Published online Aug 24, 2021. doi: 10.5306/wjco.v12.i8.702
Peer-review started: May 10, 2021
First decision: June 5, 2021
Revised: June 18, 2021
Accepted: July 12, 2021
Article in press: July 12, 2021
Published online: August 24, 2021
Processing time: 105 Days and 3.6 Hours
Currently, the detection of PIK3CA mutations is of special interest in personalized medicine because it is frequently found in triple-negative breast cancer (TNBC). The PI3KCA mutation is an independent negative prognostic factor for survival in metastatic breast cancer, and its prognostic value in liquid biopsy as a biomarker of treatment and early relapse is under investigation, both for metastatic disease and neoadjuvant scenario with curative intent.
A 54-year-old female patient with TNBC clinical stage IIIA, who, after receiving neoadjuvant chemotherapy (based on anthracyclines and taxanes), surgery, radiotherapy, and adjuvant capecitabine, was detected with a PI3KCA mutation in tissue and peripheral blood (ctDNA in liquid biopsy). After 10 mo, the patient had disease relapse of left cervical node disease.
The detection of PIK3CA mutation in TNBC after neoadjuvant treatment might be associated with early relapse or rapid disease progression.
Core Tip: This case report evaluates the detection of a PIK3CA mutation in liquid biopsy and tumor tissue in a patient with locally-advanced triple-negative breast cancer after receiving conventional oncological therapy and its association with early relapse and progression disease. This case highlights the importance of detecting the PIK3CA mutation as a potential biomarker of early relapse. In addition, the PIK3CA mutation could lead to additional interventions to detect metastatic disease earlier in the follow-up. There is limited information of liquid biopsy studies after surgery. Furthermore, there is very limited data about trials of PIK3CA mutations in Peruvian patients with non-metastatic breast cancer.
- Citation: Valencia GA, Rioja P, Morante Z, Araujo JM, Vallejos HD, Guerra H, Gomez HL. PIK3CA mutation in non-metastatic triple-negative breast cancer as a potential biomarker of early relapse: A case report. World J Clin Oncol 2021; 12(8): 702-711
- URL: https://www.wjgnet.com/2218-4333/full/v12/i8/702.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i8.702
Triple-negative breast cancer (TNBC) is a highly heterogeneous disease, with limited options of medical treatment. Currently, there is a great interest in the need to investigate the presence of predictive and/or prognostic biomarkers, as well as new personalized therapies. Despite the initial sensitivity to neoadjuvant chemotherapy, TNBC patients are more likely than other subtypes to develop relapse within the first 3 years after diagnosis, both distantly and locally, usually with visceral and brain metastases[1].
The phosphatidylinositol-3-kinase (PI3K)/AKT signaling pathway is activated in some cancers (including breast cancer) and is important not only as a biomarker, but also as a possible therapeutic target in TNBC. Large-scale genomic analyses have characterized the heterogeneous nature of this subtype, identifying a subgroup with activation of the PI3K/AKT pathway through somatic mutations in the PIK3CA gene, which induces tumorigenesis and angiogenesis[2]. PIK3CA mutations are found in a 28%-40% of patients with breast cancer, while in the luminal androgen receptor TNBC subtype (associated with high mutational load), it presents mutations enriched in PIK3CA up to a 55%[3-5]. The complexity of the PI3K pathway, as well as the presence of multiple mediators within the same signaling pathway, explain part of the resistance to treatment, as well as the risk of disease relapse. The PIK3CA mutation is considered an independent negative prognostic factor in advanced breast cancer. It can be detected using tumor tissue or liquid biopsy[6].
Fifty-four-year-old female patient, postmenopausal, with a family history of a mater
Patient detected a tumor in her left breast of 3 years of evolution, without pain, without nipple involvement nor changes in color.
The patient has controlled hypertension and diabetes mellitus.
Clinical examination showed an Eastern Cooperative Oncology Group (ECOG) per
The mammography and breast ultrasound showed signs suggestive of left breast multicentric compromise associated with ipsilateral nodal involvement. The Breast Imaging-Reporting and Data System (BIRADS) score was 6.
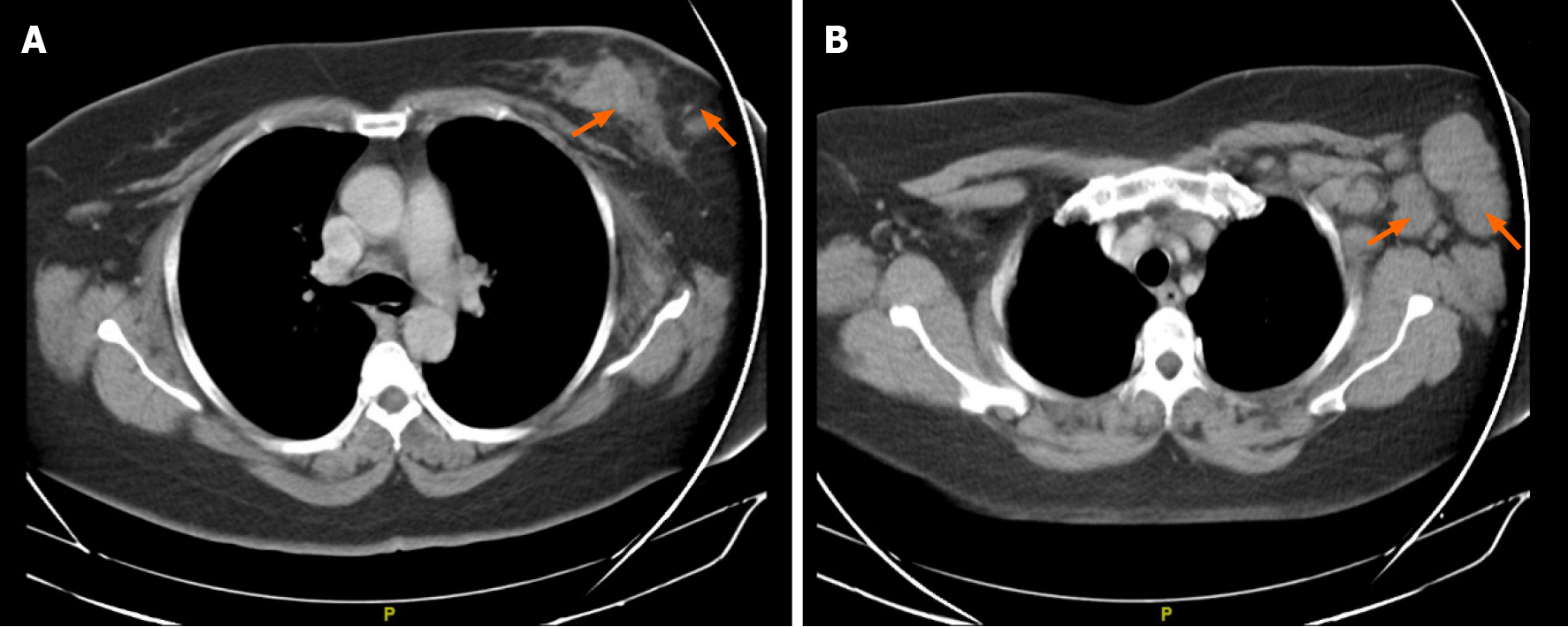
Chest computer tomography (CT) showed signs suggestive of a malignant neo
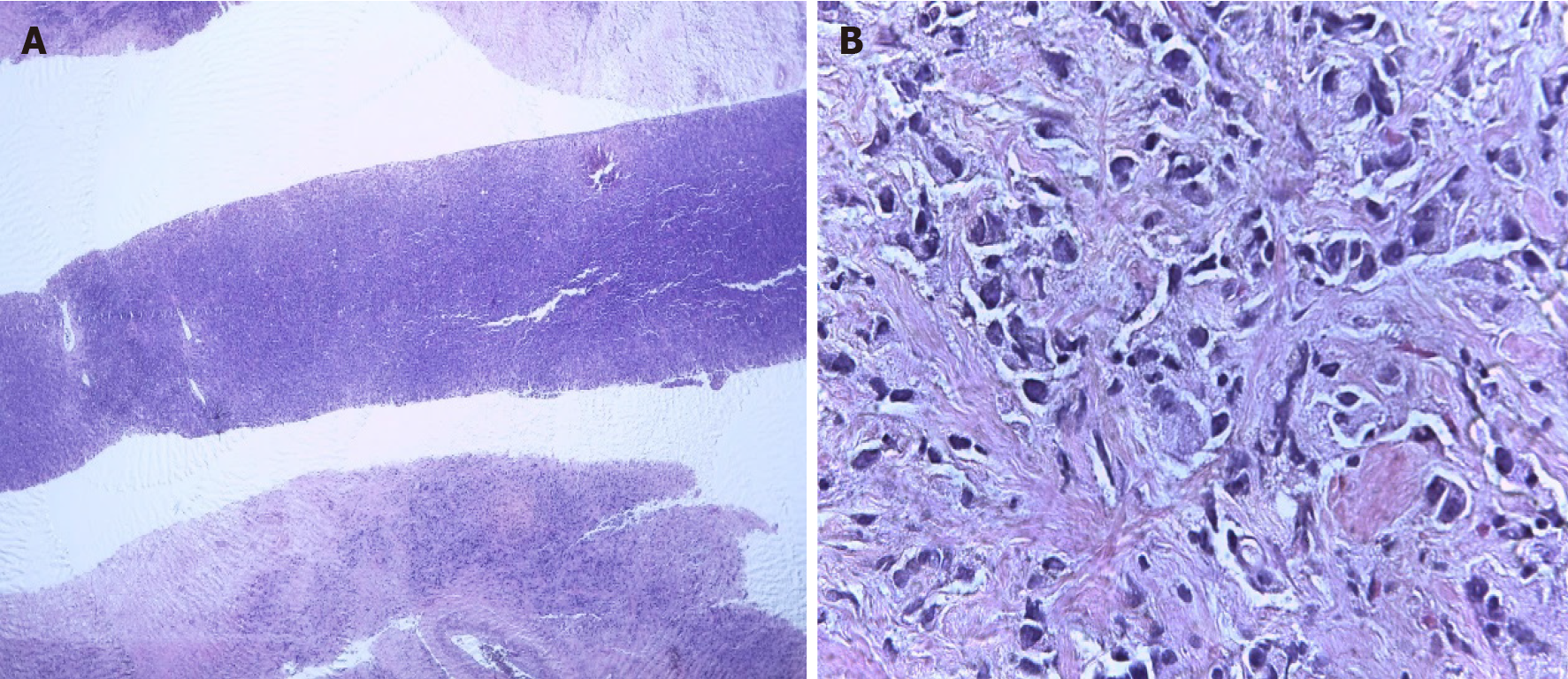
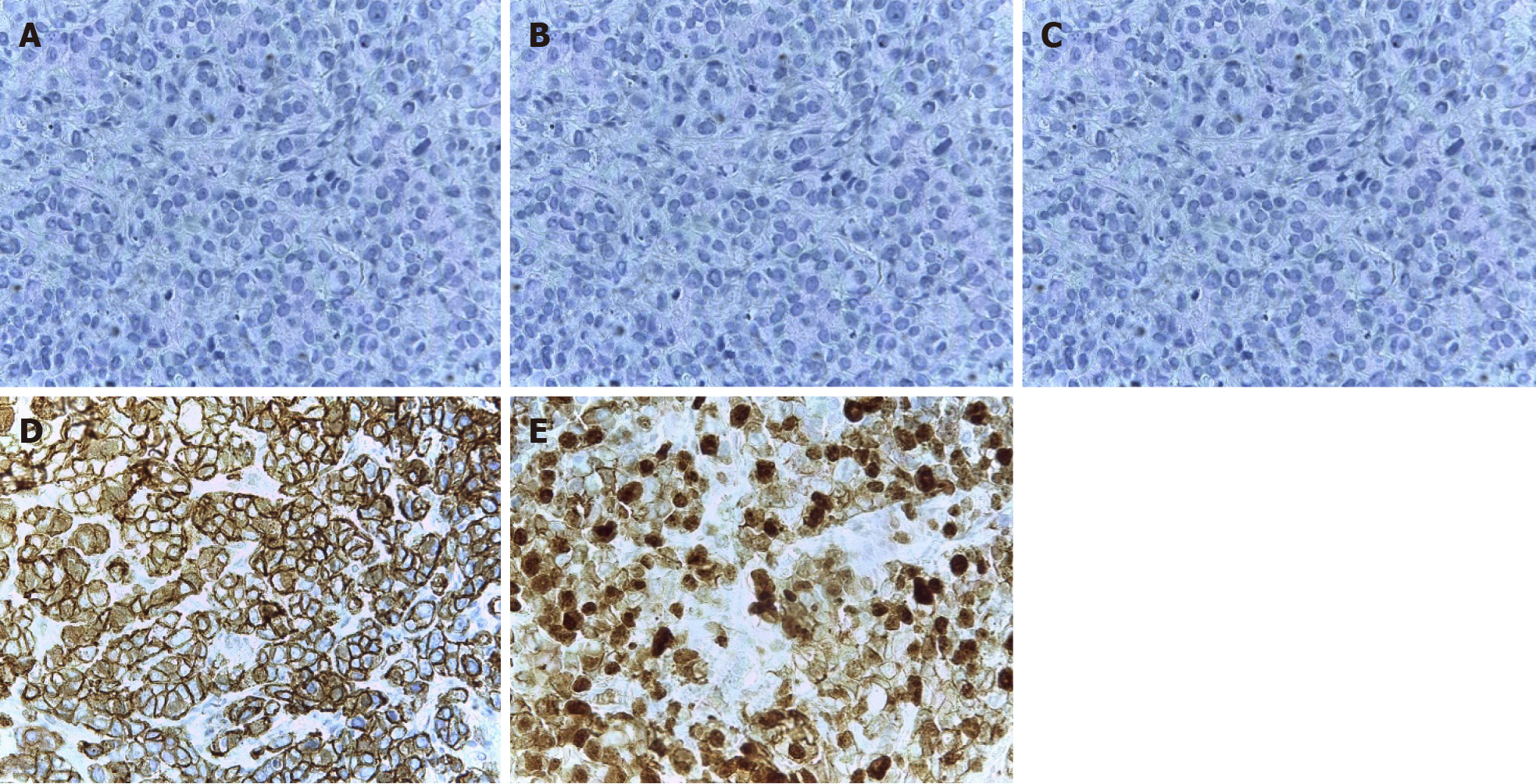
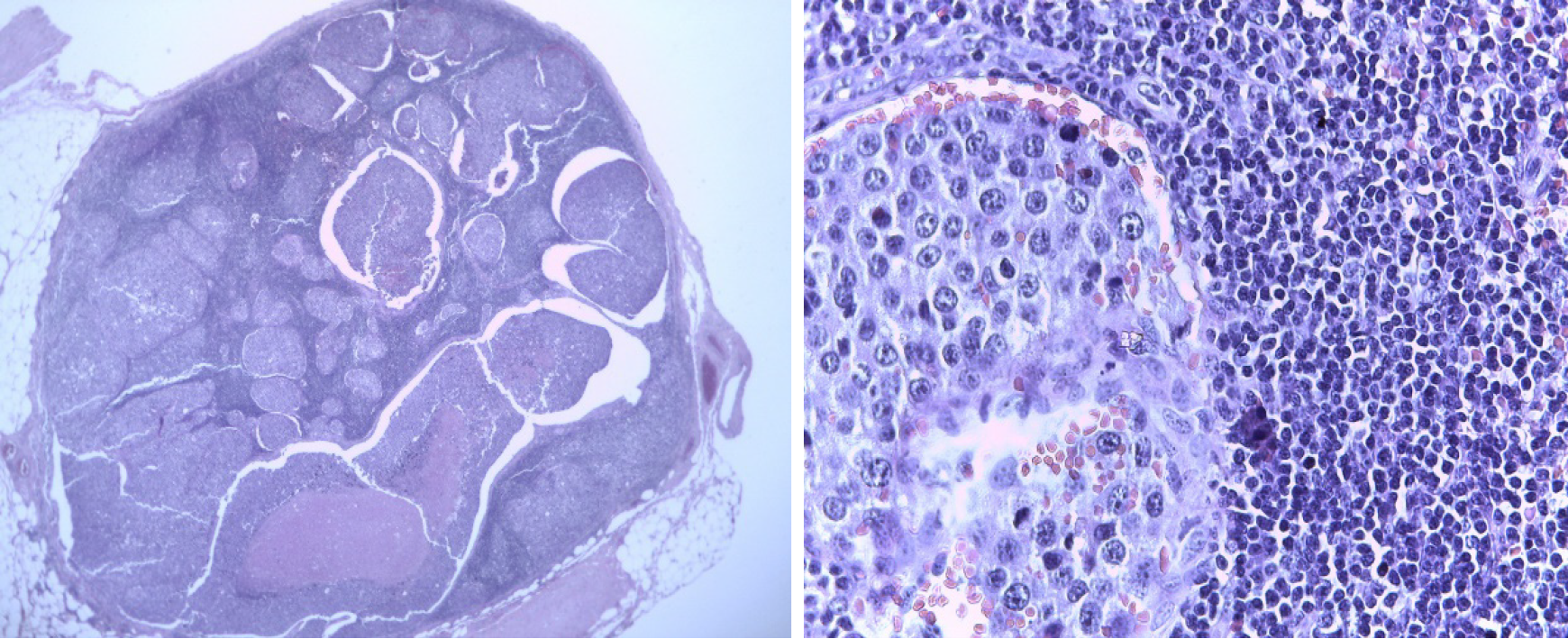
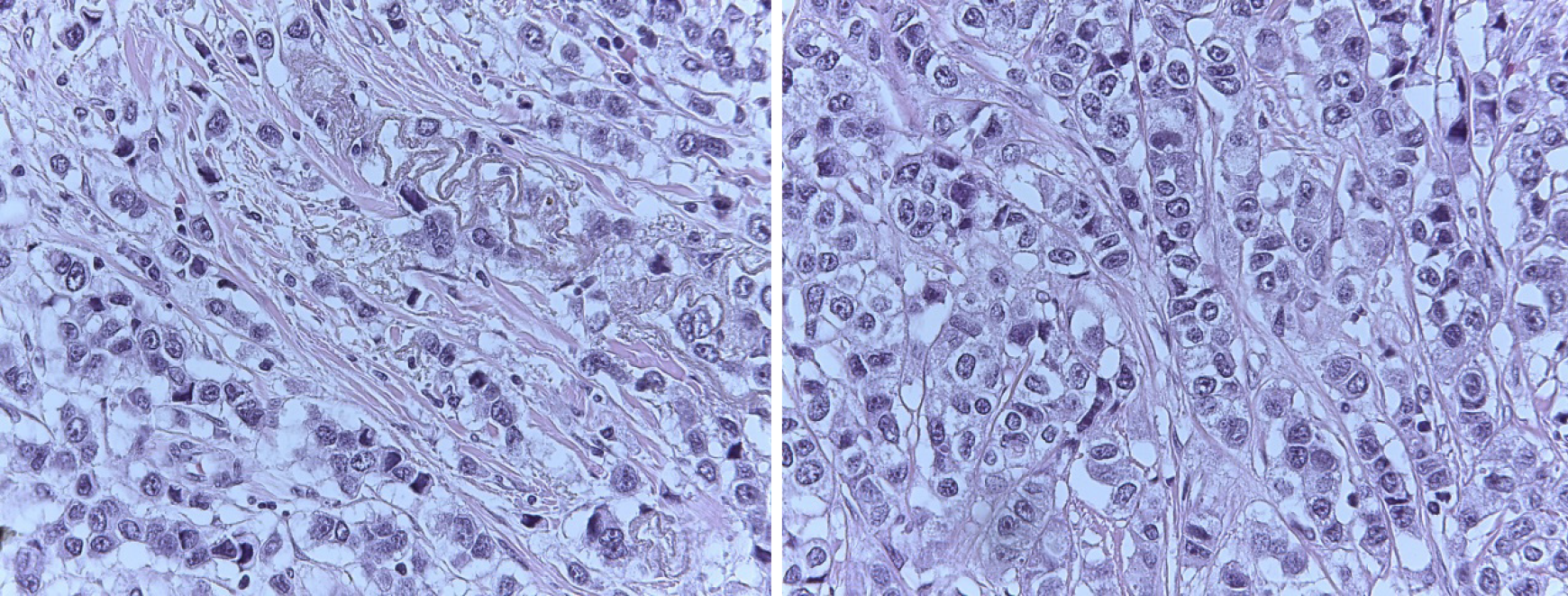
The core biopsy of the left breast reported the following: The R12 lesion (Figure 2) corresponds to an infiltrating carcinoma of the breast, invasive ductal carcinoma not otherwise specified (NOS)/invasive breast carcinoma of no special type (NST) with lobular pattern infiltration areas, and histological grade noted high, lymphovascular invasion was present, but perineural invasion was not observed. Immunohistochemistry analysis determined that the tumor was negative for estrogen receptor (ER), progesterone receptor (PR), cerbB2 (or human epidermal growth factor, HER2) , positive for e-cadherin, and had 60% positive for ki67 (Figure 3). The lesion in R2 corresponds to an infiltrating carcinoma of the breast, NOS/NST with areas of infiltration of lobular pattern, and histological grade noted that neither high, lymphovas
The final diagnosis was TNBC IIIA with left breast multicentric compromise.
The patient began standard neoadjuvant therapy (NAT) (doxorubicin-cyclophosphamide for 4 courses, followed by weekly paclitaxel for 12 wk) reaching partial clinical response (R12: tumor 4 cm × 2 cm, R2: 1.5 cm tumor, left axillary node: 2 cm × 2 cm).
The patient underwent a modified radical mastectomy with radical axillary dis
Subsequently, the patient received external radiotherapy at a dose of 5000 cGy in 25 sessions at the level of the left rib cage and ipsilateral supraclavicular region, and continued with adjuvant capecitabine, completing 6 mo of treatment. She has con
After surgery, she was included in two biomarker local studies. The first research study, called “Evaluation of prognostic markers of survival and predictive of treat
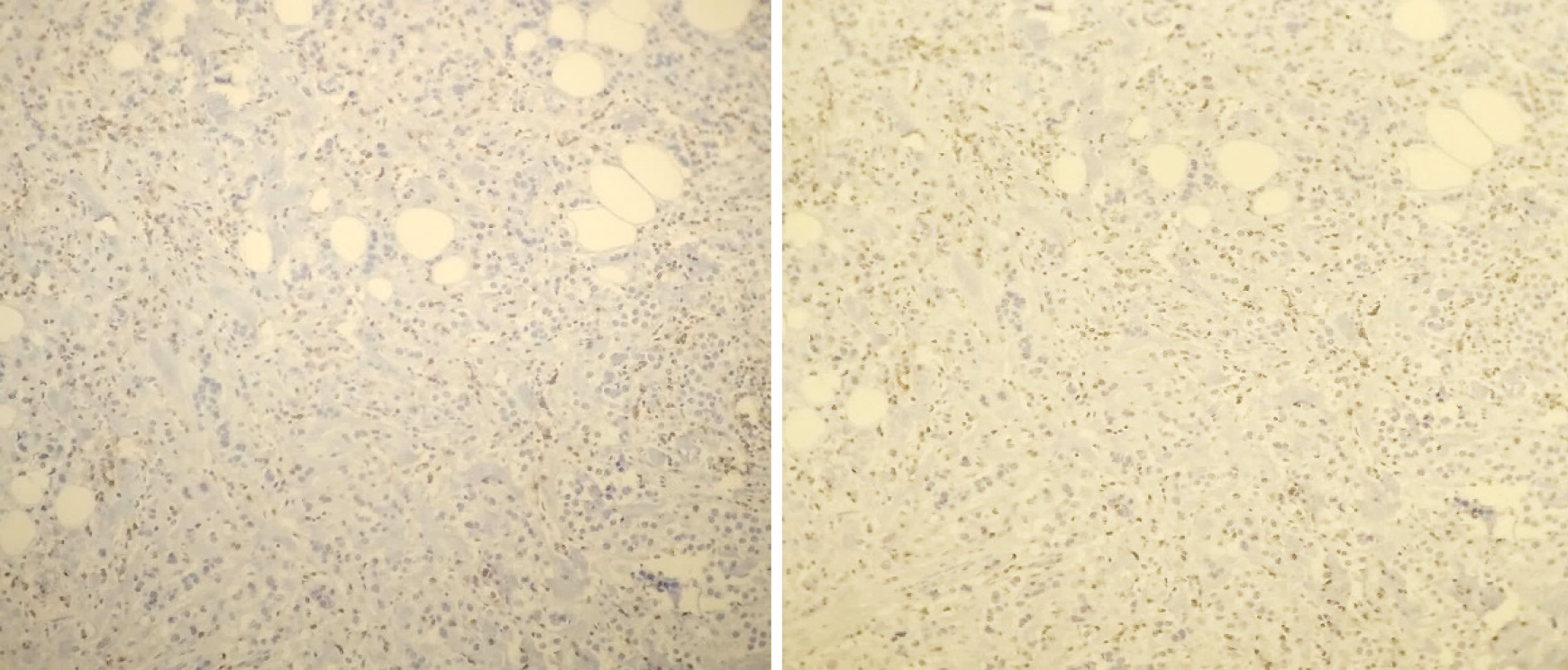
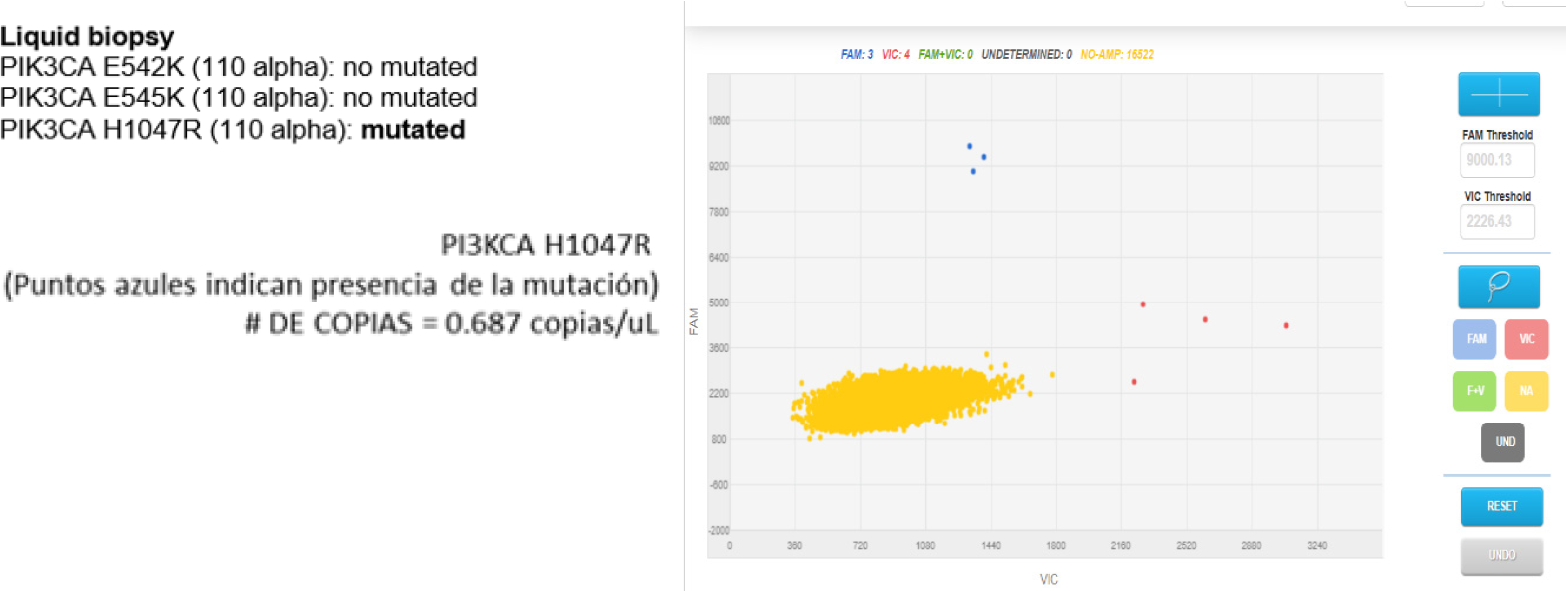
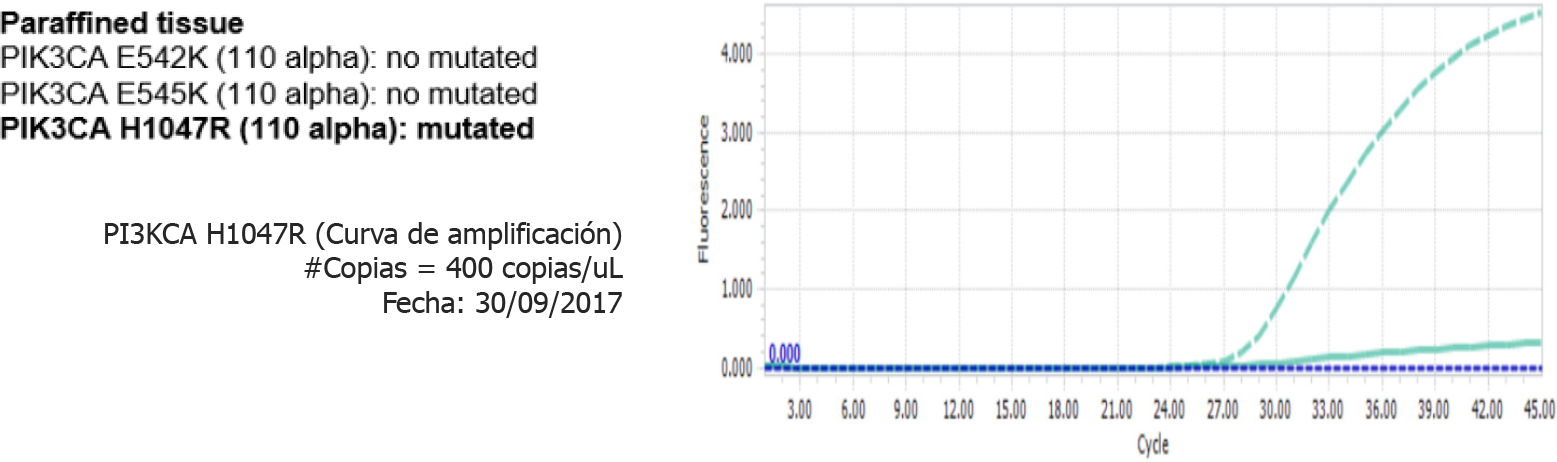
The other study was conducted using a liquid biopsy and called: “Circulating tumor DNA as biomarkers in triple negative breast cancer”, resulted in the detection of PIK3CA H1047R (110 alpha), which was mutated, both in liquid biopsy (Figure 9) and in paraffin tissue (Figure 10)[8].
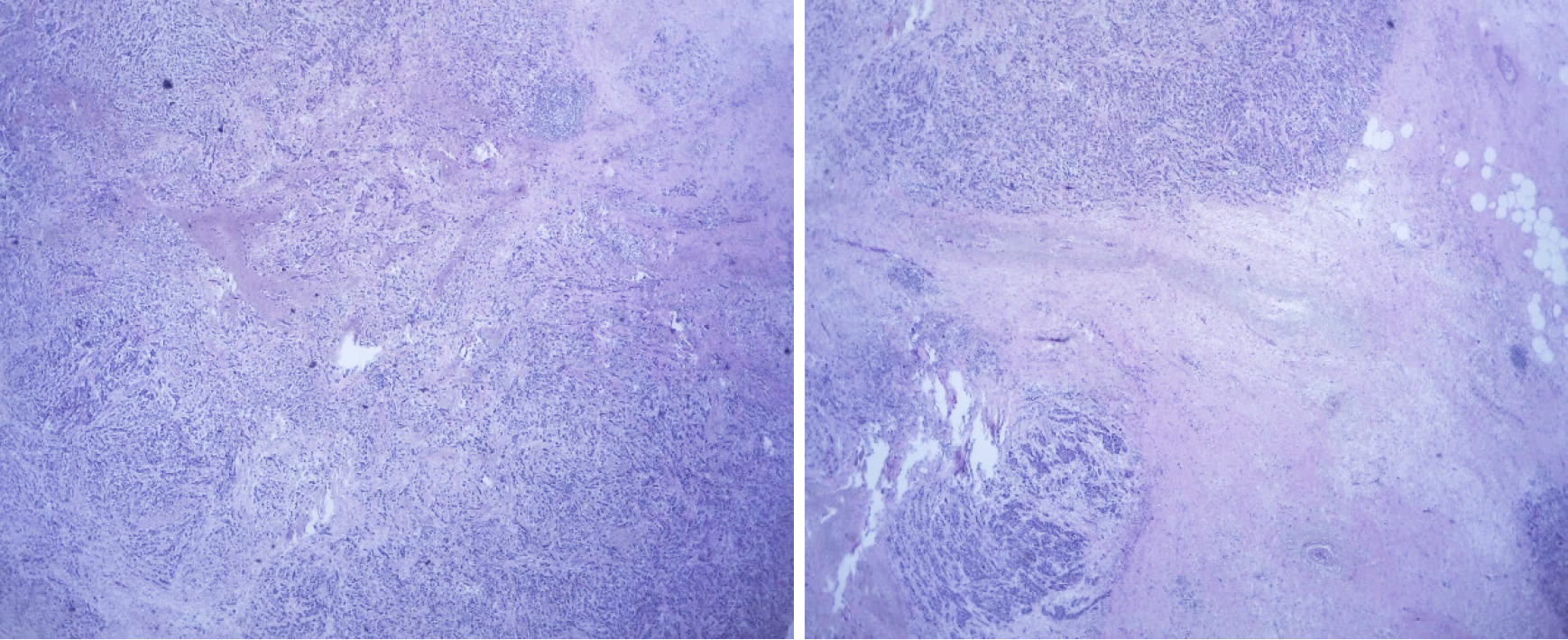
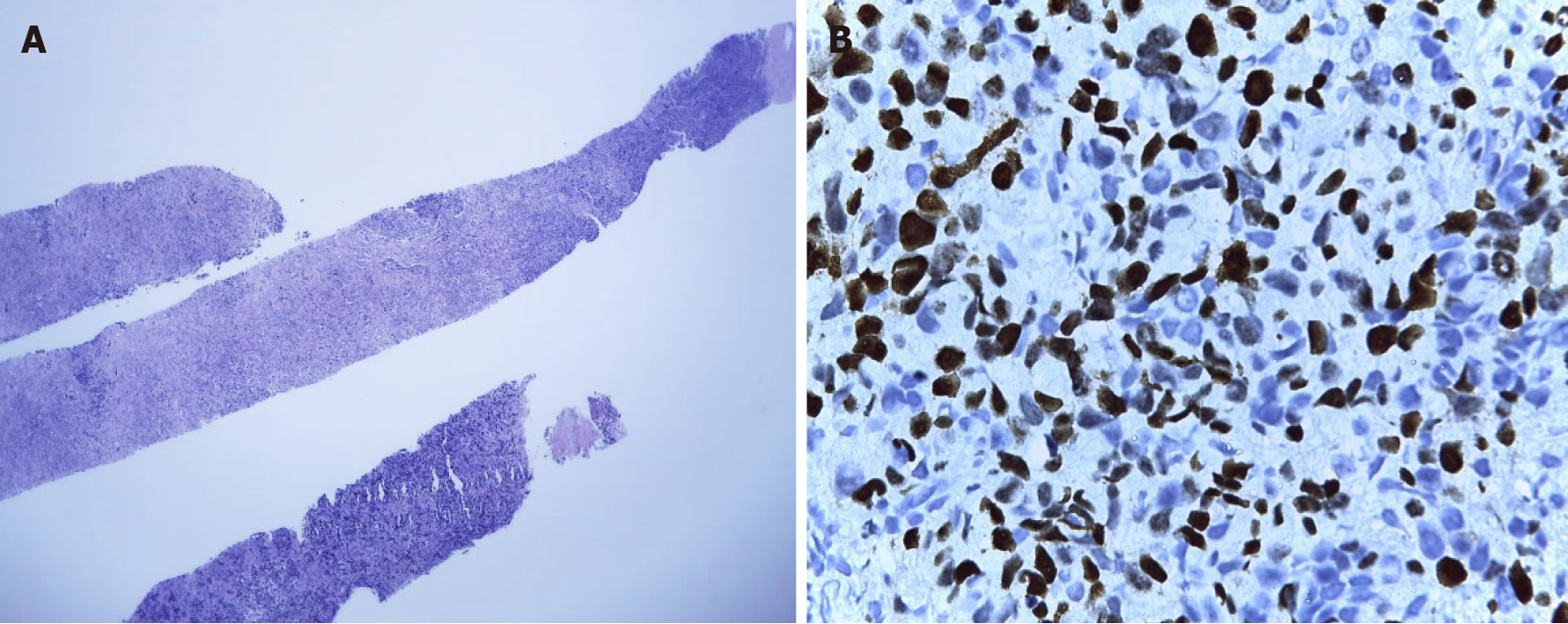
In March 2019, after a 10-mo disease-free interval, the patient’s disease relapsed with the presence of multiple lymphadenopathies in the left cervical region, the largest at 3 cm. Biopsy of the left cervical node indicated metastases of infiltrating primary breast carcinoma with an intermediate histological grade (Figure 11A), and immunohistochemistry analysis in relation to TNBC finding 60%-70% positive cells for ki67 (Figure 11B). However, chest and abdomen tomography without signs of visceral recurrence. Genetic counseling was requested, but the patient was negative for the BRCA mutation. The patient started chemotherapy with carboplatin-gemcitabine for 6 courses, reaching a complete clinical response and reassessment imaging without evidence of disease. She continued maintenance with gemcitabine.
Patient with TNBC IIIA with left breast multicentric compromise, who, after receiving standard neoadjuvant treatment, followed by surgery, radiotherapy, and adjuvant capecitabine, presents cervical lymph node recurrence after a 10-mo disease-free interval. After surgery, she participated in 2 biomarker research studies, detecting the presence of a positive PIK3CA mutation in tumor tissue and peripheral blood (liquid biopsy).
The PI3K/AKT/mTOR pathway is an oncogenic intracellular pathway that regulates cell proliferation, metabolism, growth, survival, and apoptosis. The PIK3CA mutation, being the most common of the PI3K/AKT signaling pathway amplifications, is present in all molecular subtypes of breast cancer, and is detected in 20%-25% of all cases[2]. Although these individual mutations are rare, the combined activated mutations in PIK3CA and AKT1, with inactivating mutations in PTEN (a tumor suppressor gene that is inactivated when mutations are detected), occur in 25%-30% of metastatic TNBC (mTNBC)[2]. It has been determined that the PIK3CA mutation has an independent negative prognostic value of survival, as a systematic review has shown that patients with breast cancer and high levels of ctDNA after receiving neoadjuvant treatment have early disease relapse[9-11].
Liquid biopsy is being evaluated in breast cancer as a potential tool to capture tumor evolution in real time, to guide and monitor systemic treatment, as well as being a promising method to identify drug resistance mechanisms through the de
Novel trials has shown that ctDNA has a prognostic value in TNBC, predicting minimal residual disease (MRD) and early relapse after neoadjuvant chemotherapy with curative intent with high specificity[16,17]. ctDNA detection is useful for mo
Although there is a positive PIK3CA mutation result in this case report, to date, there are no standardized procedures for PIK3CA detection in breast cancer in Peru. Therefore, the application of ctDNA should be based on defined protocols to ensure reproducibility of results, due to mostly undetectable levels of ctDNA in early breast cancer[20]. Finally, it is not known whether the presence of the PIK3CA mutation in peripheral blood and tumor tissue confers a worse prognosis compared to having the mutation in 1 of the 2 samples evaluated (blood, tissue). These findings could increase the interval of control visits or earlier approaches for follow-up to detect local or distant relapse.
From the perspective of Personalized Medicine, it is important to characterize the genetic profile of tumors, either in liquid biopsy and/or tumor tissue, in order to detect potential biomarkers and subsequently new targeted therapies. In the case report, the detection of the PIK3CA mutation in a patient with locally advanced TNBC after neoadjuvant treatment and surgery may be associated with an early relapse of disease. Many trials have shown the value of detecting the PIK3CA mutation in liquid biopsy (ctDNA) in patients with TNBC as a potential biomarker for identifying early relapse and disease progression. The detection of PIK3CA mutations might help identify patients at risk of recurrence, and it could also generate closer therapeutic or follow-up behaviors in order to detect micro metastatic disease/MRD and distant disease.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Peru
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Teragawa H S-Editor: Wang LL L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Khosravi-Shahi P, Cabezón-Gutiérrez L, Aparicio Salcedo MI. State of art of advanced triple negative breast cancer. Breast J. 2019;25:967-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Millis SZ, Gatalica Z, Winkler J, Vranic S, Kimbrough J, Reddy S, O'Shaughnessy JA. Predictive Biomarker Profiling of > 6000 Breast Cancer Patients Shows Heterogeneity in TNBC, With Treatment Implications. Clin Breast Cancer. 2015;15:473-481.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Bareche Y, Venet D, Ignatiadis M, Aftimos P, Piccart M, Rothe F, Sotiriou C. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann Oncol. 2018;29:895-902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 270] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 4. | Lehmann BD, Bauer JA, Schafer JM, Pendleton CS, Tang L, Johnson KC, Chen X, Balko JM, Gómez H, Arteaga CL, Mills GB, Sanders ME, Pietenpol JA. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res. 2014;16:406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 259] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 5. | Jiang YZ, Ma D, Suo C, Shi J, Xue M, Hu X, Xiao Y, Yu KD, Liu YR, Yu Y, Zheng Y, Li X, Zhang C, Hu P, Zhang J, Hua Q, Hou W, Ren L, Bao D, Li B, Yang J, Yao L, Zuo WJ, Zhao S, Gong Y, Ren YX, Zhao YX, Yang YS, Niu Z, Cao ZG, Stover DG, Verschraegen C, Kaklamani V, Daemen A, Benson JR, Takabe K, Bai F, Li DQ, Wang P, Shi L, Huang W, Shao ZM. Genomic and Transcriptomic Landscape of Triple-Negative Breast Cancers: Subtypes and Treatment Strategies. Cancer Cell. 2019;35:428-440.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 603] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 6. | Sobhani N, Roviello G, Corona SP, Scaltriti M, Ianza A, Bortul M, Zanconati F, Generali D. The prognostic value of PI3K mutational status in breast cancer: A meta-analysis. J Cell Biochem. 2018;119:4287-4292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 7. | Castaneda C. Evaluation of prognostic markers of survival and predictive of response to treatment in a population with cancer using the first platform in Peru for the detection and determination of gene expression without DNA or RNA amplification. INNOVATE- PERU EC-4-P-028-17. Executive Summaries of Research Project Grants. 2013-2018. Peru [Internet]. Available from: http://portal.concytec.gob.pe/images/publicaciones/informes/subvenciones_proyectos_fondecyt_resumenes_ejecutivos_2013-2018-2-350_merged.pdf. |
| 8. | Castaneda C. Circulating tumor cells and circulating tumor DNA as biomarkers in triple-negative breast cancer. FONDECYT N198-2015. Executive Summaries of Research Project Grants. 2013-2018. Peru [Internet]. Available from: http://portal.concytec.gob.pe/images/publicaciones/informes/subvenciones_proyectos_fondecyt_resumenes_ejecutivos_2013-2018-2-350_merged.pdf. |
| 9. | Rossi G, Ignatiadis M. Promises and Pitfalls of Using Liquid Biopsy for Precision Medicine. Cancer Res. 2019;79:2798-2804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 10. | Schiavon G, Hrebien S, Garcia-Murillas I, Cutts RJ, Pearson A, Tarazona N, Fenwick K, Kozarewa I, Lopez-Knowles E, Ribas R, Nerurkar A, Osin P, Chandarlapaty S, Martin LA, Dowsett M, Smith IE, Turner NC. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med. 2015;7:313ra182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 459] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 11. | Koo KM, Mainwaring PN. The role of circulating tumor DNA testing in breast cancer liquid biopsies: getting ready for prime time. Breast Cancer Manag. 2020;9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Loibl S, Majewski I, Guarneri V, Nekljudova V, Holmes E, Bria E, Denkert C, Schem C, Sotiriou C, Loi S, Untch M, Conte P, Bernards R, Piccart M, von Minckwitz G, Baselga J. PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab. Ann Oncol. 2016;27:1519-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 13. | Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 972] [Cited by in RCA: 1064] [Article Influence: 177.3] [Reference Citation Analysis (0)] |
| 14. | Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, Kinzler KW, Vogelstein B, Diaz LA Jr. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2240] [Cited by in RCA: 2102] [Article Influence: 123.6] [Reference Citation Analysis (0)] |
| 15. | Chen YH, Hancock BA, Solzak JP, Brinza D, Scafe C, Miller KD, Radovich M. Next-generation sequencing of circulating tumor DNA to predict recurrence in triple-negative breast cancer patients with residual disease after neoadjuvant chemotherapy. NPJ Breast Cancer. 2017;3:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | Garcia-Murillas I, Schiavon G, Weigelt B, Ng C, Hrebien S, Cutts RJ, Cheang M, Osin P, Nerurkar A, Kozarewa I, Garrido JA, Dowsett M, Reis-Filho JS, Smith IE, Turner NC. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med. 2015;7:302ra133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 796] [Cited by in RCA: 858] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 17. | Buono G, Gerratana L, Bulfoni M, Provinciali N, Basile D, Giuliano M, Corvaja C, Arpino G, Del Mastro L, De Placido S, De Laurentiis M, Cristofanilli M, Puglisi F. Circulating tumor DNA analysis in breast cancer: Is it ready for prime-time? Cancer Treat Rev. 2019;73:73-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 18. | Zhao W, Qiu Y, Kong D. Class I phosphatidylinositol 3-kinase inhibitors for cancer therapy. Acta Pharm Sin B. 2017;7:27-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 19. | Radovich M. Detection of circulating tumor DNA (ctDNA) after neoadjuvant chemotherapy is significantly associated with disease recurrence in early-stage triple-negative breast cancer (TNBC): Preplanned correlative results from clinical trial BRE12-158. Presented at the San Antonio Breast Cancer Symposium, San Antonio, Texas; December 10-14. 2019. Abstract: GS5-02 [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT02101385. |
| 20. | Benesova L, Belsanova B, Suchanek S, Kopeckova M, Minarikova P, Lipska L, Levy M, Visokai V, Zavoral M, Minarik M. Mutation-based detection and monitoring of cell-free tumor DNA in peripheral blood of cancer patients. Anal Biochem. 2013;433:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |