phases of SEMS as BTS: (1) Relieving obstruction by SEMS; (2) recovering the patient’s condition and mechanically preparing the colon; and (3) definitive elective surgery. It has been 30 years since the introduction of colonic stenting. Palliative SEMS placement is established as a preferred option in incurable malignant colonic obstruction because it confers superior quality of life by avoiding stoma and is associated with shorter time to initiation of chemotherapy than palliative surgery[21]. In contrast, the role of SEMS as BTS is still controversial, with concerns of adverse oncologic outcomes after SEMS insertion limiting its application as BTS.
There are different types of SEMS materials including stainless steel, elgiloy, and nitinol[31]. Endoscopists should be aware of the characteristics of each stent. Stainless steel stents are relatively stiff and interfere with magnetic resonance imaging (MRI) examination. Meanwhile, elgiloy stents have better elasticity and flexibility and do not interfere with MRI assessment. Nitinol stents are made of nickel-titanium and have poorer fluoroscopic visualization compared with elgiloy stents. Therefore, radiopaque markers, such as gold or silver markers, are added to both ends of these stents. Nitinol stents have superior flexibility and better memory to hold the original shape than stainless steel and elgiloy stents; consequently, nitinol stents are popular worldwide.
SEMS is classified as covered or uncovered. Covered SEMS has a silicone membrane on bare wires, preventing tumor ingrowth. For obstructed CRC, uncovered SEMS is recommended for both curative and palliative settings[21]. A recent meta-analysis, including one randomized controlled trial (RCT) and nine observational studies, compared covered and uncovered SEMS in curative and palliative settings. The study found that uncovered SEMS was associated with fewer complications (relative risk [RR]: 0.57; 95%CI: 0.44-0.74; P < 0.001), tumor ingrowth (RR: 0.29; 95%CI: 0.09-0.93; P = 0.040), and SEMS migration (RR: 0.29; 95%CI: 0.17-0.48; P < 0.001)[32]. Meanwhile, there was limited evidence regarding the optimal SEMS diameter[21]. Previous studies showed no association between SEMS diameter and success or perforation rate[33,34]. However, a few studies suggested an association between SEMS diameter < 24 mm and adverse events, especially migration[35-37]. Regarding SEMS length, it is recommended that the SEMS should be long enough to extend at least 1.5-2 cm on each side of the lesion, and the degree of SEMS shortening after deployment must be considered[21].
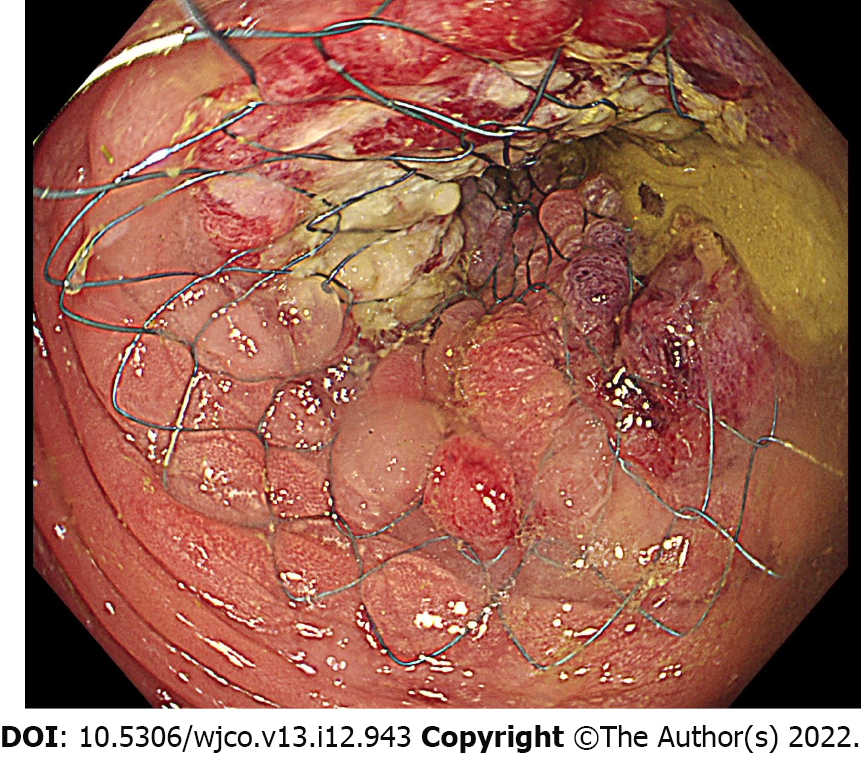
Colonic stenting can be performed endoscopically (through-the-scope technique) or fluoroscopically (over-the-wire technique). Several studies showed comparable technical success rate between endoscopic and fluoroscopic methods, but a combined endoscopic-fluoroscopic method showed the highest success rate[38-41]. Therefore, the European Society of Gastrointestinal Endoscopy (ESGE) guideline recommends that colonic stenting should be performed with the combined use of endoscopy and fluoroscopy[21]. For the combined technique, a soft-tipped hydrophilic guidewire is passed through the strictured lumen. Contrast injection helps to delineate the stenosis and to confirm guidewire placement under fluoroscopy. The SEMS is then passed over the guidewire and deployed under endoscopic visualization and fluoroscopic guidance[42] (Figure 1). Stricture dilation should not be performed either before or after colonic stenting as it increases the risk of perforation[21,43]. The recommended interval to curative resection after BTS stenting is approximately 2 wk[21].
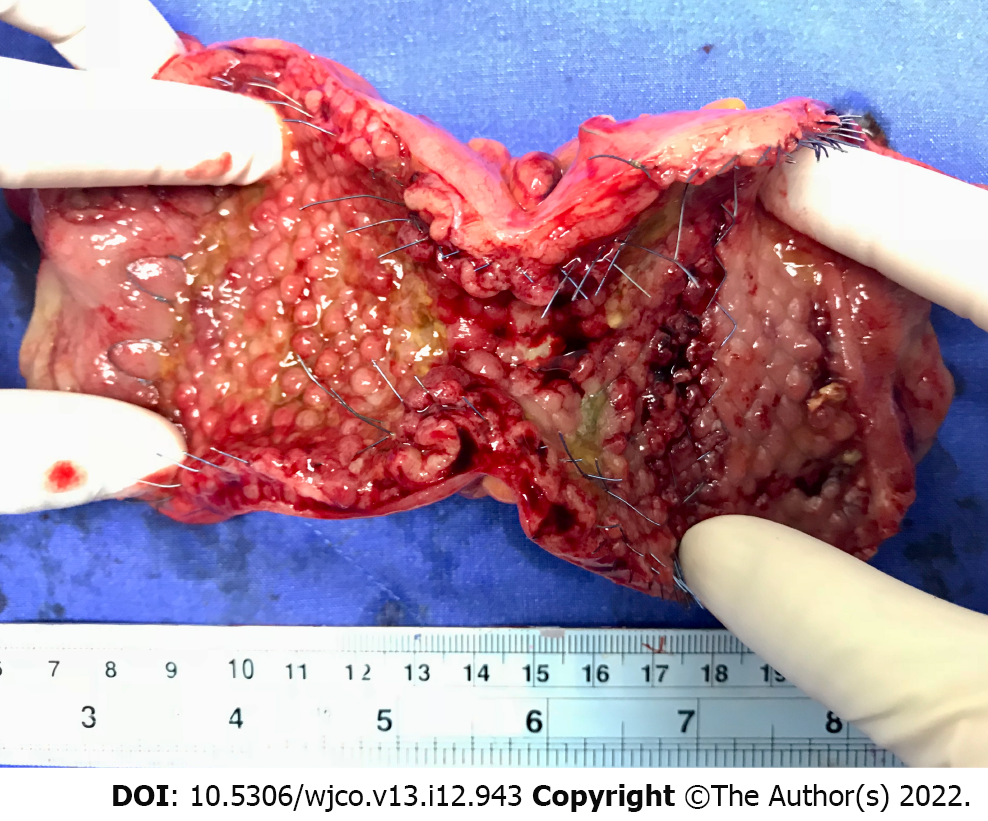
Owing to the high morbidity (45%-50%) and mortality (15%-20%) of ES for obstructed CRC, the BTS concept of avoiding an emergent situation is appealing[44]. After relieving the obstruction by SEMS placement, the clinicians can have time to stabilize the patients, improve their nutrition, correct electrolyte imbalance, and mechanically prepare the colon before definite resection. In addition, it is crucial that surgeons gain the ability to perform laparoscopic resection after BTS stenting (Figure 2). Compared with open resection, laparoscopic resection is associated with lower postoperative pain, earlier recovery of bowel function, and shorter hospital stay[45].
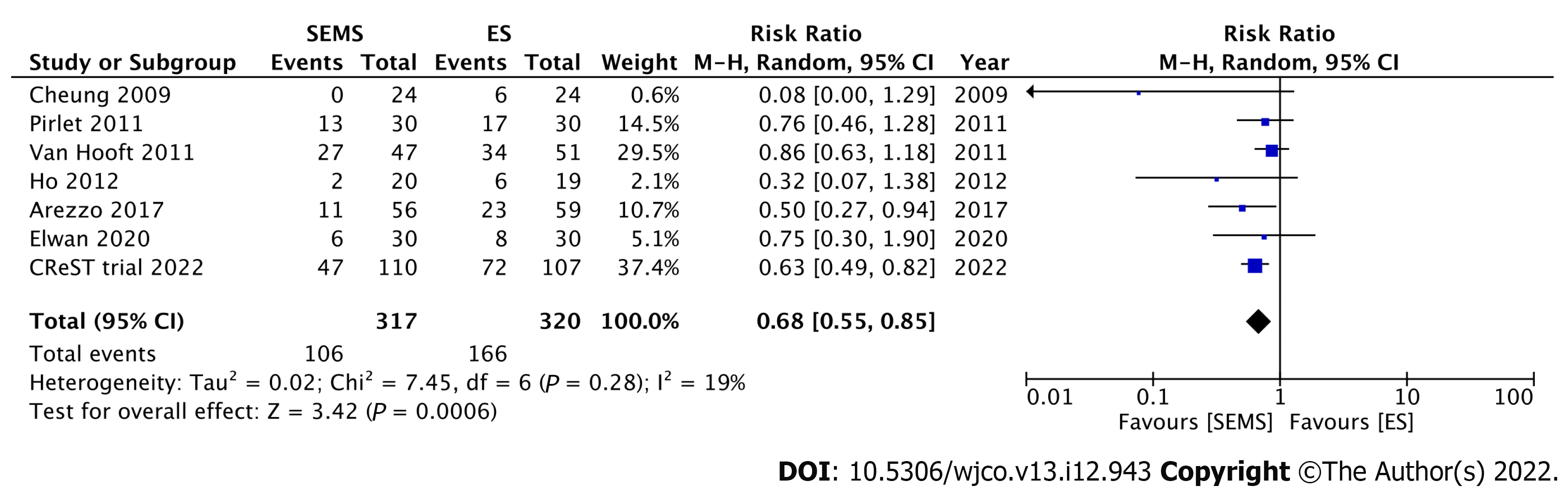
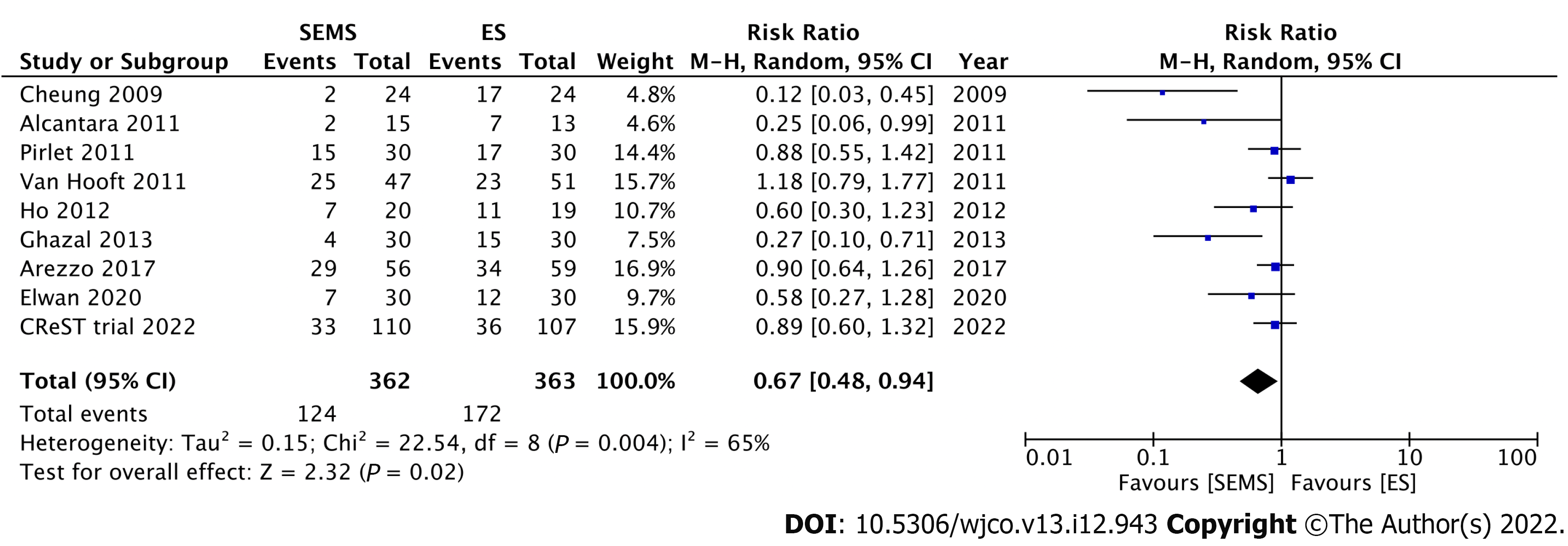
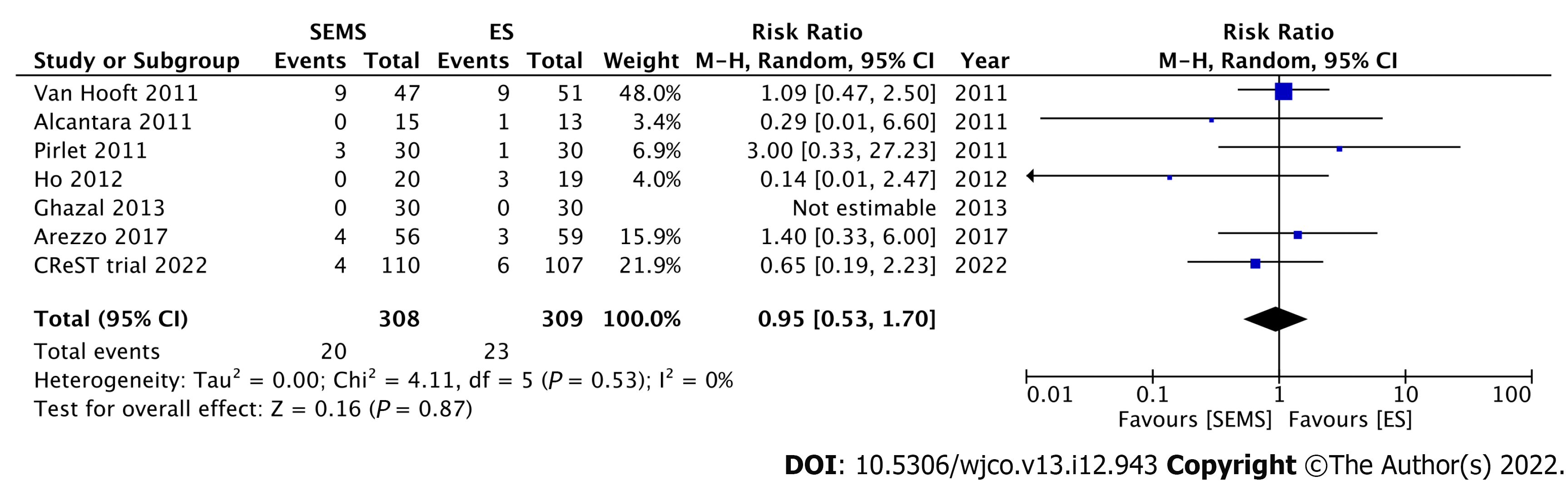
Nine RCTs have investigated the short-term outcomes of SEMS placement as BTS in comparison with those of ES for LMCO (Table 1). Notably, perforation after SEMS insertion and the success rate influenced postoperative outcomes. Four RCTs without perforation showed that SEMS insertion as BTS had a lower morbidity rate than ES[46-49]. Meanwhile, 1 RCT with a low stent success rate (70%) and 3 RCTs with 6.6%-12% perforation rate showed no difference in morbidity between SEMS insertion as BTS and ES[15,50-52]. Additionally, RCTs with low perforation rate also showed a significantly lower rate of postoperative stoma[15,46,53]. Stoma is well recognized to adversely affect quality of life. We conducted meta-analyses that included these nine RCTs. Of these, seven, nine, and seven studies reported the stoma rates, postoperative morbidity, and mortality rates, respectively. In the SEMS group, the stoma rate (RR: 0.68; 95%CI: 0.55-0.85, I2 = 19%) and postoperative morbidity (RR: 0.67; 95%CI: 0.48-0.94, I2 = 65%) were significantly lower than those in the ES group (Figures 3 and 4). There were no differences in mortality rates between the SEMS and ES groups (RR: 0.95; 95%CI: 0.53-1.70, I2 = 0%) (Figure 5).
| Ref. | Year | n | Perforation rate (%) | Stoma rate (%) | Morbidity (%) | Mortality (%) |
| Cheung et al[46] | 2009 | 48 | 0 | SEMS, 0; ES, 25, (P = 0.03)a | SEMS, 8; ES, 70; (P = N/A) | N/A |
| Van Hooft et al[51] | 2011 | 98 | 12 | SEMS, 57; ES, 66; (P = 0.35) | SEMS, 53; ES, 45; (P = 0.43) | SEMS, 19; ES, 17; (P = 0.84) |
| Pirlet et al[52] | 2011 | 60 | 6.6 | SEMS, 43; ES, 56; (P = 0.3) | SEMS, 50; ES, 56; (P = 1) | SEMS, 10; ES, 3; (P = N/A) |
| Alcántara et al[47] | 2011 | 28 | 0 | N/A | SEMS, 13; ES, 54; (P = 0.042)a | SEMS, 0; ES, 8; (P = 0.46) |
| Ho et al[50] | 2012 | 39 | 0 | SEMS, 10; ES, 31; (P = 0.12) | SEMS, 35; ES, 58; (P = 0.15) | SEMS, 0; ES, 16; (P = 0.1) |
| Ghazal et al[48] | 2013 | 60 | 0 | N/A | SEMS, 13; ES, 50; (P = 0.012)a | SEMS, 0; ES, 0 |
| Arezzo et al[15] | 2017 | 115 | 8.9 | SEMS, 22; ES, 39; (P = 0.031)a | SEMS, 52; ES, 58; (P = 0.529) | SEMS, 7; ES, 5; (P = 0.943) |
| Elwan et al[49] | 2020 | 601 | 0 | SEMS, 20; ES, 27; (P = N/A) | SEMS, 23; ES, 40; (P = 0.029)a | N/A |
| CReST trial[53] | 2022 | 2172 | 3.33 | SEMS, 43; ES, 67; (P < 0.001)a | SEMS, 34; ES, 35; (P = 0.930) | SEMS, 4; ES, 6; (P = 0.480) |
In 2021, Cirocchi et al[54] conducted a systematic review and meta-analysis of RCTs and found that compared with ES, SEMS placement as BTS had a higher rate of successful primary anastomosis (RR: 1.26; 95%CI: 1.01-1.57), lower stoma rate (RR: 0.62; 95%CI: 0.45-0.85), and lower postoperative complication (RR: 0.61; 95%CI: 0.45-0.85). The mortality rate was comparable between the two modalities. To conclude, SEMS placement as BTS clearly has short-term benefits of higher primary anastomosis, lower stoma rate, and lower morbidity than ES. In this context, low perforation and high stenting success rates are needed to benefit from SEMS.
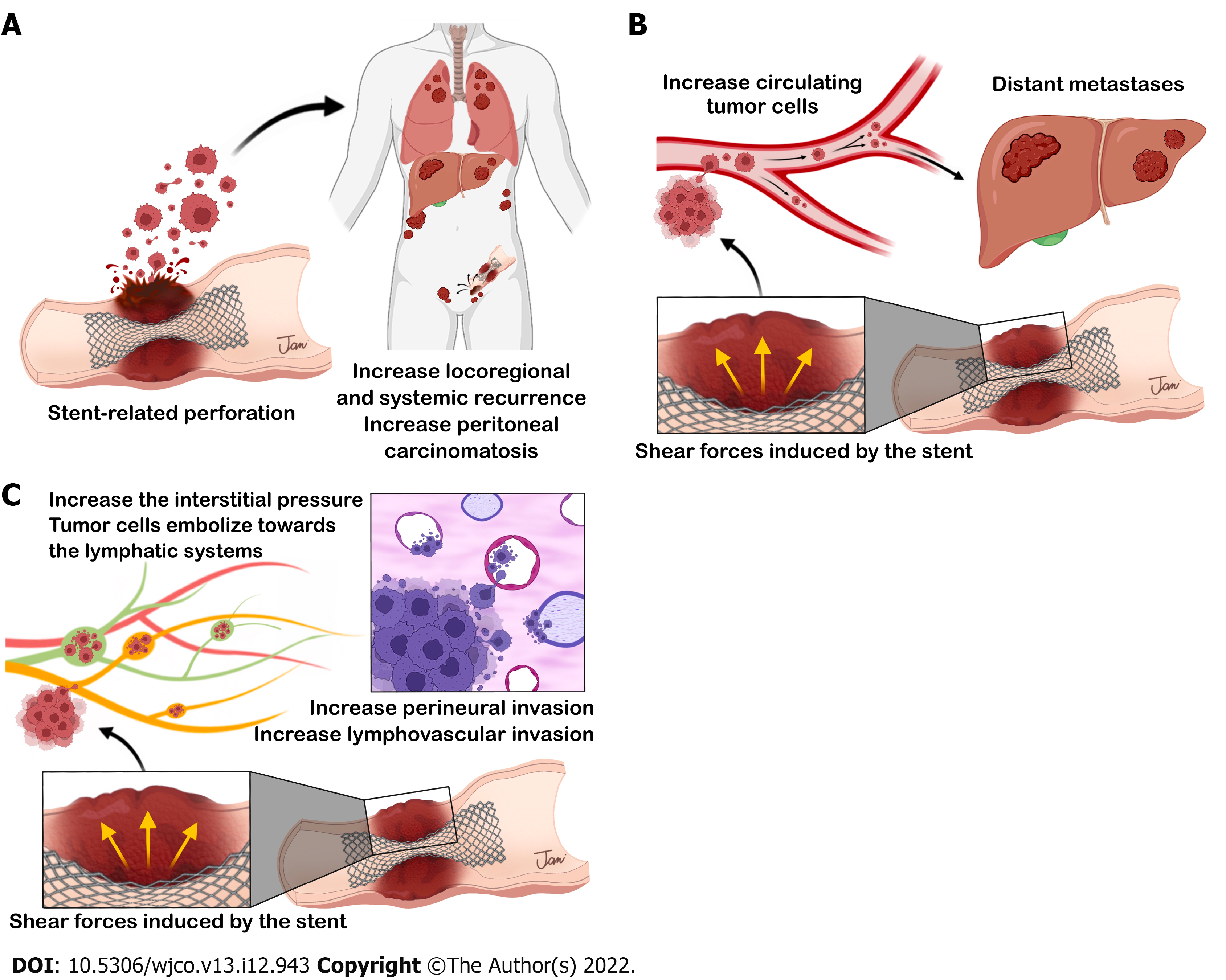
Despite the impressive short-term results of SEMS placement as BTS, its application has been debated due to concerns about adverse long-term oncologic outcomes. Theoretically, shear forces created by SEMS might lead to cancer cell dissemination through the following three possible mechanisms (Figure 6): (1) SEMS-related perforation; (2) increased circulating tumor cells; and (3) aggressive pathological features after SEMS placement.
In obstructed CRC, manipulation of the ulcerated and necrotic tissue through SEMS may cause tumor perforation, which is the most feared complication of SEMS insertion. Perforation causes tumor dissemination into the peritoneal cavity and increases locoregional recurrence and peritoneal carcinomatosis, which affects long-term outcomes[55]. Perforation can be classified into clinical and silent perforation. Interestingly, some studies showed that silent perforation in SEMS as BTS can occur in up to 6%-27% of patients[43,51,52,56]. Further, this rate may still be underestimated because silent perforation can be diagnosed only from pathological assessment of the surgical specimen. Given that there have been sparse reports of silent perforation in the literature, its impact on oncologic outcomes is difficult to verify; however, it should not be disregarded.
In the Dutch Stent-In 2 trial, Sloothaak et al[57] found that 83% of patients with SEMS-related perforation have recurrence. Moreover, Gorissen et al[11] suggested that local recurrence was higher in patients who underwent SEMS placement as BTS than in those who underwent ES (32% vs 8%, P = 0.04). In this study, all patients with perforation had recurrence. Sabbagh et al[58] found that SEMS-related perforation was an independent risk factor for poor overall survival. Sensitivity analyses revealed that 3-year overall survival was better in studies with < 8% SEMS-related perforation rate than in those with ≥ 8%[59]. Balciscueta et al[55] recently conducted a systematic review and meta-analysis of 13 studies (1 RCT, 4 prospective studies, 8 retrospective studies) with long-term oncologic outcomes. The overall rate of SEMS-related perforation was 8.9%. The locoregional recurrence rate was higher in patients with perforation than in those without perforation (26.6% vs 12.5%; OR: 2.41; 95%CI: 1.33-4.34; P = 0.04), while the systemic recurrence rate was comparable.
In summary, SEMS-related perforation influences the occurrence of adverse oncologic outcomes; therefore, an endoscopist’s experience and expertise are crucial with respect to deciding between SEMS placement as BTS or ES. The ESGE guideline recommends a shared decision-making discussion with the patient that should include the availability of stenting expertise and the risk of perforation in the endoscopy unit[21].
SEMS placement could impair oncologic outcomes despite the absence of perforation. SEMS exerts shear forces on the tumor and makes tumor cells disseminate throughout the body[60]. Maruthachalam et al[9] found a more significant rise in cytokeratin 20 messenger RNA expression in peripheral circulation after SEMS placement than after conventional colonoscopy. The presence of messenger RNA coding for epithelial markers indicates the presence of tumor cells or shed debris in the circulation. Furthermore, Yamashita et al[10] found that SEMS placement induces tumor cell dissemination into the peripheral circulation. Using circulating cell-free DNA (cfDNA) and circulating tumor DNA (ctDNA) as indicators, Takahashi et al[61] recently found that SEMS insertion may cause massive cellular and tumor damage. The patients who underwent SEMS placement had higher postoperative plasma levels of both cfDNA and ctDNA than did those who underwent transanal tube decompression. On the contrary, Ishibashi et al[62] found that the increase of circulating tumor cells after SEMS insertion may be temporary, as in most cases, the number of circulating tumor cells decreased 4 d after SEMS placement. Although evidence of tumor cell dissemination after SEMS placement exists, there is inadequate clinical evidence of its negative effects on survival and prognosis.
SEMS insertion leads to a sudden increase in interstitial pressure inside the tumor mass, possibly causing detachment of cells and tumor embolization towards the lymphatic systems and resulting in lymphatic invasion[63]. Hayashi et al[64] noted that the tumor pressure is important, not only for the number of tumor cells shed, but also for the size of emboli shedding into lymphatics around the tumor. Several studies revealed that SEMS insertion might promote perineural invasion found in surgical specimens, although these studies failed to translate higher perineural invasion into poorer oncologic outcomes[18,65-67]. Meanwhile, various studies found that SEMS had no significant effect on the incidence of perineural invasion compared with ES[8,68-70]. Conflicting findings with respect to other adverse pathological features such as lymphovascular and vascular invasion have also been reported[13,18,19,65,67,70-72]; therefore, cumulative data are needed. Balciscueta et al[73] recently conducted a meta-analysis of 1273 patients from 10 retrospective cohort studies and found higher perineural invasion (OR: 1.98; 95%CI: 1.22-3.21; P = 0.006) and lymphatic invasion (OR: 1.45; 95%CI: 1.10-1.90; P = 0.008) after SEMS insertion than after ES. Therefore, the use of SEMS as BTS should be carefully considered due to an increase in adverse pathological characteristics, although the long-term adverse oncological effects have not been demonstrated.
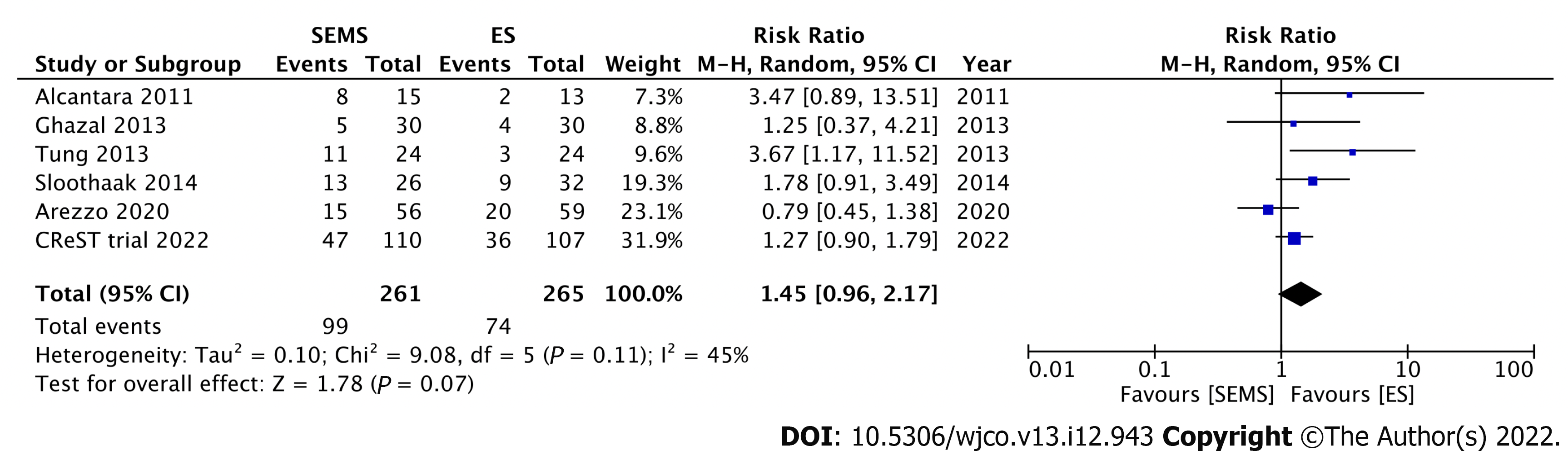
Six RCTs have reported long-term oncologic outcomes after SEMS placement as BTS compared with those of ES (Table 2). For the studies without SEMS-related perforation, the recurrence and survival outcomes are not significantly different between the two modalities[47,48,74]. In contrast, the Dutch Stent-In 2 trial, which had a high perforation rate of 23%, reported poorer disease-free survival in patients who underwent SEMS insertion as BTS than in those underwent ES[57]. This study underlined the strong association between SEM-related perforation and adverse oncologic outcomes. The long-term follow-up outcomes of the ESCO and CReST trials have been recently published. The ESCO trial reported comparable oncologic outcomes between the two modalities, with an 8.9% rate of SEMS-related perforation rate[75]. Similarly, the CReST trial found a comparable 3-year recurrence rate and overall survival between SEMS as BTS and ES, with a low SEMS-related perforation rate of 3.3%[53]. The latest systematic review and meta-analysis of five RCTs by Cirocchi et al[54] revealed comparable recurrence rates and oncologic outcomes between SEMS placement as BTS and ES. We conducted a meta-analysis of these six RCTs that reported the recurrence rate. There was no significant difference in recurrence rates between the SEMS and ES groups (RR: 1.45; 95%CI: 0.96-2.17, I2 = 45%) (Figure 7).
| Ref. | Year | n | Perforation rate (%) | Median F/U time (mo) | Recurrence (%) | Overall survival (OS, %) | Disease-free survival (DFS, %) |
| Alcántara et al[47] | 2011 | 28 | 0 | 38 | SEMS, 53; ES, 15; (P = 0.055) | 5-yr OS: SEMS, 60; ES, 68; (P = 0.843) | Disease-free period (mo): SEMS, 25; ES, 27; (P = 0.096) |
| Tung et al[74] | 2013 | 48 | 0 | 32 | SEMS, 46; ES, 13; (P = 0.400) | 5-yr OS: SEMS, 48; ES, 27; (P = 0.076) | 5-yr DFS: SEMS, 52; ES, 48; (P = 0.630) |
| Ghazal et al[48] | 2013 | 60 | 0 | 18 | SEMS, 17; ES, 13; (P = 0.228) | N/A | N/A |
| Sloothaak et al[57] | 2014 | 58 | 23 | 43 | SEMS, 50; ES, 28; (P = N/A) | 4-yr OS: SEMS, 58; ES, 67; (P = 0.478) | 4-yr DFS: SEMS, 30; ES, 49; (P=0.007)a |
| Arezzo et al[75] | 2020 | 115 | 8.9 | 37 | SEMS, 28; ES, 36; (P = N/A) | 3-yr OS: SEMS, 63; ES, 68; (P = 0.822) | 3-yr DFS: SEMS, 50; ES, 56; (P = 0.972) |
| CReST trial[53] | 2022 | 2171 | 3.32 | N/A | 3-yr recurrence: SEMS, 43; ES, 34; (P = 0.340) | 3-yr OS: SEMS, 46; ES, 37; (P = 0.560) | N/A |
These clinical studies show that among the three proposed mechanisms of cancer cell dissemination by SEMS, only SEMS-related perforation clearly influences adverse oncologic outcomes. Sensitivity analyses showed that a < 8% perforation rate is the oncologically safe cut-off point for SEMS insertion[59]. Therefore, endoscopy units that aim to perform SEMS as BTS should audit and improve their SEMS-related perforation rate to be lower than 8%. Two other mechanisms, including increased circulating tumor cells and aggressive pathological features, failed to produce consistent clinical evidence in decreased overall and disease-free survival in the SEMS group. Therefore, further studies are needed to validate the clinical impact of these hypotheses.
The mainstay treatment of the potentially curable colon cancer is complete oncologic resection. However, one of the challenges is the risk of local and distant recurrence, which is estimated at 20%-30% in locally advanced colon cancer (defined as: T3 tumors with ≥ 5 mm invasion beyond the muscularis propria; T4; or extensive regional lymph node involvement without distant metastases)[76]. Recently, there was increasing evidence to support the use of neoadjuvant chemotherapy in locally advanced colon cancer[77-81]. The theoretical advantages include the early treatment of micrometastases, increased likelihood of clear resection (R0) margin, and ability to evaluate the chemosensitivity of the tumor[76]. Gosavi et al[76] conducted a meta-analysis of two RCTs and reported that neoadjuvant chemotherapy increased the likelihood of R0 resection in locally advanced colon cancer (RR 0.47; 95%CI: 0.47-0.96) without an increase in complications (anastomotic leak, wound infection, or re-operation). However, the safety of neoadjuvant chemotherapy after SEMS placement in obstructive colon cancer is also a concern.
There were a few studies using neoadjuvant chemotherapy after SEMS placement in obstructive colon cancer. The FOxTROT trial showed a significant decrease in R1 resection rate in patients who received neoadjuvant chemotherapy for locally advanced colon cancer. However, only a few patients in this trial underwent SEMS placement as BTS; therefore, a conclusion about SEMS safety could not be drawn[79]. Recently, Han et al[82] conducted a comparative study investigating the safety of neoadjuvant chemotherapy after SEMS placement. They found that the adverse events of preoperative chemotherapy were well-tolerated, and the neoadjuvant chemotherapy did not increase SEMS-related complications (P = 0.13). Moreover, this study revealed that patients who received neoadjuvant chemotherapy had better overall survival than those who received postoperative chemotherapy (mean overall survival, 53 vs 47 mo, respectively, P = 0.02). However, well-designed RCTs with larger sample size and long-term follow-up are needed to confirm the safety and potential survival benefit of neoadjuvant chemotherapy after SEMS placement.
Patients with incurable stage IV colon cancer benefit from SEMS placement by avoiding palliative surgery and early initiation of chemotherapy. However, there is a concern that chemotherapy during SEM placement might induce complications. For palliative SEMS placement, many studies (including patients with and without chemotherapy) reported perforation rates of 7%-13%[83-88]. Therefore, the decision to perform SEM insertion in patients with incurable stage IV colon cancer must consider the risks of long-term SEMS-related complications weighted against SEMS benefits[89].
The administration of antiangiogenic agents (e.g., bevacizumab) in patients who underwent SEMS placement was found to increase the risk of SEMS-related perforation. A retrospective study reported 3-fold higher perforation rate in patients who received bevacizumab after SEMS placement than in those who did not receive bevacizumab[90]. In a large retrospective study of 1008 patients who received bevacizumab for incurable colon cancer, Bong et al[91] found that SEMS placement is a significant risk factor for complications requiring surgery in patients who received bevacizumab (HR 5.69, 95%CI 2.37-13.64, P < 0.001). In contrast, a retrospective study reported no significant difference in perforation rate in patients who received chemotherapy with and without bevacizumab (7.3% vs 7.0%, respectively, P = 0.925)[92]. The updated 2020 ESGE guideline recommends chemotherapy as a safe treatment in patients who have undergone palliative SEMS insertion. However, SEMS placement should not be performed while patients are receiving antiangiogenic therapy[21].
Although many RCTs and prospective and retrospective studies have investigated the role of SEMS placement as BTS in comparison with that of ES in LMCO, current evidence is still conflicting, and the international guidelines are also dynamic and discordant[20,21]. In 2014, the ESGE guideline did not recommend using SEMS insertion as BTS based on previous studies with low success and high perforation rates[20]. Nevertheless, many comparative studies and one RCT published thereafter[13-19,93] reported impressive short- and long-term oncologic outcomes. As such, the updated ESGE guideline released in 2020 considers SEMS placement as BTS a valid treatment option in patients with LMCO. The guideline emphasized that the medical team has to discuss the risks and benefits of SEMS with patients and SEMS insertion should be performed or directly supervised by a competent endoscopist[21].
The proficiency of the endoscopist is crucial when SEMS as BTS is considered. Previous RCTs with low perforation rate showed appreciable short-term outcomes of SEMS, including lower stoma rate, higher primary anastomosis, lower morbidity, and comparable oncologic outcomes to ES[15,46-49,74,75]. Moreover, current evidence clearly demonstrates the association between SEMS-related perforation and negative oncologic outcomes. Therefore, SEMS placement as BTS is a valid option for competent endoscopists.
Impaired oncological outcomes after SEMS placement that result from increased circulating tumor and adverse pathological characteristics remain a concern. However, current evidence could not demonstrate adverse long-term oncological effects. Further well-designed collaborative studies are needed to investigate the association among the alteration of circulating tumors, adverse pathological characteristics, and oncologic outcomes.
Colonic obstruction is a common presentation of CRC that needs emergency intervention. SEMS placement as BTS, converting an emergency situation to an elective one, improves short-term outcomes in LMCO, including higher primary anastomosis, lower stoma rate, and lower postoperative morbidity, compared with ES. However, there remain concerns on adverse oncologic outcomes from shear forces induced by SEMS. There are three possible mechanisms of tumor dissemination from SEMS: (1) SEMS-related perforation; (2) increased circulating tumor cells; and (3) aggressive pathological features after SEMS placement. However, among these, only SEM-related perforation clearly influences adverse oncologic outcomes. Consistent clinical evidence supporting the association of the other two mechanisms with decreased overall and disease-free survival is lacking. Therefore, further well-designed collaborative studies are needed to validate the clinical impact of these mechanisms. Current guidelines consider SEMS placement as BTS a valid treatment option in patients with LMCO, but it should be performed by competent endoscopists.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Society for Gastrointestinal Endoscopy, 162994.
Specialty type: Oncology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen D, China; Sterpetti AV, Italy S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 2. | Cao Y, Gu J, Deng S, Li J, Wu K, Cai K. Long-term tumour outcomes of self-expanding metal stents as 'bridge to surgery' for the treatment of colorectal cancer with malignant obstruction: a systematic review and meta-analysis. Int J Colorectal Dis. 2019;34:1827-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Fielding LP, Phillips RK, Hittinger R. Factors influencing mortality after curative resection for large bowel cancer in elderly patients. Lancet. 1989;1:595-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 101] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Mulcahy HE, Skelly MM, Husain A, O'Donoghue DP. Long-term outcome following curative surgery for malignant large bowel obstruction. Br J Surg. 1996;83:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Pisano M, Zorcolo L, Merli C, Cimbanassi S, Poiasina E, Ceresoli M, Agresta F, Allievi N, Bellanova G, Coccolini F, Coy C, Fugazzola P, Martinez CA, Montori G, Paolillo C, Penachim TJ, Pereira B, Reis T, Restivo A, Rezende-Neto J, Sartelli M, Valentino M, Abu-Zidan FM, Ashkenazi I, Bala M, Chiara O, De' Angelis N, Deidda S, De Simone B, Di Saverio S, Finotti E, Kenji I, Moore E, Wexner S, Biffl W, Coimbra R, Guttadauro A, Leppäniemi A, Maier R, Magnone S, Mefire AC, Peitzmann A, Sakakushev B, Sugrue M, Viale P, Weber D, Kashuk J, Fraga GP, Kluger I, Catena F, Ansaloni L. 2017 WSES guidelines on colon and rectal cancer emergencies: obstruction and perforation. World J Emerg Surg. 2018;13:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 192] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 6. | Ellis CT, Maykel JA. Defining Anastomotic Leak and the Clinical Relevance of Leaks. Clin Colon Rectal Surg. 2021;34:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 7. | Gainant A. Emergency management of acute colonic cancer obstruction. J Visc Surg. 2012;149:e3-e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Khomvilai S, Pattarajierapan S. Comparison of Long-term Outcomes of Colonic Stenting as a "Bridge to Surgery" and Emergency Surgery in Patients With Left-Sided Malignant Colonic Obstruction. Ann Coloproctol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 9. | Maruthachalam K, Lash GE, Shenton BK, Horgan AF. Tumour cell dissemination following endoscopic stent insertion. Br J Surg. 2007;94:1151-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 10. | Yamashita S, Tanemura M, Sawada G, Moon J, Shimizu Y, Yamaguchi T, Kuwai T, Urata Y, Kuraoka K, Hatanaka N, Yamashita Y, Taniyama K. Impact of endoscopic stent insertion on detection of viable circulating tumor cells from obstructive colorectal cancer. Oncol Lett. 2018;15:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Gorissen KJ, Tuynman JB, Fryer E, Wang L, Uberoi R, Jones OM, Cunningham C, Lindsey I. Local recurrence after stenting for obstructing left-sided colonic cancer. Br J Surg. 2013;100:1805-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 12. | Katsuki R, Jo T, Yasunaga H, Ishimaru M, Sakamoto T. Outcomes of self-expandable metal stent as bridge to surgery vs emergency surgery for left-sided obstructing colon cancer: A retrospective cohort study. Am J Surg. 2021;221:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Yang SY, Park YY, Han YD, Cho MS, Hur H, Min BS, Lee KY, Kim NK. Oncologic Outcomes of Self-Expandable Metallic Stent as a Bridge to Surgery and Safety and Feasibility of Minimally Invasive Surgery for Acute Malignant Colonic Obstruction. Ann Surg Oncol. 2019;26:2787-2796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Saito S, Yoshida S, Isayama H, Matsuzawa T, Kuwai T, Maetani I, Shimada M, Yamada T, Tomita M, Koizumi K, Hirata N, Kanazawa H, Enomoto T, Sekido H, Saida Y. A prospective multicenter study on self-expandable metallic stents as a bridge to surgery for malignant colorectal obstruction in Japan: efficacy and safety in 312 patients. Surg Endosc. 2016;30:3976-3986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Arezzo A, Balague C, Targarona E, Borghi F, Giraudo G, Ghezzo L, Arroyo A, Sola-Vera J, De Paolis P, Bossotti M, Bannone E, Forcignanò E, Bonino MA, Passera R, Morino M. Colonic stenting as a bridge to surgery vs emergency surgery for malignant colonic obstruction: results of a multicentre randomised controlled trial (ESCO trial). Surg Endosc. 2017;31:3297-3305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 16. | Amelung FJ, Borstlap WAA, Consten ECJ, Veld JV, van Halsema EE, Bemelman WA, Siersema PD, Ter Borg F, van Hooft JE, Tanis PJ; Dutch Snapshot Research Group. Propensity score-matched analysis of oncological outcome between stent as bridge to surgery and emergency resection in patients with malignant left-sided colonic obstruction. Br J Surg. 2019;106:1075-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Lara-Romero C, Vilches Á, Caunedo-Álvarez Á, Hergueta-Delgado P, Lavín-Castejón I, Andrade-Bellido R, Alcaín-Martínez G. Better recurrence-free survival after stent bridge to surgery compared to emergency surgery for obstructive left-sided colonic cancer in patients with stage III status of the American Joint Committee on Cancer (AJCC): a bicentric retrospective study. Int J Colorectal Dis. 2019;34:1241-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Kang SI, Oh HK, Yoo JS, Ahn S, Kim MH, Kim MJ, Son IT, Kim DW, Kang SB, Park YS, Yoon CJ, Shin R, Heo SC, Lee IT, Youk EG, Chang TY, Park SC, Sohn DK, Oh JH, Park JW, Ryoo SB, Jeong SY, Park KJ; Seoul Colorectal Group (SECOG). Oncologic outcomes of preoperative stent insertion first vs immediate surgery for obstructing left-sided colorectal cancer. Surg Oncol. 2018;27:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Park J, Lee HJ, Park SJ, Hur H, Min BS, Cheon JH, Kim TI, Kim NK, Kim WH. Long-term outcomes after stenting as a bridge to surgery in patients with obstructing left-sided colorectal cancer. Int J Colorectal Dis. 2018;33:799-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | van Hooft JE, van Halsema EE, Vanbiervliet G, Beets-Tan RG, DeWitt JM, Donnellan F, Dumonceau JM, Glynne-Jones RG, Hassan C, Jiménez-Perez J, Meisner S, Muthusamy VR, Parker MC, Regimbeau JM, Sabbagh C, Sagar J, Tanis PJ, Vandervoort J, Webster GJ, Manes G, Barthet MA, Repici A; European Society of Gastrointestinal Endoscopy (ESGE). Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Gastrointest Endosc. 2014;80:747-61.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 21. | van Hooft JE, Veld JV, Arnold D, Beets-Tan RGH, Everett S, Götz M, van Halsema EE, Hill J, Manes G, Meisner S, Rodrigues-Pinto E, Sabbagh C, Vandervoort J, Tanis PJ, Vanbiervliet G, Arezzo A. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2020. Endoscopy. 2020;52:389-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 198] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 22. | Vogel JD, Eskicioglu C, Weiser MR, Feingold DL, Steele SR. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Treatment of Colon Cancer. Dis Colon Rectum. 2017;60:999-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 23. | Ferrada P, Patel MB, Poylin V, Bruns BR, Leichtle SW, Wydo S, Sultan S, Haut ER, Robinson B. Surgery or stenting for colonic obstruction: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2016;80:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | van de Velde CJ, Boelens PG, Borras JM, Coebergh JW, Cervantes A, Blomqvist L, Beets-Tan RG, van den Broek CB, Brown G, Van Cutsem E, Espin E, Haustermans K, Glimelius B, Iversen LH, van Krieken JH, Marijnen CA, Henning G, Gore-Booth J, Meldolesi E, Mroczkowski P, Nagtegaal I, Naredi P, Ortiz H, Påhlman L, Quirke P, Rödel C, Roth A, Rutten H, Schmoll HJ, Smith JJ, Tanis PJ, Taylor C, Wibe A, Wiggers T, Gambacorta MA, Aristei C, Valentini V. EURECCA colorectal: multidisciplinary management: European consensus conference colon & rectum. Eur J Cancer. 2014;50:1.e1-1.e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 307] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 25. | Trompetas V. Emergency management of malignant acute left-sided colonic obstruction. Ann R Coll Surg Engl. 2008;90:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Zarnescu Vasiliu EC, Zarnescu NO, Costea R, Rahau L, Neagu S. Morbidity after reversal of Hartmann operation: retrospective analysis of 56 patients. J Med Life. 2015;8:488-491. [PubMed] |
| 27. | Hallam S, Mothe BS, Tirumulaju R. Hartmann's procedure, reversal and rate of stoma-free survival. Ann R Coll Surg Engl. 2018;100:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 28. | Dohmoto M, Hünerbein M, Schlag PM. Application of rectal stents for palliation of obstructing rectosigmoid cancer. Surg Endosc. 1997;11:758-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Spinelli P, Dal Fante M, Mancini A. Self-expanding mesh stent for endoscopic palliation of rectal obstructing tumors: a preliminary report. Surg Endosc. 1992;6:72-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Tejero E, Mainar A, Fernández L, Tobío R, De Gregorio MA. New procedure for the treatment of colorectal neoplastic obstructions. Dis Colon Rectum. 1994;37:1158-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 175] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Seo SY, Kim SW. Endoscopic Management of Malignant Colonic Obstruction. Clin Endosc. 2020;53:9-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Mashar M, Mashar R, Hajibandeh S. Uncovered vs covered stent in management of large bowel obstruction due to colorectal malignancy: a systematic review and meta-analysis. Int J Colorectal Dis. 2019;34:773-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Lee YJ, Yoon JY, Park JJ, Park SJ, Kim JH, Youn YH, Kim TI, Park H, Kim WH, Cheon JH. Clinical outcomes and factors related to colonic perforations in patients receiving self-expandable metal stent insertion for malignant colorectal obstruction. Gastrointest Endosc. 2018;87:1548-1557.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 34. | Tomita M, Saito S, Makimoto S, Yoshida S, Isayama H, Yamada T, Matsuzawa T, Enomoto T, Kyo R, Kuwai T, Hirata N, Shimada M, Hirakawa T, Koizumi K, Saida Y. Self-expandable metallic stenting as a bridge to surgery for malignant colorectal obstruction: pooled analysis of 426 patients from two prospective multicenter series. Surg Endosc. 2019;33:499-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Small AJ, Coelho-Prabhu N, Baron TH. Endoscopic placement of self-expandable metal stents for malignant colonic obstruction: long-term outcomes and complication factors. Gastrointest Endosc. 2010;71:560-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 36. | Kim BC, Han KS, Hong CW, Sohn DK, Park JW, Park SC, Kim SY, Baek JY, Choi HS, Chang HJ, Kim DY, Oh JH. Clinical outcomes of palliative self-expanding metallic stents in patients with malignant colorectal obstruction. J Dig Dis. 2012;13:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Manes G, de Bellis M, Fuccio L, Repici A, Masci E, Ardizzone S, Mangiavillano B, Carlino A, Rossi GB, Occhipinti P, Cennamo V. Endoscopic palliation in patients with incurable malignant colorectal obstruction by means of self-expanding metal stent: analysis of results and predictors of outcomes in a large multicenter series. Arch Surg. 2011;146:1157-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 38. | Geraghty J, Sarkar S, Cox T, Lal S, Willert R, Ramesh J, Bodger K, Carlson GL. Management of large bowel obstruction with self-expanding metal stents. A multicentre retrospective study of factors determining outcome. Colorectal Dis. 2014;16:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Kim JW, Jeong JB, Lee KL, Kim BG, Jung YJ, Kim W, Kim HY, Ahn DW, Koh SJ, Lee JK. Comparison of clinical outcomes between endoscopic and radiologic placement of self-expandable metal stent in patients with malignant colorectal obstruction. Korean J Gastroenterol. 2013;61:22-29. [PubMed] |
| 40. | Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol. 2004;99:2051-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 411] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 41. | de Gregorio MA, Laborda A, Tejero E, Miguelena JM, Carnevale FC, de Blas I, Gimenez M, Maynar M, D'Agostino H. Ten-year retrospective study of treatment of malignant colonic obstructions with self-expandable stents. J Vasc Interv Radiol. 2011;22:870-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Ribeiro IB, de Moura DTH, Thompson CC, de Moura EGH. Acute abdominal obstruction: Colon stent or emergency surgery? World J Gastrointest Endosc. 2019;11:193-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 43. | van Halsema EE, van Hooft JE, Small AJ, Baron TH, García-Cano J, Cheon JH, Lee MS, Kwon SH, Mucci-Hennekinne S, Fockens P, Dijkgraaf MG, Repici A. Perforation in colorectal stenting: a meta-analysis and a search for risk factors. Gastrointest Endosc. 2014;79:970-82.e7; quiz 983.e2, 983.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 44. | Tekkis PP, Kinsman R, Thompson MR, Stamatakis JD; Association of Coloproctology of Great Britain, Ireland. The Association of Coloproctology of Great Britain and Ireland study of large bowel obstruction caused by colorectal cancer. Ann Surg. 2004;240:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 217] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 45. | Pascual M, Salvans S, Pera M. Laparoscopic colorectal surgery: Current status and implementation of the latest technological innovations. World J Gastroenterol. 2016;22:704-717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 46. | Cheung HY, Chung CC, Tsang WW, Wong JC, Yau KK, Li MK. Endolaparoscopic approach vs conventional open surgery in the treatment of obstructing left-sided colon cancer: a randomized controlled trial. Arch Surg. 2009;144:1127-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 47. | Alcántara M, Serra-Aracil X, Falcó J, Mora L, Bombardó J, Navarro S. Prospective, controlled, randomized study of intraoperative colonic lavage vs stent placement in obstructive left-sided colonic cancer. World J Surg. 2011;35:1904-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 48. | Ghazal AH, El-Shazly WG, Bessa SS, El-Riwini MT, Hussein AM. Colonic endolumenal stenting devices and elective surgery vs emergency subtotal/total colectomy in the management of malignant obstructed left colon carcinoma. J Gastrointest Surg. 2013;17:1123-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 49. | Elwan TH, Zaher NA. Endoscopic stenting as a bridge to elective surgery vs emergency laparotomy for patients with acute malignant large bowel obstruction. Egypt J Surg. 2020;39:529-535. [DOI] [Full Text] |
| 50. | Ho KS, Quah HM, Lim JF, Tang CL, Eu KW. Endoscopic stenting and elective surgery vs emergency surgery for left-sided malignant colonic obstruction: a prospective randomized trial. Int J Colorectal Dis. 2012;27:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 51. | van Hooft JE, Bemelman WA, Oldenburg B, Marinelli AW, Lutke Holzik MF, Grubben MJ, Sprangers MA, Dijkgraaf MG, Fockens P; collaborative Dutch Stent-In study group. Colonic stenting vs emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol. 2011;12:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 313] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 52. | Pirlet IA, Slim K, Kwiatkowski F, Michot F, Millat BL. Emergency preoperative stenting vs surgery for acute left-sided malignant colonic obstruction: a multicenter randomized controlled trial. Surg Endosc. 2011;25:1814-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 53. | CReST Collaborative Group. Colorectal Endoscopic Stenting Trial (CReST) for obstructing left-sided colorectal cancer: randomized clinical trial. Br J Surg. 2022;109:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 54. | Cirocchi R, Arezzo A, Sapienza P, Crocetti D, Cavaliere D, Solaini L, Ercolani G, Sterpetti AV, Mingoli A, Fiori E. Current Status of the Self-Expandable Metal Stent as a Bridge to Surgery Versus Emergency Surgery in Colorectal Cancer: Results from an Updated Systematic Review and Meta-Analysis of the Literature. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Balciscueta I, Balciscueta Z, Uribe N, García-Granero E. Long-term outcomes of stent-related perforation in malignant colon obstruction: a systematic review and meta-analysis. Int J Colorectal Dis. 2020;35:1439-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Fryer E, Gorissen KJ, Wang LM, Guy R, Chetty R. Spectrum of histopathological changes encountered in stented colorectal carcinomas. Histopathology. 2015;66:480-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Sloothaak DA, van den Berg MW, Dijkgraaf MG, Fockens P, Tanis PJ, van Hooft JE, Bemelman WA; collaborative Dutch Stent-In study group. Oncological outcome of malignant colonic obstruction in the Dutch Stent-In 2 trial. Br J Surg. 2014;101:1751-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 58. | Sabbagh C, Browet F, Diouf M, Cosse C, Brehant O, Bartoli E, Mauvais F, Chauffert B, Dupas JL, Nguyen-Khac E, Regimbeau JM. Is stenting as "a bridge to surgery" an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction? Ann Surg. 2013;258:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 59. | Amelung FJ, Burghgraef TA, Tanis PJ, van Hooft JE, Ter Borg F, Siersema PD, Bemelman WA, Consten ECJ. Critical appraisal of oncological safety of stent as bridge to surgery in left-sided obstructing colon cancer; a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2018;131:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 60. | Matsuda A, Miyashita M, Matsumoto S, Matsutani T, Sakurazawa N, Takahashi G, Kishi T, Uchida E. Comparison of long-term outcomes of colonic stent as "bridge to surgery" and emergency surgery for malignant large-bowel obstruction: a meta-analysis. Ann Surg Oncol. 2015;22:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 61. | Takahashi G, Yamada T, Iwai T, Takeda K, Koizumi M, Shinji S, Uchida E. Oncological Assessment of Stent Placement for Obstructive Colorectal Cancer from Circulating Cell-Free DNA and Circulating Tumor DNA Dynamics. Ann Surg Oncol. 2018;25:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 62. | Ishibashi R, Yoshida S, Odawara N, Kishikawa T, Kondo R, Nakada A, Hakuta R, Takahara N, Tanaka E, Sekiba K, Seimiya T, Ohnaga T, Otsuka M, Koike K. Detection of circulating colorectal cancer cells by a custom microfluid system before and after endoscopic metallic stent placement. Oncol Lett. 2019;18:6397-6404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 63. | Lemoine L, Sugarbaker P, Van der Speeten K. Pathophysiology of colorectal peritoneal carcinomatosis: Role of the peritoneum. World J Gastroenterol. 2016;22:7692-7707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 64. | Hayashi K, Jiang P, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Moossa AR, Bouvet M, Hoffman RM. Real-time imaging of tumor-cell shedding and trafficking in lymphatic channels. Cancer Res. 2007;67:8223-8228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 65. | Kim MK, Kye BH, Lee IK, Oh ST, Ahn CH, Lee YS, Lee SC, Kang WK. Outcome of bridge to surgery stenting for obstructive left colon cancer. ANZ J Surg. 2017;87:E245-E250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Kim HJ, Choi GS, Park JS, Park SY, Jun SH. Higher rate of perineural invasion in stent-laparoscopic approach in comparison to emergent open resection for obstructing left-sided colon cancer. Int J Colorectal Dis. 2013;28:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 67. | Haraguchi N, Ikeda M, Miyake M, Yamada T, Sakakibara Y, Mita E, Doki Y, Mori M, Sekimoto M. Colonic stenting as a bridge to surgery for obstructive colorectal cancer: advantages and disadvantages. Surg Today. 2016;46:1310-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | Cao Y, Yang M, Yan L, Deng S, Gu J, Mao F, Wu K, Liu L, Cai K. Colon metal stents as a bridge to surgery had no significant effects on the perineural invasion: a retrospective study. World J Surg Oncol. 2020;18:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Tamini N, Angrisani M, Aldè S, Nespoli L, Oldani M, Braga M, Gianotti L. Does preoperative stent positioning in obstructive left sided colon cancer increase the risk of perineural invasion? Updates Surg. 2021;73:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 70. | Kim SJ, Kim HW, Park SB, Kang DH, Choi CW, Song BJ, Hong JB, Kim DJ, Park BS, Son GM. Colonic perforation either during or after stent insertion as a bridge to surgery for malignant colorectal obstruction increases the risk of peritoneal seeding. Surg Endosc. 2015;29:3499-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Chen XQ, Xue CR, Hou P, Lin BQ, Zhang JR. Lymphocyte-to-monocyte ratio effectively predicts survival outcome of patients with obstructive colorectal cancer. World J Gastroenterol. 2019;25:4970-4984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 72. | Veld JV, Amelung FJ, Borstlap WAA, van Halsema EE, Consten ECJ, Siersema PD, Ter Borg F, van der Zaag ES, de Wilt JHW, Fockens P, Bemelman WA, van Hooft JE, Tanis PJ; Dutch Snapshot Research Group. Comparison of Decompressing Stoma vs Stent as a Bridge to Surgery for Left-Sided Obstructive Colon Cancer. JAMA Surg. 2020;155:206-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 73. | Balciscueta I, Balciscueta Z, Uribe N, García-Granero E. Perineural invasion is increased in patients receiving colonic stenting as a bridge to surgery: a systematic review and meta-analysis. Tech Coloproctol. 2021;25:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 74. | Tung KL, Cheung HY, Ng LW, Chung CC, Li MK. Endo-laparoscopic approach vs conventional open surgery in the treatment of obstructing left-sided colon cancer: long-term follow-up of a randomized trial. Asian J Endosc Surg. 2013;6:78-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 75. | Arezzo A, Forcignanò E, Bonino MA, Balagué C, Targarona E, Borghi F, Giraudo G, Ghezzo L, Passera R, Morino M; collaborative ESCO study group. Long-term Oncologic Results After Stenting as a Bridge to Surgery Versus Emergency Surgery for Malignant Left-sided Colonic Obstruction: A Multicenter Randomized Controlled Trial (ESCO Trial). Ann Surg. 2020;272:703-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 76. | Gosavi R, Chia C, Michael M, Heriot AG, Warrier SK, Kong JC. Neoadjuvant chemotherapy in locally advanced colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2021;36:2063-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 77. | Karoui M, Rullier A, Piessen G, Legoux JL, Barbier E, De Chaisemartin C, Lecaille C, Bouche O, Ammarguellat H, Brunetti F, Prudhomme M, Regimbeau JM, Glehen O, Lievre A, Portier G, Hartwig J, Goujon G, Romain B, Lepage C, Taieb J; for PRODIGE 22 investigators/collaborators. Perioperative FOLFOX 4 Versus FOLFOX 4 Plus Cetuximab Versus Immediate Surgery for High-Risk Stage II and III Colon Cancers: A Phase II Multicenter Randomized Controlled Trial (PRODIGE 22). Ann Surg. 2020;271:637-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 78. | Arredondo J, Baixauli J, Pastor C, Chopitea A, Sola JJ, González I, A-Cienfuegos J, Martínez P, Rodriguez J, Hernández-Lizoain JL. Mid-term oncologic outcome of a novel approach for locally advanced colon cancer with neoadjuvant chemotherapy and surgery. Clin Transl Oncol. 2017;19:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 79. | Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol. 2012;13:1152-1160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 347] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 80. | Liu F, Yang L, Wu Y, Li C, Zhao J, Keranmu A, Zheng H, Huang D, Wang L, Tong T, Xu J, Zhu J, Cai S, Xu Y. CapOX as neoadjuvant chemotherapy for locally advanced operable colon cancer patients: a prospective single-arm phase II trial. Chin J Cancer Res. 2016;28:589-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 81. | Jakobsen A, Andersen F, Fischer A, Jensen LH, Jørgensen JC, Larsen O, Lindebjerg J, Pløen J, Rafaelsen SR, Vilandt J. Neoadjuvant chemotherapy in locally advanced colon cancer. A phase II trial. Acta Oncol. 2015;54:1747-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 82. | Han JG, Wang ZJ, Dai Y, Li XR, Qian Q, Wang GY, Zhai ZW, Zeng WG. Short-term Outcomes of Elective Surgery Following Self-Expandable Metallic Stent and Neoadjuvant Chemotherapy in Patients With Left-Sided Colon Cancer Obstruction. Dis Colon Rectum. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 83. | Lee HJ, Hong SP, Cheon JH, Kim TI, Min BS, Kim NK, Kim WH. Long-term outcome of palliative therapy for malignant colorectal obstruction in patients with unresectable metastatic colorectal cancers: endoscopic stenting vs surgery. Gastrointest Endosc. 2011;73:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 84. | Ahn HJ, Kim SW, Lee SW, Lim CH, Kim JS, Cho YK, Park JM, Lee IS, Choi MG. Long-term outcomes of palliation for unresectable colorectal cancer obstruction in patients with good performance status: endoscopic stent vs surgery. Surg Endosc. 2016;30:4765-4775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 85. | Pattarajierapan S, Manomayangoon C, Tipsuwannakul P, Khomvilai S. Comparison of colonic stenting and stoma creation as palliative treatment for incurable malignant colonic obstruction. JGH Open. 2022;6:630-636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 86. | Cézé N, Charachon A, Locher C, Aparicio T, Mitry E, Barbieux JP, Landi B, Dorval E, Moussata D, Lecomte T. Safety and efficacy of palliative systemic chemotherapy combined with colorectal self-expandable metallic stents in advanced colorectal cancer: A multicenter study. Clin Res Hepatol Gastroenterol. 2016;40:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 87. | Karoui M, Charachon A, Delbaldo C, Loriau J, Laurent A, Sobhani I, Tran Van Nhieu J, Delchier JC, Fagniez PL, Piedbois P, Cherqui D. Stents for palliation of obstructive metastatic colon cancer: impact on management and chemotherapy administration. Arch Surg. 2007;142:619-23; discussion 623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 88. | Pacheco-Barcia V, Mondéjar R, Martínez-Sáez O, Longo F, Moreno JA, Rogado J, Donnay O, Santander C, Carrato A, Colomer R. Safety and Oncological Outcomes of Bevacizumab Therapy in Patients With Advanced Colorectal Cancer and Self-expandable Metal Stents. Clin Colorectal Cancer. 2019;18:e287-e293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 89. | Matsuda A, Yamada T, Matsumoto S, Shinji S, Ohta R, Sonoda H, Takahashi G, Iwai T, Takeda K, Sekiguchi K, Yoshida H. Systemic Chemotherapy is a Promising Treatment Option for Patients with Colonic Stents: A Review. J Anus Rectum Colon. 2021;5:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Imbulgoda A, MacLean A, Heine J, Drolet S, Vickers MM. Colonic perforation with intraluminal stents and bevacizumab in advanced colorectal cancer: retrospective case series and literature review. Can J Surg. 2015;58:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 91. | Bong JW, Lee JL, Kim CW, Yoon YS, Park IJ, Lim SB, Yu CS, Kim TW, Kim JC. Risk Factors and Adequate Management for Complications of Bevacizumab Treatment Requiring Surgical Intervention in Patients With Metastatic Colorectal Cancer. Clin Colorectal Cancer. 2018;17:e639-e645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 92. | Park YE, Park Y, Park SJ, Cheon JH, Kim WH, Kim TI. Outcomes of stent insertion and mortality in obstructive stage IV colorectal cancer patients through 10 year duration. Surg Endosc. 2019;33:1225-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 93. | Sterpetti AV, Sapienza P, Fiori E, Marzo LD, Lamazza A. Improved results for left-sided malignant colorectal obstruction with a proper selection for self expandable metal stent placement, surgical resection or diverting stoma. Eur J Surg Oncol. 2020;46:2064-2067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |