Published online Oct 24, 2022. doi: 10.5306/wjco.v13.i10.813
Peer-review started: March 16, 2022
First decision: July 12, 2022
Revised: July 22, 2022
Accepted: September 21, 2022
Article in press: September 21, 2022
Published online: October 24, 2022
Processing time: 217 Days and 8.6 Hours
Severe oral mucositis associated with cancer therapy is a frequent complication that may affect a patient's systemic condition, resulting in interruption and/or prolongation of cancer therapy. Dentoxol® is a medical solution in the form of a mouthwash that has been shown to result in statistically significant improvement in the prevention of severe oral mucositis. However, knowing the measures of the clinical significance of this therapy is important for accurate decision-making.
To describe the clinical impact of Dentoxol® use in severe oral mucositis.
Clinical significance was measured using the results obtained in a randomized controlled clinical trial previously conducted by the same group of researchers. The measures of clinical significance evaluated were the absolute risk or incidence, relative risk, absolute risk reduction, relative risk reduction, number needed to treat, and odds ratio.
The data obtained show that the impact of Dentoxol® on reducing the severity of oral mucositis has important clinical relevance.
The results of this study justify the incorporation of Dentoxol® mouth rinse into clinical protocols as a complement to cancer therapy to prevent and/or treat oral mucositis secondary to radio
Core Tip: Oral mucositis associated with cancer therapy is a frequent complication. Dentoxol® is a medical solution that has been shown to prevent severe oral mucositis. The clinical significance of Dentoxol® was measured using the results obtained in a randomized controlled clinical trial previously conducted by the same group of researchers. The data obtained show that the clinical impact of Dentoxol® on oral mucositis justifies its incorporation into clinical protocols as a complement to cancer therapy to prevent and/or treat oral mucositis.
- Citation: Solé S, Becerra S, Carvajal C, Bettolli P, Letelier H, Santini A, Vargas L, Cifuentes A, Larsen F, Jara N, Oyarzún J, Bustamante E, Martínez B, Rosenberg D, Galván T. Clinical relevance of the use of Dentoxol® for oral mucositis induced by radiotherapy: A phase II clinical trial. World J Clin Oncol 2022; 13(10): 813-821
- URL: https://www.wjgnet.com/2218-4333/full/v13/i10/813.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i10.813
Oral mucositis is a complication that arises from cancer treatment (chemotherapy and/or radiotherapy) and manifests as erythematous and ulcerative lesions of the oral mucosa. These lesions cause considerable pain and functional impairment that can compromise nutritional status and prevent adequate oral hygiene in patients, increasing the risk of local infection and systemic spread. Additionally, in some cases, it can limit the dose or continuity of cancer therapy[1-3].
The available scientific evidence indicates that between 94%-96% of patients treated with head and neck radiotherapy develop some degree of oral mucositis, while 66% present with severe oral mucositis[4,5].
The pain caused by lesions often compromises a patient’s ability to eat, frequently leading to the need for via nasogastric or gastrostomy tubes, which can impact the general condition of the patient due to weight loss 5%[6] as well as the overall cost of therapy by requiring hospitalization. Approximately 16% of patients with head and neck radiotherapy require hospitalization due to oral mucositis. In addition, 11% of patients who received radiotherapy for head and neck cancer had unplanned interruptions in radiotherapy due to severe oral mucositis[7].
The pathogenesis of oral mucositis is complex and involves different pathways. One of the events involved in the development of mucositis is the inflammatory response of tissues to cancer therapy[8,9]. Within these tissues, the participation of proinflammatory cytokines such as TNF- and IL-1 plays key roles in both the onset of tissue damage and acceleration of the process[10-13]. Likewise, these cytokines induce the expression of cyclooxygenase-2, which is responsible for the production of proinflammatory prostanoids such as prostaglandin E2 and prostacyclin I2 and for tissue injury and pain at the inflammation site[14-16].
Additionally, ulcers caused by oral mucositis can be colonized by bacteria from the patient's own oral flora. This secondary colonization may aggravate the clinical picture of mucositis through the release of bacterial products (lipopolysaccharides) capable of generating greater tissue damage and inhibiting the healing process[13]. Sobue et al[17] evaluated the growth of and inflammatory responses against Candida albicans, Candida glabrata, and 2 streptococcal species of the mitis group (S. oralis and S. mitis), which are frequently associated with oral mucositis, in an organotypic model to represent chemotherapy-induced mucositis. Although a nonsignificant increase in growth was observed for the species studied, the authors reported an exacerbated proinflammatory response to C. albicans, C. glabrata, and S. oralis[17]. Recently, a positive correlation was found between ≥ grade 2 oral mucositis and the presence of Bacteroidales G2, Capnocytophaga, Eikenella, Mycoplasma, Sneathia, and the periodontopathogens Porphyromonas and Tannerella. Additionally, a large amount of Fusobacterium, Haemophilus, Tannerella, Porphyromonas, and Eikenella on buccal mucosa influenced oral mucositis susceptibility[18]. Bacteriome disturbance has been shown to have a strong and independent association with oral mucositis severity through decreases in commensal organisms such as those belonging to the Streptococcus, Actinomyces, Gemella, Granulicatella, and Veillonella genera and increases in gram-negative bacteria such as Fusobacterium nucleatum and Prevotella oris[19].
The complex nature of oral mucositis requires a comprehensive preventive and therapeutic approach that can address the different pathways involved to achieve a successful outcome[20]. Managing only inflammation or overinfection is not sufficient for efficient and adequate control.
In this context, Dentoxol®, an aqueous solution used as a mouthwash, whose main mode of action is mechanical sloughing of the superficial epithelial cell layer of the oral mucosa, thus stimulating local regeneration of the epithelium, was developed. The interaction of its components (purified water, xylitol, sodium bicarbonate, eugenol, camphor, parachlorophenol, and peppermint essence) in specific concentrations sloughs and eliminates cells damaged by radio/chemotherapy as well as particles and detritus present in the oral cavity, such as bacteria and organic debris. The clinical effect observed is a result of the interaction of its components acting on the different aspects of the physiopathogenesis of oral mucositis (antioxidant, bacteriostatic, bactericidal, anti-inflammatory, and moisturizing properties and mucosal regenerative stimulation). As a result, Dentoxol® can prevent oral mucositis by physically moisturizing and lubricating the oral mucosa to provide flexibility and resistance. Accordingly, it affects several pathways that influence the severity of oral mucositis[21].
Recently, a randomized controlled clinical trial conducted by this research team evaluated the effect of Dentoxol® mouthwash on the prevalence of severe oral mucositis and found statistically significant results regarding the prevention and reduction in the severity of oral mucositis[21]. Many clinical studies present their results based on statistical significance. However, clinical measures of significance are essential for evaluating the relevance and usefulness of a therapy in daily clinical practice[22].
Considering the high incidence of oral mucositis in patients undergoing head and neck cancer therapy as well as the relevant impact of this pathology on patient morbidity and quality of life, in addition to the associated economic costs, the clinical significance of an agent that can successfully treat oral mucositis needs to be analyzed. The aim of the present study is to objectively and clearly present the clinical impact of Dentoxol® on affected tissues based on statistical results obtained in a previously conducted clinical trial, with the aim of providing a clearer picture of the impact that clinicians responsible for managing this pathology should expect in their daily work when using this preventive and therapeutic tool to manage and control oral mucositis.
Severe oral mucositis: Grade 3 or 4 mucositis based on the scale described by the World Health Organization (Table 1).
| Week | Dentoxol® (n = 55) | Control (n = 53) | |||||||
| No severe oral mucositis | Severe oral mucositis | No severe oral mucositis | Severe oral mucositis | P value | |||||
| n | % | n | % | n | % | n | % | ||
| 1 | 49 | 94.2 | 3 | 5.8 | 49 | 98.0 | 1 | 2.0 | 0.327 |
| 2 | 49 | 100 | 0 | 0.0 | 42 | 95.5 | 2 | 4.5 | 0.131 |
| 3 | 47 | 95.9 | 2 | 4.1 | 30 | 76.9 | 9 | 23.1 | 0.007a |
| 4 | 42 | 91.3 | 4 | 8.7 | 29 | 70.7 | 12 | 29.3 | 0.013a |
| 5 | 33 | 73.3 | 12 | 26.7 | 19 | 52.8 | 17 | 47.2 | 0.055 |
| 6 | 29 | 64.4 | 16 | 35.6 | 15 | 46.9 | 17 | 53.1 | 0.125 |
| 7 | 22 | 57.9 | 16 | 42.1 | 11 | 44.0 | 14 | 56.0 | 0.280 |
| 8 | 11 | 57.7 | 6 | 35.3 | 4 | 44.4 | 5 | 55.6 | 0.320 |
A descriptive study was conducted on the clinical significance of Dentoxol® in treating oral mucositis based on results obtained in a randomized controlled clinical trial with a parallel arm design (1:1) evaluating the effect of Dentoxol® mouthwash (test group) versus a placebo mouthwash (control group) on the incidence of severe oral mucositis associated with cancer therapy. The full methodology of the clinical trial was previously published by Lalla et al[21].
A total of 108 patients older than 18 years (Dentoxol® group = 55 and control group = 53) participated in the study.
Once the statistical results of the clinical trial were obtained, clinical significance measures such as the absolute risk (AR), relative risk (RR), absolute risk reduction (ARR), relative risk reduction, number necessary to treat (NNT), and odds ratio were calculated using a contingency table (Table 2).
| Severe oral mucositis | No severe oral mucositis | Total | |
| Dentoxol® | a | b | a + b |
| Control | c | d | c + d |
| Total | a + c | b + d | a + b + c + d |
A total of 108 patients were considered for the analysis of the outcomes of the randomized controlled clinical trial evaluating the use of Dentoxol®.
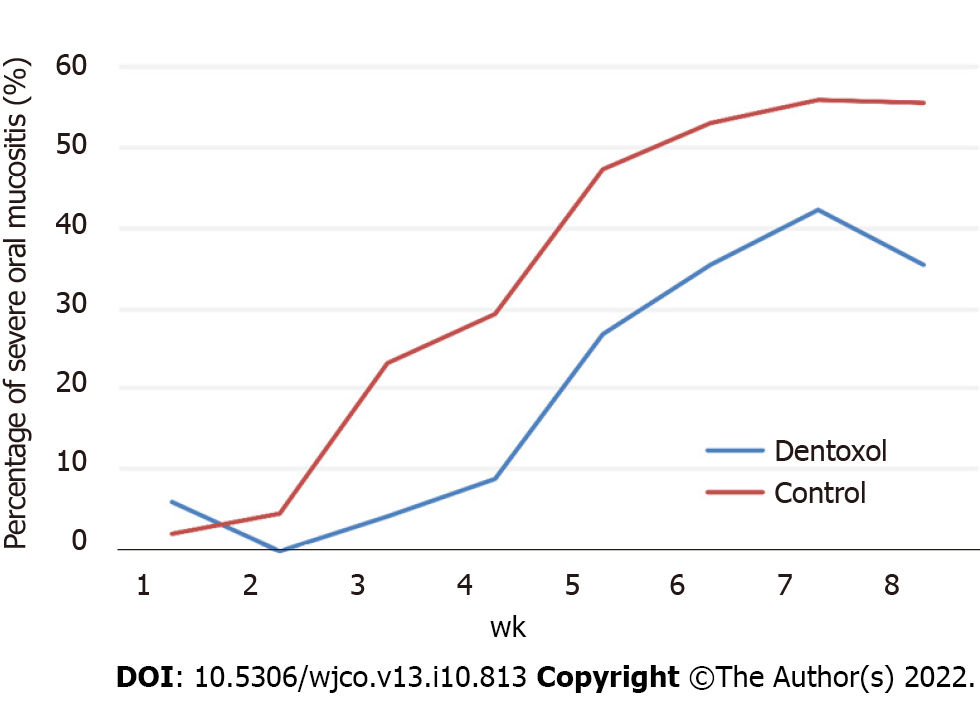
Table 1 shows the number and percentage of patients who presented with severe oral mucositis in each treatment group. The Dentoxol® and control groups showed a progressive increase in the frequency of severe oral mucositis, with a peak at seven weeks.
Compared with the control group, the Dentoxol® group presented a lower number of patients with severe oral mucositis every week except for the first week, with a statistically significant difference observed at weeks 3 and 4 of the follow-up (see Table 1).
Table 2 shows the measures of clinical significance. The ARs of severe oral mucositis in the Dentoxol® group were 0.04 and 0.09 or 4% and 9% for weeks 3 and 4, respectively, versus 0.23 and 0.29 or 23% and 29%, respectively, in the control group. Additionally, from week 2 onward, the relative risk of severe oral mucositis in the Dentoxol® group was less than 1, indicating that Dentoxol® use acted as a protective factor.
Dentoxol® use was positively associated with a reduction in severe oral mucositis from week 2 onward, showing ARR values greater than 0. The values at weeks 3 and 4, ARR = 0.19 or 19% and 0.21 or 21%, respectively, indicate that if 100 patients were treated with Dentoxol®, 19 and 21, respectively, fewer cases of severe mucositis would occur compared to the control group. Likewise, during weeks 3 and 4, when statistically significant differences between the groups were noted, 5 patients (NNT) would need to be treated with Dentoxol® to prevent 1 additional case of severe oral mucositis (Table 3).
| Week | AR | RR (95%CI) | ARR | RRR | NNT | OR (95%CI) | |
| Dentoxol (%) | Control (%) | Dentoxol® | Dentoxol® (%) | Dentoxol® (%) | Dentoxol® | Dentoxol® | |
| 1 | 5.77 | 2 | 2.88 (0.31-26.82) | -4 | -188 | -27 | 3 (0.30-29.85) |
| 2 | 0 | 4.55 | - | 5 | 100 | 22 | - |
| 3 | 4.08 | 23.08 | 0.18 (0.04-0.77) | 19 | 82.31 | 5 | 0.14 (0.03-0.70) |
| 4 | 8.7 | 29.27 | 0.3 (0.1-0.85) | 21 | 70.29 | 5 | 0.23 (0.07-0.78) |
| 5 | 26.67 | 47.22 | 0.56 (0.31-1.02) | 21 | 43.53 | 5 | 0.41 (0.16-1.03) |
| 6 | 35.56 | 53.13 | 0.67 (0.4-1.12) | 18 | 33.07 | 6 | 0.49 (0.29-1.23) |
| 7 | 42.11 | 56 | 0.75 (0.45-1.25) | 14 | 24.81 | 7 | 0.57 (0.21-1.58) |
| 8 | 35.29 | 55.56 | 0.64 (0.27-1.52) | 20 | 36.47 | 5 | 0.44 (0.08-2.27) |
Measures of clinical significance allow making well-founded decisions when evaluating a therapy and can be applied in daily clinical practice and especially in recommendations for massive clinical protocols because through these measures, expected results with a real impact on the population can be obtained.
In the present study, the group that used Dentoxol® showed a lower incidence (AR) of severe oral mucositis than the control group (Figure 1) from the 2nd week of evaluation to the 4th week, representing the greatest difference between the groups (Table 2). The results shown in Table 2 demonstrate the strong potential of Dentoxol® to lower treatment complications. Treating 5 patients with Dentoxol® will prevent 1 additional case of severe oral mucositis that may need percutaneous endoscopic gastrostomy tubes, liquid diet supplements, pain medications, etc. Moreover, the results in Table 1 show that Dentoxol® delayed the onset of severe mucositis such that even patients with a severe grade who received it had the complication for a shorter period, with benefits on cost and quality of life.
Another important conclusion of the clinical trial was that the most beneficial effects of therapy with Dentoxol® are observed in patients who follow the instructions to rinse 4 times a day (Figure 1). Therefore, following the recommended instructions specifying that more frequent rinses yield better clinical results is important. Furthermore, the rinsing time should be longer than 1 min to allow the product to exert its effects on the oral mucosa. This is not a minor point because cancer patients very often have nausea and vomiting, which should be controlled to allow rinses at the appropriate frequency and time. A clinically recognized effective strategy is to begin with rinses days or ideally weeks before starting cancer therapy to prepare the mucosa for toxic effects and their consequences and thus more effectively prevent the onset of mucositis. Therefore, continuing to study different clinical protocols based on the experience of clinicians regarding this aspect through well-designed controlled clinical trials is essential.
With respect to the latter, the literature contains multiple studies evaluating different products and protocols to reduce the onset and severity of oral mucositis[23-25]. Properly designed studies allow their results to be comparable in terms of clinical effectiveness for correct decision-making. In this sense, 1 multicenter clinical trial evaluated the ability of Caphosol®, an electrolyte solution with concentrated calcium phosphate, to reduce oral mucositis in patients who received radiotherapy for head and neck cancer[26]. When observing the percentages of severe oral mucositis, 29.3% and 42.4% of grade 3 mucositis occurred during the 3rd and 4th weeks, respectively, and 8.6% and 18.6% of grade 4 mucositis occurred during the same weeks. If we compare these results with those obtained in the Dentoxol® trial, at the 3rd and 4th weeks, only 4.1% and 8.7% of patients who rinsed with Dentoxol® had severe oral mucositis, respectively (grades 3 and 4, respectively). Another more recent clinical trial showed no reduction in the incidence or duration of severe oral mucositis with Caphosol® use in patients with head and neck cancer versus the control group (64.1% vs 65.4%)[24]. Given the results obtained in these studies, the benefit of Caphosol® is not clear. On the other hand, Dentoxol® showed a statistically significant clinical benefit for patients undergoing radiotherapy for head and neck cancer.
Systematic reviews are also useful for comparing the different applications and clinical effectiveness of multiple therapeutic alternatives. Accordingly, a 2017 Cochrane Library review evaluated the effect of cytokines and growth factors in the prevention of oral mucositis[25]. The main agent evaluated was keratinocyte growth factor (KGF). The results indicated that KGF decreased the risk of severe oral mucositis in patients undergoing head and neck cancer therapy, with an RR = 0.79 and a 95% confidence interval (CI) = 0.69-0.90 (obtained from 3 studies), and that 7 patients (95%CI = 5-15) would need to be treated to prevent 1 case of severe mucositis[25]. If we compare those findings with the results for Dentoxol®, the latter agent had a lower RR from the 3rd week of follow-up, with RR values = 0.18 to 0.75, and between 5 and 7 patients (depending on the week of follow-up) would be required to prevent an additional case of severe oral mucositis. Although these results may seem similar, notably, KGF is a drug with important limitations: it is not indicated for solid tumors because it may enhance their growth; the cost is much higher; and it must be administered by IV infusion. Other products used for similar clinical conditions could be considered for comparative evaluations[27].
To better present the results of the Dentoxol® study and to facilitate comparisons with other results, we must note that the placebo used in the clinical trial from which the analysis of the present study was performed was not a totally inactive agent. Due to ethical reasons, the control group could not be deprived of minimum protection; therefore, the placebo used was a mouthwash composed of an aqueous solution of sodium bicarbonate and xylitol, thus reducing the actual difference between the Dentoxol® group and the control group. Therefore, the benefit provided by rinses with Dentoxol® is even greater in reality.
In conclusion, more well-designed controlled clinical trials are needed to increase scientific evidence and test different clinical protocols and therapeutic strategies to offer patients effective solutions based on scientific evidence. To facilitate comparisons with other interventions, the type of cancer presented by the patients, the type of therapy (chemo- and/or radiotherapy), the frequency, dose, starting point, and duration of therapy for oral mucositis, etc., should be considered. Additionally, the timing of evaluation considering the pathogenesis of mucositis is also an important factor.
In this study, the safety and clinical efficacy of Dentoxol® were demonstrated for the prevention and treatment of severe oral mucositis, an unwanted pathology that is a complication of treatments for much more serious diseases, including cancer. However, this complication can impact the costs and continuity of cancer treatment and, above all, the quality of life of patients. In this study, the effects of Dentoxol® were clinically evident and detectable in a small number of treated patients; therefore, the inclusion of Dentoxol® in clinical protocols is highly recommended for the management and control of the side effects of cancer treatments, which is as important as the other components of the therapeutic arsenal for cancer.
Oral mucositis is a complication that arises from cancer treatment (chemotherapy and/or radiotherapy) and manifests as erythematous and ulcerative lesions of the oral mucosa. The pathogenesis of oral mucositis is complex and involves different pathways. One of the events involved in the development of mucositis is the inflammatory response of tissues to cancer therapy The complex nature of oral mucositis requires a comprehensive preventive and therapeutic approach that can address the different pathways involved to achieve a successful outcome. Managing only inflammation or overinfection is not sufficient for efficient and adequate control. In this context, Dentoxol®, an aqueous solution used as a mouthwash, whose main mode of action is mechanical sloughing of the superficial epithelial cell layer of the oral mucosa, thus stimulating local regeneration of the epithelium, was developed. Recently, a randomized controlled clinical trial conducted by this research team evaluated the effect of Dentoxol® mouthwash on the prevalence of severe oral mucositis and found statistically significant results regarding the prevention and reduction in the severity of oral mucositis.
Many clinical studies present their results based on statistical significance. However, clinical measures of significance are essential for evaluating the relevance and usefulness of a therapy in daily clinical practice.
The aim of the present study is to objectively and clearly present the clinical impact of Dentoxol® on affected tissues based on statistical results obtained in a previously conducted clinical trial, with the aim of providing a clearer picture of the impact that clinicians responsible for managing this pathology should expect in their daily work when using this preventive and therapeutic tool to manage and control oral mucositis.
Once the statistical results of the clinical trial were obtained, clinical significance measures such as the absolute risk (AR), relative risk (RR), absolute risk reduction (ARR), relative risk reduction, number necessary to treat (NNT), and odds ratio were calculated using a contingency table.
The ARs of severe oral mucositis in the Dentoxol® group were 0.04 and 0.09 or 4% and 9% for weeks 3 and 4, respectively, versus 0.23 and 0.29 or 23% and 29%, respectively, in the control group. Additionally, from week 2 onward, the relative risk of severe oral mucositis in the Dentoxol® group was less than 1, indicating that Dentoxol® use acted as a protective factor. Dentoxol® use was positively associated with a reduction in severe oral mucositis from week 2 onward, showing ARR values greater than 0. The values at weeks 3 and 4, ARR = 0.19 or 19% and 0.21 or 21%, respectively, indicate that if 100 patients were treated with Dentoxol®, 19 and 21, respectively, fewer cases of severe mucositis would occur compared to the control group. Likewise, during weeks 3 and 4, when statistically significant differences between the groups were noted, 5 patients (NNT) would need to be treated with Dentoxol® to prevent 1 additional case of severe oral mucositis.
In this study, the effects of Dentoxol® were clinically evident and detectable in a small number of treated patients; therefore, the inclusion of Dentoxol® in clinical protocols is highly recommended for the management and control of the side effects of cancer treatments, which is as important as the other components of the therapeutic arsenal for cancer.
We extend our most sincere gratitude to Felipe Galván and Dr. Rajesh Lalla for their constant support in the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Chile
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aoun G, Lebanon; Boon CS, United Kingdom; Gursel B, Turkey; Hasan S, India; Heboyan A, Armenia S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Lalla RV, Peterson DE. Oral mucositis. Dent Clin North Am. 2005;49:167-184, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent Clin North Am. 2008;52:61-77, viii. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 351] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 3. | Treister N, Sonis S. Mucositis: biology and management. Curr Opin Otolaryngol Head Neck Surg. 2007;15:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Le QT, Kim HE, Schneider CJ, Muraközy G, Skladowski K, Reinisch S, Chen Y, Hickey M, Mo M, Chen MG, Berger D, Lizambri R, Henke M. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: a randomized, placebo-controlled study. J Clin Oncol. 2011;29:2808-2814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Henke M, Alfonsi M, Foa P, Giralt J, Bardet E, Cerezo L, Salzwimmer M, Lizambri R, Emmerson L, Chen MG, Berger D. Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2011;29:2815-2820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys. 2007;68:1110-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 348] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 7. | Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, Komaroff E, Nalysnyk L, Zilberberg MD. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 822] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 8. | Lalla RV, Schubert MM, Bensadoun RJ, Keefe D. Anti-inflammatory agents in the management of alimentary mucositis. Support Care Cancer. 2006;14:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Sonis ST, Peterson RL, Edwards LJ, Lucey CA, Wang L, Mason L, Login G, Ymamkawa M, Moses G, Bouchard P, Hayes LL, Bedrosian C, Dorner AJ. Defining mechanisms of action of interleukin-11 on the progression of radiation-induced oral mucositis in hamsters. Oral Oncol. 2000;36:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 167] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Hong JH, Chiang CS, Tsao CY, Lin PY, McBride WH, Wu CJ. Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. Int J Radiat Biol. 1999;75:1421-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 131] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Epperly MW, Gretton JA, DeFilippi SJ, Greenberger JS, Sikora CA, Liggitt D, Koe G. Modulation of radiation-induced cytokine elevation associated with esophagitis and esophageal stricture by manganese superoxide dismutase-plasmid/liposome (SOD2-PL) gene therapy. Radiat Res. 2001;155:2-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Xun CQ, Thompson JS, Jennings CD, Brown SA, Widmer MB. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood. 1994;83:2360-2367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 248] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Sonis ST. Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol. 1998;34:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 345] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2755] [Cited by in RCA: 2713] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 15. | Seibert K, Masferrer JL. Role of inducible cyclooxygenase (COX-2) in inflammation. Receptor. 1994;4:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Mertz PM, DeWitt DL, Stetler-Stevenson WG, Wahl LM. Interleukin 10 suppression of monocyte prostaglandin H synthase-2. Mechanism of inhibition of prostaglandin-dependent matrix metalloproteinase production. J Biol Chem. 1994;269:21322-21329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 123] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Sobue T, Bertolini M, Thompson A, Peterson DE, Diaz PI, Dongari-Bagtzoglou A. Chemotherapy-induced oral mucositis and associated infections in a novel organotypic model. Mol Oral Microbiol. 2018;33:212-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Vesty A, Gear K, Biswas K, Mackenzie BW, Taylor MW, Douglas RG. Oral microbial influences on oral mucositis during radiotherapy treatment of head and neck cancer. Support Care Cancer. 2020;28:2683-2691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Hong BY, Sobue T, Choquette L, Dupuy AK, Thompson A, Burleson JA, Salner AL, Schauer PK, Joshi P, Fox E, Shin DG, Weinstock GM, Strausbaugh LD, Dongari-Bagtzoglou A, Peterson DE, Diaz PI. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome. 2019;7:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 20. | Peterson DE, Lalla RV. Oral mucositis: the new paradigms. Curr Opin Oncol. 2010;22:318-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Lalla RV, Solé S, Becerra S, Carvajal C, Bettoli P, Letelier H, Santini A, Vargas L, Cifuentes A, Larsen F, Jara N, Oyarzún J, Feinn R, Bustamante E, Martínez B, Rosenberg D, Galván T. Efficacy and safety of Dentoxol® in the prevention of radiation-induced oral mucositis in head and neck cancer patients (ESDOM): a randomized, multicenter, double-blind, placebo-controlled, phase II trial. Support Care Cancer. 2020;28:5871-5879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Kraemer HC, Morgan GA, Leech NL, Gliner JA, Vaske JJ, Harmon RJ. Measures of clinical significance. J Am Acad Child Adolesc Psychiatry. 2003;42:1524-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 239] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | Rao NG, Trotti A, Kim J, Schell MJ, Zhao X, Amdur RJ, Brizel DM, Chambers MS, Caudell JJ, Miyamoto C, Rosenthal DI. Phase II multicenter trial of Caphosol for the reduction of mucositis in patients receiving radiation therapy for head and neck cancer. Oral Oncol. 2014;50:765-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Wong KH, Kuciejewska A, Sharabiani MTA, Ng-Cheng-Hin B, Hoy S, Hurley T, Rydon J, Grove L, Santos A, Ryugenji M, Bhide SA, Nutting CM, Harrington KJ, Newbold KL. A randomised controlled trial of Caphosol mouthwash in management of radiation-induced mucositis in head and neck cancer. Radiother Oncol. 2017;122:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Riley P, Glenny AM, Worthington HV, Littlewood A, Fernandez Mauleffinch LM, Clarkson JE, McCabe MG. Interventions for preventing oral mucositis in patients with cancer receiving treatment: cytokines and growth factors. Cochrane Database Syst Rev. 2017;11:CD011990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Manterola DC, Otzen HT. Valoración clínica del riesgo, interpretación y utilidad práctica. Int. J. Morphol. 2015; 33: 842-849. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Heboyan A, Avetisyan A, Skallevold HE, Rokaya D, Marla V, Vardanyan A. Occurrence of Recurrent Aphthous Stomatitis (RAS) as a Rare Oral Manifestation in a Patient with Gilbert's Syndrome. Case Rep Dent. 2021;2021:6648729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |