Published online May 24, 2021. doi: 10.5306/wjco.v12.i5.367
Peer-review started: December 24, 2020
First decision: March 17, 2021
Revised: March 17, 2021
Accepted: April 22, 2021
Article in press: April 22, 2021
Published online: May 24, 2021
Processing time: 150 Days and 1.7 Hours
In the first studies of electrochemotherapy (ECT), small cutaneous metastases were treated and only mild or moderate pain was observed; therefore, pain was not considered a significant issue. As the procedure began to be applied to larger cutaneous metastases, pain was reported more frequently. For that reason, reduction of both muscle contractions and pain have been investigated over the years.
To present an overview of different protocols described in literature that aim to reduce muscle contractions and pain caused by the electroporation (EP) effect in both ECT and irreversible EP treatments.
Thirty-three studies published between January 1999 and November 2020 were included. Different protocol designs and electrode geometries that reduce patient pain and the number of muscle contractions and their intensity were analysed.
The analysis showed that both high frequency and bipolar/biphasic pulses can be used to reduce pain and muscle contractions in patients who undergo EP treat
Pain reduction in EP-based treatments can be achieved by appropriately defining the protocol parameters and electrode design. Most results can be achieved with high frequency and/or bipolar/biphasic pulses. However, the efficacy of these alternative protocols remains a crucial point to be assessed further.
Core Tip: This is an overview of different published protocols that aim to reduce muscle contractions and pain due to the electroporation (EP) effect. The analysis showed that both high frequency and bipolar/biphasic pulses can be used to reduce pain and muscle contractions. Moreover, appropriate electrode design can lower EP-related morbidity.
- Citation: Fusco R, Di Bernardo E, D'Alessio V, Salati S, Cadossi M. Reduction of muscle contraction and pain in electroporation-based treatments: An overview. World J Clin Oncol 2021; 12(5): 367-381
- URL: https://www.wjgnet.com/2218-4333/full/v12/i5/367.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i5.367
Electrochemotherapy (ECT) is a locoregional anti-tumour therapy that combines a low dose of a chemotherapy drug with high-intensity electric pulses to induce cell membrane electroporation (EP). Consequently, the drugs enter the tumour cells and exert their cytotoxicity[1-4]. Unlike other antitumour treatments based on physical phenomena, ECT is able to exert a specific effect at the cellular level, causing the death of the treated tumour cells. Because it is highly effective in treating cutaneous and subcutaneous tumours regardless of histology[5-7], ECT treatment has been extended to more deeply located tumours[8-13].
To increase the efficacy of EP treatment, the voltage amplitude and the duration or the number of electric pulses are often increased, as long as the required current does not exceed the limit set by the pulse generator. The standard operating procedures[6] for ECT define the electric protocol that, combined with intra-tumour or intravenous delivery of bleomycin or cisplatin[14-19], guarantees an adequate efficacy of the therapy: A train of eight high voltage 100 μs monopolar electric pulses with a repetition frequency of either 1 Hz or 5 kHz is often used. However, the application of high voltage monopolar pulses may cause pain and muscle contractions[20]. For that reason, the use of muscle relaxants and general anaesthesia[21-23] are often required.
In the first studies on ECT, small cutaneous metastases were treated with the observation of only mild or moderate pain was[24-26]; therefore, pain was not consi
Repetition frequency of electric pulses has a close relationship with muscle contraction, which leads to a painful burning sensation and patient complaints[19,20]. An increase in repetition frequency by reducing the pulse-to-pulse pause, seems to reduce unpleasant sensations that occur during ECT[24,35-42]. Moreover, many authors reported that electric pulses lasting microseconds at a high repetition frequency do not decrease ECT antitumour efficacy[35,38]. However, although the pulse frequency is related to muscle contraction, the pain sensation also depends on other pulse characteristics such as voltage amplitude and pulse number, duration and shape[36]. The electrodes used for ECT treatment can also affect the onset of pain. Particularly, needle length, diameter and configuration that changes the distance between needles can be optimised so that the muscle volume crossed by the current is reduced as much as possible. Electrodes with a smaller distance between needles are less painful because they require lower voltages. However, they only treat small portions of tissue, and thus must be applied multiple times to cover the entire lesion.
More recently, it has been demonstrated that the use of high frequency irreversible electroporation (H-FIRE)[28-33], namely bursts of short high frequency bipolar pulses, can further reduce muscle contraction and the subsequent pain caused by the electric pulses. Treatment with H-FIRE pulses, however, may require an electric field intensity higher than the standard electric protocol, both for ECT and for irreversible electroporation (IRE), to reach an equivalent treatment efficacy. An additional disadvantage is delivering pulses at considerably higher voltage amplitudes[34].
The aim of this review is to present an overview of the different protocols proposed in the literature to reduce muscle contractions and pain caused by the EP effect in both ECT and IRE treatments. The main findings of a number of researchers are reported in the results section. The impact of different electrode designs is also considered, as the reduction of muscle contraction and patient morbidity can also be obtained with an appropriate electrode design.
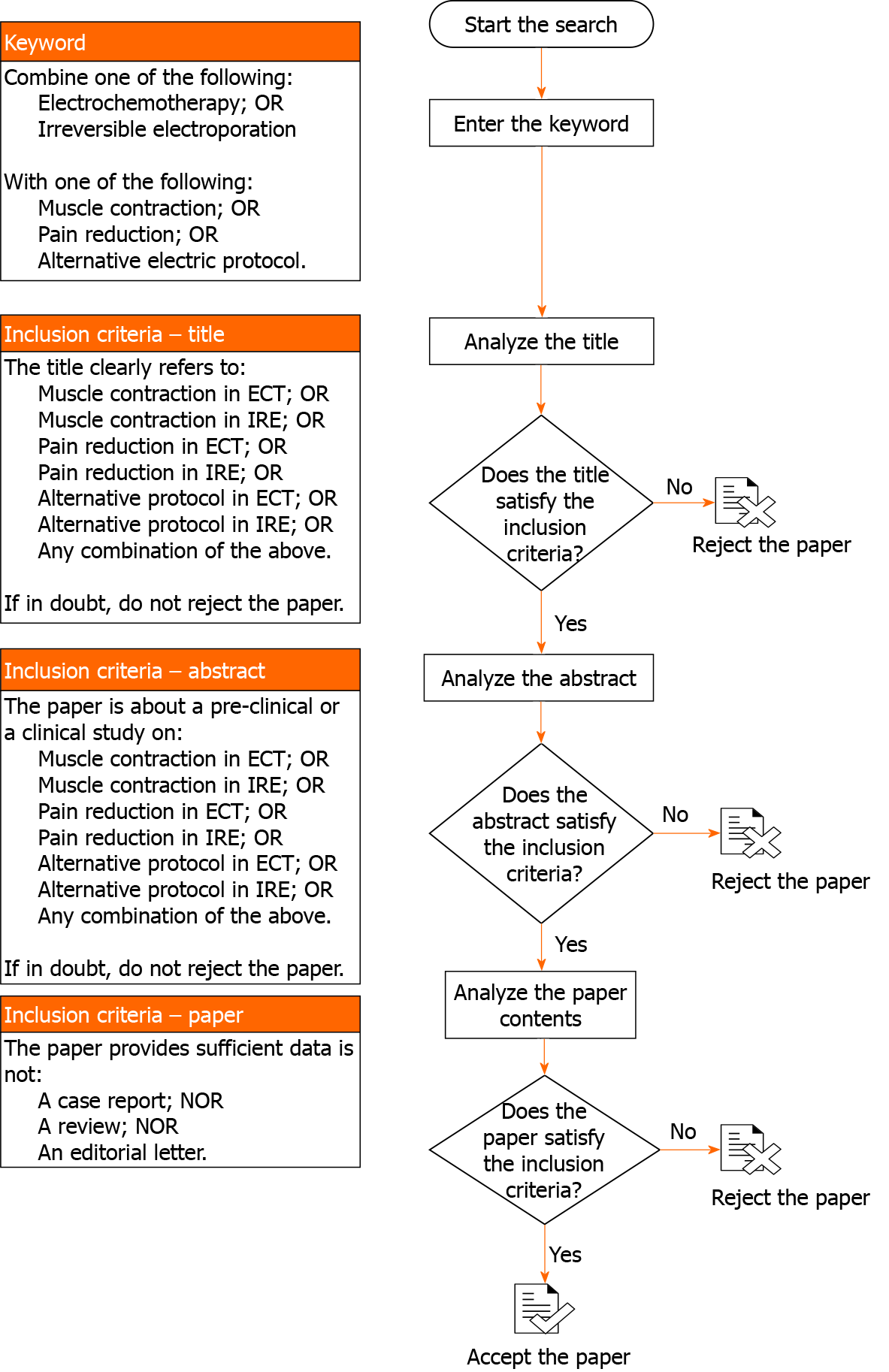
This review is the result of a self-study without protocol or a registration number. In order to ensure an adequate variety of the assessed studies, several electronic databases were searched: PubMed (United States National Library of Medicine, http://www.ncbi.nlm.nih.gov/PubMed); Scopus (Elsevier, http://www.scopus.com/); the Web of Science (Thomson Reuters, http://apps.webofknowledge.com/); and Google Scholar (https://scholar.goo-gle.it/). Only studies published between January 1999 and November 2020 were analysed because that time window is consistent with recent developments in the fields of ECT and IRE. Papers not indexed in the electronic databases were evaluated through the references of included studies. The systematic search for papers of interest is shown in the flow chart in Figure 1. The inclusion criteria evaluated the article title, abstract and contents and included pre-clinical and clinical studies that examined pain or muscle contractions caused by reversible or IRE treatments. Only articles written in English were included. Studies with insufficient reported data, case reports, reviews or letters to the editor were excluded Four investigators carried out data extraction from the included papers, focusing on the type of study (i.e. numerical analysis, in vitro, in vivo or ex vivo), the type of EP (ECT or IRE), the pulse characteristics and the main results regarding reduction of muscle contraction and pain.
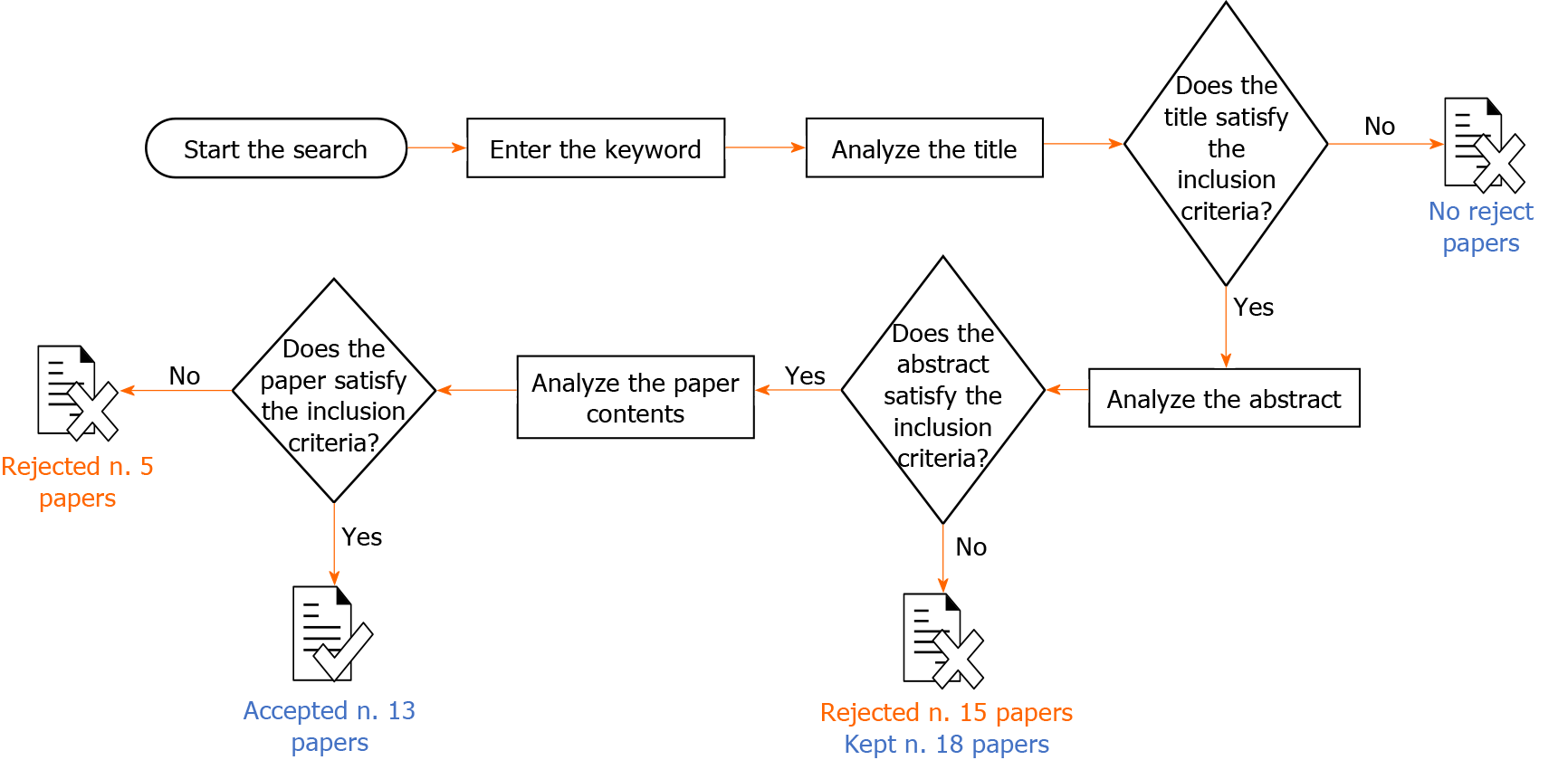
A considerable number of protocols that aim at reducing muscle contractions and pain, caused by the EP effect were found in the literature. The research was conducted with the aim of identifying the parameters that are most responsible for the perception of pain and the stimulation of muscle contraction in patients undergoing EP treatment. Thirty-three studies published between January 1999 and November 2020 were retrieved and papers not indexed in the electronic databases were identified in their reference lists. As per the approach described in Figure 1, 15 studies did not meet the abstract inclusion criteria and were therefore rejected. Five papers were found to be case reports, reviews or editorial letters, did not satisfy the inclusion criteria and were not included in the analysis. The remaining thirteen articles[29,30,33,34,37-45] were included in this manuscript, as they met all the required criteria (Figure 2). Four papers described treatment of cutaneous and subcutaneous tumours[37,40,41,44], two papers included sarcomas[38,42] and pancreatic tumours[43,44] and six were conducted in healthy subjects or phantoms[29,30,33,34,39,45].
In a study published in 1999, Daskalov et al[36] compared monophasic and biphasic pulses in vivo. The monophasic pulse protocol consisted of eight exponentially or rectangular-shaped pulses of 100 μs with a frequency of 1 Hz. In the biphasic pulse protocol, a rectangular pulse of 50 + 50 μs without intra-pulse delay was used in two different ways: (1) Eight pulses with a 1 s interval; and (2) A single burst of eight pulses spaced at 1 ms, with a total duration of 7.1 ms. In both protocols, the selected pulse amplitude ranged from 750 V to 1250 V, depending on the tumour size, with a resulting electrical field strength varying between 330 V/cm and 1250 V/cm. The study showed that the two pulse protocols (monophasic or biphasic) provided the same effect in terms of treatment result. However, the biphasic pulses were better tolerated by the patients. Particularly, the second biphasic mode, a single burst of eight pulses, was considered more acceptable than the first, which comprised eight separate stimuli. Thus, applying the pulses in a rapid sequence was as effective as the use of a larger inter-pulse interval and was better tolerated by the patients. The results of this study[36] were also confirmed by Melzack[46] who previously noted that increasing the number, (N) of applied pulses led to a better effect compared with increasing the pulse duration (T), provided that N × T was constant. In a later study, Miklavcic et al[37] demonstrated that pulse frequencies above the frequency of tetanic contraction (100 Hz) gradually reduced the number of individual muscle contractions. They identified muscle contractions associated with high voltage pulses as the main source of pain for patients undergoing ECT. When the pulse frequency was relatively low, the patient experienced separate muscle contractions associated with each delivered pulse. For that reason, the authors investigated the relationship between muscle contraction and pulse characteristics; particularly, repetition frequency and pulse amplitude. A train of eight 100 μs rectangular pulses at either low or high voltage amplitudes was used. At a low voltage (70 V), the measurements were performed at pulse repetition frequencies of 1, 10, 20, 50, 100, 200, 500, 1000, 2000 and 5000 Hz. At a high voltage (250 V), the measurements were performed at 1, 100, 500, 1000 and 5000 Hz. To investigate the effect of both frequency and amplitude on muscle contraction, they measured the muscle torque in response to electric pulses. They also studied the antitumour efficacy of ECT at different pulse repetition frequencies to be sure that the pain reduction did not lead to a loss of treatment efficacy. Measurements of muscle torque confirmed that high frequency pulses reduced the number of individual contractions to a single muscle contraction. More precisely, with increasing pulse frequency, muscle torque increased up to the frequency of 100 or 200 Hz, reaching a maximum value of 0.16-0.24 nm; however, a further increase of frequency above 200 Hz reduced the muscle torque regardless of the pulse amplitude, with a mean value of about 0.07 nm at 5 kHz, a value similar to that observed during the application of 1 Hz pulse trains. Moreover, by increasing the frequency of electric pulses above the frequency of tetanic contrac
The relationship of pulse frequency and muscle contraction and subsequent patient pain, was successively studied by Zupanic et al[38]. A train of eight electric pulses, of 1 Hz and 5 kHz repetition frequencies, was delivered to 40 healthy patients. After the conclusion of each protocol, the subjects completed the short-form McGill Pain Questionnaire[47] with separate visual analogue scales for pain intensity and unpleasantness. Their results confirmed what Miklavcic et al[37] had previously demonstrated, by finding that muscle contractions, which contribute to the discomfort felt by the subjects during the delivery of electric pulses, are strictly related to pulse frequency. When evaluating the sensorial and affective quality of pain (in the short-form McGill Pain Questionnaire, the most frequently selected pain descriptors were stabbing (80%), cramping (57.5%), throbbing (60%), shooting (60%) and hot-burning (53.8%). However, while both protocols of EP received similar average intensity scores for most descriptors (1.4 for stabbing, 1.0 for cramping, 1.1 for throbbing, 1.1-0.9 for shooting and 1.0-0.7 for hot-burning), treatment with 5 kHz electric pulses was less unpleasant. Therefore, the latter (P = 0.017) was preferred over the standard 1 Hz pulses, even though the perceived pain intensity, ranging from 6 mm to 94 mm, with similar visual analogic scores, was almost the same regardless of the frequency.
In a 2014 study, Spugnini et al[39] analysed the effects of biphasic pulse length on both treatment efficacy and morbidity. The authors investigated two different protocols of trains of eight biphasic pulses, at a voltage of 1300 V/cm. The standard protocol consisted of pulses lasting 50 + 50 ms each, with a frequency of 1 Hz and with 1 ms intra- and inter-pulse intervals. The investigational protocol consisted of pulses lasting 50 + 50 μs each, with a frequency of 1 Hz and with 10 μs intra- and inter-pulse intervals. The protocols were tested both in vitro (human lung cancer cell line A549) and in vivo (mice xenografts; privately owned rabbits with spontaneous tumours). Three of the mice treated with the standard protocol had a strong (grade 4) muscular contraction and the other four had a moderate (grade 3) muscular contraction. Mice treated with the investigational protocol had muscular contractions reported as flicker (grade 1, two mice), weak (grade 2, four mice) and moderate (one mouse). The rabbits treated with the investigational protocol had muscular contractions graded as flicker (two rabbits), weak (three rabbits) and moderate (one rabbit). Given the results obtained from the study, it was concluded that the investigational protocol substantially reduced the morbidity associated with the delivery of electric pulses and achieved a significantly higher efficacy compared with the standard protocol.
In a more recent in vitro study in mouse skin melanoma (B16-F1) cells, Scuderi et al[40] delivered the electric pulses after adding 1–330 μM cisplatin. Two pulse protocols were evaluated: (1) Eight 100 μs monopolar pulses, 0.4–1.2 kV/cm, 1 Hz (standard ECT protocol); and (2) Eight bursts at 1 Hz, consisting of 50 bipolar pulses with 1 + 1 μs width, 0.5–5 kV/cm, 1 μs intra-pulse delay [high frequency EP (HF-EP)]. The analysis of the results was conducted by evaluating the difference between the two protocols (monopolar or bipolar pulses), focusing on their effect on both the efficacy of the treatment and the associated cytotoxicity. First, the results showed that both monopolar and bipolar pulse protocols, in combination with cisplatin, achieved the desired efficacy in killing cells. However, as the onset of membrane permeabilisation was higher in the HF-EP (2 kV/cm) protocol than in the ECT (0.8 kV/cm, P = 0.036) protocol, the bipolar pulse protocol needed a higher electric field (2 kV/cm, P < 0.001 vs 1.2 kV/cm, P < 0.001). Second, the results obtained suggest that HF-EP could be used in ECT with potential alleviation of muscle contractions and pain. In fact, even if the pain was not evaluated, it has been previously demonstrated that a short negative pulse delivered after a positive pulse accelerates passive repolarisation that abolishes the action potential. That means that fewer muscle contractions, and thus less pain, can be expected with HF-EP than with the classic 100 μs pulses. As the authors themselves concluded, although it is still at the in vitro testing stage, the clinical use of HF-EP pulses for ECT could potentially decrease the discomfort associated with muscle contractions and pain, and simplify the treatment procedure by lowering the dose of muscle relaxants and anaesthesia and avoiding synchronisation with the electrocardiogram.
Finally, in 2020, García-Sánchez et al[41] assessed the ability of sine waves to perform ECT. They compared the classic ECT protocol (eight squared unipolar pulses of 100 μs and 1 Hz repetition frequency, electric field of 1300 V/cm) with both bipolar square pulses and sinusoidal bursts. The analysed protocols (bipolar and sinusoidal) were made for pulses with no intra- nor inter-pulse pauses. The bursts were delivered at various frequencies between 10 and 100 kHz and with electric fields of at least 1300 V/cm, and the duration and number of pulses varied depending on the experiment. The authors also carried out a computer simulation to calculate the electric field distribution and the temperature increase during the delivery of the treatment. Furthermore, verification of the effectiveness of the treatment was essential in the comparison between the different protocols, which was taken into account by considering the tumour response. Specifically, the efficacy of the treatment was assessed by comparing sinusoidal bursts at three frequencies (10, 50 and 100 kHz) and two electric field intensities (1300 and 1600 V/cm). Their results showed that sinusoidal pulses reduced both the extent of muscle contractions and skin damage. The effects were significantly lower when a high frequency wave was applied and when the square bipolar pulse was used. However, there was a clear loss of efficacy with the increase in frequency, confirming that the external electric field should be increased to 1600 V/cm in to achieve an equivalent EP effect, thus allowing for a tumour volume growth of less than 200 mm2 within a 25 d follow-up period.
Golberg and Rubinsky[28] performed a numerical analysis to evaluate the influence of the electrode geometry in the reduction of pain and muscle contractions. The numerical analysis considered various electrode configurations. For each experimental setup, a single pulse of 400 V and 100 μs was delivered. The results showed that conventional EP protocols and electrode design could generate muscle contraction, inducing electric fields in surprisingly large volumes of non-target tissue surrounding the EP-treated tissue. They also found that electrode placement in a structure referred to as a “current cage” substantially reduced the volume of non-target tissue exposed to electric fields above the threshold of muscle contraction. Furthermore, in an experimental study using a tissue phantom, they compared a commercial two parallel needle EP system with the current cage design. They found that a certain arrangement of needle electrodes limited the amount of tissue exposed to electric fields that above the muscle contraction threshold, while having a minimal impact on the extent of EP. The design consisted of a central, energised electrode surrounded by an array of grounded electrodes. Similar geometries have been used successfully for cardiac defibrillation and ECT. Interestingly, by having 16 or more grounded electrodes and by reducing the insertion depth of the central energised electrode relative to the grounded electrodes, the predicted amount of tissue experiencing muscle contractions fell dramatically. In fact, the analysis revealed that the ratio of the volume affected by the muscle contraction (Vmc) and that affected by the EP phenomenon (Vep) using a commercial parallel eight-electrode array, was 135 and was 410, with an electric field of than 600 V/cm and 1120 V/cm. The corresponding ratios were 73 and 26 when the 26-electrode current cage was used. Moreover, the total Vmc was 15.09 mm2 when the commercial parallel eight-electrode array was used, compared with 2.90 mm2 when using the 26-electrode current cage.
In 2011, Arena et al[32] used a combination of analytical, numerical and experimental techniques to investigate H-FIRE. In their in vivo protocols, they compared a standard IRE pulse protocol to H-FIRE. In both protocols, 180 bursts were delivered, with each burst lasting 200 μs and being delivered at a frequency of 1 Hz. In the IRE protocol, each burst consisted of a single pulse of 200 μs width. In the H-FIRE protocol, each burst consisted of (1) 50 bipolar pulses at 250 kHz and a single polarity duration of 2 μs; and (2) 100 bipolar pulses at 500 kHz with single polarity duration of 1 μs. No visual or tactile evidence of muscle contraction was seen during H-FIRE, but all IRE protocols resulted in detectable muscle contractions. The mean peak accelerations (0.8 g, 0.4 g and 0.1 g) during IRE treatments at the cervicothoracic junction for each applied voltage (200 V, 100 V and 50 V) were significantly different from each other. On the other hand, H-FIRE resulted in no detectable acceleration at the cervicothoracic junction. The in vivo experiments also showed that H-FIRE produced an ablative effect on brain tissue comparable to that obtained in non-thermal IRE treatments. Specifically, there was complete uniformity of tissue death within the targeted areas. A sharp transition zone was present between lesions and normal brain tissue.
In 2014, Sano et al[42] studied the effects of bipolar pulses on both muscle contractions and cell viability using an IRE protocol. Each monopolar waveform typical of the standard protocol was replaced with a burst of alternating polarity pulses; the total energised burst time was the same as that used in the standard protocol (100 μs). The bipolar protocol consisted of 80 bursts at a frequency of 1 Hz; in each burst, the positive/negative wavelength varied from 250 ns to 50 μs, with an intra-pulse delay fixed at 2 μs. The authors showed that, at 1500 V/cm, only treatments with bursts containing 50 + 50 μs pulses (Table 1) resulted in an interesting compromise between low viability (below 10%) and muscle contraction reduction that less undesirable than those associated with longer monopolar pulses. Sano et al[43]. analysed muscle contraction in a murine model when using different pulse protocols[43]. Treatment efficacy was also tested in an in vitro tumour model using PPT8182 murine primary pancreatic tumour cells. To facilitate comparison between groups, the authors applied the following simplified electrical dose formula:
Dose = V2 × Tp × n × N
Where V is the applied voltage, Tp is the pulse width, n is the number of pulses per burst, and N is the number of bursts per treatment, which was typically 80. Thanks to the use of a custom pulse generation system, bursts of bipolar pulses with constitutive pulse widths of 250 ns, 500 ns, 1 μs, 2 μs, 5 μs, 10 μs and 50 μs were delivered. They also used custom-made electrodes with 1.27 mm diameter dispensing needles and a 2.0 mm edge-to-edge separation distance. Given the formula above, the lethal electric field thresholds were found to be 2022, 1687, 1070, 755, 640, 629 and 531 V/cm for bursts containing 0.25, 0.5, 1, 2, 5, 10 and 50 μs pulses, respectively. Qualitatively, the results showed that muscle contractions occurred to a lesser extent in treatments with bipolar bursts of pulses between 1µs and 5 μs, compared with those in treatments with standard IRE protocol (100 μs monopolar pulses at 200 V). At 400 V, the 100-μs pulses induced such strong muscle contractions that complete anaesthesia was necessary to carry out the procedure. In contrast, 1000 V treatments with bursts of 5 μs pulses were well tolerated with light sedation and local anaesthesia.
Similarly, in 2016, Sweeney et al[33] carried out a quantitative comparison between different pulsing schemes. They compared trains of 100 μs monopolar pulses conventionally used in IRE and ECT, with pulse trains containing bursts or evenly spaced 1 μs bipolar pulses. They assessed both the reduction of muscle contractions and the cell permeability obtained with the different pulsed electric field protocols. Cell per
In 2017, Yao et al[44] explored the effect of IRE ablation on muscle contractions. The authors studied how to reduce muscle contractions by acting both on the frequency of monopolar pulses and on the nature of the electrodes used. The study was conducted with rabbit liver tissue. The H-FIRE protocol consisted of a series of 90 bursts. Each burst had a repetition frequency of 1 Hz and comprised 50, 20, 10, 4 or 2 monopolar pulses with individual pulse widths of 2, 5, 10, 20 or 50 μs. The total energised time was 100 μs. The experiments were conducted with both traditional and insulated needle electrodes with the aim of investigating how the electrode design influenced the muscle contractions. Each pair of electrodes were separated by a fixed distance of 10 mm. A finite element model was also used to establish the lethal thresholds of H-FIRE protocols; consequently, the pulse voltage amplitude range was set from 800 V to 2000 V. An accelerometer was used to measure muscle contractions. The authors observed that the H-FIRE protocol reduced muscle contractions. The muscle contraction strength increased with the increase in voltage amplitude and pulse width. A quite linear increase in acceleration occurred when the voltage was increased, regardless of the pulse duration. For example, a 10 μs pulse produced an acceleration of about 1.5 g at 1000 V, and about 4 g at 2000 V Conversely, at a fixed voltage, a consistent increase in the acceleration value was observed when the pulse length was also increased (e.g., less than 1 g of acceleration for 2 μs pulses at 1500 V vs more than 7 g of acceleration for 100 μs pulses at 1500 V). Moreover, fewer muscle contractions were detected when using insulated needle electrodes and the ablation area was smaller than that obtained with traditional needle electrodes (e.g., about 5 g of acceleration with 50 μs pulses at 1500 V using insulated needles vs about 6.5 g of acceleration with 50 μs pulses at 1500 V using non-insulated needles).
Sano et al[29] compared the effect on muscle contraction associated with IRE to those associated with different H-FIRE protocols. The experiments were conducted ex vivo and muscle contractions were measured with an accelerometer. In order to make the comparison consistent, the total energised time in H-FIRE protocols was ensured to be equal to one of the standard IRE protocols. The traditional IRE protocols consisted of five monopolar pulses lasting 25, 50, 75 and 100 μs, with a repetition frequency of 0.5 or 1 Hz and with an amplitude of 3000 V. To examine alternative strategies, high-energy bipolar bursts with energised times between 100 μs and 200 μs and voltages between 3000 V and 4500 V were delivered. The investigated H-FIRE protocols were split into three subgroups: (1) Symmetric 2 + 2 μs high frequency pulses with an intra-pulse delay of 2 μs, voltages of from 500 to 5000 V and total energised times of 100 or 200 μs; (2) Symmetric 2 + 2 μs high frequency pulses with an intra-pulse delay of 5 μs or 10 μs, a voltage of 5000 V and a total energised time of 100 μs; and (3) Asymmetric high frequency pulses with a 2 μs positive wave, an intra-pulse of 2 μs and negative waves of 0.25, 0.5 or 1 μs (voltage of 3000 V). An energised time of 100 μs with 2–2–2 H-FIRE pulses produced muscle contractions that increased with the voltage (accelerations of 0.005 g and 0.210 g for voltages of 500 and 5000 V, respectively). When the voltage was set at 3000 V, the acceleration peak obtained in the symmetrical H-FIRE protocol was 9-12 times smaller than that seen with traditional IRE pulses (0.72 g with a 75 μs pulse length). Moreover, symmetrical high frequency pulses enabled the delivery of substantially higher voltages and energised times while producing smaller accelerations than traditional IRE pulses. In fact, the acceleration values remained relatively constant when the total energised time was increased from 100 μs to 200 μs, and even when the applied voltage was increased to 4500 V. Conversely, both symmetric pulses with a variable intra-pulse delay and asymmetric pulses produced significantly greater muscle contractions. However, asymmetrical H-FIRE produced significantly greater (α < 0.001) muscle contractions at 3000 V compared with the symmetrical waveforms. The maximum peak acceleration (0.80 g) comparable to that achieved with the traditional IRE pulses, was achieved with the 2–2–0.25 waveform. The authors concluded that muscle contractions can be reduced with H-FIRE pulses when the voltage and energised time are held constant (3000 V, 100 μs). Additionally, high voltage and high-energy H-FIRE treatments produced less intense muscle contractions. However, since the experiments were conducted ex vivo, it is reasonable to consider that muscle contractions in vivo may be greater than those observed in this study. Ablation efficacy should also be assessed.
| Electroporation protocol | ||||||||
| Ref. | Type of pulse | Number of pulses | Pulse duration | Pulse frequency | Electric field | Muscle contraction/pain reduction | ||
| Daskalov et al[36], 1999 | In vivo | ECT | Monophasic | 1 burst of 8 pulses | 100 μs | 1 Hz | 0.33-1.25 kV/cm | Achievable with biphasic pulses |
| Biphasic | 1 burst of 8 pulses | 50-0-50 μs | 1 Hz | |||||
| Biphasic | 1 burst of 8 pulses | 50-0-50 μs | Approximately 909 Hz | |||||
| Miklavcic et al[37], 2005 | In vivo | ECT | Monopolar | 1 burst of 8 pulses | 100 μs | 1 to 5000 Hz (ten or five steps) | 88 or 313 V/cm | Achievable with high frequency pulses |
| Zupanic et al[38], 2007 | In vivo | ECT | Monopolar | 1 burst of 8 pulses | 100 μs | 1 Hz | 600 V/cm | Achievable with high frequency pulses |
| Monopolar | 1 burst of 8 pulses | 100 μs | 5000 Hz | |||||
| Spugnini et al[39], 2014 | In vitro | ECT | Biphasic | 1 burst of 8 pulses | 50-10-50 μs | 9 kHz | 1.3 kV/cm | Achievable with biphasic pulses |
| In vivo | ||||||||
| Scuderi et al[40], 2019 | In vitro | ECT | Monopolar | 1 burst of 8 pulses | 100 μs | 1 Hz | 1.2 kV/cm | Achievable with bipolar HF-EP |
| Bipolar | 8 bursts of 50 pulses | 1-1-1 μs | 250 kHz | 3 kV/cm | ||||
| García-Sánchez et al[41], 2020 | In vivo | ECT | Unipolar | Bursts of 8 pulses | 100 μs | 1 Hz | 1.3 kV/cm | Achievable with sinusoidal pulses |
| Bipolar | Number of bursts and pulses depend on experiments and frequency | 100 μs-5 ms | 10-100 kHz | > 1.3 kV/cm | ||||
| Sinusoidal | ||||||||
| Golberg and Rubinsky[28], 2012 | Numerical | - | Monopolar | 1 pulse | 100 μs | - | > 800 V/cm | Achievable with an appropriate electrode design and arrangement |
| Arena et al[32], 2011 | In vivo | IRE | Monopolar | 90-180 pulses | 200 μs | 1 Hz | 0.5-2 kV/cm | Achievable with H-FIRE |
| Bipolar | 180 bursts of 50 pulses | 2-0-2 μs | 250 kHz | 1-4 kV/cm | ||||
| Bipolar | 180 bursts of 100 pulses | 1-0-1 μs | 500 kHz | 4 kV/cm | ||||
| Sano et al[42], 2014 | In vitro | IRE | Bipolar | 80 bursts of 1 pulse | 50-2-50 μs | 1 Hz | 1.5 kV/cm | Achievable with biphasic pulses |
| Sano et al[43], 2015 | In vitro | IRE | Monopolar | 1 pulse | 100 μs | 1 Hz | 1.5 kV/cm | Achievable with bipolar pulses |
| In vivo | Bipolar | 8-120 bursts of 1-200 pulses | Pulse width: 250 ns-50 μs | 20 to 20000 kHz (seven steps) | Approximately 0.5-2 kV/cm | |||
| Sweeney et al[33], 2016 | In vitro | IRE | Monopolar | 1 burst of 200 pulses | 100 μs | 2 kHz | 750-1250 V/cm | Achievable with high-frequency bipolar pulses |
| Bipolar | 200 bursts of 50 pulses | 1 -1-1 μs | 250 kHz | 1250 V/cm | ||||
| Bipolar | 200 bursts of 50 pulses | 1-4-1 μs | 100 kHz | 1250 V/cm | ||||
| Yao et al[44], 2017 | In vivo | IRE | Bipolar | 90 bursts of 50 pulses | 2-2-2 μs | 250 kHz | 1-2 kV/cm | Achievable with H-FIRE and insulated needle electrodes |
| Bipolar | 90 bursts of 20 pulses | 5-2-5 μs | Approximately 143 kHz | |||||
| Bipolar | 90 bursts of 10 pulses | 10-2-10 μs | Approximately 83 kHz | |||||
| Bipolar | 90 bursts of 4 pulses | 25-2-25 μs | Approximately 37 kHz | 1–1.75 kV/cm | ||||
| Bipolar | 90 bursts of 2 pulses | 50-2-50 μs | Approximately 20 kHz | 1–1.5 kV/cm | ||||
| Monopolar | 90 bursts of 1 pulse | 100 μs | 1 Hz | 0.8–1.5 kV/cm | ||||
| Sano et al[29], 2018 | Ex vivo | IRE | Monopolar | 5 pulses | 100 μs | 1 Hz | 1 kV/cm | Achievable with (symmetric) H-FIRE pulses |
| Bipolar | 5 bursts of 1-200 pulses | 2-2-2 μs | Not available | 0.17-1.7 kV/cm | ||||
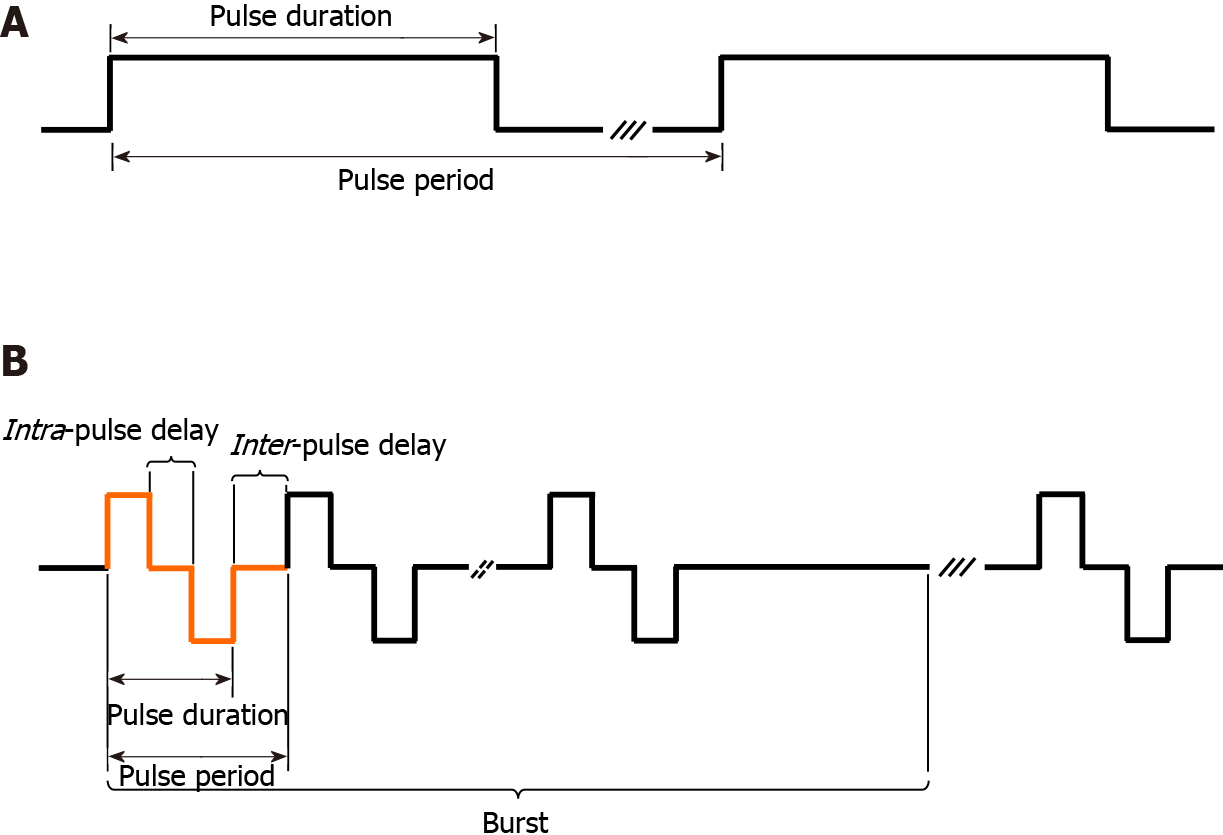
Table 1 summarises the outcomes of the literature analysis. The type of study (numerical analysis, in vitro, in vivo or ex vivo), the type of electroporation protocol (ECT or IRE), the pulse characteristics and the main results are reported. With reference to the pulse characteristics, it was considered of particular interest to report: (1) The type of pulse; (2) The number of pulses (i.e. the number of bursts and the number of pulses per burst); (3) The pulse duration (when bipolar/biphasic, as positive pulse width–intra-pulse delay–negative pulse width); (4) The pulse frequency (i.e. the inverse of a single monopolar/monophasic or bipolar/biphasic pulse period); and (5) The electric field applied (Figure 3).
Muscle contractions and pain are the main undesirable effects associated with EP treatments, both ECT and IRE. Many authors have investigated different protocol designs and electrode geometries in order to reduce patient pain, the number of muscle contractions and their intensity. As this review shows, particular importance was given to the length, frequency and type of the delivered pulses. Less attention was paid to the influence of the electrode design even though it does affect the portion of the muscle through which the current flows. As reported by Miklavcic et al[37] the reduction of voltage amplitude, does not result in an appreciable reduction of patient discomfort. Moreover, even if it is widely accepted that a decrease in the pulse amplitude can be balanced by an increase in the pulse duration, there is still dis
The reduction of pain due to muscle contractions can be obtained by increasing the pulse repetition frequency above that of tetanic contraction (100 Hz)[38]. When the frequency was higher than 2 kHz, patients experienced a single muscle contraction rather than multiple muscle contractions after every single pulse[38,39]. Moreover, treatment efficacy was not altered by an increase in frequency of up to 5 kHz[38,39]. That was confirmed by Yang et al[47], who observed that steep pulsed electric fields with a given frequency and appropriate electric field intensity achieved a cytotoxicity of close to 100%. However, even though the total number of muscle contractions per treatment was reduced, the intensity of the contractions remained similar to that observed in standard protocols.
When altering the pulse repetition frequency, attention should be paid to the choice of pulse numbers and amplitude[48,49]. In fact, the relationship between the pulse parameters for ECT and treatment efficacy, assessed by the cell cytotoxicity rate, can display a highly linear behaviour up to a certain number of pulses and/or field intensity. Thereafter, an exponential model is more appropriate. That is consistent with a recent study by García-Sánchez et al[41], which found that convenient, reversible EP and efficient ECT of subcutaneous tumours and a remarkable reduction of muscle contraction could be achieved by applying sinusoidal fields. However, the frequency of sine waves has been shown to significantly affect ECT effectiveness. At 100 kHz, a clear loss of efficacy was observed[41]. In order to achieve a tumour regression similar to that obtained at 10 kHz, the electric field intensity should be theoretically increased 1.56 times. Those results highlight the charge-dependent nature of the EP phenomenon, where the cell membrane must be charged at the minimum induced transmembrane voltage in order to achieve effective electro-permeabilisa
Several authors have investigated reduction in morbidity achieved with bipolar/bi
Sano et al[43] reported that bursts of bipolar pulses resulted in both instantaneous and delayed cell death and that an inverse relationship existed between pulse width and toxicity, despite the delivery of equal quantities of energy. However, 1500 V/cm bursts containing 50 + 50 μs pulses resulted in a viability below 10% and low muscle contractions, which was less undesirable than those induced by longer monopo
Finally, the protocols described by Scuderi et al[40] and Sweeney et al[33] achieved the results obtained with high frequency pulses together with those achieved with bipolar pulses in ECT and IRE. They found that bipolar pulses at a high frequency were able to mitigate both undesirable muscle contraction and patient pain in EP therapies. Additional reduction was achieved when the bipolar pulse had a symme
Reductions of muscle contraction and morbidity can be achieved with an appropriate electrode design. Fewer and less intense muscle contractions were reported by Yao et al[44] when using insulated needle electrodes. A more sophisticated electrode design was proposed by Golberg and Rubinsky[28] where a central energised electrode was surrounded by at least 16 grounded electrodes, obtaining significant pain reduction. However, the impact of the new electrode designs on treatment efficacy remains to be more deeply evaluated.
This review found that both pulse frequency and shape can be modified to reduce pain and muscle contractions in patients who undergo EP treatments. Furthermore, a combination of high frequency pulses with bipolar/biphasic ones were shown to enhance this capability. However, simply ensuring that equivalent energy is admi
In electrochemotherapy (ECT), pain and muscular contractions were reported as the most frequent drawbacks.
To review aimed to assess the literature describing technical advances intended to reduce muscle contraction and pain associated with electroporation (EP) effects.
The objective was to present an overview of different protocols proposed in the literature that aim to reduce muscle contraction in both ECT and irreversible EP treatments.
Thirty-three published studies reporting different protocol designs and electrode geometries were selected for analysis.
Both high frequency and bipolar/biphasic pulses can be used to reduce pain and muscle contractions in patients who undergo EP treatments. Moreover, adequate electrode design can lower EP-related morbidity.
Pain reduction in EP-based treatments can be achieved by appropriately defining the protocol parameters and the electrode design.
The desired results can be achieved with high frequency and/or bipolar/biphasic pulses.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aureliano M, Gadzijev EM S-Editor: Fan JR L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Tozon N, Sersa G, Cemazar M. Electrochemotherapy: potentiation of local antitumour effectiveness of cisplatin in dogs and cats. Anticancer Res. 2001;21:2483-2488. [PubMed] |
| 2. | Jaroszeski MJ, Dang V, Pottinger C, Hickey J, Gilbert R, Heller R. Toxicity of anticancer agents mediated by electroporation in vitro. Anticancer Drugs. 2000;11:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 116] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Orlowski S, Belehradek J Jr, Paoletti C, Mir LM. Transient electropermeabilization of cells in culture. Increase of the cytotoxicity of anticancer drugs. Biochem Pharmacol. 1988;37:4727-4733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 303] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | Sersa G, Cemazar M, Miklavcic D. Antitumor effectiveness of electrochemotherapy with cis-diamminedichloroplatinum(II) in mice. Cancer Res. 1995;55:3450-3455. [PubMed] |
| 5. | Mali B, Jarm T, Snoj M, Sersa G, Miklavcic D. Antitumor effectiveness of electrochemotherapy: a systematic review and meta-analysis. Eur J Surg Oncol. 2013;39:4-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 236] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 6. | Marty M, Sersa G, Garbay JR, Gehl J, Collins CG, Snoj M, Billard V, Geertsen PF, Larkin JO, Miklavcic D, Pavlovic I, Paulin-Kosir SM, Cemazar M, Morsli N, Soden DM, Rudolf Z, Robert C, O’Sullivan GC, Mir LM: Electrochemotherapy – an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases. Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur J Cancer. 2006;S4:3-13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 548] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 7. | Campana LG, Mocellin S, Basso M, Puccetti O, De Salvo GL, Chiarion-Sileni V, Vecchiato A, Corti L, Rossi CR, Nitti D. Bleomycin-based electrochemotherapy: clinical outcome from a single institution’s experience with 52 patients. Ann Surg Oncol. 2009;16:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | Miklavčič D, Serša G, Brecelj E, Gehl J, Soden D, Bianchi G, Ruggieri P, Rossi CR, Campana LG, Jarm T. Electrochemotherapy: technological advancements for efficient electroporation-based treatment of internal tumors. Med Biol Eng Comput. 2012;50:1213-1225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | Edhemovic I, Brecelj E, Gasljevic G, Marolt Music M, Gorjup V, Mali B, Jarm T, Kos B, Pavliha D, Grcar Kuzmanov B, Cemazar M, Snoj M, Miklavcic D, Gadzijev EM, Sersa G. Intraoperative electrochemotherapy of colorectal liver metastases. J Surg Oncol. 2014;110:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Tarantino L, Busto G, Nasto A, Fristachi R, Cacace L, Talamo M, Accardo C, Bortone S, Gallo P, Tarantino P, Nasto RA, Di Minno MN, Ambrosino P. Percutaneous electrochemotherapy in the treatment of portal vein tumor thrombosis at hepatic hilum in patients with hepatocellular carcinoma in cirrhosis: A feasibility study. World J Gastroenterol. 2017;23:906-918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 11. | Tarantino L, Busto G, Nasto A, Nasto RA, Tarantino P, Fristachi R, Cacace L, Bortone S. Electrochemotherapy of cholangiocellular carcinoma at hepatic hilum: A feasibility study. Eur J Surg Oncol. 2018;44:1603-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Granata V, Fusco R, Piccirillo M, Palaia R, Petrillo A, Lastoria S, Izzo F. Electrochemotherapy in locally advanced pancreatic cancer: Preliminary results. Int J Surg. 2015;18:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Miklavčič D, Mali B, Kos B, Heller R, Serša G. Electrochemotherapy: from the drawing board into medical practice. Biomed Eng Online. 2014;13:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 14. | Mir LM, Tounekti O, Orlowski S. Bleomycin: revival of an old drug. Gen Pharmacol. 1996;27:745-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 144] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Tounekti O, Pron G, Belehradek J Jr, Mir LM. Bleomycin, an apoptosis-mimetic drug that induces two types of cell death depending on the number of molecules internalized. Cancer Res. 1993;53:5462-5469. [PubMed] |
| 16. | Spreckelmeyer S, Orvig C, Casini A. Cellular transport mechanisms of cytotoxic metallodrugs: an overview beyond cisplatin. Molecules. 2014;19:15584-15610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 17. | Mir LM, Gehl J, Serša G, Collins CG, Garbay J-R, Billard V, Geertsend PF, Rudolf 17. Gerald C. O’Sullivan GC, Marty M. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. Eur J Cancer Suppl. 2006;4:14-25. [RCA] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 364] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 18. | Gehl J, Sersa G, Matthiessen LW, Muir T, Soden D, Occhini A, Quaglino P, Curatolo P, Campana LG, Kunte C, Clover AJP, Bertino G, Farricha V, Odili J, Dahlstrom K, Benazzo M, Mir LM. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018;57:874-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 246] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 19. | Arena CB, Davalos RV. Advances in therapeutic electroporation to mitigate muscle contractions. J Membr Sci Technol. 2012;2:1-3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Ball C, Thomson KR, Kavnoudias H. Irreversible electroporation: a new challenge in “out of operating theatre” anesthesia. Anesth Analg. 2010;110:1305-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Mali B, Jarm T, Corovic S, Paulin-Kosir MS, Cemazar M, Sersa G, Miklavcic D. The effect of electroporation pulses on functioning of the heart. Med Biol Eng Comput. 2008;46:745-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Deodhar A, Dickfeld T, Single GW, Hamilton WC Jr, Thornton RH, Sofocleous CT, Maybody M, Gónen M, Rubinsky B, Solomon SB. Irreversible electroporation near the heart: ventricular arrhythmias can be prevented with ECG synchronization. AJR Am J Roentgenol. 2011;196:W330-W335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 23. | Larkin JO, Collins CG, Aarons S, Tangney M, Whelan M, O’Reily S, Breathnach O, Soden DM, O’Sullivan GC. Electrochemotherapy: aspects of preclinical development and early clinical experience. Ann Surg. 2007;245:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Landström FJ, Nilsson CO, Crafoord S, Reizenstein JA, Adamsson GB, Löfgren LA. Electroporation therapy of skin cancer in the head and neck area. Dermatol Surg. 2010;36:1245-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Campana LG, Valpione S, Falci C, Mocellin S, Basso M, Corti L, Balestrieri N, Marchet A, Rossi CR. The activity and safety of electrochemotherapy in persistent chest wall recurrence from breast cancer after mastectomy: a phase-II study. Breast Cancer Res Treat. 2012;134:1169-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Matthiessen LW, Johannesen HH, Hendel HW, Moss T, Kamby C, Gehl J. Electrochemotherapy for large cutaneous recurrence of breast cancer: a phase II clinical trial. Acta Oncol. 2012;51:713-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Cabula C, Campana LG, Grilz G, Galuppo S, Bussone R, De Meo L, Bonadies A, Curatolo P, De Laurentiis M, Renne M, Valpione S, Fabrizio T, Solari N, Guida M, Santoriello A, D’Aiuto M, Agresti R. Electrochemotherapy in the Treatment of Cutaneous Metastases from Breast Cancer: A Multicenter Cohort Analysis. Ann Surg Oncol. 2015;22 Suppl 3:S442-S450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Golberg A, Rubinsky B. Towards electroporation based treatment planning considering electric field induced muscle contractions. Technol Cancer Res Treat. 2012;11:189-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Sano MB, Fan RE, Cheng K, Saenz Y, Sonn GA, Hwang GL, Xing L. Reduction of Muscle Contractions during Irreversible Electroporation Therapy Using High-Frequency Bursts of Alternating Polarity Pulses: A Laboratory Investigation in an Ex Vivo Swine Model. J Vasc Interv Radiol. 2018;29:893-898.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Latouche EL, Arena CB, Ivey JW, Garcia PA, Pancotto TE, Pavlisko N, Verbridge SS, Davalos RV, Rossmeisl JH. High-Frequency Irreversible Electroporation for Intracranial Meningioma: A Feasibility Study in a Spontaneous Canine Tumor Model. Technol Cancer Res Treat. 2018;17:1533033818785285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 31. | Dong S, Wang H, Zhao Y, Sun Y, Yao C. First Human Trial of High-Frequency Irreversible Electroporation Therapy for Prostate Cancer. Technol Cancer Res Treat. 2018;17:1533033818789692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 32. | Arena CB, Sano MB, Rossmeisl JH Jr, Caldwell JL, Garcia PA, Rylander MN, Davalos RV. High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction. Biomed Eng Online. 2011;10:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 225] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 33. | Sweeney DC, Reberšek M, Dermol J, Rems L, Miklavčič D, Davalos RV. Quantification of cell membrane permeability induced by monopolar and high-frequency bipolar bursts of electrical pulses. Biochim Biophys Acta. 2016;1858:2689-2698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Pucihar G, Mir LM, Miklavcic D. The effect of pulse repetition frequency on the uptake into electropermeabilized cells in vitro with possible applications in electrochemotherapy. Bioelectrochemistry. 2002;57:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Zhang L, Rabussay DP. Clinical evaluation of safety and human tolerance of electrical sensation induced by electric fields with non-invasive electrodes. Bioelectrochemistry. 2002;56:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Daskalov I, Mudrov N, Peycheva E. Exploring new instrumentation parameters for electrochemotherapy. Attacking tumors with bursts of biphasic pulses instead of single pulses. IEEE Eng Med Biol Mag. 1999;18:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Miklavcic D, Pucihar G, Pavlovec M, Ribaric S, Mali M, Macek-Lebar A, Petkovsek M, Nastran J, Kranjc S, Cemazar M, Sersa G. The effect of high frequency electric pulses on muscle contractions and antitumor efficiency in vivo for a potential use in clinical electrochemotherapy. Bioelectrochemistry. 2005;65:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Zupanic A, Ribaric S, Miklavcic D. Increasing the repetition frequency of electric pulse delivery reduces unpleasant sensations that occur in electrochemotherapy. Neoplasma. 2007;54:246-250. [PubMed] |
| 39. | Spugnini EP, Melillo A, Quagliuolo L, Boccellino M, Vincenzi B, Pasquali P, Baldi A. Definition of novel electrochemotherapy parameters and validation of their in vitro and in vivo effectiveness. J Cell Physiol. 2014;229:1177-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Scuderi M, Rebersek M, Miklavcic D, Dermol-Cerne J. The use of high-frequency short bipolar pulses in cisplatin electrochemotherapy in vitro. Radiol Oncol. 2019;53:194-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | García-Sánchez T, Mercadal B, Polrot M, Muscat A, Sarnago H, Lucia O, Mir LM. Successful Tumor Electrochemotherapy Using Sine Waves. IEEE Trans Biomed Eng. 2020;67:1040-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Sano MB, Arena CB, DeWitt MR, Saur D, Davalos RV. In-vitro bipolar nano- and microsecond electro-pulse bursts for irreversible electroporation therapies. Bioelectrochemistry. 2014;100:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 43. | Sano MB, Arena CB, Bittleman KR, DeWitt MR, Cho HJ, Szot CS, Saur D, Cissell JM, Robertson J, Lee YW, Davalos RV. Bursts of Bipolar Microsecond Pulses Inhibit Tumor Growth. Sci Rep. 2015;5:14999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 44. | Yao C, Dong S, Zhao Y, Lv Y, Liu H, Gong L, Ma J, Wang H, Sun Y. Bipolar Microsecond Pulses and Insulated Needle Electrodes for Reducing Muscle Contractions During Irreversible Electroporation. IEEE Trans Biomed Eng. 2017;64:2924-2937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 45. | Orlowski S, Mir LM. Cell electropermeabilization: a new tool for biochemical and pharmacological studies. Biochim Biophys Acta. 1993;1154:51-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 169] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 46. | Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4782] [Cited by in RCA: 4428] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 47. | Yang XJ, Li J, Sun CX, Zheng FY, Hu LN. The effect of high frequency steep pulsed electric fields on in vitro and in vivo antitumor efficiency of ovarian cancer cell line skov3 and potential use in electrochemotherapy. J Exp Clin Cancer Res. 2009;28:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | García-Sánchez T, Leray I, Ronchetti M, Cadossi R, Mir LM. Impact of the number of electric pulses on cell electrochemotherapy in vitro: Limits of linearity and saturation. Bioelectrochemistry. 2019;129:218-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Silve A, Leray I, Leguèbe M, Poignard C, Mir LM. Cell membrane permeabilization by 12-ns electric pulses: Not a purely dielectric, but a charge-dependent phenomenon. Bioelectrochemistry. 2015;106:369-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 50. | Kotnik T, Pucihar G, Miklavcic D. Induced transmembrane voltage and its correlation with electroporation-mediated molecular transport. J Membr Biol. 2010;236:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 51. | Arena CB, Szot CS, Garcia PA, Rylander MN, Davalos RV. A three-dimensional in vitro tumor platform for modeling therapeutic irreversible electroporation. Biophys J. 2012;103:2033-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |