Published online Oct 24, 2022. doi: 10.5306/wjco.v13.i10.762
Peer-review started: March 16, 2021
First decision: April 27, 2021
Revised: May 22, 2021
Accepted: October 2, 2022
Article in press: October 2, 2022
Published online: October 24, 2022
Processing time: 583 Days and 6.1 Hours
Gastrointestinal (GI) cancers are a set of diverse diseases affecting many parts/ organs. The five most frequent GI cancer types are esophageal, gastric cancer (GC), liver cancer, pancreatic cancer, and colorectal cancer (CRC); together, they give rise to 5 million new cases and cause the death of 3.5 million people annually. We provide information about molecular changes crucial to tumorigenesis and the behavior and prognosis. During the formation of cancer cells, the genomic changes are microsatellite instability with multiple chromosomal arrangements in GC and CRC. The genomically stable subtype is observed in GC and pancreatic cancer. Besides these genomic subtypes, CRC has epigenetic modification (hypermethylation) associated with a poor prognosis. The pathway information highlights the functions shared by GI cancers such as apoptosis; focal adhesion; and the p21-activated kinase, phosphoinositide 3-kinase/Akt, transforming growth factor beta, and Toll-like receptor signaling pathways. These pathways show survival, cell proliferation, and cell motility. In addition, the immune response and inflammation are also essential elements in the shared functions. We also retrieved information on protein-protein interaction from the STRING database, and found that proteins Akt1, catenin beta 1 (CTNNB1), E1A binding protein P300, tumor protein p53 (TP53), and TP53 binding protein 1 (TP53BP1) are central nodes in the network. The protein expression of these genes is associated with overall survival in some GI cancers. The low TP53BP1 expression in CRC, high EP300 expression in esophageal cancer, and increased expression of Akt1/TP53 or low CTNNB1 expression in GC are associated with a poor prognosis. The Kaplan Meier plotter database also confirmed the association between expression of the five central genes and GC survival rates. In conclusion, GI cancers are very diverse at the molecular level. However, the shared mutations and protein pathways might be used to understand better and reveal diagnostic/prognostic or drug targets.
Core Tip: We highlight the genomic mutations and cellular pathways in gastrointestinal (GI) cancers. These are responsible for the cell’s behavior, allowing unlimited cell replication and invasion of other tissues. Using the STRING database, we found that Akt1, catenin beta 1, E1A binding protein p300, tumor protein p53 (TP53), and TP53 binding protein 1 are central nodes in the GI cancer protein network. Their expression is associated with poor survival in some GI cancers, which was confirmed by the Kaplan Meier plotter database. This information points to crucial and shared aspects of the most frequent GI cancers.
- Citation: Bispo IMC, Granger HP, Almeida PP, Nishiyama PB, de Freitas LM. Systems biology and OMIC data integration to understand gastrointestinal cancers. World J Clin Oncol 2022; 13(10): 762-778
- URL: https://www.wjgnet.com/2218-4333/full/v13/i10/762.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i10.762
In 2020, the number of cancer cases in the digestive system was 5 million and 3.5 million deaths worldwide[1,2]; the physiologic system with the highest number of cases and among the highest percentage of deaths[3] (Table 1). The cancer types in this system can be classified as organ origin and cell type. The most frequent are esophageal cancer (EC), gastric cancer (GC), liver cancer, pancreatic cancer, and colorectal cancer (CRC)[2,3]. GC, liver cancer, and CRC are among the most common causes of cancer deaths annually[2]. Gastrointestinal (GI) cancers also have specific molecular changes in genetic/genome, epigenetics, gene expression, and cellular pathways contributing to tumor behavior. This information might be helpful in diagnosis, prognosis, and new drug development.
EC has two subtypes: esophageal squamous cell cancer (ESCC) and esophageal adenocarcinoma (EAC)[4]. The incidence of ESCC increases globally and predominantly in Eastern Asia and Eastern/Southern Africa[4-7]. However, the ESCC decreases while EAC increases in the United States and a few European countries[5]. The ESSC and EAC incidence differences are geographically observed in sex and ethnic patterns[4,5].
There is also a well-established genetic factor associated with sex, and although it is still not well understood, it is known that the ratio between men to women is 2.5-4.4:1[4,6]. Studies indicate a protective effect of female sex hormones, including a lower risk of cancer for women previously breastfed. Nevertheless, environmental factors also influence this prevalence as, for example, men tend to abuse alcohol and tobacco, which are primary risk factors for the manifestation of EC[4,8].
The risk factors for ESCC are smoking, a low vegetables/fruit diet, and alcohol consumption[9], whereas for EAC, the risk factors are obesity and gastroesophageal reflux disease[9,10]. When alcohol and tobacco are used together, there is an increased risk. This combination is believed to be responsible for 70%-90% of cases, mainly because they cause chronic irritation and inflammation of the esophageal mucosa. In the case of obesity, the greater the abdominal circumference, the greater the intra-abdominal pressure increases the probability of developing gastroesophageal reflux[4,6,11-14].
Early diagnosis is fundamental to improving prognosis. However, dysplasia usually is asymptomatic[4,11,12,15] and manifests at an average age of 67 years, when there is a high incidence of metastasis, mainly in lymph nodes, liver, lungs, and bones[11,12]. These features make the EC an aggressive malignancy with a 15%-23% 5-year survival rate[9,10].
GC has the fourth highest incidence and mortality worldwide[1,2]. The primary risk factors for GC are genetics, diet (high amount of salt and low consumption of fruits and vegetables), Helicobacter pylori or Epstein–Barr virus infection, smoking, alcohol intake, and sedentary life[16-19]. The principal risk factor for GC is H. pylori infection, accounting for 80% of the cases. Although the incidence of H. pylori infection is decreasing, GC deaths are still high. While the primary risk factor is H. pylori infection, many genes are associated with GC[16,18,20], and some genetic variations that can interact with H. pylori increase the GC risk[21,22]. The incidence of GC is higher in males (1.32-2.2) and in Eastern/Central Asia and Latin America[16,18].
Obesity can induce inflammation of the stomach lining through tumor necrosis factor (TNF), interleukin 6 (IL-6), and C-C motif chemokine ligand 2. By contrast, a diet rich in fruits and vegetables has proven to be an ally in cancer prevention because it contains numerous antioxidants that prevent metabolic damage, especially vitamin C[18].
A relevant factor in the decline of GC has been the successful prevention and treatment of infections by H. pylori[18]. According to the International Agency for Research on Cancer, this is a carcinogen from group 1, meaning there is sufficient evidence of human carcinogenicity[23,24]. H. pylori infection affects more than half of the world’s population, and its eradication may considerably decrease the chances of stomach cancer. However, it would increase the chances of esophageal adenocarcinoma. However, it is unknown how this esophageal protection mechanism occurs[18,24,25].
There are about 1 million new cases of liver cancer each year, with hepatocellular carcinoma (HCC) responsible for most patients (90%) and the second most common cause of cancer death worldwide[26,27].
HCC presents a poor prognosis due to a late diagnosis. Multiple different tumors may occur in a single patient, leading to intra-tumor and intra-patient heterogeneity, which makes it difficult to establish a treatment line for HCC[27,28]. This heterogeneity can be caused by environmental factors and genomic and biological changes caused by the tumor lesion[27].
Cirrhosis and non-alcoholic fatty liver disease are risk factors associated with alcohol abuse and obesity that can lead to the onset of HCC. Genetic factors such as diabetes, exposure to carcinogens (aflatoxins), and biological factors, especially hepatitis virus infection, can be highlighted[28].
The HCC development is a multistep process. It starts as a chronic liver disease that leads to inflammation, fibrosis, or aberrant hepatocyte regeneration. This set of conditions can progress to cirrhosis and later malignancy. The causes of this inflammation can be hepatitis B virus/hepatitis C virus infection, fatty liver disease, excessive alcohol intake, and aflatoxin consumption[26,29]. The outcome of this inflammation can be influenced by epigenetics and the immunological response in the tumor microenvironment to create a preneoplastic lesion until producing cells with highly proliferative, invasive, and survival skills[26].
The geographic regions most affected by HCC are Southeast Asia and sub-Saharan Africa, where there is endemic infection by the hepatitis virus and high exposure to aflatoxin, which are responsible for 70%-90% of cases in these places[28]. Currently, there is no line of therapy based on biomarkers suitable for HCC, although some candidate genes already exist[30].
Pancreatic cancer, characterized by pancreatic ductal adenocarcinoma (PDAC), is the seventh leading cause of cancer-related deaths worldwide[31]. Its incidence is higher in Europe, followed by North America and Oceania, mainly in people over 70-years-old. Incidence and mortality increase with aging and are more common in men than women[32].
It is highly fatal because it presents aggressive growth and a lack of symptoms in the disease’s initial stage. As the tumor progresses, a picture of nonspecific symptoms begins, including jaundice, weight loss, abdominal pain, and fatigue[32]. About 80% of diagnoses are made in the advanced clinical stages, leading to a low 5-year prognosis of survival after surgery[33]. Surgical resection is the single strategy capable of curing pancreatic cancer. Besides, using chemotherapy concomitantly improves survival rates[34].
The main risk factors for the onset of pancreatic adenocarcinoma are smoking, alcohol, obesity, H. pylori, and type 2 diabetes[34]. Other factors, such as fat infiltration into the pancreas, have been associated with developing intraepithelial neoplasms. Pancreatic cancer can also arise from genetic factors that can cause familial syndromes, such as Peutz-Jeghers syndrome[31]. A history of pancreatic cancer in first-degree relatives leads to a 2- to 3-fold increase in incidence risk due to inherited genetic predispositions[35].
CRC is the second most deadly cancer worldwide (1.3 million) and is the third leading cause of cancer-related deaths (540000) annually[2]. CRC is responsible for about 10% of cancer-related deaths worldwide, and in the last 45 years, there has been an increase in this mortality rate[36]. Its incidence is higher in developed countries such as Australia and New Zealand, followed by countries in Europe, East Asia, and North America. The frequency increases as individuals age, usually appearing in people over 50 years[37].
The tumor can originate in both the colon and the rectum. However, usually fuse because they have similar clinical and biological characteristics, with adenocarcinoma as the primary cell type of the tumor[37]. Many factors are associated with this increase in the diagnosis/mortality rate, such as an increase in life expectancy, poor dietary habits, and risk factors: smoking, red meat consumption, sedentary lifestyle, obesity, alcohol intake, and genetics[36,38-40]. These factors change the genetic/molecular in colon epithelial cells deactivating suppressor tumor genes and activating oncogenes to create aggressive and malignant behavior[40].
In the early stages, the disease has no clinical manifestation. The patient may be asymptomatic for years, but as the disease progresses, it advances to a more severe condition, with symptoms such as changes in intestinal motility, hidden or evident colorectal bleeding, cramps, loss of weight, weakness, and fatigue are manifesting[37].
There are several generalized genomic changes when esophageal carcinoma cells are analyzed. The most evident is a somatic mutation in tumor protein p53 (TP53) that appears in about 83% of cells. The p53 protein is a tumor suppressor and one of the most important transcription factors for regulating proliferation, apoptosis, autophagy, and cell cycle. However, this gene has a high mutation percentage in cancer cases, reaching 75% in tumor cells[12,41].
There are also changes in genes that control cell cycle and differentiation, including cyclin-dependent kinase inhibitor 2A (CDKN2A), nuclear factor erythroid derived 2-like 2, checkpoint kinase 1/2, and Notch1/3. Others may appear overexpressed such as cyclin D1 (CCND1) and CDK4/6[12,42-44]. The B cell translocation gene 3 protein can regulate the cell cycle’s progression; its low expression is related to the appearance of esophageal adenocarcinoma, and its expression level is directly correlated with lymph node metastasis[12,45].
The presence of mutations in the growth factors in cancer cells is well documented in the literature. Overexpression of epidermal growth factor receptor (EGFR) in carcinoma cells is associated with lymph node metastasis, and its expression level also influences the patient’s clinical stage. Another growth factor correlated with esophageal carcinoma is vascular endothelial growth factor C (VEGFC), encoded by the Fms related receptor tyrosine kinase 1 gene, and its levels in the tissues correlate with tumor stages and metastasis state[12,41].
Using next-generation sequencing, frequent mutations in carcinoma cells have been observed in the lysine methyltransferase 2D (KMT2D), SET domain containing 2 histone lysine methyltransferase, Notch1, retinoblastoma 1, CDKN2A, BRCA1-associated protein-1, forkhead box O3, and MutS homolog 6 (MSH6) genes compared to adenocarcinoma. It was also observed that some copy number variations in fibroblast growth factor 3 (FGF3), FGF4, FGF19, and CCND1 are more expressed in carcinoma compared to adenocarcinoma[46].
Besides the infectious causes, the genetic data have helped to classify the GC into three additional subtypes: microsatellite instability (21.7%), genome stability (19.6%), and chromosome instability tumors (49.1%)[47].
Although infection is environmental, GC caused by infection is associated with genetic modifications such as phosphoinositide 3-kinase catalytic subunit (PIK3CA) mutations or gene amplification of Janus kinase (JAK), programmed death-ligand 1/2, or ERBB2. The infectious pathogen can also induce epigenetic modifications in this type of GC as DNA methylation in the phosphatase and tensin homolog (PTEN) gene promoter[48] and tumor-suppressor gene adenomatous polyposis coli (APC)[49]. Microsatellite instability is more associated with many truncating or missense mutations. The genes with the highest number of mutations in microsatellite instability GC are EGFR, ERBB3, KRAS/NRAS, and PIK3CA[50].
Genomically stable tumors present many mutations, especially genes well associated with cancer. The gene Ras homolog family member A works as signal transduction inducing cell proliferation, actin cytoskeleton structure, and cell movement associated with metastasis[51,52]. The genes claudin 18 and Rho-GTPase-activating proteins are frequently translocated in genomically stable GC tumors. The gene cadherin 1 (CDH1) encodes a cell-cell adhesion protein, which is also currently mutated in this type of cancer[53]. Furthermore, CDH1 has a role in cell proliferation, invasive behavior, and migration[54-56]. In the CDH1 gene, autosomal dominant mutations increase stomach cancer risk, especially when one of its copies is lost, generating a scenario of diffuse hereditary GC[18].
The chromosomal alterations involve gene amplification of EGFR, ERBB2/3, KRAS/NRAS, and RASA1; gene deletion of PTEN. These genetic modifications probably would result in gene activation or deactivation, which would result in tumor cell phenotypes. EGFR, ERBB2/3, JAK2, FGFR2, MET, KRAS/NRAS, and PIK3CA are predicted to be active, while RASA1, PTEN, and PIK3R1 would be inactive.
Numerous genetic changes in HCC cells, including mutations, changes in the number of copies, and chromosomal rearrangements, lead to a very complex genomic picture. Its complexity is further aggravated when etiological factors that precede the tumor development for years are considered[57].
Some genes play a fundamental role in cancer development, which is why they appear more frequently as TP53, MYC, WNT, and CTNNB1. Also highlighted are genes related to the cell cycle, such as CCND1 and CDKN2A[57].
A study integrating RNA sequencing, DNA sequencing, T cell receptor sequencing, and single nucleotide polymorphism array was carried out to investigate the space-time interactions between cancer and immune cells. A difference in the interaction of the adaptive immune system was detected in different regions of the same tumor. The TP53 and CTNNB1 genes expressed clonal mutations. High-level amplifications have been reported for CCND1, FGF19, and VEGFA. Mutations related to environmental risk factors such as smoking and alcohol were found in telomerase reverse transcriptase (TERT), CTNNB1, TP53, axin 1, and AT-rich interactive domain-containing protein 1A (ARID1A). There were also mutations without an apparent etiological factor in TERT, KMT2B, CCNA2, and CCNE1[58].
HCC results from of a multistep process involving genetic, epigenetic, and transcriptomic interactions. Among these interactions, epigenetics is among the most affected, leading to profound gene expression changes that can facilitate tumor formation The most common form of epigenetic silencing of tumor suppressor genes is hypermethylation of DNA. This epigenetic change usually occurs in CpG islands of gene-promoting regions such as deleted in liver cancer 1, tissue factor pathway inhibitor 2, CDKN2A, and PTEN[30].
The etiology of PDAC is mainly related to genetic predisposition, environmental factors such as smoking, obesity, and poor nutritional diet. These factors lead to chromosomal instability, affecting cell cycle pathways, chromatin remodeling, WNT, MYC, NOTCH signaling, and DNA damage repairs[35,59]. Among the mutated genes, the one that appears most frequently is KRAS[60]. It is also possible to highlight mutations in MLH1, MSH2, PMS2, and MSH6 responsible for Lynch Syndrome and mutations in the germ lines of PALB26, 11, 12, and ATM7, 12, 13[35].
Pancreatic cancer genome analyses showed a homogenous profile with somatic mutations in a few genes shared KRAS, TP53, CDKN2A, and SMAD4. However, other less frequent genes are also involved including mitogen-activated protein kinase kinase 4 (MAP2K4), lysine demethylase 6A, ring finger protein 43, ARID1A, transforming growth factor beta receptor 2 (TGFβR2), GNAS, Ras responsive element binding protein 1, and Polybromo 1[61-63]. These mutations can vary, and it is observed that non-silent mutations, gene amplification (> 8 copies, deletions, and structural variants)[63]. The set of genes that appear often mutated in pancreatic cancer plays a role in oncogenes, DNA damage repair, and chromatin modification[61,64]. The pancreatic cancer genome has chromosomal rearrangements classified into four subtypes: stable, locally rearranged, scattered, and unstable[61]. The mutation event more frequent is non-silent single nucleotide variants and copy number change (loss)[61]. The pancreatic cancer stable subtype was found in 20% of samples and had very few structural rear
Most CRC cases are sporadic (70%), and only 30% are inherited[38]. The genes most affected are DNA mismatch-repair genes, APC, or mutY DNA glycosylase[39,40]. The DNA mismatch-repair proteins malfunctioning creates the condition of genetic mutation accumulation and tumor cells rising.
The CRC has three genetic subtypes based on their genomic alterations. The genomic alterations are chromosome or microsatellite instability or epigenetic changes of CpG islands (CpG island methylator phenotype - CIMP)[65,66]. Chromosomal instability is the most frequent in CRC, present in 71%-85%[65,66]. The genetic differences also lead to overall survival differences in CRC. The CIMP subtype is associated with poor prognosis, followed by chromosome instability, and microsatellite instability showed the best survival[66-68]. The CIMP's poor prognosis indicates the importance of CpG meth
The genetic/genomic diversity in GI cancers shows the importance of molecular characterization to improve the treatment and prognosis.
The cellular pathways show the main activities and functions present in a cell when proteins work together. The cancer pathways are responsible for the cell’s behavior, allowing unlimited cell replication, survival, and tissue invasion. The pathways also are responsible for the molecular changes driving tumorigenesis. Understanding how a set of proteins work together to develop a cancer cell might point to the target proteins to block these processes.
The pathways most present among the GI cancers discussed here are apoptosis, focal adhesion, and p21-activated kinase (PAK), PI3K/Akt, TGF-β, and Toll-like receptor (TLR) signaling pathways (Table 2)[69-93].
Apoptosis plays a role in maintaining the balance in cell division and death during development and life. The unbalance of apoptosis leads to survival and uncontrolled division in tumorigenesis[94]. The apoptosis pathway is triggered by irreparable DNA damage, and it has many proteins that can fail and be blocked to inhibit cell death. The intrinsic process is mediated by mitochondria releasing cytochrome C after BH3 proteins activate B-cell lymphoma 2 (Bcl-2)-associated X protein and Bcl-2 homologous antagonist/killer. The cytochrome C and apoptotic protease activating factor 1, and caspase-9 create the apoptosome to continue the apoptosis process. The extrinsic process has death receptor ligands (cluster of differentiation 95 ligand [CD95L], TNF-related apoptosis-inducing ligand, and TNFα), death receptors, and associated proteins (Fas-associated death domain and TNF receptor 1-associated death domain protein) that transduce the death signal until caspase-8. Both intrinsic and extrinsic processes act on caspase-3/6/7 to induce the apoptosis cascade. Cell death by apoptosis results in a non-inflammatory process, which attracts research to the development of therapies that use apoptosis to treat cancer[95-97].
The PAK1 signaling pathway has six members divided into two groups and induces proliferation, survival, and motility[98]. PAK1 participates in cancer tumorigenesis after being highly expressed. The crosstalk of PAK1 with the MAPK/extracellular signal-regulated kinase (ERK) and PI3K/Akt pathways induces proliferation and survival, respectively[99]. PAK1 also connects with the Wnt signaling pathway through CTNNB1 and continues to stimulate growth and metastasis[98]. PAK1 expression protects the cell from apoptosis after interaction with Raf, which inactivates Bcl-2 family members (BCL2 associated agonist of cell death [BAD]) in mitochondria[98,100].
TLRs are part of the family of pattern knowledge receptors and operate on innate immunity, participating in the body’s first line of defense against invasion of microbial pathogens, tissue damage, and cancer. Its signaling pathway controls immune cell activation, maturation, and immune functions, especially the secretion of cytokines, influencing the tumor’s metabolism, proliferation, and spread[101]. They are expressed by several immune cells such as macrophages, dendritic cells, B lymphocytes, natural killer cells, non-immune cells such as epithelial cells, and cancer-associated fibroblasts[102]. When expressed in the tumor, TLRs can release cytokines and chemokines into the tumor environment to recruit other immune cells to release more proinflammatory cytokines, pro-angiogenic factors, and growth factors[101].
The TGF-β signaling pathways are pleiotropic, regulating multiple functions such as cell growth, differentiation, apoptosis, angiogenesis, motility, invasion, and immune response. Modifications in this pathway might play an essential role in developing tumors and metastasis. These modifications can affect not only the tumor cells but also the environment. At this level, the TGF-β generates an environment conducive to tumor growth and metastasis at all carcinogenesis stages. TGF-β has a contradictory behavior at the cellular level, acting as a suppressor and a tumor promoter[103,104]. Initially, the TGF-β pathway promotes cell cycle arrest and apoptosis. It promotes cancer cell motility, invasion, tumor progression, and metastasis in advanced stages. Thus, the accumulation of mutations is responsible for guiding the evolution from a suppressor pathway to a tumor promoter[105].
The HCC RNA sequencing study identified four subtypes of HCC using 212 samples. The pathway analyses using the expression data reveal the enriched pathways metabolism RNA processes such as RNA processing, binding, and splicing. Although all the samples are from HCC, this result indicates different gene expression, cell activity, and behaviors. These enriched processes are not shared by the four HCC groups funded. However, at least three groups shared translation, ribosome, metabolism of proteins, and cytoplasm ribosomal proteins[106]. The microarray analysis using 25 HCC samples identified thousands of differentially expressed genes, and the pathways of cell cycle response, DNA damage response, cell survival, and apoptosis were identified. In addition, it was also linked to pathway terms and poor prognosis clinical parameters. These results also agree with RNA sequencing study point transcriptional regulation, RNA processing, and cell cycle regulation. The single-cell RNA sequencing analysis indicates 119 genes associated with HCC. The pathways analysis using Gene Ontology showed an acute inflammatory response, oxidative stress, and humoral response. Simultaneously, the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways indicate IL-17 and TNF signaling pathways, infectious disease, and rheumatoid arthritis. These samples present more immunological functions[107]. According to the OncoVar database, the KEGG pathways associated with HCC are mainly cancer pathways, viral infection, cell longevity (growth and death), antineoplastic drug resistance, and transduction signaling pathways (Wnt and Hippo signaling pathways)[108]. The molecular pathways in HCC are not entirely understood, and these results showed a notable variation of response in the differentially expressed genes working together to express a function.
Analysis combining CRC and endometrial cancer microarray samples identified 139 genes upregulated in both studies. These genes operate in the cellular functions of cell proliferation, Wnt signaling pathway, fatty acid beta-oxidation, transcription, exocytosis, dopaminergic neuron differentiation, and platelet degranulation. The KEGG pathways enriched were tight junctions, rheumatoid arthritis, renal cell carcinoma, and cancer pathways signaling. The rheumatoid arthritis pathway was enriched in more than one study with the genes (ATP6V0D1, ATP6V1D, CD28, CTLA4, CTSK, FOS, IL-18, and JUN)[109]. Other microarray meta-analysis studies using CRC samples point to also the KEGG pathways related to the cell cycle, pathways in cancer, and the Wnt signaling pathway. These pathways are linked; as a result, they share proliferation and block apoptosis[65]. Together, these processes induce the normal cell to convert to a tumor cell.
The number of GI cancer projects in different OMIC levels found many genes working in tumorigenesis. The GI cancers discussed here sum 178 different genes with associated mutations. The number of genes with mutations associated with GI cancers ranges from 41 to 89 genes in HCC and GC.
Each of these cancers has variation and can be classified into subtypes according to cell origin, chromosomal structural rearrangements, gene expression, and cell behaviors. However, there are 46 genes shared by at least two types of cancers. These genes should be investigated to understand better how they assist in the cell transformations to tumors, biomarkers of tumor cells, and potential drug or therapy targets. The genes present in all five types of cancers are activin A receptor type 2A, APC, ARID1A, and CTNNB1.
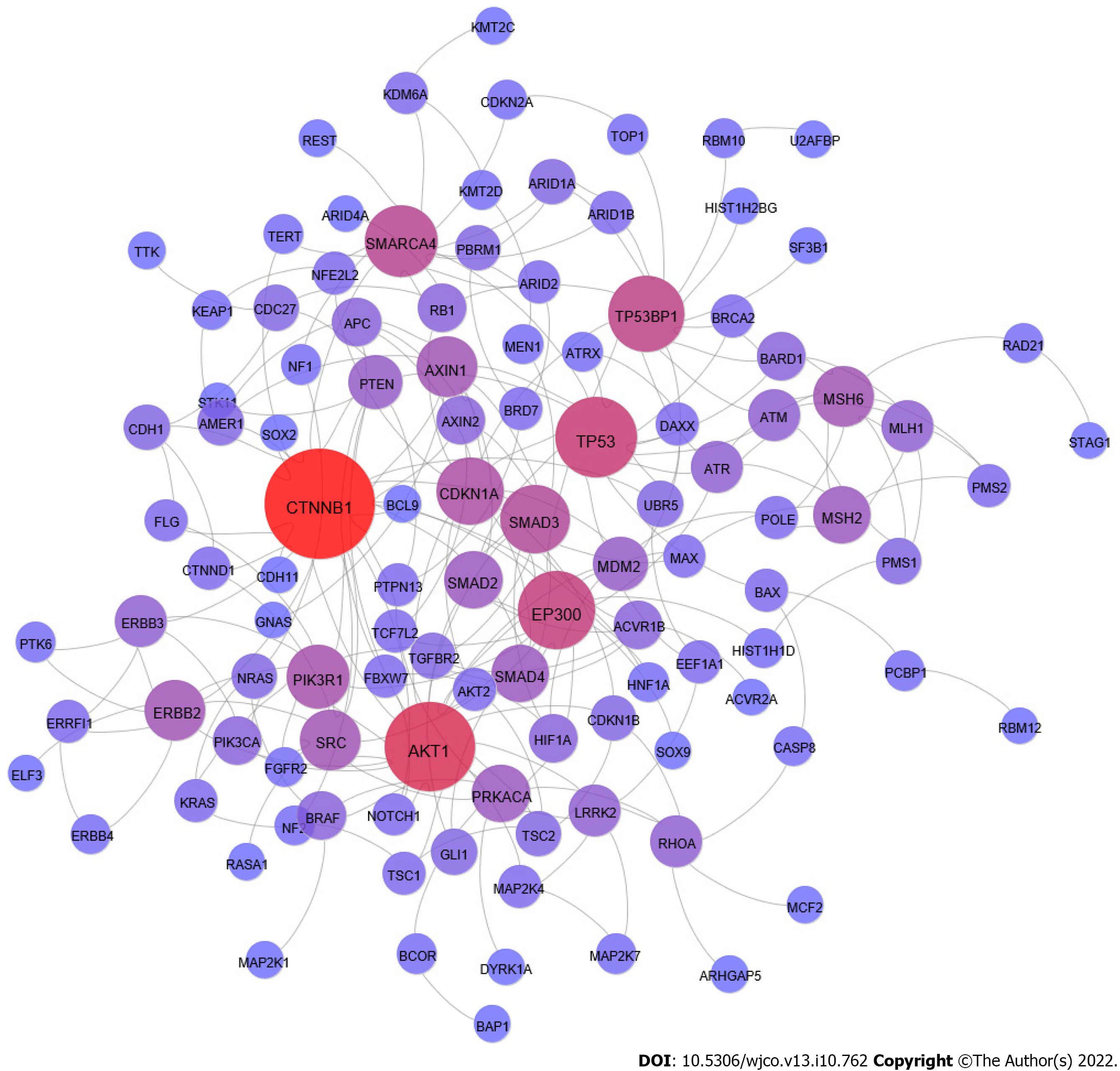
We used information from STRING database to check the protein-protein interaction (PPI) from these 178 genes. We used the experimental information only to build this PPI network. The PPI investigation allows for building a network with 111 genes connected (Figure 1)[110]. The number of nodes in the PPI network indicates that these genes work together in GI cancer tumorigenesis.
We analyzed the GI cancer network to identify in this PPI most connected protein (high degree) as central nodes. The proteins CTNNB1, Akt1, TP53, EP300, and TP53-binding protein 1 (53BP1) are the central nodes with the highest degree.
The CTNNB1 gene encodes a beta-catenin protein expressed in the adherens junctions[53]. The beta-catenin is a cytoplasm protein that works in the adhesion between cells. The beta-catenin binds the actin in the cytoskeleton and the E-cadherin protein in the cell membrane, connecting neighboring cells[111]. The beta-catenin is also a mediator in the Wnt signaling pathway. When activated, the Wnt signaling pathway induces the accumulation of beta-catenin in the nucleus, activating target genes' transcription[53]. The WNT protein binds the receptor in the membrane and induces beta-catenin to accumulate, promoting cell survival and proliferation[65]. The mutations in CTNNB1 gene are frequently found in HCC (13%)[112,113], CRC (6%)[114], and it is mutated in 4% of GC[47].
The Akt1 is a central protein in cell transduction signaling, which, when induced by PI3K, induces process cell proliferation, survival, and angiogenesis. The activation of the mammalian target of rapamycin (mTOR) complex by ATK is investigated as a drug target to treat PDAC[115-117]. The Epstein-Barr virus and H. pylori induce inflammation and the expression of Akt in GC. The outcome is cell proliferation and telomerase activation[118,119]. The investigation of blockage of Akt in GC resulted in suppression of growth and metastasis[120]. The investigation of critical proteins in HCC PPI identified several functions crucial in tumorigenesis, cell proliferation, anti-apoptosis, and metastasis. The PPI network showed Akt1 as a potential drug target[104]. These results indicate Akt1 central position in tumorigenesis and a potential drug target.
The 53BP1 protein has a role in DNA damage response and cycle arrest, triggering the expression of p53; the malfunctioning of this protein might lead to the development of genomic instability and molecular diseases. The lack of function of 53BP1 is associated with poor prognosis, angiogenesis, and metastasis[121]. The decreased expression of 53BP1 in CRC induces radiotolerance and chemoresistance. Moreover, CRC cells with lower expression of 53BP1 have a higher proliferating rate, decreased apoptosis, and poor prognosis[122-124]. The 53BP1 also interacts with p53, as indicated in CRC and EC, when the reduction of 53BP1 induces the downregulation of p53[122,123,125]. The 53BP1 is expressed as soon as DNA damage treatment occurs in human pancreatic cells[126]. The 53BP1 might also influence tumor outcome in pancreatic cancer, as shown when the variation of 53BP1 expression changes the association of carbohydrate 19-9, a well-known pancreatic cancer marker, and overall survival[100].
The p300 protein (encoded by the EP300 gene) is a histone acetyltransferase that participates in chromatin remodeling and interacts with basal transcriptional machinery to improve DNA binding, affecting gene transcription in normal and cancer cells[127]. The EP300 mutations are common in CRC and GC by frameshift in microsatellite regions[128]. The mutation in EP300 is frequent in EC (10%), and it correlates with a poor prognosis, associated with cell proliferation, migration, and invasion (metastasis)[129,130]. The role of p300 in remodeling the chromatin makes it appropriate to investigate epigenetic therapies, and the use of natural nutrients as potential prevention and treatment has already been discussed with GC[131].
All GI cancers discussed here have a low 5-year survival rate, except CRC (Table 1). The esophagus, liver, and pancreas have the lowest 5-year survival rate. The late diagnosis, metastasis, and aggressive behavior are associated with a low 5-year survival rate. Many studies describe the poor prognosis as associated with gene expression[97,122,129,132-135].
The expression levels are crucial information that might work as a prognostic factor in GI cancers. The association between TP53BP1 expression and overall survival analyses in CRC indicate a connection with low expression and low survival in the I-IIA stage, T3-T4, and N0[122]. Again, this protein has an essential role in CRC, not only to a high degree but also as a prognostic marker. The EP300 gene has high expression associated with poor survival in ESCC[129]. The long non-coding RNAs (lncRNAs) have a critical role in cancer development, and the high expression of ANRIL and homeobox A11-antisense RNA (HOXA11-AS) lncRNA is associated with poor survival in GC[132,133]. The overexpression of lncRNA ANRIL is significantly associated with GC progression and can serve as an independent predictor of patient survival[136]. The high expression of ANRIL combined with polycomb repressive complex 2 significantly silences microRNA 99a (miR-99a) and miR-449a at the transcriptional level, which increases the expression of mTOR, CDK6, and E2 transcription factor 1[132]. The HOXA11-AS gene reduces the expression of suppressor tumor genes Krüppel-like Factor 2 (KLF2) and protease serine 8 at the transcriptional level[133]. KLF2 downregulation is associated with migration, invasion, and poor survival[137,138]. KLF2 inhibits growth and migration and induces pancreatic cancer cells to senescence.
ESCC has poor survival when low esophageal cancer-related gene 4 expression occurs compared to the high-expression group[139]. EAC has worse overall survival when IL11 expression increases. Poor survival is also observed in a low expression of neuronal pentraxin 1, inositol 1,4,5-trisphosphate receptor type 1, and platelet derived growth factor D[140].
PDAC analyses show that high expression of the centromere protein F, sciellin, serpin family B member 5, solute carrier family 2 member 1 (SLC2A1), SLC6A14, transmembrane channel like 7, and transmembrane serine protease 4 is associated with a lower probability of survival compared to the same genes in low expression[141].
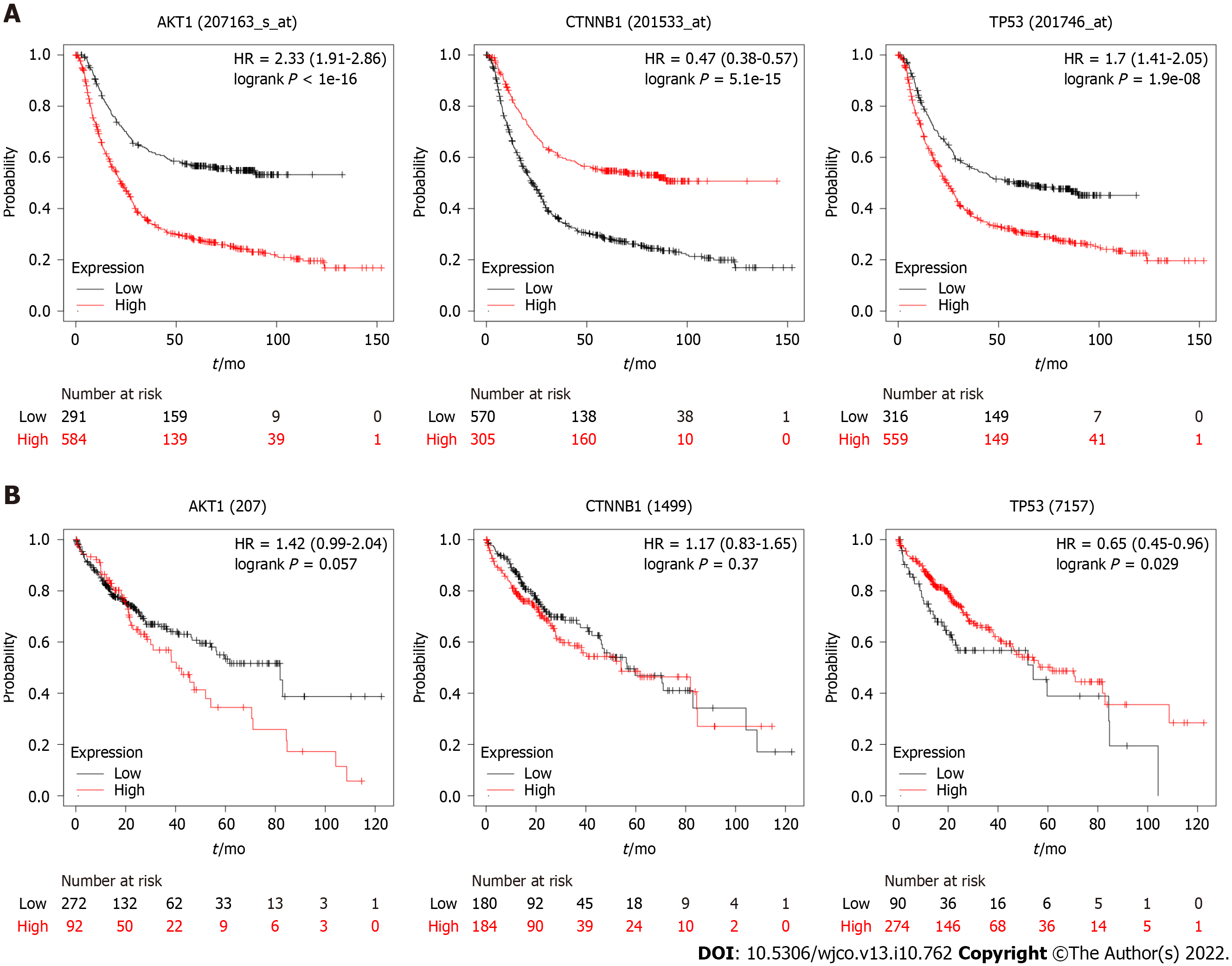
We investigated the gene expression and overall survival of the central genes present in the PPI network (Figure 1). We used information from the Kaplan Meier plotter (https://kmplot.com)[142] to investigate the potential prognosis of the central genes. Three of the five genes investigated have gene expression associated with survival (Akt1, TP53, and CTNNB1) (Figure 2).
The high expression of Akt1 and TP53 in GC is associated with a poor prognosis. In contrast, low CTNNB1 expression is correlated with reduced survival. The expression values and survival curves for TP53 (mRNA) in the Kaplan Meier plotter agree with tumor protein p53 expression in GC[143,144]. The TP53 expression is low and has a short half-life in normal cells, whereas in tumor cells, this gene has high expression and a long half-file[145]. The higher expression of TP53 is indicative of the worst prognosis. Akt1 expression was not indicative of prognosis[146]. However, they found that EGFR and Akt1 expression are mutually exclusive and associated with poor survival. This result might be due to the two proteins acting in the same pathway. The phosphorylated Akt1 and CTNNB1 high expression are associated with poor survival[147,148].
There is no significant difference between Akt1 or CTNNB1 high and low expression groups in liver cancer. Regarding the TP53 gene, the differences in expression are not significant in the initial stage of carcinoma. However, this high expression predicts a poor prognosis and a higher mortality rate than a low expression. The results are not according to the TP53 gene expression for HCC, where TP53 high expression is present in poor prognosis groups[149].
However, the prognosis markers based on expression have limitations, and the result must be taken together with other markers.
The OMIC information about GI cancer is very complex, and each organ/region has subtypes and particularities. We presented information about and brought to light the most common genomic changes among these cancers. The pathways shared by these molecular diseases also point to the standard functions and the crosstalk of these pathways and the PAK1 pathway centrality, connecting to MAPK/ERK, PI3K/Akt, apoptosis, and Wnt signaling pathways. The PPI network pointed to five central genes, and the literature corroborates the crucial role in GI cancer with expression and poor prognosis association. This information might help in the target choice of drug and therapy research.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wu ZQ, China S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | Ferlay J. Global Cancer Observatory: Cancer Today. 2020 [cited 2021-02-05] Available from: https://gco.iarc.fr/today/about. |
| 2. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 4877] [Article Influence: 696.7] [Reference Citation Analysis (1)] |
| 3. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15289] [Article Influence: 3057.8] [Reference Citation Analysis (4)] |
| 4. | Malhotra GK, Yanala U, Ravipati A, Follet M, Vijayakumar M, Are C. Global trends in esophageal cancer. J Surg Oncol. 2017;115:564-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 235] [Article Influence: 29.4] [Reference Citation Analysis (1)] |
| 5. | Gupta B, Kumar N. Worldwide incidence, mortality and time trends for cancer of the oesophagus. Eur J Cancer Prev. 2017;26:107-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 6. | Arnold M, Laversanne M, Brown LM, Devesa SS, Bray F. Predicting the Future Burden of Esophageal Cancer by Histological Subtype: International Trends in Incidence up to 2030. Am J Gastroenterol. 2017;112:1247-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 302] [Article Influence: 37.8] [Reference Citation Analysis (2)] |
| 7. | Middleton DRS, Bouaoun L, Hanisch R, Bray F, Dzamalala C, Chasimpha S, Menya D, Mbalawa CG, N'Da G, Woldegeorgis MA, Njie R, Koulibaly M, Buziba N, Ferro J, Nouhou H, Ogunbiyi F, Wabinga HR, Chokunonga E, Borok MZ, Korir AR, Mwasamwaja AO, Mmbaga BT, Schüz J, McCormack VA. Esophageal cancer male to female incidence ratios in Africa: A systematic review and meta-analysis of geographic, time and age trends. Cancer Epidemiol. 2018;53:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology. 2018;154:360-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1152] [Article Influence: 164.6] [Reference Citation Analysis (1)] |
| 9. | Abbas G, Krasna M. Overview of esophageal cancer. Ann Cardiothorac Surg. 2017;6:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 10. | Alsop BR, Sharma P. Esophageal Cancer. Gastroenterol Clin North Am. 2016;45:399-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 11. | Hesari A, Azizian M, Sheikhi A, Nesaei A, Sanaei S, Mahinparvar N, Derakhshani M, Hedayt P, Ghasemi F, Mirzaei H. Chemopreventive and therapeutic potential of curcumin in esophageal cancer: Current and future status. Int J Cancer. 2019;144:1215-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Huang FL, Yu SJ. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J Surg. 2018;41:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 535] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 13. | Salimian KJ, Waters KM, Eze O, Pezhouh MK, Tarabishy Y, Shin EJ, Canto MI, Voltaggio L, Montgomery EA. Definition of Barrett Esophagus in the United States: Support for Retention of a Requirement for Goblet Cells. Am J Surg Pathol. 2018;42:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Solanky D, Krishnamoorthi R, Crews N, Johnson M, Wang K, Wolfsen H, Fleischer D, Ramirez FC, Katzka D, Buttar N, Iyer PG. Barrett Esophagus Length, Nodularity, and Low-grade Dysplasia are Predictive of Progression to Esophageal Adenocarcinoma. J Clin Gastroenterol. 2019;53:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Horie Y, Yoshio T, Aoyama K, Yoshimizu S, Horiuchi Y, Ishiyama A, Hirasawa T, Tsuchida T, Ozawa T, Ishihara S, Kumagai Y, Fujishiro M, Maetani I, Fujisaki J, Tada T. Diagnostic outcomes of esophageal cancer by artificial intelligence using convolutional neural networks. Gastrointest Endosc. 2019;89:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 271] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 16. | Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 839] [Article Influence: 167.8] [Reference Citation Analysis (0)] |
| 17. | Boland CR, Yurgelun MB. Historical Perspective on Familial Gastric Cancer. Cell Mol Gastroenterol Hepatol. 2017;3:192-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 719] [Article Influence: 102.7] [Reference Citation Analysis (1)] |
| 19. | Yusefi AR, Bagheri Lankarani K, Bastani P, Radinmanesh M, Kavosi Z. Risk Factors for Gastric Cancer: A Systematic Review. Asian Pac J Cancer Prev. 2018;19:591-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 161] [Reference Citation Analysis (0)] |
| 20. | Sánchez-Zauco N, Torres J, Gómez A, Camorlinga-Ponce M, Muñoz-Pérez L, Herrera-Goepfert R, Medrano-Guzmán R, Giono-Cerezo S, Maldonado-Bernal C. Circulating blood levels of IL-6, IFN-γ, and IL-10 as potential diagnostic biomarkers in gastric cancer: a controlled study. BMC Cancer. 2017;17:384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Ramis IB, Vianna JS, Gonçalves CV, von Groll A, Dellagostin OA, da Silva PEA. Polymorphisms of the IL-6, IL-8 and IL-10 genes and the risk of gastric pathology in patients infected with Helicobacter pylori. J Microbiol Immunol Infect. 2017;50:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Arachchi PS, Fernando N, Weerasekera MM, Senevirathna B, Weerasekera DD, Gunasekara CP. Proinflammatory Cytokine IL-17 Shows a Significant Association with Helicobacter pylori Infection and Disease Severity. Gastroenterol Res Pract. 2017;2017:6265150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | International Agency for Research on Cancer (IARC). Infection with Helicobacter pylori. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group, Lyon, France. 1994: 177-240. |
| 24. | Moss SF. The Clinical Evidence Linking Helicobacter pylori to Gastric Cancer. Cell Mol Gastroenterol Hepatol. 2017;3:183-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 205] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 25. | Choi IJ, Kim CG, Lee JY, Kim YI, Kook MC, Park B, Joo J. Family History of Gastric Cancer and Helicobacter pylori Treatment. N Engl J Med. 2020;382:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 252] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 26. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3155] [Article Influence: 525.8] [Reference Citation Analysis (37)] |
| 27. | Liu J, Dang H, Wang XW. The significance of intertumor and intratumor heterogeneity in liver cancer. Exp Mol Med. 2018;50:e416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 28. | Rao CV, Asch AS, Yamada HY. Frequently mutated genes/pathways and genomic instability as prevention targets in liver cancer. Carcinogenesis. 2017;38:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 29. | Tunissiolli NM, Castanhole-Nunes MMU, Biselli-Chicote PM, Pavarino EC, da Silva RF, da Silva RC, Goloni-Bertollo EM. Hepatocellular Carcinoma: a Comprehensive Review of Biomarkers, Clinical Aspects, and Therapy. Asian Pac J Cancer Prev. 2017;18:863-872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |
| 30. | Chen M, Wei L, Law CT, Tsang FH, Shen J, Cheng CL, Tsang LH, Ho DW, Chiu DK, Lee JM, Wong CC, Ng IO, Wong CM. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology. 2018;67:2254-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 971] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 31. | Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1650] [Article Influence: 330.0] [Reference Citation Analysis (1)] |
| 32. | Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10:10-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 1511] [Article Influence: 251.8] [Reference Citation Analysis (1)] |
| 33. | Pietrasz D, Pécuchet N, Garlan F, Didelot A, Dubreuil O, Doat S, Imbert-Bismut F, Karoui M, Vaillant JC, Taly V, Laurent-Puig P, Bachet JB. Plasma Circulating Tumor DNA in Pancreatic Cancer Patients Is a Prognostic Marker. Clin Cancer Res. 2017;23:116-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 194] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 34. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1338] [Cited by in RCA: 1254] [Article Influence: 179.1] [Reference Citation Analysis (39)] |
| 35. | Chaffee KG, Oberg AL, McWilliams RR, Majithia N, Allen BA, Kidd J, Singh N, Hartman AR, Wenstrup RJ, Petersen GM. Prevalence of germ-line mutations in cancer genes among pancreatic cancer patients with a positive family history. Genet Med. 2018;20:119-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 36. | Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14:89-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 1059] [Article Influence: 176.5] [Reference Citation Analysis (1)] |
| 37. | Mattiuzzi C, Sanchis-Gomar F, Lippi G. Concise update on colorectal cancer epidemiology. Ann Transl Med. 2019;7:609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 38. | Hadjipetrou A, Anyfantakis D, Galanakis CG, Kastanakis M, Kastanakis S. Colorectal cancer, screening and primary care: A mini literature review. World J Gastroenterol. 2017;23:6049-6058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 39. | Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 520] [Cited by in RCA: 900] [Article Influence: 112.5] [Reference Citation Analysis (2)] |
| 40. | Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ, Watanabe T. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1014] [Cited by in RCA: 1115] [Article Influence: 111.5] [Reference Citation Analysis (0)] |
| 41. | Wang Z, Li C, Li Y, Guo X, Yan Z, Gao F. DpdtbA-Induced Growth Inhibition in Human Esophageal Cancer Cells Involved Inactivation of the p53/EGFR/AKT Pathway. Oxid Med Cell Longev. 2019;2019:5414670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Cuzziol CI, Castanhole-Nunes MMU, Pavarino ÉC, Goloni-Bertollo EM. MicroRNAs as regulators of VEGFA and NFE2L2 in cancer. Gene. 2020;759:144994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Li Y, Xiao F, Li W, Hu P, Xu R, Li J, Li G, Zhu C. Overexpression of Opa interacting protein 5 increases the progression of liver cancer via BMPR2/JUN/CHEK1/RAC1 dysregulation. Oncol Rep. 2019;41:2075-2088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Natsuizaka M, Whelan KA, Kagawa S, Tanaka K, Giroux V, Chandramouleeswaran PM, Long A, Sahu V, Darling DS, Que J, Yang Y, Katz JP, Wileyto EP, Basu D, Kita Y, Natsugoe S, Naganuma S, Klein-Szanto AJ, Diehl JA, Bass AJ, Wong KK, Rustgi AK, Nakagawa H. Interplay between Notch1 and Notch3 promotes EMT and tumor initiation in squamous cell carcinoma. Nat Commun. 2017;8:1758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 45. | Zhang F, Zhang M. Oleuropein inhibits esophageal cancer through hypoxic suppression of BTG3 mRNA. Food Funct. 2019;10:978-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Salem ME, Puccini A, Xiu J, Raghavan D, Lenz HJ, Korn WM, Shields AF, Philip PA, Marshall JL, Goldberg RM. Comparative Molecular Analyses of Esophageal Squamous Cell Carcinoma, Esophageal Adenocarcinoma, and Gastric Adenocarcinoma. Oncologist. 2018;23:1319-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 47. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4833] [Article Influence: 439.4] [Reference Citation Analysis (2)] |
| 48. | Ebrahimi V, Soleimanian A, Ebrahimi T, Azargun R, Yazdani P, Eyvazi S, Tarhriz V. Epigenetic modifications in gastric cancer: Focus on DNA methylation. Gene. 2020;742:144577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 49. | Zhou X, Jiao D, Dou M, Zhang W, Hua H, Chen J, Li Z, Li L, Han X. Association of APC gene promoter methylation and the risk of gastric cancer: A meta-analysis and bioinformatics study. Medicine (Baltimore). 2020;99:e19828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Matsuoka T, Yashiro M. Biomarkers of gastric cancer: Current topics and future perspective. World J Gastroenterol. 2018;24:2818-2832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 230] [Cited by in RCA: 317] [Article Influence: 45.3] [Reference Citation Analysis (7)] |
| 51. | Kong R, Yi F, Wen P, Liu J, Chen X, Ren J, Li X, Shang Y, Nie Y, Wu K, Fan D, Zhu L, Feng W, Wu JY. Myo9b is a key player in SLIT/ROBO-mediated lung tumor suppression. J Clin Invest. 2015;125:4407-4420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 52. | Chen Y, Yang Z, Meng M, Zhao Y, Dong N, Yan H, Liu L, Ding M, Peng HB, Shao F. Cullin mediates degradation of RhoA through evolutionarily conserved BTB adaptors to control actin cytoskeleton structure and cell movement. Mol Cell. 2009;35:841-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 222] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 53. | Tang Y, Liu Z, Zhao L, Clemens TL, Cao X. Smad7 stabilizes beta-catenin binding to E-cadherin complex and promotes cell-cell adhesion. J Biol Chem. 2008;283:23956-23963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | Lin G, Aranda V, Muthuswamy SK, Tonks NK. Identification of PTPN23 as a novel regulator of cell invasion in mammary epithelial cells from a loss-of-function screen of the 'PTP-ome'. Genes Dev. 2011;25:1412-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 55. | Lorger M, Moelling K. Regulation of epithelial wound closure and intercellular adhesion by interaction of AF6 with actin cytoskeleton. J Cell Sci. 2006;119:3385-3398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Jesse S, Koenig A, Ellenrieder V, Menke A. Lef-1 isoforms regulate different target genes and reduce cellular adhesion. Int J Cancer. 2010;126:1109-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Castelli G, Pelosi E, Testa U. Liver Cancer: Molecular Characterization, Clonal Evolution and Cancer Stem Cells. Cancers (Basel). 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 58. | Sia D, Villanueva A, Friedman SL, Llovet JM. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology. 2017;152:745-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 829] [Article Influence: 103.6] [Reference Citation Analysis (2)] |
| 59. | Knudsen ES, Balaji U, Mannakee B, Vail P, Eslinger C, Moxom C, Mansour J, Witkiewicz AK. Pancreatic cancer cell lines as patient-derived avatars: genetic characterisation and functional utility. Gut. 2018;67:508-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 60. | Waters AM, Der CJ. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb Perspect Med. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 595] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 61. | Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, Quinn MC, Robertson AJ, Fadlullah MZ, Bruxner TJ, Christ AN, Harliwong I, Idrisoglu S, Manning S, Nourse C, Nourbakhsh E, Wani S, Wilson PJ, Markham E, Cloonan N, Anderson MJ, Fink JL, Holmes O, Kazakoff SH, Leonard C, Newell F, Poudel B, Song S, Taylor D, Waddell N, Wood S, Xu Q, Wu J, Pinese M, Cowley MJ, Lee HC, Jones MD, Nagrial AM, Humphris J, Chantrill LA, Chin V, Steinmann AM, Mawson A, Humphrey ES, Colvin EK, Chou A, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Pettitt JA, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N, Jamieson NB, Graham JS, Niclou SP, Bjerkvig R, Grützmann R, Aust D, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Falconi M, Zamboni G, Tortora G, Tempero MA; Australian Pancreatic Cancer Genome Initiative, Gill AJ, Eshleman JR, Pilarsky C, Scarpa A, Musgrove EA, Pearson JV, Biankin AV, Grimmond SM. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2130] [Cited by in RCA: 1982] [Article Influence: 198.2] [Reference Citation Analysis (1)] |
| 62. | Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grützmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM; Australian Pancreatic Cancer Genome Initiative, Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Waddell N, Biankin AV, Grimmond SM. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2848] [Cited by in RCA: 2539] [Article Influence: 282.1] [Reference Citation Analysis (0)] |
| 63. | Collisson EA, Bailey P, Chang DK, Biankin AV. Molecular subtypes of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2019;16:207-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 589] [Article Influence: 98.2] [Reference Citation Analysis (0)] |
| 64. | Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2017;32:185-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1377] [Article Influence: 172.1] [Reference Citation Analysis (0)] |
| 65. | Centelles JJ. General aspects of colorectal cancer. ISRN Oncol. 2012;2012:139268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 66. | Simons CC, Hughes LA, Smits KM, Khalid-de Bakker CA, de Bruïne AP, Carvalho B, Meijer GA, Schouten LJ, van den Brandt PA, Weijenberg MP, van Engeland M. A novel classification of colorectal tumors based on microsatellite instability, the CpG island methylator phenotype and chromosomal instability: implications for prognosis. Ann Oncol. 2013;24:2048-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 67. | Phipps AI, Limburg PJ, Baron JA, Burnett-Hartman AN, Weisenberger DJ, Laird PW, Sinicrope FA, Rosty C, Buchanan DD, Potter JD, Newcomb PA. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology. 2015;148:77-87.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 324] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 68. | Wielandt AM, Hurtado C, Moreno C M, Villarroel C, Castro M, Estay M, Simian D, Martinez M, Vial MT, Kronberg U, López-Köstner F. Characterization of Chilean patients with sporadic colorectal cancer according to the three main carcinogenic pathways: Microsatellite instability, CpG island methylator phenotype and Chromosomal instability. Tumour Biol. 2020;42:1010428320938492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 69. | Dai F, Mei L, Meng S, Ma Z, Guo W, Zhou J, Zhang J. The global expression profiling in esophageal squamous cell carcinoma. Genomics. 2017;109:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Zhang C, Peng L, Zhang Y, Liu Z, Li W, Chen S, Li G. The identification of key genes and pathways in hepatocellular carcinoma by bioinformatics analysis of high-throughput data. Med Oncol. 2017;34:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 71. | Zhou W, Wu J, Zhang J, Liu X, Guo S, Jia S, Zhang X, Zhu Y, Wang M. Integrated bioinformatics analysis to decipher molecular mechanism of compound Kushen injection for esophageal cancer by combining WGCNA with network pharmacology. Sci Rep. 2020;10:12745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 72. | Nam S, Park T. Pathway-based evaluation in early onset colorectal cancer suggests focal adhesion and immunosuppression along with epithelial-mesenchymal transition. PLoS One. 2012;7:e31685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 73. | Gu C, Wang X, Long T, Zhong Y, Ma Y, Hu Z, Li Z. FSTL1 interacts with VIM and promotes colorectal cancer metastasis via activating the focal adhesion signalling pathway. Cell Death Dis. 2018;9:654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 74. | Donahue TR, Tran LM, Hill R, Li Y, Kovochich A, Calvopina JH, Patel SG, Wu N, Hindoyan A, Farrell JJ, Li X, Dawson DW, Wu H. Integrative survival-based molecular profiling of human pancreatic cancer. Clin Cancer Res. 2012;18:1352-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 75. | Badea L, Herlea V, Dima SO, Dumitrascu T, Popescu I. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology. 2008;55:2016-2027. [PubMed] |
| 76. | Wu J, Zhao X, Lin Z, Shao Z. A system level analysis of gastric cancer across tumor stages with RNA-seq data. Mol Biosyst. 2015;11:1925-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 77. | Akbari F, Peymani M, Salehzadeh A, Ghaedi K. Integrative in silico and in vitro transcriptomics analysis revealed new lncRNAs related to intrinsic apoptotic genes in colorectal cancer. Cancer Cell Int. 2020;20:546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Zhang W, Liu S, Zhan H, Yan Z, Zhang G. Transcriptome sequencing identifies key pathways and genes involved in gastric adenocarcinoma. Mol Med Rep. 2018;18:3673-3682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 79. | Hao Y, Zhang J, Shan G, Zhang N, Jin W, Nan K. Establishment of optimal regulatory network of colorectal cancer based on p42.3 protein. Saudi J Biol Sci. 2017;24:1781-1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 80. | Mahlamäki EH, Kauraniemi P, Monni O, Wolf M, Hautaniemi S, Kallioniemi A. High-resolution genomic and expression profiling reveals 105 putative amplification target genes in pancreatic cancer. Neoplasia. 2004;6:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 81. | Fang ZP, Jiang BG, Gu XF, Zhao B, Ge RL, Zhang FB. P21-activated kinase 5 plays essential roles in the proliferation and tumorigenicity of human hepatocellular carcinoma. Acta Pharmacol Sin. 2014;35:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Chen H, Miao J, Li H, Wang C, Li J, Zhu Y, Wang J, Wu X, Qiao H. Expression and prognostic significance of p21-activated kinase 6 in hepatocellular carcinoma. J Surg Res. 2014;189:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 83. | Huang S, Zhu Y, Wang C, Li X, Cui X, Tu S, You L, Fu J, Chen Z, Hu W, Gong W. PAK5 facilitates the proliferation, invasion and migration in colorectal cancer cells. Cancer Med. 2020;9:4777-4790. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Ausborn NL, Wang T, Wentz SC, Washington MK, Merchant NB, Zhao Z, Shyr Y, Chakravarthy AB, Xia F. 53BP1 expression is a modifier of the prognostic value of lymph node ratio and CA 19-9 in pancreatic adenocarcinoma. BMC Cancer. 2013;13:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 85. | Ding X, Duan H, Luo H. Identification of Core Gene Expression Signature and Key Pathways in Colorectal Cancer. Front Genet. 2020;11:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 86. | Wang WF, Xie Y, Zhou ZH, Qin ZH, Wu JC, He JK. PIK3CA hypomethylation plays a key role in activation of the PI3K/AKT pathway in esophageal cancer in Chinese patients. Acta Pharmacol Sin. 2013;34:1560-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 87. | Javle M, Li Y, Tan D, Dong X, Chang P, Kar S, Li D. Biomarkers of TGF-β signaling pathway and prognosis of pancreatic cancer. PLoS One. 2014;9:e85942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 88. | Li CW, Chang PY, Chen BS. Investigating the mechanism of hepatocellular carcinoma progression by constructing genetic and epigenetic networks using NGS data identification and big database mining method. Oncotarget. 2016;7:79453-79473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 89. | Zang S, Guo R, Xing R, Zhang L, Li W, Zhao M, Fang J, Hu F, Kang B, Ren Y, Zhuang Y, Liu S, Wang R, Li X, Yu Y, Cheng J, Lu Y. Identification of differentially-expressed genes in intestinal gastric cancer by microarray analysis. Genomics Proteomics Bioinformatics. 2014;12:276-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 90. | Meng XR, Lu P, Mei JZ, Liu GJ, Fan QX. Expression analysis of miRNA and target mRNAs in esophageal cancer. Braz J Med Biol Res. 2014;47:811-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 91. | Cui X, Xin H, Peng H, Chen Y. Comprehensive bioinformatics analysis of the mRNA profile of PLCE1 knockdown in esophageal squamous cell carcinoma. Mol Med Rep. 2017;16:5871-5880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 92. | Fels Elliott DR, Perner J, Li X, Symmons MF, Verstak B, Eldridge M, Bower L, O'Donovan M, Gay NJ; OCCAMS Consortium, Fitzgerald RC. Impact of mutations in Toll-like receptor pathway genes on esophageal carcinogenesis. PLoS Genet. 2017;13:e1006808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 93. | Zali H, Rezaei-Tavirani M, Vafaee R. Gastric cardia adenocarcinoma pathway analysis. Gastroenterol Hepatol Bed Bench. 2013;6:S11-S18. [PubMed] |
| 94. | Jan R, Chaudhry GE. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv Pharm Bull. 2019;9:205-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 533] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 95. | Khan KH, Blanco-Codesido M, Molife LR. Cancer therapeutics: Targeting the apoptotic pathway. Crit Rev Oncol Hematol. 2014;90:200-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 96. | Baig S, Seevasant I, Mohamad J, Mukheem A, Huri HZ, Kamarul T. Potential of apoptotic pathway-targeted cancer therapeutic research: Where do we stand? Cell Death Dis. 2016;7:e2058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 209] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 97. | Ashkenazi A. Targeting the extrinsic apoptotic pathway in cancer: lessons learned and future directions. J Clin Invest. 2015;125:487-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 192] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 98. | Tse EY, Ching YP. The role of p21-activated kinases in hepatocellular carcinoma metastasis. J Mol Signal. 2014;9:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | He H, Huynh N. p21-activated kinase family: promising new drug targets. Res Reports Biochem. 2015;5:119-128. [DOI] [Full Text] |
| 100. | Jin S, Zhuo Y, Guo W, Field J. p21-activated Kinase 1 (Pak1)-dependent phosphorylation of Raf-1 regulates its mitochondrial localization, phosphorylation of BAD, and Bcl-2 association. J Biol Chem. 2005;280:24698-24705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 101. | Cen X, Liu S, Cheng K. The Role of Toll-Like Receptor in Inflammation and Tumor Immunity. Front Pharmacol. 2018;9:878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 102. | Smith M, García-Martínez E, Pitter MR, Fucikova J, Spisek R, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Toll-like receptor agonists in cancer immunotherapy. Oncoimmunology. 2018;7:e1526250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 103. | Lebrun JJ. The Dual Role of TGFβ in Human Cancer: From Tumor Suppression to Cancer Metastasis. ISRN Mol Biol. 2012;2012:381428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 255] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 104. | Sun N, Taguchi A, Hanash S. Switching Roles of TGF-β in Cancer Development: Implications for Therapeutic Target and Biomarker Studies. J Clin Med. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 105. | Neuzillet C, Tijeras-Raballand A, Cohen R, Cros J, Faivre S, Raymond E, de Gramont A. Targeting the TGFβ pathway for cancer therapy. Pharmacol Ther. 2015;147:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 492] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 106. | Xue H, Luo L, Yao YT, Wei LL, Deng SP, Huang XL. Integrated analysis of the RNA-Seq data of liver hepatocellular carcinoma. Neoplasma. 2018;65:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 107. | Zhang S, Liu Z, Wu D, Chen L, Xie L. Single-Cell RNA-Seq Analysis Reveals Microenvironmental Infiltration of Plasma Cells and Hepatocytic Prognostic Markers in HCC With Cirrhosis. Front Oncol. 2020;10:596318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 108. | Wang T, Ruan S, Zhao X, Shi X, Teng H, Zhong J, You M, Xia K, Sun Z, Mao F. OncoVar: an integrated database and analysis platform for oncogenic driver variants in cancers. Nucleic Acids Res. 2021;49:D1289-D1301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 109. | Rahman F, Mahmud P, Karim R, Hossain T, Islam F. Determination of novel biomarkers and pathways shared by colorectal cancer and endometrial cancer via comprehensive bioinformatics analysis. Informatics Med Unlocked. 2020;20:100376. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 110. | Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607-D613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10161] [Cited by in RCA: 11676] [Article Influence: 1946.0] [Reference Citation Analysis (1)] |
| 111. | Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans. 2008;36:149-155. [RCA] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 308] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 112. | Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C, Shinde J, Soysouvanh F, Calatayud AL, Pinyol R, Pelletier L, Balabaud C, Laurent A, Blanc JF, Mazzaferro V, Calvo F, Villanueva A, Nault JC, Bioulac-Sage P, Stratton MR, Llovet JM, Zucman-Rossi J. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 1336] [Article Influence: 133.6] [Reference Citation Analysis (0)] |
| 113. | Kim S, Jeong S. Mutation Hotspots in the β-Catenin Gene: Lessons from the Human Cancer Genome Databases. Mol Cells. 2019;42:8-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 67] [Reference Citation Analysis (0)] |
| 114. | Alomar SY, Mansour L, Abuderman A, Alkhuriji A, Arafah M, Alwasel S, Harrath AH, Almutairi M, Trayhyrn P, Dar JA. β-Catenin accumulation and S33F mutation of CTNNB1 gene in colorectal cancer in Saudi Arabia. Pol J Pathol. 2016;67:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 115. | Conway JR, Herrmann D, Evans TJ, Morton JP, Timpson P. Combating pancreatic cancer with PI3K pathway inhibitors in the era of personalised medicine. Gut. 2019;68:742-758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 116. | Jiang BH, Liu LZ. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim Biophys Acta. 2008;1784:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 117. | Ebrahimi S, Hosseini M, Shahidsales S, Maftouh M, Ferns GA, Ghayour-Mobarhan M, Hassanian SM, Avan A. Targeting the Akt/PI3K Signaling Pathway as a Potential Therapeutic Strategy for the Treatment of Pancreatic Cancer. Curr Med Chem. 2017;24:1321-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 118. | Fattahi S, Amjadi-Moheb F, Tabaripour R, Ashrafi GH, Akhavan-Niaki H. PI3K/AKT/mTOR signaling in gastric cancer: Epigenetics and beyond. Life Sci. 2020;262:118513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 246] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 119. | Sasaki T, Yamashita Y, Kuniyasu H. AKT plays a crucial role in gastric cancer. Oncol Lett. 2015;10:607-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 120. | Ye B, Jiang LL, Xu HT, Zhou DW, Li ZS. Expression of PI3K/AKT pathway in gastric cancer and its blockade suppresses tumor growth and metastasis. Int J Immunopathol Pharmacol. 2012;25:627-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 121. | Mirza-Aghazadeh-Attari M, Mohammadzadeh A, Yousefi B, Mihanfar A, Karimian A, Majidinia M. 53BP1: A key player of DNA damage response with critical functions in cancer. DNA Repair (Amst). 2019;73:110-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 122. | Bi J, Huang A, Liu T, Zhang T, Ma H. Expression of DNA damage checkpoint 53BP1 is correlated with prognosis, cell proliferation and apoptosis in colorectal cancer. Int J Clin Exp Pathol. 2015;8:6070-6082. [PubMed] |
| 123. | Xiao Y, Zheng X, Huang A, Liu T, Zhang T, Ma H. Deficiency of 53BP1 inhibits the radiosensitivity of colorectal cancer. Int J Oncol. 2016;49:1600-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 124. | Yao J, Huang A, Zheng X, Liu T, Lin Z, Zhang S, Yang Q, Zhang T, Ma H. 53BP1 loss induces chemoresistance of colorectal cancer cells to 5-fluorouracil by inhibiting the ATM-CHK2-P53 pathway. J Cancer Res Clin Oncol. 2017;143:419-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 125. | Yang J, Jing L, Liu CJ, Bai WW, Zhu SC. 53BP1 regulates cell cycle arrest in esophageal cancer model. Eur Rev Med Pharmacol Sci. 2019;23:604-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 126. | Miwa S, Tome Y, Yano S, Hiroshima Y, Uehara F, Mii S, Kimura H, Hayashi K, Tsuchiya H, Bouvet M, Efimova EV, Hoffman RM. Single cell time-lapse imaging of focus formation by the DNA damage-response protein 53BP1 after UVC irradiation of human pancreatic cancer cells. Anticancer Res. 2013;33:1373-1377. [PubMed] |
| 127. | Attar N, Kurdistani SK. Exploitation of EP300 and CREBBP Lysine Acetyltransferases by Cancer. Cold Spring Harb Perspect Med. 2017;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 128. | Kim MS, Lee SH, Yoo NJ. Frameshift mutations of tumor suppressor gene EP300 in gastric and colorectal cancers with high microsatellite instability. Hum Pathol. 2013;44:2064-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 129. | Bi Y, Kong P, Zhang L, Cui H, Xu X, Chang F, Yan T, Li J, Cheng C, Song B, Niu X, Liu X, Xu E, Hu X, Qian Y, Wang F, Li H, Ma Y, Yang J, Liu Y, Zhai Y, Wang Y, Zhang Y, Liu H, Liu J, Wang J, Cui Y, Cheng X. EP300 as an oncogene correlates with poor prognosis in esophageal squamous carcinoma. J Cancer. 2019;10:5413-5426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 130. | Gao YB, Chen ZL, Li JG, Hu XD, Shi XJ, Sun ZM, Zhang F, Zhao ZR, Li ZT, Liu ZY, Zhao YD, Sun J, Zhou CC, Yao R, Wang SY, Wang P, Sun N, Zhang BH, Dong JS, Yu Y, Luo M, Feng XL, Shi SS, Zhou F, Tan FW, Qiu B, Li N, Shao K, Zhang LJ, Xue Q, Gao SG, He J. Genetic landscape of esophageal squamous cell carcinoma. Nat Genet. 2014;46:1097-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 549] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 131. | Calcagno DQ, Wisnieski F, Mota ERDS, Maia de Sousa SB, Costa da Silva JM, Leal MF, Gigek CO, Santos LC, Rasmussen LT, Assumpção PP, Burbano RR, Smith MA. Role of histone acetylation in gastric cancer: implications of dietetic compounds and clinical perspectives. Epigenomics. 2019;11:349-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 132. | Zhang EB, Kong R, Yin DD, You LH, Sun M, Han L, Xu TP, Xia R, Yang JS, De W, Chen Jf. Long noncoding RNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing of miR-99a/miR-449a. Oncotarget. 2014;5:2276-2292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 307] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 133. | Sun M, Nie F, Wang Y, Zhang Z, Hou J, He D, Xie M, Xu L, De W, Wang Z, Wang J. LncRNA HOXA11-AS Promotes Proliferation and Invasion of Gastric Cancer by Scaffolding the Chromatin Modification Factors PRC2, LSD1, and DNMT1. Cancer Res. 2016;76:6299-6310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 404] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 134. | Mori Y, Ishiguro H, Kuwabara Y, Kimura M, Mitsui A, Kurehara H, Mori R, Tomoda K, Ogawa R, Katada T, Harata K, Fujii Y. Expression of ECRG4 is an independent prognostic factor for poor survival in patients with esophageal squamous cell carcinoma. Oncol Rep. 2007;18:981-985. [PubMed] |
| 135. | Chen HW, Huang XD, Li HC, He S, Ni RZ, Chen CH, Peng C, Wu G, Wang GH, Wang YY, Zhao YH, Zhang YX, Shen AG, Wang HM. Expression of FOXJ1 in hepatocellular carcinoma: correlation with patients' prognosis and tumor cell proliferation. Mol Carcinog. 2013;52:647-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 136. | Hao NB, He YF, Li XQ, Wang K, Wang RL. The role of miRNA and lncRNA in gastric cancer. Oncotarget. 2017;8:81572-81582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 187] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 137. | Zhang J, Li G, Feng L, Lu H, Wang X. Krüppel-like factors in breast cancer: Function, regulation and clinical relevance. Biomed Pharmacother. 2020;123:109778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 138. | Wang B, Liu M, Song Y, Li C, Zhang S, Ma L. KLF2 Inhibits the Migration and Invasion of Prostate Cancer Cells by Downregulating MMP2. Am J Mens Health. 2019;13:1557988318816907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 139. | Ge S, Xu Y, Wang H, Sun Y, Tian X, Cao Z, Lin X, Xu J, Wang Q. Downregulation of esophageal cancer-related gene 4 promotes proliferation and migration of hepatocellular carcinoma. Oncol Lett. 2017;14:3689-3696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 140. | Zhao M, Wang J, Yuan M, Ma Z, Bao Y, Hui Z. Multivariate gene expression-based survival predictor model in esophageal adenocarcinoma. Thorac Cancer. 2020;11:2896-2908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 141. | Cheng Y, Wang K, Geng L, Sun J, Xu W, Liu D, Gong S, Zhu Y. Identification of candidate diagnostic and prognostic biomarkers for pancreatic carcinoma. EBioMedicine. 2019;40:382-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 142. | Lánczky A, Győrffy B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J Med Internet Res. 2021;23:e27633. [PubMed] [DOI] [Full Text] |
| 143. | Hiralal Sankalecha T, Jagdish Gupta S, Rangrao Gaikwad N, Narayanrao Ughade S. Correlation of P53 Expression with Various Clinicopathological Parameters of Gastric Carcinoma and Its Relationship with Survival. J Gastroenterol Hepatol Res. 2017;6:2370-2375. [DOI] [Full Text] |
| 144. | Hwang HJ, Nam SK, Park H, Park Y, Koh J, Na HY, Kwak Y, Kim WH, Lee HS. Prediction of TP53 mutations by p53 immunohistochemistry and their prognostic significance in gastric cancer. J Pathol Transl Med. 2020;54:378-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 145. | Ozaki T, Nakagawara A. Role of p53 in Cell Death and Human Cancers. Cancers (Basel). 2011;3:994-1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 321] [Cited by in RCA: 476] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 146. | Petrini I, Lencioni M, Vasile E, Fornaro L, Belluomini L, Pasquini G, Ginocchi L, Caparello C, Musettini G, Vivaldi C, Caponi S, Ricci S, Proietti A, Fontanini G, Naccarato AG, Nardini V, Santi S, Falcone A. EGFR and AKT1 overexpression are mutually exclusive and associated with a poor survival in resected gastric adenocarcinomas. Cancer Biomark. 2018;21:731-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 147. | Zheng CH, Wang JB, Lin MQ, Zhang PY, Liu LC, Lin JX, Lu J, Chen QY, Cao LL, Lin M, Tu RH, Xie JW, Li P, Huang CM. CDK5RAP3 suppresses Wnt/β-catenin signaling by inhibiting AKT phosphorylation in gastric cancer. J Exp Clin Cancer Res. 2018;37:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 148. | Di Bartolomeo M, Pietrantonio F, Pellegrinelli A, Martinetti A, Mariani L, Daidone MG, Bajetta E, Pelosi G, de Braud F, Floriani I, Miceli R. Osteopontin, E-cadherin, and β-catenin expression as prognostic biomarkers in patients with radically resected gastric cancer. Gastric Cancer. 2016;19:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 149. | Li Z, Han C, Feng J. Relationship of the expression levels of XIAP and p53 genes in hepatocellular carcinoma and the prognosis of patients. Oncol Lett. 2017;14:4037-4042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |