Published online Jul 24, 2021. doi: 10.5306/wjco.v12.i7.565
Peer-review started: February 27, 2021
First decision: May 4, 2021
Revised: May 17, 2021
Accepted: June 18, 2021
Article in press: June 18, 2021
Published online: July 24, 2021
Processing time: 143 Days and 20 Hours
BRCA1/2 pathogenic variants are widely known as major risk factors mainly for breast and ovarian cancer, while their role in gastrointestinal (GI) malignancies such as colorectal cancer (CRC), gastric cancer and oesophageal cancer (OeC) is still not well established. The main objective of this review is to summarise the available evidence on this matter. The studies included in the review were selected from PubMed/GoogleScholar/ScienceDirect databases to identify published articles where BRCA1/2 pathogenic variants were assessed either as a risk factor or a prognostic/predictive factor in these malignancies. Our review suggests that BRCA1/2 might have a role as a risk factor for colorectal, gastric and OeC, albeit with differences among these diseases: In particular BRCA1 seems to be much more frequently mutated in CRC whereas BRCA2 appears to be much more closely associated with gastric and OeC. Early-onset cancer seems to be also associated with BRCA1/2 mutations and a few studies suggest a positive prog
Core Tip: BRCA1/2 pathogenic mutations, other than increasing the risk of breast and ovarian cancer, may increase the risk of developing other types of cancers, such as prostate and pancreatic cancers. Our review highlights that BRCA mutations could also have a predisposing role to gastrointestinal tumours, in particular gastric, esophageal and colorectal cancer, thus playing an important role in terms of surveillance proce
- Citation: Maccaroni E, Giampieri R, Lenci E, Scortichini L, Bianchi F, Belvederesi L, Brugiati C, Pagliaretta S, Ambrosini E, Berardi R. BRCA mutations and gastrointestinal cancers: When to expect the unexpected? World J Clin Oncol 2021; 12(7): 565-580
- URL: https://www.wjgnet.com/2218-4333/full/v12/i7/565.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i7.565
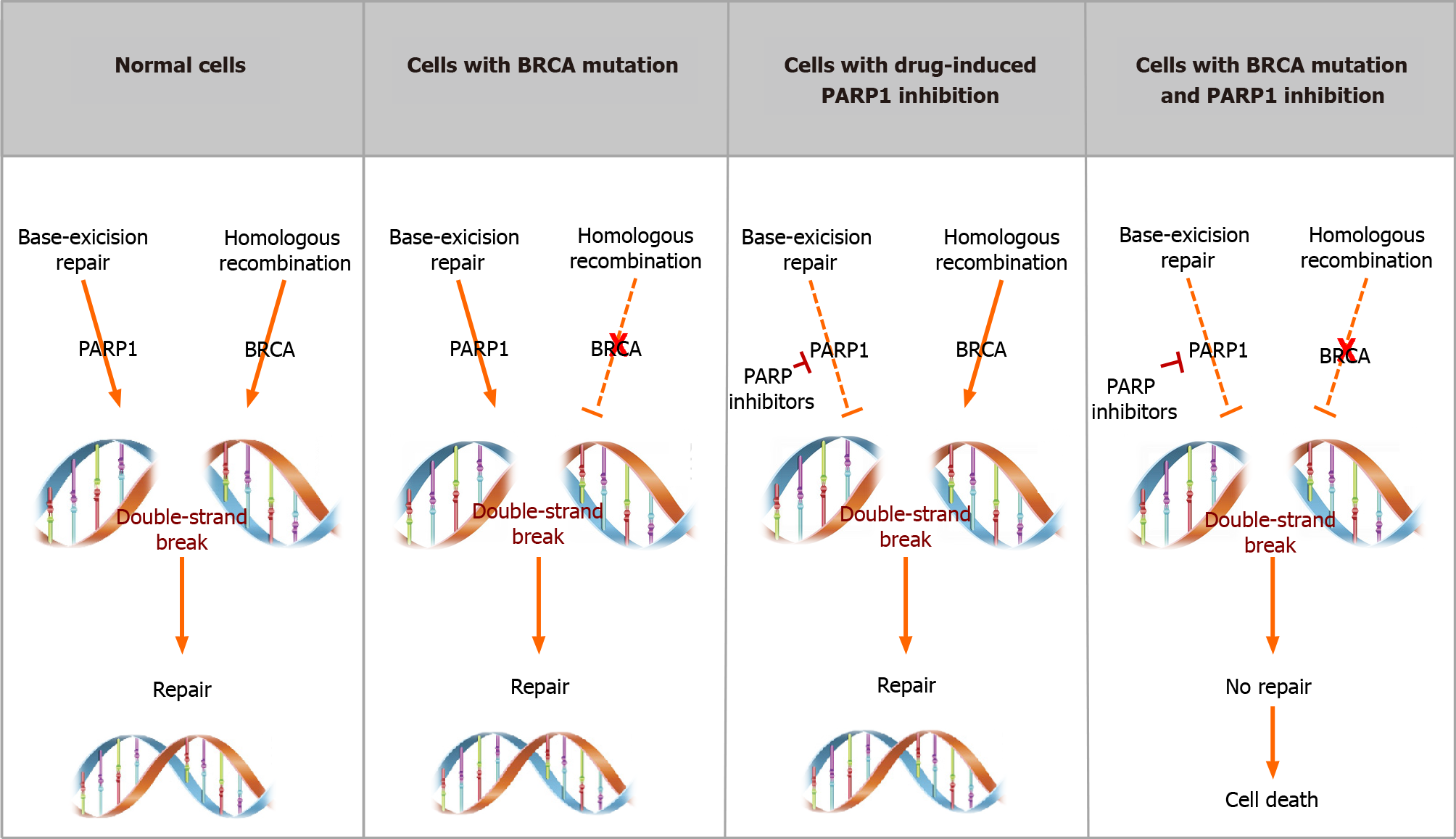
BRCA mutations are defined as pathogenic variants in either the BRCA1 or BRCA2 gene: These two tumour suppressor genes are involved in different crucial pathways including DNA repair, cell proliferation control and apoptosis. In particular, BRCA1 and BRCA2 genes are mainly involved in the homologous recombination (HR) process, responsible for maintenance of genome integrity through an error-free repair pathway for DNA double-strand breaks in response to DNA damage[1,2]. Loss-of-function mutations in BRCA1/2 may lead to accumulation of DNA double-strand breaks and result in genomic instability and tumour development.
BRCA1 and BRCA2-associated hereditary breast and ovarian cancer syndrome is characterised by an increased risk of breast cancer and ovarian cancer (including fallopian tube and primary peritoneal tumours), up to 84% for breast cancer and 40% for ovarian cancer[3-5]. Moreover, higher risk of male breast cancer has been reported especially in BRCA2 carriers[6-9].
In BRCA2 pathogenic variant carriers, familial cancer types different from breast and ovarian, such as prostate, pancreatic cancer, and melanoma, have also been described[10-14].
In addition to that, case-control and family-based studies seem to suggest that BRCA1/2 pathogenic germline carriers might be at increased risk of other malignan
These studies might be susceptible to selection bias; furthermore, misclassification can be present, due to the fact that GI cancer diagnoses were based only on infor
Moreover, prospective evidence estimating GI cancer incidence in BRCA1/2 pathogenic variants carriers are still rare[17]. Predicting GI cancer incidence rates among BRCA carriers might convey meaningful implications both for genetic coun
Furthermore, BRCA1 and BRCA2 pathogenic variants have an emerging role for novel target therapies. BRCA1/2 have essential functions in the HR DNA repair pathway, and genomic alterations in BRCA genes lead to impaired DNA repair, called homologous recombination deficiency (HRD). Platinum compounds [such as oxali
In BRCA-mutant GI cancer, the role of HRD alterations is still widely unknown and only few data about their clinical impact are available.
The aim of this review is to highlight a possible association between BRCA pathogenic variants and GI cancer risk and also to investigate the role of BRCA mutations as a prognostic and/or predictive factor in this setting of patients. As the role of BRCA mutations (particularly BRCA2) has already been clarified in pancreatic cancer patients and in such cases PARPi[20] are already used in everyday clinical practice, we decided to focus only on gastric (GC), oesophageal cancer (OeC) and colorectal cancer (CRC).
The studies included in the present review were selected from PubMed/Google
CRC is the third most common malignancy in both sexes: It accounts for 10% of new cancer diagnoses and it represents the second estimated leading cause of cancer-related death in 2020 worldwide[22].
One out of 20 CRC patients has a hereditary predisposition. Lynch syndrome (LS, hereditary non-polyposis CRC) is the most common hereditary disorder, justifying 3% of all cases; LS is an autosomal dominant syndrome that causes alterations in the DNA repairing system known as mismatch repair (MMR) proteins. These proteins are able to restore insertions, deletions, as well as A-G and T-C mismatches[23]. Lack of function variants in one of the four genes (hMLH1, hMSH2, hMSH6, PMS2) that encodes the MMR proteins determines, as a final effect, an increase in replication errors in specific areas of the genome where single or double-nucleotide repeats are present, known as microsatellite regions. An increase or decrease in microsatellite length results in what is known as microsatellite instability (MSI) and increases DNA susceptibility to further mutation in oncogenes and tumour suppressor genes. Twelve to fifteen percent of all CRCs harbour a deficient MMR system and the percentage is lower (5%) if we only consider patients with metastatic involvement (mCRCs)[24]. Among these cases, 2/3 are due to sporadic mutations, while only 1/3 of cases are due to germline loss-of-function mutations. Because of this fact, patients with MSI-high status CRC diagnosis should be advised genetic counselling and testing so as not to overlook further diagnosis of LS. Other clinical factors that might lead to suspected LS are Amsterdam I/II criteria and revised Bethesda guidelines[25,26]: Although these criteria might suggest the need for pedigree analysis, it must be considered that genomic profiling remains the gold standard for diagnosis of LS either in patients who fit these criteria or those screened because of a MSI-high CRC tumour.
While the risk of developing CRC in LS is well known, the same does not apply to BRCA1/2 mutation carriers; several efforts have been made to evaluate the lifetime susceptibility of BRCA1/2 mutation carriers to develop CRC and have yielded con
Mauri et al[27] suggested that BRCA1/2 mutations might determine an increase in CRC diagnoses, particularly in young patients.
Another study of Kim et al[28] suggested that even first and second-degree relatives of high-risk BRCA mutated (BRCAm) breast cancer patients are at increased risk of non-breast/ovarian cancer.
Phelan et al[17] conducted a multicentre prospective study involving a cohort of 7105 female patients with the aim of assessing the CRC incidence in women carrying pathogenic BRCA1/2 mutations. Enrolment was conducted in fifty centres among five countries in Canada, United States and Europe, from 1992 to 2010; the median follow-up was 5.5 years.
When newly diagnosed CRC were matched with population-specific incidence rates, no statistically significant differences were found: Authors reported 21 new CRC diagnoses between mutation carriers, while 23.6 cases were expected. Among them, 16 cases occurred in BRCA1m (vs 17.4 expected), and 5 cases in BRCA2m (vs 6.1 expected). Although this study failed to show a higher prevalence in all mutated patients, subgroup analysis of BRCA1 carriers showed that early-onset (30-49 years) CRC diagnosis was four times greater than controls. The large population of patients enrolled and the prospective design of this study support these findings; however, the lack of male patients narrows these results to only the female population. Moreover, the relatively small number of incident cases and the ability to retrieve only 2/3 of the pathological reports, may represent another limitation of the study.
Similarly, Oh et al[29] presented a meta-analysis of 14 out of 18 studies initially selected, as to estimate the CRC risk in BRCAm carriers. Using a random-effects model, the authors observed a statistically significant increase in the odds of CRC in BRCAm carriers [odd ratio (OR) = 1.24, 95%CI: 1.02 to 1.51, P = 0.03]. When the authors focused on specific BRCA1 or BRCA2 carriers, they highlighted that BRCA1 carriers were at a higher risk of developing CRC. On the other hand, they did not observe a higher risk of early onset CRC as previously stated by Phelan et al[17].
In contrast, in their systematic review and meta-analysis Cullinane et al[30] did not observe any statistically significant difference in terms of CRC risk among BRCAm carriers, regardless of the gene involved. Neither did adjustment for age modify these findings, nor when the analysis was conducted only in the Ashkenazi Jewish heritage population.
It is well established that BRCA1/2 mutations incidence is broader in some ethnic groups, such as the Ashkenazi Jewish population; in this ethnicity there is a high population frequency (approximately 2%) of three founder mutations: BRCA1 185delAG, BRCA1 5382insC, and BRCA2 6174delT[31]. Kirchhoff et al[31] collected data from 586 Ashkenazi Jewish patients with pathologically confirmed diagnosis of CRC and from 5012 healthy subjects (controls), to assess whether having one of the three founder mutations could translate into an increased CRC risk. Mutations incidence in the two groups was matched and after adjusting for age at diagnosis and sex, it was observed that harbouring BRCA1/2 mutations was not associated with higher CRC risk[31].
Similarly, Niel et al[32], assessed whether positive breast cancer family history could represent a risk factor for developing other tumour types. The authors performed a case-control study comparing data from 1422 patients with pathologically confirmed CRC, coming from five major hospitals in northern Israel, with 1585 control subjects. They observed that neither BRCA1/2 founder mutations nor breast cancer family history were significantly correlated with increased CRC risk.
Another interesting topic to analyse is whether BRCA-related CRC could be characterised by peculiar clinico-pathological features. Xu et al[33] described CRC phenotype differences among suspected LS patients with MMR and BRCA/BRCA-like carrying variants. Among 22833 patients receiving radical surgery for cancer at Fudan University Shanghai Cancer Center, 202 underwent multigene testing covering 139 genes: 42 were carriers of a pathogenic MMR-gene variant, while 20 carried BRCA or a BRCA-like variant. The most relevant differences between the two groups of patients were that the mean CRC age at diagnosis was lower in the MMR group (44.95 years vs 56.45 years); furthermore, BRCA/BRCA-like variants were associated with a sig
Based on histological and molecular features, CRC is actually a heterogeneous group of tumours, characterised by different prognosis and response to medical treatment. Mucinous carcinoma (MC) and adenocarcinoma (AC) are the two most common histological subtypes, accounting for 10%-15% and 85%-90% of cases respectively. In particular, MC is usually associated with worse prognosis: It is more frequently diagnosed in young patients and it is usually diagnosed later than AC, due to the late occurrence of symptoms; most of these tumours occur in the proximal colon. MC is also described as having quicker disease progression. Harpaz et al[34] in their paper assessed the correlation between MC and BRCAm CRCs.
One thousand one hundred thirty-four mCRCs were analyzed: A statistically significant higher incidence of BRCA1/2 mutations in MC than in AC patients was observed (14.8% vs 4.1%, respectively). Moreover, when considering the “mutation count”, defined as “somatic non-synonymous variants in encoding genes by exome sequencing” for each sample supplied by the database, they also observed that somatic BRCAm CRC has a higher mutation count than wild-type BRCA (BRCAwt); mucinous histology was also the strongest predictor of mutation count, independently of MSI as well as other variables too.
The authors conducted a prospective case-control study to confirm the results of their previous retrospective study: MC and AC CRC patients treated at Hadassah Medical Center in Jerusalem were prospectively enrolled. Thirty out of 53 MC cases were included, together with 40 controls: Authors observed a higher frequency of BRCAm patients in MC (40% vs 27%, respectively, P = 0.2705 by chi-squared test). The difference was not statistically significant. Lack of concordance with their previous results might be due to the small cohort and to other limitations: In particular, mutation analysis revealed that variants frequently seen in MC histology such as KRAS, BRAF and PI3K mutations were not observed in this cohort of patients. However, analyzing the BRCA2m group, they noted a trend toward higher frequency of mucinous histology. Moreover, they confirmed the higher tumour mutational burden (TMB) in BRCAm compared to BRCAwt carriers.
While the effects of BRCAm are well known in females, studies focused on males are rarer; indeed, it has been hypothesised that male carriers of BRCA mutations are more likely to develop several types of malignancies, including melanomas, breast, prostatic and pancreatic cancer. However, clinicians used to test far fewer male than female patients for BRCAm, thus limiting our actual knowledge of clinic-pathological and prognostic implications of these kinds of tumours.
Sun et al[35] conducted a retrospective pan-tumour survey to identify tumour characteristics in BRCA1/2 male mutation carriers; clinical data of mutated male patients was compared with female patients carrying the same mutation, as well as BRCAwt male patients. Results of the analysis showed that CRC incidence was higher in BRCAm males than in BRCAm female patients and non-BRCAm males. In par
Five-years OS rate for patients with mCRC remains poor, around 14%. Standard chemotherapy for these patients includes 5-fluorouracil (5-FU) in association with irinotecan (FOLFIRI), oxaliplatin (FOLFOX) or both (FOLFOXIRI). These regimens are usually associated, particularly in first and second-line settings, with anti-EGFR or anti-VEGF targeted drugs, on the basis of tumour BRAF/RAS mutational status[36].
It has been previously described that BRCA1/2m cancers should have increased sensitivity to platinum compounds; on this basis, a few published case reports suggest that BRCA1/2 mutational testing, particularly in younger CRC patients might guide treatment selection in earlier settings of the disease. Soyano et al[37] described the case of a young man with locally advanced rectal cancer [T3N1M0 according to tumour-node-metastasis (TNM) staging]: He was tested for BRCA1/2 mutation and was found to be a BRCA1m carrier. After multidisciplinary evaluation, he started standard chemoradiotherapy with capecitabine as a radiosensitizer; however, when a BRCA1 mutation was found, oxaliplatin was added to standard chemoradiotherapy. It was also decided to opt for a total neoadjuvant strategy as in giving additional two cycles of chemotherapy (mFOLFOX6), after the end of chemoradiotherapy, just before surgery. The pathological report after surgery revealed complete pathological res
Likewise, Lin et al[38] described the case of a 25 years old man affected with locally advanced (cT3N2M0) rectal cancer who had an excellent response to oxaliplatin-based chemotherapy. The patient was due to receive neoadjuvant concurrent standard radio-chemotherapy, but given the increased risk of radiation-induced infertility he refused any radiotherapy; 4 cycles of chemotherapy 5-FU and oxaliplatin-based (FOLFOX6) were then administered. After surgical primary tumour resection performed by laparoscopic total mesorectal excision (TME), the histological report showed complete response. Owing to this excellent but unexpected reaction to oxaliplatin-based treatment, the authors decided to perform next-generation sequencing in both DNA samples taken from the primary tumour sample and the peripheral blood sample; somatic BRCA2 variant was found, as well as high TMB.
This data suggest that the assessment of BRCA-status might be performed when a particularly deep response to platinum-based chemotherapy is reached.
It has already been explained how, in cells that harbour HR system mutations leading to defective DNA double-strand break repair, as in BRCAm patients, genome stability is maintained through the activity of BER and PARP enzymes. However, Paviolo et al[39] demonstrated that in PARPi-treated BRCA-defective CRC samples, even though DSBs are generated, they are not directly responsible for cell death. Indeed, more than DSBs, it was proven that rapid and anomalous repair of these defects, through means of DNA repair mechanisms different from HRD, leads to severe chromosomic aberrations which are the cause of cell death.
In clinical practice, several PARPi, such as olaparib, niraparib, rucaparib, are widely used in treating ovarian, breast, pancreatic and prostate cancer harbouring BRCA mutations or HRD signature, and they are under investigation in many other neoplasms.
Veliparib (ABT-888) was the first PARPi to be studied in CRC treatment: Prior works demonstrated its efficacy in BRCA-deficient compared to proficient cells and proved that combining platinum-based compounds with PARPi provided increased response[40]. Harpaz et al[34] described the use of veliparib as maintenance therapy in a patient carrying a germline pathogenic BRCA1m, affected by metastatic KRAS and BRAF wild type rectal adenocarcinoma with pelvic and lung metastases. He un
Niraparib, a potent and selective PARP1/2i, has shown in vitro and in vivo efficacy in BRCA mutant cell lines; sensitivity to niraparib monotherapy is 10-fold in BRCA mutant cell lines compared to BRCAwt cells[41].
A phase 2, multicentre, open-label study evaluating rucaparib, an orally available PARP1i, in patients with unresectable, locally advanced or metastatic solid tumours with deleterious mutations in HR repair genes is currently still recruiting; the trial enrols patients with either somatic or germline variants and BRCA1 and BRCA2 mutations are allowed. (NCT04171700). Primary outcome is the best overall response rate[42].
In addition to that, a clinical trial evaluating the use of immune checkpoint inhibitors in solid tumours with BRCA mutation is still ongoing[43] (Table 1).
| Study title | Design | Status | Trial description | |
| NCT03337087 | Liposomal irinotecan, fluorouracil, leucovorin calcium, and rucaparib in treating patients with metastatic pancreatic, colorectal, gastroesophageal, or biliary cancer | Phase I | Recruiting | Experimental: nal-IRI, leucovorin, fluorouracil, rucaparib |
| NCT02286687 | Talazoparib in treating patients with recurrent, refractory, advanced, or metastatic cancers and alterations in the BRCA genes | Phase IIa | Recruiting | Experimental: Talazoparib |
| NCT03428802 | Pembrolizumab in treating participants with metastatic, recurrent or locally advanced cancer and genomic instability | Phase IIa | Recruiting | Experimental: Pembrolizumab |
| NCT04503265 | A trial of AMXI-5001 for treatment in patients with advanced malignancies | Phase I-IIa | Recruiting | Experimental: AMXI-5001 (oral PARP and microtubule polymerization inhibitor) |
| NCT04171700 | A study to evaluate rucaparib in patients with solid tumors and with deleterious mutations in HRR genes (LODESTAR) | Phase IIa | Recruiting | Experimental: Rucaparib |
| NCT03415659 | Phase I clinical study of HWH340 tablet in patients with advanced solid tumors | Phase I | Recruiting | Experimental: HWH340 monotherapy |
| NCT02723864 | Veliparib (ABT-888), an Oral PARP inhibitor, and VX-970, an ATR inhibitor, in combination with cisplatin in people with refractory solid tumors | Phase I | Active, not recruiting | Experimental: VX-970 at Days 2 and 9 of each 21-d cycle; veliparib twice a day (BID) days 1-3 and 8-10 of each cycle; cisplatin at day 1 (and day 8 from DL3 onwards) of each cycle |
| NCT00516373 | A study to assess the safety and pharmacokinetics of an inhibitor of Poly ADP-Ribose Polymerase-1 (PARP) | Phase I | Active, not recruiting | Experimental: KU-0059436 (oral PARP inhibitor) |
| NCT04439227 | Testing AZD1775 as a potential targeted treatment in cancers with BRCA genetic changes (MATCH-Subprotocol Z1I) | Phase II | Active, not recruiting | Experimental: Adavosertib |
| NCT03565991 | Javelin BRCA/ATM: Avelumab plus talazoparib in patients with BRCA or ATM mutant solid tumors | Phase II | Active, not recruiting | Experimental: Combination of avelumab and talazoparib |
| NCT01482715 | A study of oral rucaparib in patients with a solid tumor (Phase I) or with gBRCA mutation ovarian cancer (Phase II) | Phase I-IIa | Completed | Experimental: Rucaparib |
| NCT01989546 | Pilot trial of BMN 673, an oral PARP Inhibitor, in patients with advanced solid tumors and deleterious BRCA mutations | Phase I-IIa | Completed | Experimental: Talazoparib (BMN 673) |
| NCT01286987 | Study of Talazoparib, a PARP Inhibitor, in patients with advanced or recurrent solid tumors | Phase I | Completed | Experimental: Talazoparib |
| NCT03767075 | A modular multi-basket trial to improve personalized medicine in cancer patients (BoB) | Phase II | Recruiting | Experimental: Atezolizumab |
Despite these premises, at the moment there is still no evidence-based data that supports the use of these drugs in CRC patients; because of this it is suggested that PARPi-based clinical trials should be enforced in BRCAm carriers to further validate their use in this setting.
Furthermore, whole-genome sequencing in BRCA1/2m patients allowed identification of different signatures correlated with BRCA status; this BRCA-ness signature was present even in cells without BRCA1/2 mutations, suggesting that the signature might be more likely to predict HRD compared with simple assessment of BRCA mutational status[40]. Should the signature be more widely used, it can be expected that it will be tested to predict PARPi sensitivity.
Worldwide, GC is one of the most common malignancies and the third leading cause of cancer-related mortality[44]. Approximately 8%-30% of patients have a positive family history[45], but only 1%-3% of GCs are truly hereditary[46]. GC predisposition has been linked to familial cancer syndromes, including hereditary diffuse GC[47], LS[48], Peutz-Jeghers syndrome[49], Li-Fraumeni syndrome[50], familial adenomatous polyposis syndrome[51], adenocarcinoma and proximal polyposis syndrome of the stomach[52].
The association between germline mutation of BRCA genes and risk of GC was investigated in several studies, although some aspects remain unclear.
Historically, carriers of germline BRCA1/2 mutations have shown a four to six-fold increased risk of developing GC compared to the general population[53,54] and the risk appears to be higher in patients with BRCA1 mutations than in patients with BRCA2 mutations[54,55].
Regarding BRCA2 carriers, the Polish study by Jakubowska et al[56] assessed the importance of a family history of GC to predict the presence of BRCA2 mutations in ovarian cancer patients. In this study, BRCA2 mutation was found in 8 of 34 women with ovarian cancer and a family history of GC vs 3 of 75 women with OR and a family history of ovarian cancer, but not of GC (OR = 7.4; 95%CI: 1.8-30; P = 0.004); this finding would confirm that GC is a BRCA2-related tumour.
Remarkably, a study conducted by the same group of authors, suggested that founder BRCA1 mutations reported in Polish breast/ovarian cancer patients do not contribute to increased GC risk[57].
Furthermore, Lorenzo Bermejo et al[58] demonstrated that GC before the age of 70 was twice as frequent in families with breast and ovarian cancers as in the general population; similarly, Schlebusch et al[59] found a high prevalence of GC in the BRCA2-positive families compared with the general population.
Nevertheless, in a study performed in the Netherlands, 139 BRCA2 families with 66 different pathogenic mutations were analysed and significantly increased risk of developing GC was not observed[16].
These conflicting data could be partially justified by the high prevalence of Ashkenazi Jews in the studies of Schlebusch et al[59] and Jakubowska et al[56]; in fact, ovarian, pancreatic and gastric cancer as well as non-Hodgkin’s lymphoma have a higher incidence rate in the Ashkenazi population[60].
To further assess the impact of founder mutations in the Ashkenazi population, Figer et al[61] focused on a founder BRCA2 variant that is present in about 1.5% of the general Ashkenazi population and rarely in non-Ashkenazi Jews. In order to evaluate the contribution of this mutation to non-CRC GI cancer, they tested 70 consecutive unselected Ashkenazi Jews with GI malignancies that carried this specific mutation. They concluded that the rate of the Ashkenazi Jews with BRCA2 founder mutation in patients with GC was 5.7%, approximately five times higher than the general popu
It has previously been reported that GC in BRCA2 carriers may be sex-related, as it seems to be more frequent in males[6,58]; in an interesting article, Cavanagh et al[62] argue that women carrying BRCA1/2 mutations usually develop early-onset breast and ovarian cancer and therefore may not survive long enough to develop GC at an older age; this would explain the high prevalence of GC (and other cancers) in male BRCA-carriers.
Regarding BRCA-associated tumours in males, Sun et al[35] recently conducted a retrospective pan-tumour survey on 346 cases of BRCA-associated tumours including GC in males and a comparative analysis among male and female BRCA carriers (349 cases), as well as in male patients who were not BRCA carriers (4577 cases); similar incidences of BRCA mutations (6.0% vs 6.6%) and age at diagnosis of BRCA-related tumour (median, 65 vs 60 years) were observed both in male and female patients.
Moreover, when evaluating both OS and PFS in patients who developed GC, interesting differences were observed: Compared with females, BRCA mutations in males were associated with decreased OS and PFS; however, subgroup analysis demonstrated that BRCA mutation was associated with increased OS in GC (HR for OS: 0.60 P = 0.05). Considering the limited data about the outcome of gastric cancer in BRCA carriers, these results are of particular note. However, data regarding the prognostic impact of BRCA pathogenic variants in GC patients are still scarce.
Another study supporting these results was conducted by Halpern et al[63], where ten GC patients with BRCA mutations were assessed; 6/10 patients had metastatic disease. Median OS of all ten GC patients was 47.5 mo. Median OS for patients diagnosed with operable disease was 55.5 mo and was 32 mo for patients with metastatic disease. Particularly, patients with metastatic disease have a 1-, 2- and 3-year survival rate of 100%, 83.3% and 50%, respectively. Albeit the number of patients included in the analysis is rather limited, it is noteworthy, as it seems to support the idea that BRCA mutations might be associated with better survival: Nowadays median OS of patients with metastatic gastric cancer in western countries is usually around 10-12 mo.
In order to clarify the real incidence and outcome in BRCA patients with GC, a trial is currently active; in this prospective trial the investigators aim to evaluate the incidence of BRCA loss in patients with advanced GC and to observe the treatment outcome and the possibility of BRCA loss as a predictive and prognostic factor[64].
As previously discussed, existing clinical studies have suggested that some BRCA-associated tumours are sensitive to PARPi[18]. Therefore, a phase I/II study investigating side effects and best dose of lyposomal irinotecan and rucaparib when given together with fluorouracil and leucovorin calcium is currently available for BRCA patients with pancreatic, colorectal, gastroesophageal or respiratory cancer biliary.
Other basket trials available for patients with BRCA mutations are summarised in Table 1.
Finally, several cancers in BRCA carriers have been shown to be particularly sensitive to platinum-based chemotherapy[65,66], but studies evaluating the chemosensitivity of patients with BRCA germline mutations and GC are not available.
However, previous clinical and preclinical studies have reported an association between a low level of BRCA1 expression or a BRCA1 mutation and the chemosensi
According to the American Joint Committee on Cancer, patients with a negative-BRCA1 sporadic GC are more likely to have a high grade of tumour, a high TNM stage and a poorly differentiated tumour[53,67]; at the same time, other studies have revealed that BRCAnegative sporadic GC are more sensitive to platinumbased adjuvant chemotherapy compared with BRCA1positive sporadic GC[68]. These findings indicate that patients with BRCA1negative GC have a longer OS time and improved prognosis, which suggests an important association between BRCA1 expression and platinumbased chemotherapy.
OeC is the seventh most common cancer and the sixth leading cause of cancer-related deaths worldwide[69]. The incidence rate of OeC varies considering the location; particularly high frequency is present in East Asia and Eastern/Southern Africa, where oesophageal squamous cell carcinoma (SCC) represents the main histology, while adenocarcinoma is more common in Western countries[70]. Most cases of OeC are sporadic and caused by somatic mutations[71] and oesophageal lesions are rarely described in hereditary CRC syndromes as LS or familial adenomatous polyposis (FAP). However, several studies reported the occurrence of OeC in patients with attenuated FAP or Gardner’s syndrome, which are both caused by mutations in the APC gene[72]. The association between BRCA1/2 germline mutations and OeC has been explored in some studies.
Moran et al[73], in a family-based study, observed a relative risk increase of OeC (regardless of histology) (relative risk 2.9, 95%CI: 1.1-6) in families with BRCA1 muta
Conversely, BRCA2 carriers seem to have a higher risk of developing SCC than Adenocarcinoma.
In fact, contribution of BRCA2 mutations for the development of SCC has been reported in high- and low-risk Chinese populations, in the Turkmen population of Iran and in the very high risk region of northeast India. Specifically, Hu et al[74] have reported five different BRCA2 mutations in 6 out of 44 (13%) patients with SCC in high-risk Chinese population, while Akbari et al[75] observed a nonsense BRCA2 pathogenic gene variant in eight SCC cases with a family history of OeC in the Turkmen population of Iran. Instead, Kaushal et al[76] have investigated 317 cases of SCC in a high-risk region of India and they conducted, performing BRCA2 gene germline mutations, screening in 20 familial and 80 non-familial SCC patients: They found non-synonymous BRCA2 variants in 3 out of 20 patients with familial SCC, while no sequence alterations were found in 80 non-familial SCC cases. Moreover, a study to screen mutations of BRCA2 gene in 47 SCC patients from a low-risk Chinese population was conducted by Zhong et al[77]. They found 9 germline missense point mutations in apparently sporadic male patients, with a mutation frequency of 19%. In addition to that, when an additional cohort of 94 healthy controls underwent screening for the 9 mutations previously identified in SCC cases, only 2 positive individuals (mutation frequency 2%) were found.
Finally, a recent, large-scale study by Ko et al[78] included 4517 individuals: 186 familial SCC patients from a high risk region of China, while the rest were healthy East Asian individuals (3289 Henan and 1228 moderate-risk Hong Kong Chinese). They identified BRCA2 Loss-of-function mutations in 3.23% (6/186) familial SCC patients compared to 0.21% in the East Asians (OR = 15.89, P = 2.48 × 10-10).
Regarding the therapeutic implications between BRCA carriers and OeC, few data are still available in the literature. However, encouraging preclinical studies would show that PARPi in combination with a DNA damaging agent might be beneficial in this setting[79]. Furthermore, the PARPi olaparib appears to sensitise OeC cells to fractionated proton irradiation[80].
Since the discovery in the mid-nineties of both BRCA1/2 genes and their impact on breast and ovarian cancer risk, both surveillance and risk-reducing surgical procedures have been developed as effective means to reduce the risk of developing both malignancies and to overall reduce the risk of death in carriers of these variants. However, recently, BRCA carriers have been recognised to be at risk of developing other kinds of cancer types, such as prostate, pancreatic cancer and melanoma[10-14].
GI cancers are a heterogenous group of malignancies that, taken together, represent the most common tumour type worldwide. Most tumours of this group are mainly due to environmental and lifestyle risk factors, while the weight of hereditary predisposition is rather low (usually around 5%-10% of all cancers). LS represents the most common form of hereditary predisposition to these kinds of tumours (mainly for CRC), while the role of BRCA1/2 mutations is less understood.
In our review we tried to summarize the current published evidence on the role of BRCA1/2 pathogenic variants in colorectal, gastric and OeC risk and prognosis.
As for CRC, there is no definitive consensus regarding increased risk of deve
While there is no consensus on the impact of germline BRCA1/2 mutations on CRC risk, their prognostic role is more clearly defined: A series of studies have shown how, in patients with CRC, BRCA mutations might increase the likelihood of response to chemotherapy. The evidence is, however, mainly derived from case reports or small retrospective case-control studies where patients are treated with oxaliplatin-based chemotherapy. All these studies suffer from selection biases, as only patients who had particularly significant responses to oxaliplatin-based treatment (and that should have more favourable prognosis), underwent genetic testing; there is a general lack of information concerning whether BRCA mutations may influence CRC prognosis regardless of treatment received. It is hoped that, in the near future, wide screening with multi-panel molecular testing in CRC patients will give us a clearer estimate of the real prevalence and prognosis of BRCA mutations; as multi-panel molecular testing will be used more frequently as a means to identify those patients who will be eligible to receive specific targeted drugs, and we will be able to identify more accurately people that might be carriers of germline BRCA mutations that would not be suspected on the basis of their family history.
The role of BRCA mutations in gastric cancer seems to be more clearly defined compared to CRC: A few studies have indicated an increased risk of gastric cancer in families with either BRCA1 or BRCA2 mutations. Even though few papers[54] suggest higher risk in BRCA1 carriers compared to BRCA2, most papers seem to agree that BRCA2 mutations are more clearly associated with an increased GC risk[56,58,59]. As well as in CRC, most studies that have shown a positive impact of BRCA mutations on GC risk have also shown an increased likelihood of early onset (< 70 years old) GC.
As stated before, all those studies that have enrolled specific ethnic groups that have a higher risk of harbouring BRCA mutations (such as Ashkenazi jews) have met with negative results; however, when the analysis was conducted by taking into account this factor, BRCA mutations maintained their causative role. Interestingly, most data that have focused on the prognosis of GC patients harbouring BRCA mutations agree on a positive prognostic role: This might partly be due to the fact that standard treatment of gastric cancer patients includes platinum compounds; it would be interesting to assess whether GC patients who harbour BRCA mutations and that do not receive any kind of platinum-based treatment (as those treated with fluoropyri
Surprisingly, although OeC is usually considered as a cancer type where environmental and lifestyle factors predominantly influence the risk of the onset of disease, a lot of published evidence can be found concerning the role of BRCA mutations in this disease. Out of the two main histologies, SCC seems to be much more closely associated with BRCA mutations compared with adenocarcinoma. All papers seem to suggest that BRCA2 mutations are more frequently observed in this tumour type compared with BRCA1 mutations. Although studies that focused on the prognostic or predictive role of BRCA1/2 are lacking, it is expected that, based on the standard treatment options for this kind of disease that include platinum compounds and radiotherapy, more favourable outcome for patients who are carriers of these muta
The retrospective nature of these studies represents a major limitation; it is however interesting to notice that most of them indicate a positive correlation of germline BRCA1/2 mutations and an increased GI cancer risk. BRCA1/2 variant assessment will soon become one of the most promising fields of research in these kinds of diseases: Most of these tumours are treated with platinum compounds (either oxa
In addition to that, it is actually recognised that, in CRC, GC and OeC, completely different clinical presentations are seen: While older patients have experienced in the last decade an improvement in survival (mainly due to introduction of screening procedures and advances in adjuvant therapy[36], younger patients are still those whose survival has not changed; most papers that have focused on BRCA1/2 mutations came to the conclusion that BRCA germline carriers are those who have the highest likelihood of early onset GI presentation. We believe that further research on early onset GI cancer might identify a greater number of patients where germline defects rather than environmental risk factors are the main causes of disease; this might also be associated with differences in treatment response. Moreover, it would advocate different surveillance procedures, as younger patients are not usually taken into account in most screening programmes.
In conclusion, we believe that this review has highlighted the importance of BRCA1/2 germline mutations as risk factors in GI malignancies and has drawn attention to future applications of this knowledge in clinical practice.
Manuscript source: Invited manuscript
Specialty type: Genetics and heredity
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng HW, da Costa AC S-Editor: Zhang H L-Editor: A P-Editor: Yuan YY
| 1. | Artusio JF Jr. The numenon and the phenomenon. Anesth Analg. 1975;54:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 928] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 2. | Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001;7:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 744] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 3. | Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, Eccles DM, Tang N, Olah E, Anton-Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tulinius H, Thorlacius S, Eerola H, Nevanlinna H, Syrjäkoski K, Kallioniemi OP, Thompson D, Evans C, Peto J, Lalloo F, Evans DG, Easton DF. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2571] [Cited by in RCA: 2555] [Article Influence: 116.1] [Reference Citation Analysis (0)] |
| 4. | Chen S, Iversen ES, Friebel T, Finkelstein D, Weber BL, Eisen A, Peterson LE, Schildkraut JM, Isaacs C, Peshkin BN, Corio C, Leondaridis L, Tomlinson G, Dutson D, Kerber R, Amos CI, Strong LC, Berry DA, Euhus DM, Parmigiani G. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol. 2006;24:863-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 249] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 5. | Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, Bishop DT, Weber B, Lenoir G, Chang-Claude J, Sobol H, Teare MD, Struewing J, Arason A, Scherneck S, Peto J, Rebbeck TR, Tonin P, Neuhausen S, Barkardottir R, Eyfjord J, Lynch H, Ponder BA, Gayther SA, Zelada-Hedman M. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2043] [Cited by in RCA: 1974] [Article Influence: 73.1] [Reference Citation Analysis (1)] |
| 6. | Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1111] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 7. | Thompson D, Easton DF; Breast Cancer Linkage Consortium. Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 768] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 8. | Tai YC, Domchek S, Parmigiani G, Chen S. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:1811-1814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 248] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 9. | Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, Jervis S, van Leeuwen FE, Milne RL, Andrieu N, Goldgar DE, Terry MB, Rookus MA, Easton DF, Antoniou AC; BRCA1 and BRCA2 Cohort Consortium; McGuffog L, Evans DG, Barrowdale D, Frost D, Adlard J, Ong KR, Izatt L, Tischkowitz M, Eeles R, Davidson R, Hodgson S, Ellis S, Nogues C, Lasset C, Stoppa-Lyonnet D, Fricker JP, Faivre L, Berthet P, Hooning MJ, van der Kolk LE, Kets CM, Adank MA, John EM, Chung WK, Andrulis IL, Southey M, Daly MB, Buys SS, Osorio A, Engel C, Kast K, Schmutzler RK, Caldes T, Jakubowska A, Simard J, Friedlander ML, McLachlan SA, Machackova E, Foretova L, Tan YY, Singer CF, Olah E, Gerdes AM, Arver B, Olsson H. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA. 2017;317:2402-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1718] [Cited by in RCA: 1855] [Article Influence: 231.9] [Reference Citation Analysis (0)] |
| 10. | Holter S, Borgida A, Dodd A, Grant R, Semotiuk K, Hedley D, Dhani N, Narod S, Akbari M, Moore M, Gallinger S. Germline BRCA Mutations in a Large Clinic-Based Cohort of Patients With Pancreatic Adenocarcinoma. J Clin Oncol. 2015;33:3124-3129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 301] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 11. | Castro E, Goh C, Olmos D, Saunders E, Leongamornlert D, Tymrakiewicz M, Mahmud N, Dadaev T, Govindasami K, Guy M, Sawyer E, Wilkinson R, Ardern-Jones A, Ellis S, Frost D, Peock S, Evans DG, Tischkowitz M, Cole T, Davidson R, Eccles D, Brewer C, Douglas F, Porteous ME, Donaldson A, Dorkins H, Izatt L, Cook J, Hodgson S, Kennedy MJ, Side LE, Eason J, Murray A, Antoniou AC, Easton DF, Kote-Jarai Z, Eeles R. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31:1748-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 617] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 12. | Mano R, Tamir S, Kedar I, Benjaminov O, Baniel J, Tabachnik T, Margel D. Malignant Abnormalities in Male BRCA Mutation Carriers: Results From a Prospectively Screened Cohort. JAMA Oncol. 2018;4:872-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Shih HA, Nathanson KL, Seal S, Collins N, Stratton MR, Rebbeck TR, Weber BL. BRCA1 and BRCA2 mutations in breast cancer families with multiple primary cancers. Clin Cancer Res. 2000;6:4259-4264. [PubMed] |
| 14. | Streff H, Profato J, Ye Y, Nebgen D, Peterson SK, Singletary C, Arun BK, Litton JK. Cancer Incidence in First- and Second-Degree Relatives of BRCA1 and BRCA2 Mutation Carriers. Oncologist. 2016;21:869-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Thompson D, Easton D; Breast Cancer Linkage Consortium. Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet. 2001;68:410-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 353] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 16. | van Asperen CJ, Brohet RM, Meijers-Heijboer EJ, Hoogerbrugge N, Verhoef S, Vasen HF, Ausems MG, Menko FH, Gomez Garcia EB, Klijn JG, Hogervorst FB, van Houwelingen JC, van't Veer LJ, Rookus MA, van Leeuwen FE; Netherlands Collaborative Group on Hereditary Breast Cancer (HEBON). Cancer risks in BRCA2 families: estimates for sites other than breast and ovary. J Med Genet. 2005;42:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 310] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 17. | Phelan CM, Iqbal J, Lynch HT, Lubinski J, Gronwald J, Moller P, Ghadirian P, Foulkes WD, Armel S, Eisen A, Neuhausen SL, Senter L, Singer CF, Ainsworth P, Kim-Sing C, Tung N, Llacuachaqui M, Chornokur G, Ping S, Narod SA; Hereditary Breast Cancer Study Group. Incidence of colorectal cancer in BRCA1 and BRCA2 mutation carriers: results from a follow-up study. Br J Cancer. 2014;110:530-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, Korach J, Huzarski T, Poveda A, Pignata S, Friedlander M, Colombo N, Harter P, Fujiwara K, Ray-Coquard I, Banerjee S, Liu J, Lowe ES, Bloomfield R, Pautier P; SOLO2/ENGOT-Ov21 investigators. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 958] [Cited by in RCA: 1313] [Article Influence: 164.1] [Reference Citation Analysis (0)] |
| 19. | Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, Wu W, Goessl C, Runswick S, Conte P. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med. 2017;377:523-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 2278] [Article Influence: 284.8] [Reference Citation Analysis (0)] |
| 20. | Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, McGuinness D, Cui KY, Schlienger K, Locker GY, Kindler HL. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1623] [Article Influence: 270.5] [Reference Citation Analysis (0)] |
| 21. | de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, Thiery-Vuillemin A, Twardowski P, Mehra N, Goessl C, Kang J, Burgents J, Wu W, Kohlmann A, Adelman CA, Hussain M. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382:2091-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 914] [Cited by in RCA: 1514] [Article Influence: 302.8] [Reference Citation Analysis (0)] |
| 22. | WHO. Global Cancer Observatory. [cited 17 May 2021]. Available from: https://gco.iarc.fr/. |
| 23. | Goyal G, Fan T, Silberstein PT. Hereditary cancer syndromes: utilizing DNA repair deficiency as therapeutic target. Fam Cancer. 2016;15:359-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Mauri G, Arena S, Siena S, Bardelli A, Sartore-Bianchi A. The DNA damage response pathway as a land of therapeutic opportunities for colorectal cancer. Ann Oncol. 2020;31:1135-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1765] [Cited by in RCA: 1690] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 26. | Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R, Hamilton SR, Hiatt RA, Jass J, Lindblom A, Lynch HT, Peltomaki P, Ramsey SD, Rodriguez-Bigas MA, Vasen HF, Hawk ET, Barrett JC, Freedman AN, Srivastava S. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2154] [Cited by in RCA: 2223] [Article Influence: 105.9] [Reference Citation Analysis (1)] |
| 27. | Mauri G, Sartore-Bianchi A, Russo AG, Marsoni S, Bardelli A, Siena S. Early-onset colorectal cancer in young individuals. Mol Oncol. 2019;13:109-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 408] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 28. | Kim H, Choi DH, Park W, Im YH, Ahn JS, Park YH, Nam SJ, Kim SW, Lee JE, Yu JH, Lee SK, Jung BY. The association between non-breast and ovary cancers and BRCA mutation in first- and second-degree relatives of high-risk breast cancer patients: a large-scale study of Koreans. Hered Cancer Clin Pract. 2019;17:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Oh M, McBride A, Yun S, Bhattacharjee S, Slack M, Martin JR, Jeter J, Abraham I. BRCA1 and BRCA2 Gene Mutations and Colorectal Cancer Risk: Systematic Review and Meta-analysis. J Natl Cancer Inst. 2018;110:1178-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (1)] |
| 30. | Cullinane CM, Creavin B, O'Connell EP, Kelly L, O'Sullivan MJ, Corrigan MA, Redmond HP. Risk of colorectal cancer associated with BRCA1 and/or BRCA2 mutation carriers: systematic review and meta-analysis. Br J Surg. 2020;107:951-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Kirchhoff T, Satagopan JM, Kauff ND, Huang H, Kolachana P, Palmer C, Rapaport H, Nafa K, Ellis NA, Offit K. Frequency of BRCA1 and BRCA2 mutations in unselected Ashkenazi Jewish patients with colorectal cancer. J Natl Cancer Inst. 2004;96:68-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Niell BL, Rennert G, Bonner JD, Almog R, Tomsho LP, Gruber SB. BRCA1 and BRCA2 founder mutations and the risk of colorectal cancer. J Natl Cancer Inst. 2004;96:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Xu Y, Li C, Wang Z, Liu F, Xu Y. Comparison of suspected Lynch syndrome patients carrying BRCA and BRCA-like variants with Lynch syndrome probands: Phenotypic characteristics and pedigree analyses. Mol Genet Genomic Med. 2020;8:e1359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Harpaz N, Gatt YE, Granit RZ, Fruchtman H, Hubert A, Grinshpun A. Mucinous Histology, BRCA1/2 Mutations, and Elevated Tumor Mutational Burden in Colorectal Cancer. J Oncol. 2020;2020:6421205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 35. | Sun P, Li Y, Chao X, Li J, Luo R, Li M, He J. Clinical characteristics and prognostic implications of BRCA-associated tumors in males: a pan-tumor survey. BMC Cancer. 2020;20:994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Associazione Italiana di Oncologia Medica. Tumor del colon. [cited 17 May 2021]. Available from: https://www.aiom.it/wp-content/uploads/2020/10/2020_LG_AIOM_Colon.pdf. |
| 37. | Soyano AE, Baldeo C, Kasi PM. BRCA Mutation and Its Association With Colorectal Cancer. Clin Colorectal Cancer. 2018;17:e647-e650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Lin Z, Liu J, Peng L, Zhang D, Jin M, Wang J, Xue J, Liu H, Zhang T. Complete pathological response following neoadjuvant FOLFOX chemotherapy in BRCA2-mutant locally advanced rectal cancer: a case report. BMC Cancer. 2018;18:1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Paviolo NS, Vega MB, Pansa MF, García IA, Calzetta NL, Soria G, Gottifredi V. Persistent double strand break accumulation does not precede cell death in an Olaparib-sensitive BRCA-deficient colorectal cancer cell model. Genet Mol Biol. 2019;43:e20190070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Reilly NM, Novara L, Di Nicolantonio F, Bardelli A. Exploiting DNA repair defects in colorectal cancer. Mol Oncol. 2019;13:681-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 41. | Genther Williams SM, Kuznicki AM, Andrade P, Dolinski BM, Elbi C, O'Hagan RC, Toniatti C. Treatment with the PARP inhibitor, niraparib, sensitizes colorectal cancer cell lines to irinotecan regardless of MSI/MSS status. Cancer Cell Int. 2015;15:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | A Study to Evaluate Rucaparib in Patients with Solid Tumors and With Deleterious Mutations in HRR Genes (LODESTAR). [accessed 2021 May 17]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT04171700 ClinicalTrials.gov Identifier: NCT04171700. |
| 43. | Guler I, Askan G, Klostergaard J, Sahin IH. Precision medicine for metastatic colorectal cancer: an evolving era. Expert Rev Gastroenterol Hepatol. 2019;13:919-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15468] [Article Influence: 2578.0] [Reference Citation Analysis (2)] |
| 45. | van der Post RS, Vogelaar IP, Manders P, van der Kolk LE, Cats A, van Hest LP, Sijmons R, Aalfs CM, Ausems MG, Gómez García EB, Wagner A, Hes FJ, Arts N, Mensenkamp AR, van Krieken JH, Hoogerbrugge N, Ligtenberg MJ. Accuracy of Hereditary Diffuse Gastric Cancer Testing Criteria and Outcomes in Patients With a Germline Mutation in CDH1. Gastroenterology. 2015;149:897-906.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 46. | Fitzgerald RC, Hardwick R, Huntsman D, Carneiro F, Guilford P, Blair V, Chung DC, Norton J, Ragunath K, Van Krieken JH, Dwerryhouse S, Caldas C; International Gastric Cancer Linkage Consortium. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet. 2010;47:436-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 433] [Cited by in RCA: 384] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 47. | van der Post RS, Vogelaar IP, Carneiro F, Guilford P, Huntsman D, Hoogerbrugge N, Caldas C, Schreiber KE, Hardwick RH, Ausems MG, Bardram L, Benusiglio PR, Bisseling TM, Blair V, Bleiker E, Boussioutas A, Cats A, Coit D, DeGregorio L, Figueiredo J, Ford JM, Heijkoop E, Hermens R, Humar B, Kaurah P, Keller G, Lai J, Ligtenberg MJ, O'Donovan M, Oliveira C, Pinheiro H, Ragunath K, Rasenberg E, Richardson S, Roviello F, Schackert H, Seruca R, Taylor A, Ter Huurne A, Tischkowitz M, Joe ST, van Dijck B, van Grieken NC, van Hillegersberg R, van Sandick JW, Vehof R, van Krieken JH, Fitzgerald RC. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet. 2015;52:361-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 395] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 48. | Capelle LG, Van Grieken NC, Lingsma HF, Steyerberg EW, Klokman WJ, Bruno MJ, Vasen HF, Kuipers EJ. Risk and epidemiological time trends of gastric cancer in Lynch syndrome carriers in the Netherlands. Gastroenterology. 2010;138:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 49. | van Lier MG, Wagner A, Mathus-Vliegen EM, Kuipers EJ, Steyerberg EW, van Leerdam ME. High cancer risk in Peutz-Jeghers syndrome: a systematic review and surveillance recommendations. Am J Gastroenterol. 2010;105:1258-64; author reply 1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 329] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 50. | Masciari S, Dewanwala A, Stoffel EM, Lauwers GY, Zheng H, Achatz MI, Riegert-Johnson D, Foretova L, Silva EM, Digianni L, Verselis SJ, Schneider K, Li FP, Fraumeni J, Garber JE, Syngal S. Gastric cancer in individuals with Li-Fraumeni syndrome. Genet Med. 2011;13:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 51. | Fornasarig M, Magris R, De Re V, Bidoli E, Canzonieri V, Maiero S, Viel A, Cannizzaro R. Molecular and Pathological Features of Gastric Cancer in Lynch Syndrome and Familial Adenomatous Polyposis. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Worthley DL, Phillips KD, Wayte N, Schrader KA, Healey S, Kaurah P, Shulkes A, Grimpen F, Clouston A, Moore D, Cullen D, Ormonde D, Mounkley D, Wen X, Lindor N, Carneiro F, Huntsman DG, Chenevix-Trench G, Suthers GK. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): a new autosomal dominant syndrome. Gut. 2012;61:774-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 53. | Chen W, Wang J, Li X, Li J, Zhou L, Qiu T, Zhang M, Liu P. Prognostic significance of BRCA1 expression in gastric cancer. Med Oncol. 2013;30:423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002;94:1365-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 487] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 55. | Tulinius H, Olafsdottir GH, Sigvaldason H, Arason A, Barkardottir RB, Egilsson V, Ogmundsdottir HM, Tryggvadottir L, Gudlaugsdottir S, Eyfjord JE. The effect of a single BRCA2 mutation on cancer in Iceland. J Med Genet. 2002;39:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Jakubowska A, Scott R, Menkiszak J, Gronwald J, Byrski T, Huzarski T, Górski B, Cybulski C, Debniak T, Kowalska E, Starzyńska T, Ławniczak M, Narod S, Lubinski J. A high frequency of BRCA2 gene mutations in Polish families with ovarian and stomach cancer. Eur J Hum Genet. 2003;11:955-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Ławniczak M, Jakubowska A, Białek A, Lubiński J, Jaworska-Bieniek K, Kaczmarek K, Starzyńska T. BRCA1 founder mutations do not contribute to increased risk of gastric cancer in the Polish population. Hered Cancer Clin Pract. 2016;14:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Lorenzo Bermejo J, Hemminki K. Risk of cancer at sites other than the breast in Swedish families eligible for BRCA1 or BRCA2 mutation testing. Ann Oncol. 2004;15:1834-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 59. | Schlebusch CM, Dreyer G, Sluiter MD, Yawitch TM, van den Berg HJ, van Rensburg EJ. Cancer prevalence in 129 breast-ovarian cancer families tested for BRCA1 and BRCA2 mutations. S Afr Med J. 2010;100:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Lynch HT, Rubinstein WS, Locker GY. Cancer in Jews: introduction and overview. Fam Cancer. 2004;3:177-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Figer A, Irmin L, Geva R, Flex D, Sulkes J, Sulkes A, Friedman E. The rate of the 6174delT founder Jewish mutation in BRCA2 in patients with non-colonic gastrointestinal tract tumours in Israel. Br J Cancer. 2001;84:478-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Cavanagh H, Rogers KM. The role of BRCA1 and BRCA2 mutations in prostate, pancreatic and stomach cancers. Hered Cancer Clin Pract. 2015;13:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 63. | Halpern N, Grinshpun A, Boursi B, Golan T, Margalit O, Aderka D, Friedman E, Laitman Y, Hubert A, Kadouri L, Hamburger T, Barnes-Kedar I, Levi Z, Ben-Aharon I, Brenner B, Goldberg Y, Peretz T, Shacham-Shmueli E. Clinical Characteristics and Prognosis of Gastric Cancer Patients with BRCA 1/2 Germline Mutations: Report of Ten Cases and a Literature Review. Onco Targets Ther. 2020;13:11637-11644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Breast Cancer Genes (BRCA) Expressions in Metastatic Gastric Cancer. [accessed 2021 May 17]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT03838406 ClinicalTrials.gov Identifier: NCT03838406. |
| 65. | Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, Thornton A, Norquist BM, Casadei S, Nord AS, Agnew KJ, Pritchard CC, Scroggins S, Garcia RL, King MC, Swisher EM. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 782] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 66. | Telli ML, Timms KM, Reid J, Hennessy B, Mills GB, Jensen KC, Szallasi Z, Barry WT, Winer EP, Tung NM, Isakoff SJ, Ryan PD, Greene-Colozzi A, Gutin A, Sangale Z, Iliev D, Neff C, Abkevich V, Jones JT, Lanchbury JS, Hartman AR, Garber JE, Ford JM, Silver DP, Richardson AL. Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple-Negative Breast Cancer. Clin Cancer Res. 2016;22:3764-3773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 788] [Article Influence: 87.6] [Reference Citation Analysis (0)] |
| 67. | Zhang ZZ, Liu YJ, Yin XL, Zhan P, Gu Y, Ni XZ. Loss of BRCA1 expression leads to worse survival in patients with gastric carcinoma. World J Gastroenterol. 2013;19:1968-1974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Kim JW, Cho HJ, Kim M, Lee KH, Kim MA, Han SW, Oh DY, Lee HJ, Im SA, Kim TY, Yang HK, Kim WH, Bang YJ. Differing effects of adjuvant chemotherapy according to BRCA1 nuclear expression in gastric cancer. Cancer Chemother Pharmacol. 2013;71:1435-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55774] [Article Influence: 7967.7] [Reference Citation Analysis (132)] |
| 70. | Malhotra GK, Yanala U, Ravipati A, Follet M, Vijayakumar M, Are C. Global trends in esophageal cancer. J Surg Oncol. 2017;115:564-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 235] [Article Influence: 29.4] [Reference Citation Analysis (1)] |
| 71. | Reid BJ, Kostadinov R, Maley CC. New strategies in Barrett's esophagus: integrating clonal evolutionary theory with clinical management. Clin Cancer Res. 2011;17:3512-3519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | van Nistelrooij AM, Dinjens WN, Wagner A, Spaander MC, van Lanschot JJ, Wijnhoven BP. Hereditary Factors in Esophageal Adenocarcinoma. Gastrointest Tumors. 2014;1:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Moran A, O'Hara C, Khan S, Shack L, Woodward E, Maher ER, Lalloo F, Evans DG. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam Cancer. 2012;11:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 218] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 74. | Hu N, Wang C, Han XY, He LJ, Tang ZZ, Giffen C, Emmert-Buck MR, Goldstein AM, Taylor PR. Evaluation of BRCA2 in the genetic susceptibility of familial esophageal cancer. Oncogene. 2004;23:852-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 75. | Akbari MR, Malekzadeh R, Nasrollahzadeh D, Amanian D, Islami F, Li S, Zandvakili I, Shakeri R, Sotoudeh M, Aghcheli K, Salahi R, Pourshams A, Semnani S, Boffetta P, Dawsey SM, Ghadirian P, Narod SA. Germline BRCA2 mutations and the risk of esophageal squamous cell carcinoma. Oncogene. 2008;27:1290-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 76. | Kaushal M, Chattopadhyay I, Phukan R, Purkayastha J, Mahanta J, Kapur S, Saxena S. Contribution of germ line BRCA2 sequence alterations to risk of familial esophageal cancer in a high-risk area of India. Dis Esophagus. 2010;23:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 77. | Zhong L, Zhu ZZ, Shen Y, Sun G, Zhao X, Zhang S, Yin X, Zhu J, Xu Z, Zhu G. Frequent germline mutation in the BRCA2 gene in esophageal squamous cell carcinoma patients from a low-risk Chinese population. Asian Pac J Cancer Prev. 2011;12:1771-1776. [PubMed] |
| 78. | Ko JM, Ning L, Zhao XK, Chai AWY, Lei LC, Choi SSA, Tao L, Law S, Kwong A, Lee NP, Chan KT, Lo A, Song X, Chen PN, Chang YL, Wang LD, Lung ML. BRCA2 loss-of-function germline mutations are associated with esophageal squamous cell carcinoma risk in Chinese. Int J Cancer. 2020;146:1042-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 79. | Secrier M, Li X, de Silva N, Eldridge MD, Contino G, Bornschein J, MacRae S, Grehan N, O'Donovan M, Miremadi A, Yang TP, Bower L, Chettouh H, Crawte J, Galeano-Dalmau N, Grabowska A, Saunders J, Underwood T, Waddell N, Barbour AP, Nutzinger B, Achilleos A, Edwards PA, Lynch AG, Tavaré S, Fitzgerald RC; Oesophageal Cancer Clinical and Molecular Stratification (OCCAMS) Consortium. Mutational signatures in esophageal adenocarcinoma define etiologically distinct subgroups with therapeutic relevance. Nat Genet. 2016;48:1131-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 288] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 80. | Kageyama SI, Junyan D, Hojo H, Motegi A, Nakamura M, Tsuchihara K, Akimoto T. PARP inhibitor olaparib sensitizes esophageal carcinoma cells to fractionated proton irradiation. J Radiat Res. 2020;61:177-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 81. | Rozen P, Lynch HT, Figer A, Rozen S, Fireman Z, Legum C, Katz L, Moy A, Kimberling W, Lynch J. Familial colon cancer in the Tel-Aviv area and the influence of ethnic origin. Cancer. 1987;60:2355-2359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |