Published online May 24, 2021. doi: 10.5306/wjco.v12.i5.323
Peer-review started: January 24, 2021
First decision: March 8, 2021
Revised: March 23, 2021
Accepted: April 23, 2021
Article in press: April 23, 2021
Published online: May 24, 2021
Processing time: 118 Days and 0.5 Hours
In 2017, immune response evaluation criteria in solid tumors (iRECIST) were introduced to validate radiologic and clinical interpretations and to better analyze tumor’s response to immunotherapy, considering the different time of following and response, between this new therapy compared to the standard one. However, even if the iRECIST are worldwide accepted, to date, different aspects should be better underlined and well reported, especially in clinical practice. Clinical experience has demonstrated that in a non-negligible percentage of patients, it is challenging to determine the correct category of response (stable disease, progression disease, partial or complete response), and consequently, to define which is the best management for those patients. Approaching radiological response in patients who underwent immunotherapy, a new uncommon kind of target lesions behavior was found. This phenomenon is mainly due to the different mechanisms of action of immunotherapeutic drug. Therefore, new groups of response have been described in clinical practice, defined as “atypical responses,” and categorized into three new groups: pseudoprogression, hyperprogression, and dissociated response. This review summarizes and reports these patterns, helping clinicians and radiologists get used to atypical responses, in order to identify patients that respond best to treatment.
Core Tip: Atypical responses are frequent events in the immunotherapy era. On these bases, it is fundamental to summarize and recap the most common and important response manifestations to help clinicians in everyday practice. Here, we present the three most common clinical and radiological patterns of response to immunotherapy: pseudoprogression, hyperprogression, and dissociated response, reporting important studies to identify the different behavior and guarantee the best management, strengthening the communication skills between specialists.
- Citation: Ippolito D, Maino C, Ragusi M, Porta M, Gandola D, Franzesi CT, Giandola TP, Sironi S. Immune response evaluation criteria in solid tumors for assessment of atypical responses after immunotherapy. World J Clin Oncol 2021; 12(5): 323-334
- URL: https://www.wjgnet.com/2218-4333/full/v12/i5/323.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i5.323
In the last few years, new therapies such as immunotherapy have been experimented with and introduced into clinical practice for the treatment of oncologic patients. Immunotherapy is a type of treatment that involves the immune system to fight cancer, targeting malignant cells and providing a precise immune response through tumor antigen recognition[1].
There are different types of immunotherapy, so different types of cancer responses can be achieved. All of them are bound by a fundamental principle: Immunotherapy is different from standard therapies (i.e. chemotherapy, radiotherapy, or oncologic surgery) because it helps the self-response to cancer[2].
For these reasons, the standard criteria for monitoring the success of therapy in oncologic patients are not sufficient. All scores, including the World Health Organization classification and the response evaluation criteria in solid tumors (RECIST 1.1.), do not consider that fighting cancer for immunotherapy requires a synergy between tumor cells and host cells[3,4]. To obviate this essential issue, since 2004, different criteria were developed to analyze these responses such as immune-related response criteria, immune-related RECIST, and finally in 2017 immuno-RECIST (iRECIST)[5-8]. These new criteria aim to consider the variety and the time of response to immunotherapy compared with standard therapy, and to standardize and validate the radiologic and clinical interpretation[9].
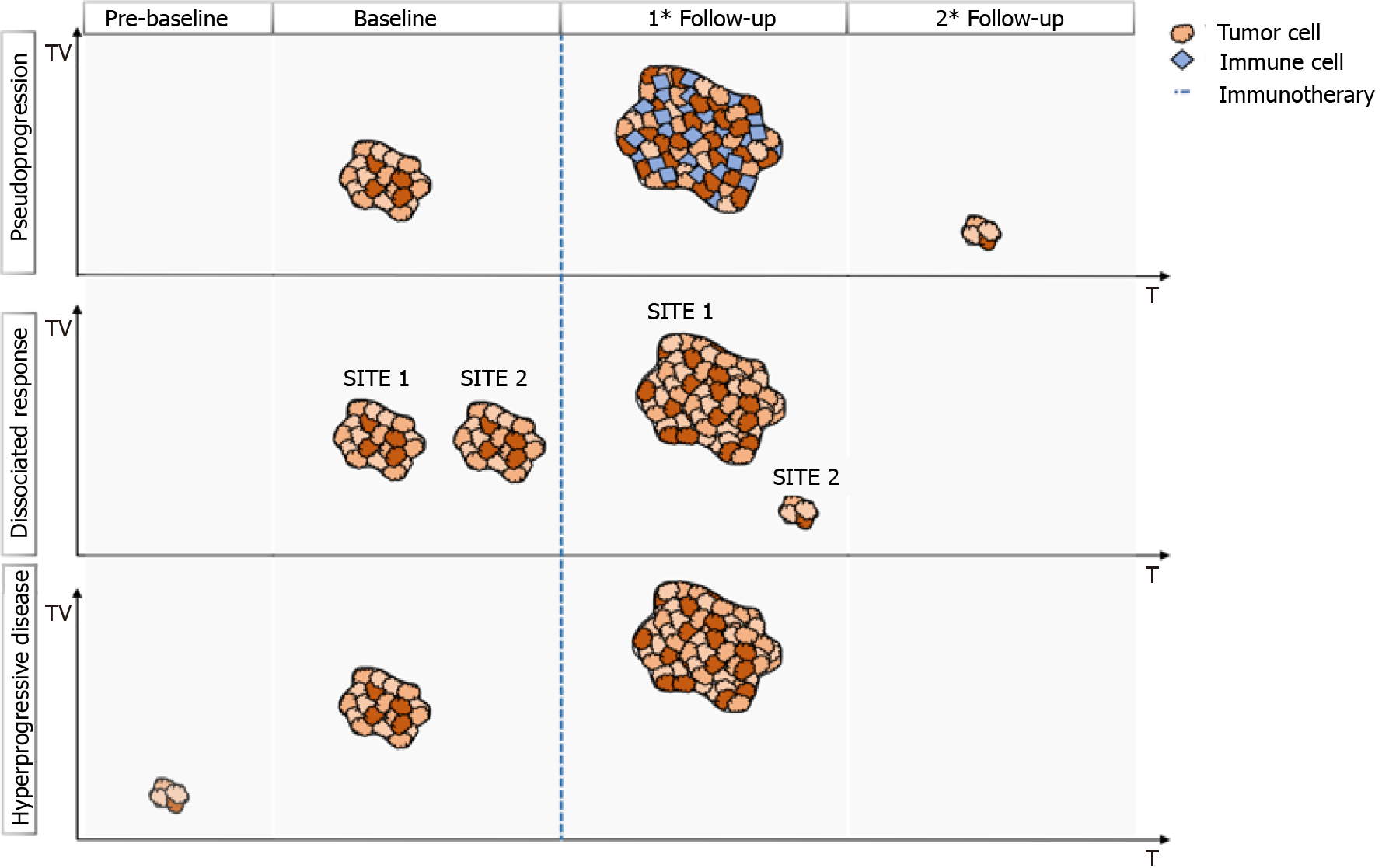
However, immunotherapy raises different questions such as: why is the target lesion increased at first control after immunotherapy and reduced at its end? Why is the target legion bigger at the end of treatment, but the patient’s conditions improve? Why do some metastases disappear, and others become bigger? These different phenomena are called pseudoprogression, hyperprogression, and dissociate response, respectively, and belong to the new lexicon of cancer response to immunotherapeutic agents[10,11].
Radiologists and clinicians should be confident with these patterns (Figure 1) and the interpretation of these data to better understand and manage oncologic patients who have undergone immunotherapy.
In this setting, the present review aims to critically analyze and summarize the most common type of responses to immunotherapy and to drive the knowledge of correct radiologic and clinical interpretation of iRECIST, strengthening the communication skills between specialists.
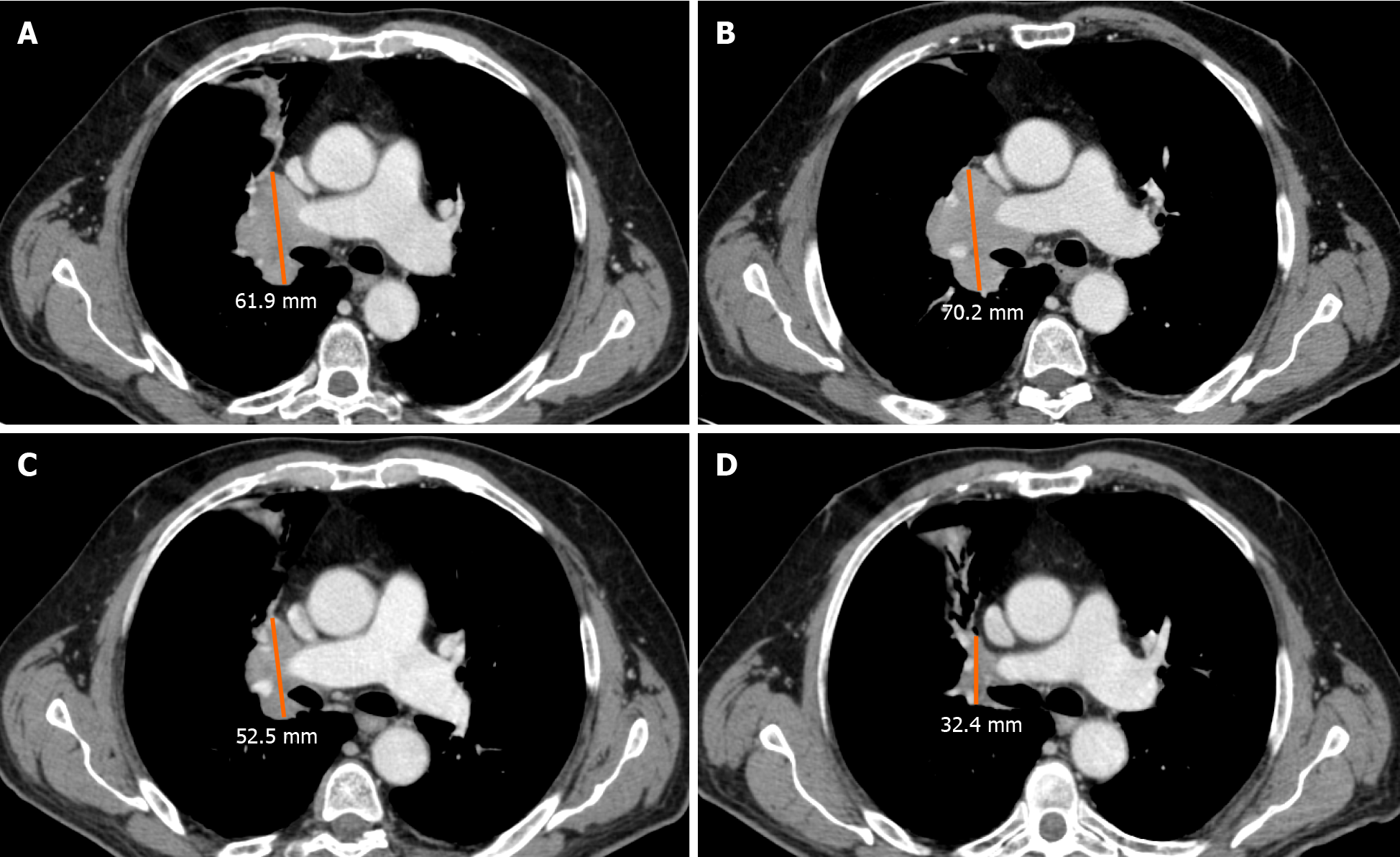
Pseudoprogression is defined as the phenomenon characterized by an initial increase in primary tumor size or new lesions appearance, after starting immunotherapy, followed by a decrease in tumor burden[12-15]. Pseudoprogression should not be considered a true tumor progression but an infiltration and recruitment of various immune cells, such as T or B lymphocytes in the tumor core[16]. Two biological hypotheses have been proposed to explain the phenomenon of pseudoprogression observed in patients treated with immuno-oncology agents. The first hypothesis concerns tumors’ continuous growth until the activation of an effective antitumoral immune response; the second one suggests that an immune-cell influx could occur in the tumoral microenvironment caused by the reactivation of the immune system, leading to inflammation and a transient increase of tumor burden[15].
A study by Cohen et al[17] described the case of a patient with melanoma brain metastasis, who was treated with pembrolizumab, presenting a pseudoprogression of brain lesions revealed through magnetic resonance imaging (MRI) and biopsy. The MRI showed an enlargement of central nervous system lesions with diffuse perilesional edema, while the histologic evaluation revealed tumor cells surrounded by reactive astrocytosis, scattered inflammatory cells, and microglial cells, which was consistent with the abovementioned response to treatment rather than tumor growth.
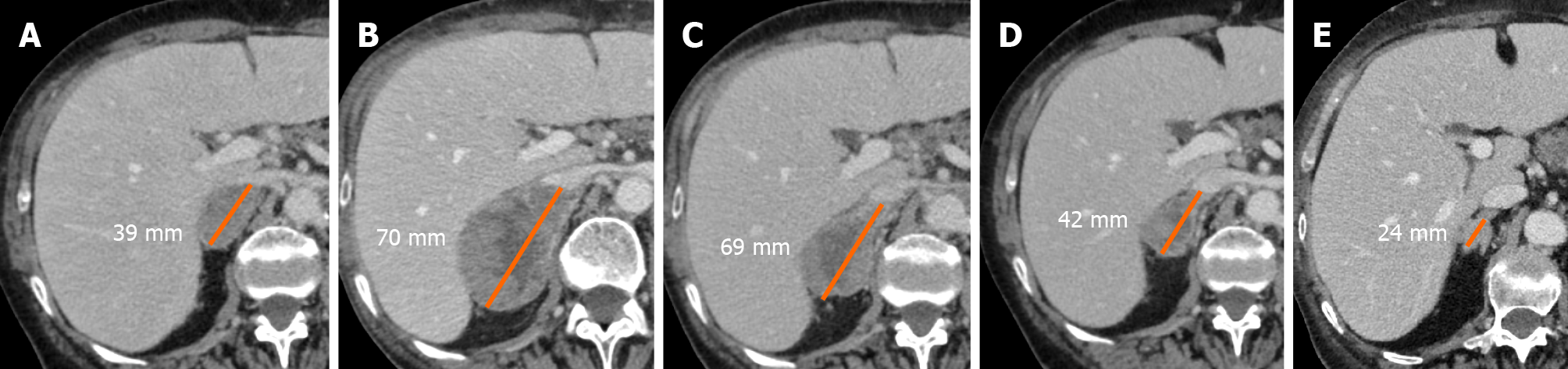
Rocha et al[18] described the case of a patient with end-stage squamous cell lung cancer, who was treated with nivolumab and exhibited pseudoprogression of the liver lesions, proved by the biopsy. The tissue sample revealed extensive areas of necrosis, no viable tumor cells, and lymphocyte infiltration. In the liver biopsy, the number of CD4-, CD8- and CD103- cells were increased, the ratio of CD4+/CD8+ T cells was decreased, and CD68+ staining indicated a higher proportion of macrophages, suggesting an inflammatory response rather than disease progression. Moreover, other cases have shown necrosis, hemorrhage, edema, and immune cell infiltration in lesions with pseudoprogression[14,15,19,20]. Therefore, the infiltration of immune cells, such as CD4+, CD8+ cells and macrophages, represents the major mechanism of pseudoprogression, consequently including edema, hemorrhage, and necrosis[12].
An unconventional pattern of response to immunotherapy was first described with the development of cytotoxic T-lymphocyte antigen 4 inhibitors in melanoma, with a patient experiencing enlargement of a cutaneous lesion during the first weeks of treatment, followed by prolonged stabilization[15]. Since then, pseudoprogression has been used to describe an objective response obtained after initial progression disease and has been observed in other cancer types[16] (Figure 2).
The occurrence of pseudoprogression was confirmed in large trials, which allows treatment beyond progression; its incidence, reported in different tumor types, has never exceeded 10% of patients[21]. However, a recent study determined that the incidence of atypical response is about 20%, including the development of new lesions, and the increase greater than 10% in the total sum of the longest dimension[22].
Pseudoprogression has been described in different types of tumors, mainly in melanoma patients but also in non-small lung cell carcinoma (NSCLC) (Figure 3), renal cancer (RCC), urothelial cancer, uveal melanoma, Merkel cell carcinoma, meso
The reported incidence of pseudoprogression in clinical trials was 2.78%-9.69% for melanoma, 1.81%-5.77% for NSCLC, 2.86%-8.82% for RCC, 1.49%-7.14% for urothelial carcinoma, 11.11% for uveal melanoma, 1.79% for HNSCC, 1.14% for Merkel cell carcinoma, and 6.90% for mesothelioma[12].
Clinical and biological characteristics of different tumors, the demographic characteristics of patients, and the different types of immunotherapy agents used might explain the different incidence of pseudoprogression in various types of solid tumors. In addition, according to some case reports, there might be some sites of pseudoprogression specific to the tumor type after immunotherapy, such as brain metastasis pseudoprogression of lung cancer and RCC[30,31].
Interestingly, for patients treated beyond progression, no increase in immune-related toxicity was reported. Furthermore, patients experiencing pseudoprogression had longer overall survival (OS) compared with standard progressive disease (PD), suggesting that patients who present with pseudoprogression can effectively obtain benefit from treatment beyond progression[23].
The iRECIST guidelines proposed two specific response patterns: unconfirmed PD (iUPD) and confirmed PD (iCPD). The iUPD is defined as PD for the RECIST v1.1 criteria that is not confirmed at the follow-up imaging assessment within 4-8 wk. The iCPD is defined as the appearance of a new lesion or further growth of the sum of measures of target lesions of 5 mm or greater at the diagnostic follow-up after the iUPD within 4-8 wk, or as an increase in a non-target lesion, that was initially categorized as iUPD. If no change in tumor size nor extent from iUPD occurs, then the time point response would again be iUPD. Complete response (iCR), partial response (iPR), and stable disease (iSD) were assigned based on the RECIST 1.1. Moreover, if after iSD, iPR, or iCR, PD takes place again, we consider it as iUPD and reset the bar again through the application of the so-called “dynamic time point”[7]. To resume, iUPD can be assigned multiple times as long as iCPD is not confirmed at the next assessment and iRECIST requires the confirmation of progression to rule out or confirm pseudoprogression.
The iRECIST guidelines proposed a status of iUPD, which would allow the continuation of treatment and follow-up more closely to better benefit patients. This approach allows the identification, understanding, and better characterization of atypical responses, such as delayed responses that occur after pseudoprogression[7].
To differentiate pseudoprogression from true progression, the iRECIST guidelines recommend that clinical trials should only include patients who are clinically stable to continue treatments until the next assessment (≥ 4 wk later). In these cases, the next imaging assessment should be performed no longer than 8 wk later, to ensure that patients remain fit for rescue therapies[7].
Among the potential useful methods to identify pseudoprogression in tumors treated with immunotherapy and to differentiate it from the true progression of the disease, the combination of biopsy and histopathologic examination is considered the gold standard, although it presents some disadvantages due to the invasive nature of the procedure. Compared to biopsy, the radiographic follow-up presents incompa
A summary of the most important studies focusing on pseudoprogression is reported in Table 1.
| Response | Cancer type | Treatment | Incidence (% range) |
| Pseudoprogression | Melanoma | Ipilimumab | 7.4-9.7 |
| Tremelimumab | 2.8-6.3 | ||
| PD1/PD-L1 inhibitors | 3.7-8.3 | ||
| Pembrolizumab | 3.7-7.3 | ||
| RCC | PD1/PD-L1 inhibitors | 4.9-14.8 | |
| Atezolizumab | 2.9 | ||
| NSCLC | PD1/PD-L1 inhibitors | 1.9-6.9 | |
| Atezolizumab | 2.8 | ||
| Urothelial | Atezolizumab | 1.5-6.8 | |
| Durvalumab | 7.1 | ||
| PD1/PD-L1 inhibitors | 8.9 | ||
| HNSCC | Pembrolizumab | 1.8 | |
| PD1/PD-L1 inhibitors | 1.3 | ||
| Mesothelioma | Tremelimumab | 6.9 | |
| Hyperprogression | NSCLC | PD1/PD-L1 inhibitors | 8.0-14.0 |
| Gastric | PD1/PD-L1 inhibitors | 21.0-29.4 | |
| RCC | PD1/PD-L1 inhibitors | 7.0-46.0 | |
| Melanoma | PD1/PD-L1 inhibitors | 1.2 | |
| Dissociated response | NSCLC | PD1/PD-L1 inhibitors | 7-5-10 |
Hyperprogressive disease (HPD) is considered fast tumor growth, after starting immunotherapy, regarding the absolute mass. However, compared with the other atypical patterns, HPD relies on its intrinsic definition in the “expected” response, and consequently, a specific description is currently missing. For example, empiric doubling of tumor volume or by using linear growth in tumor diameter have been proposed to identify the HPD and, as a matter of fact, recently published papers reported different ways to define HPD and different thresholds to stratify pa
Different cellular and genetic triggering events were studied to better define and understand HPD. The first described is linked to cytotoxic agents used before immunotherapy, probably causing a decreased effect of the last one[35] due to clones’ selection able to escape therapy. On the other hand, new immunotherapeutic agents can bind other than targeted receptors and allow rapid tumor growth. Finally, different genetic mutations, such as the most common one Janus kinase 1/2 mutation, can be directly linked to HPD, generating resistance to immunotherapy and resulting in a fast tumor volume increase. The tumor microenvironment can be strictly involved in HPD, especially by immune cell infiltration, as reported in previous papers[36-38].
From a radiological point of view, to identify HPD, at least one imaging exam should be obtained before and one after starting immunotherapy, to correctly establish an increase in tumor volume higher than the expected one[39,40].
Even if the iRECIST algorithm is the most widely applied in clinical practice, it does not suggest evaluating the pretreatment imaging data to identify the tumor growth rate (TGR), and suspected hyperprogressive patients should be followed-up for at least 12 wk for definitive confirmation[39]. The identification of HPD poses a challenge for the iRECIST, which fail to capture pre- and post-treatment tumor growth kinetics (TGK) at early times of disease, and consequently, different parameters such as “RECIST progression at the first evaluation”[39], TGR[40], TGK ratio (ratio of the slope of tumor growth before treatment and the slope of tumor growth on treatment), time to treatment failure (TTF)[41], and the combination of clinical and radiological criteria[42] have been proposed.
A recent study by Gomes da Morais et al[43], combining four different definitions for HPD previously proposed, found no overall significant differences between baseline and post-baseline tumor growth rate (P = 0.93). Finally, the authors confirmed that the progression-free survival (PFS) was shorter in patients with HPD compared with non-HPD ones.
A metanalysis published by Kim et al[44] evaluated a total of 217 HPD cases of 1519 cancer patients. Considering the lack in HPD definition, its incidence ranged from 1% to 30%, in line with Frelaut et al[23], reporting a range from 7% and 29%. Authors identified age (> 65 years), gender (female), aggressive primary tumor (high recurrence rate, > 2 metastatic sites), histological and immunological profiling (i.e. low programmed death-ligand 1 expression, epidermal growth factor receptor, mouse double minute 2 homology and DNA (cytosine-5)-methyltransferase 3A alterations) as predictive factors for HPD.
Analyzing the most important recent studies, Park et al[45] identified HPD in 18 patients (14.4%) with head and neck cancer, underlying that younger age, a primary tumor of the oral cavity, and previous locoregional irradiation are significant predictors of HPD. Moreover, patients with HPD showed a shorter median PFS and OS.
To date, different published papers have investigated the importance of HPD in lung cancer patients. Kim et al[46] observed HPD in 55 (20.9%), 54 (20.5%), and 98 (37.3%) patients according to the TGK, TGR, and TTF, underlying that HPD was associated with worse PFS and OS. The same results in terms of incidence were reported in previous retrospective studies by Ferrara et al[40] (14%), Lo Russo et al[42] (26%), Kim et al[46] (21%), as summarized in the review by Kim et al[44]. More recently, Kas et al[47], with a retrospective study including 406 patients, suggested a new definition for HPD in patients with NSCLC, based on ΔTGR.
Aoki et al[48] and Sasaki et al[49] studied the importance of HPD in gastric cancer patients reporting an incidence of 29.4% and 21% after nivolumab treatment, respectively. Both studies reported a slight decrease in PFS and OS in patients with HPD.
Kim et al[50] reported that HPD exists in a fraction of hepatocellular carcinoma (HCC) patients who received programmed cell death protein 1 (PD-1) blockade: Analyses of the baseline immune profile and on-treatment tumor growth dynamics could promote optimal patient selection and earlier identification of rapid tumor growth induced by PD-1 inhibitors in HCC patients[50].
Zheng et al[51] reviewed patients with RCC under immunotherapy, finding that the incidence of HPD ranged between 7% and 74% without any strong suggestive factors associated.
Regarding melanoma, immunotherapy treatment is not extensively reported in the literature. A recent retrospective study by Hao et al[52] and Schuiveling et al[53], enrolling 168 patients, reported a 1.2% incidence of HPD.
According to the RECIST working group, a CT scan 8 wk after the first treatment is needed to evaluate early response[7]. In line with the guidelines, if progression is not confirmed, the follow-up should be continued as previously planned, while in case of suspected progression at first-imaging follow-up, a confirmatory CT 4 wk later should be required. Moreover, considering the importance of pre-baseline imaging, a CT scan at least 1 mo before starting immunotherapy should be evaluated to define the tumor volume and consider it in further evaluations. During the anamnestic questionnaire, special attention should be addressed to pre-immunotherapy treatments, specifically regarding conventional cytotoxic agents, as aforementioned[39,40]. Radiological assessment, both CT- and MRI-based, is fundamental to determine the growth rate; however, the true positive rate can be weakened by pseudoprogression in case of pre-baseline missing, because it is not possible to distinguish between the two patterns.
On these bases, a complete assessment based on clinical and radiological findings, along with a careful evaluation of pre-baseline imaging, is needed to correctly stratify patients suspected of HPD, to define the best clinical approach possible to increase PFS and OS. The difficulties to standardize the HPD definition by using radiological criteria firstly rely on the various types of cancer to deal with and, consequently, on the different imaging techniques considered as the reference standard for staging and re-staging patients.
A summary of the most important studies focusing on hyperprogression is reported in Table 1.
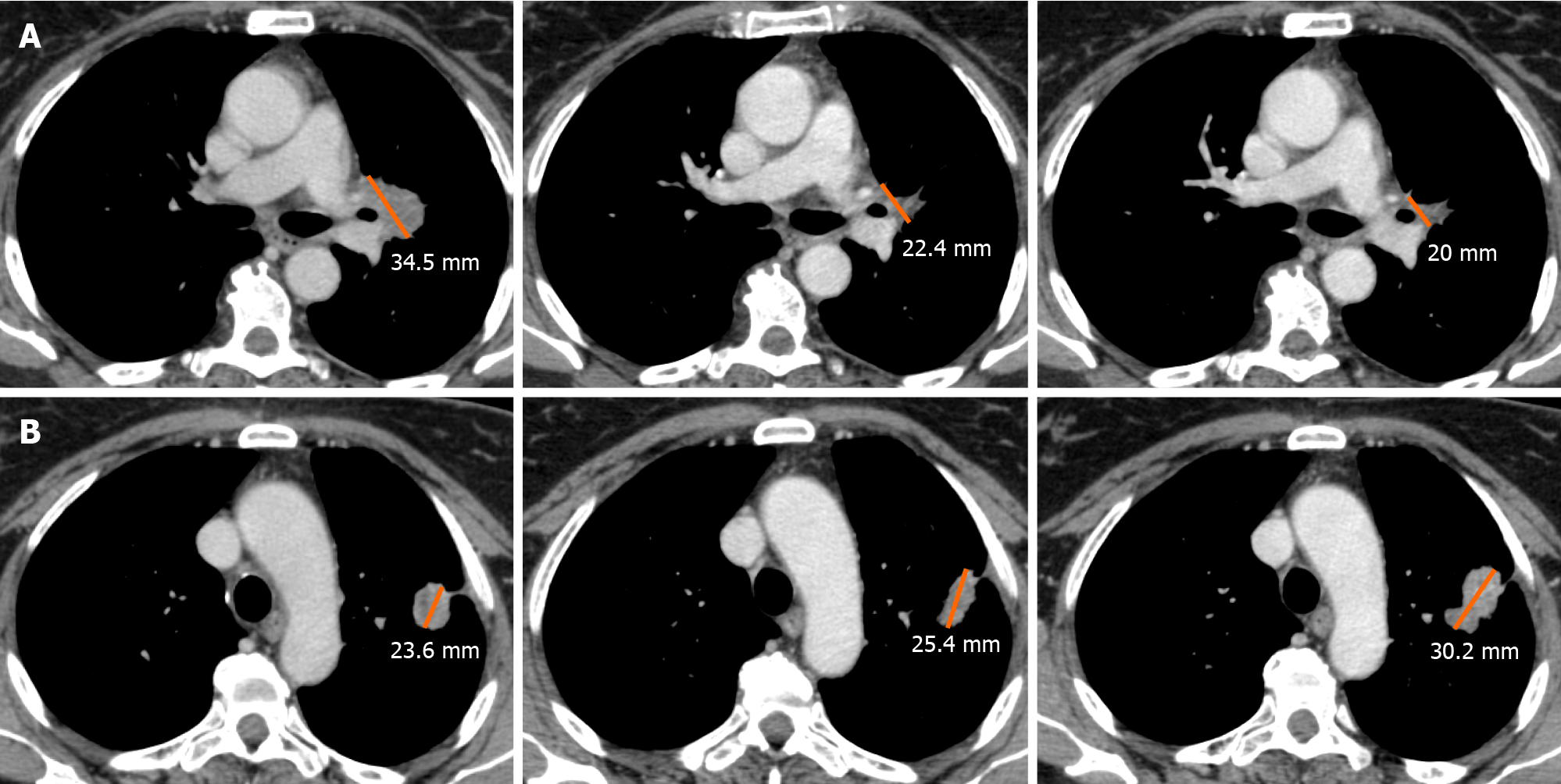
Besides the mixed pattern of response arising from traditional platinum-based chemotherapy, the development of immunotherapy has led to the introduction of the concept of dissociated response (DR).
DR has been recently described as a concomitant increase in the size of some target lesions or the appearance of new lesions, accompanied by regression of other ones[54]. A combination of factors may explain the biological mechanisms of a dissociated tumor response. Tumor heterogeneity within an individual patient and differences in tissue penetration of anti-cancer drugs have been proposed as potential reasons for DR[55]. Tumoral cells can undergo clonal evolution from a single progenitor cell into more aggressive and therapy-resistant cells, due to genomic instability of solid cancer cells.
This genotypic and phenotypic heterogeneity is an unfavorable prognostic factor for cells’ survival, and it can explain the DR, particularly when using targeted therapies due to their selective pressure on tumor evolution. Moreover, the heterogeneity of the immune environment of the lesions can actively influence therapeutic response and therefore explain different responses[56] (Figure 4).
In literature few studies reported on the incidence of DR, ranging from 7.5% to 10%[54,55,57]. Using fluorodeoxyglucose PET/CT, Humbert et al[58] recently pub
DR has been associated with different prognoses compared to progressive or non-PD. Tazdait et al[54] observed similar survival between patients with the non-PD and those with the atypical response, even if pseudoprogression and DR were not evaluated separately. On the contrary, the higher survival of patients with DR, compared to those with PD, was confirmed both by Tazdait et al[54] and Tozuka et al[55], suggesting that the prognosis of patients with DR is probably intermediate between those with PD and those with the non-PD.
In the literature, several different definitions of DR were encountered; in particular, it is still not clear if a concomitant progression and reduction of different lesions are sufficient to consider as DR, or if it is necessary to reach at least 20% of PD and 30% of PR[54,55,57]. On PET/CT, DR definition should be inspired by PET Response Criteria In solid tumor (PERCIST) and defined as a concomitant relative decrease > 30% in some tumor lesions metabolism and relative metabolic increase > 30% in others.
An important issue is the optimal duration of treatment due to the potential of late treatment effect and the rare phenomenon of pseudoprogression. Many clinicians choose to continue treatment beyond progression with immunotherapy according to the RECIST[59]. As the progressing lesions might represent pseudoprogression, the monitoring and management of patients with the DR should be similar to that of patients with pseudoprogression, if the patient is clinically stable. A recent study shows that continuing immunotherapy post-DR had significantly better survival than discontinuing therapy[57].
Besides, continuing immune checkpoint inhibitor treatment plus local ablative therapy targeted to progressing lesions could be a valid alternative to immunotherapy alone in case of single progressive lesions[56]. However, if the patient is clinically deteriorating the interruption of immune checkpoint inhibitor treatment and switching to another therapy, or clinical trial participation, should be considered[60].
The high number of atypical responses such as pseudoprogression and DR suggest that in most cases the RECIST 1.1 underestimates the benefit of treatment with immunotherapy and the new iRECIST are certainly superior in the evaluation of responses. The iRECIST consider consistently pseudoprogression, while DR is not considered[7,11], suggesting that they may not correctly describe the clinical benefit from immunotherapy[61].
Considering the different interpretations of DR given by the different authors, a more uniform definition of this phenomenon is crucial to assess the correct prognosis of patients with DR compared to progressive and non-PD after immunotherapy. As suggested by Humbert and Chardin[56], DR on CT exam should be inspired by RECIST 1.1, defined as a concomitant decrease in size > 30% in some lesions and increase in size > 20% in others (and/or presence of new lesions), while on PET/CT, DR should be motivated by PERCIST criteria, defined as a concomitant decrease > 30% in some tumor lesions metabolism and metabolic increase > 30% in others (and/or new hypermetabolic lesions).
A summary of the most important studies focusing on DR is reported in Table 1.
To conclude, DR should be considered in the iRECIST in addition to or separately from a PD, partial response, and stable disease, through radiological evaluation, for a more precise evaluation of tumor response to the immunotherapy.
iRECIST can help to correctly categorize the classes of response to immunotherapy treatment by dividing patients into four main groups (iSD, iPR, iCR, iPD), according to the radiological target lesion modifications, achieved along the time, and the standard solid response criteria (RECIST 1.1). Recently, other different kinds of response have been described in literature after immunotherapy treatment, defined as atypical responses, categorized in three patterns: pseudoprogression, hyperprogression, and dissociated response. The correct knowledge of these new atypical patterns should be correctly assessed by both radiologists and clinicians, through the deep investigation of clinical anamnesis and imaging findings to guarantee the best management.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Peck-Radosavljevic M, Rajer M, Sun Y S-Editor: Fan JR L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Liu M, Guo F. Recent updates on cancer immunotherapy. Prec Clin Med. 2018;1:65-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 2. | Kwak JJ, Tirumani SH, Van den Abbeele AD, Koo PJ, Jacene HA. Cancer immunotherapy: imaging assessment of novel treatment response patterns and immune-related adverse events. Radiographics. 2015;35:424-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 3. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21598] [Article Influence: 1349.9] [Reference Citation Analysis (1)] |
| 4. | Carter BW, Bhosale PR, Yang WT. Immunotherapy and the role of imaging. Cancer. 2018;124:2906-2922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412-7420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2311] [Cited by in RCA: 2453] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 6. | Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19:3936-3943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 386] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 7. | Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, Hodi FS, Therasse P, Hoekstra OS, Shankar LK, Wolchok JD, Ballinger M, Caramella C, de Vries EGE; RECIST working group. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143-e152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1521] [Cited by in RCA: 1693] [Article Influence: 211.6] [Reference Citation Analysis (0)] |
| 8. | Le Lay J, Jarraya H, Lebellec L, Penel N. irRECIST and iRECIST: the devil is in the details. Ann Oncol. 2017;28:1676-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Inno A, Lo Russo G, Salgarello M, Corrao G, Casolino R, Galli G, Modena A, Romano L, Pusceddu S, Greco FG, Garassino MC, Gori S. The evolving landscape of criteria for evaluating tumor response in the era of cancer immunotherapy: From Karnofsky to iRECIST. Tumori. 2018;104:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Lennartz S, Diederich S, Doehn C, Gebauer B, Grünwald V, Notohamiprodjo M, Sommer W, Schlemmer HP, Persigehl T. Radiological Monitoring of Modern Immunotherapy: A Novel Challenge for Interdisciplinary Patient Care. Rofo. 2020;192:235-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Persigehl T, Lennartz S, Schwartz LH. iRECIST: how to do it. Cancer Imaging. 2020;20:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Jia W, Gao Q, Han A, Zhu H, Yu J. The potential mechanism, recognition and clinical significance of tumor pseudoprogression after immunotherapy. Cancer Biol Med. 2019;16:655-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 13. | Nishino M. Pseudoprogression and Measurement Variability. JCO. 2016;34:3480-3481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Hochmair MJ, Schwab S, Burghuber OC, Krenbek D, Prosch H. Symptomatic pseudo-progression followed by significant treatment response in two lung cancer patients treated with immunotherapy. Lung Cancer. 2017;113:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Di Giacomo AM, Danielli R, Guidoboni M, Calabrò L, Carlucci D, Miracco C, Volterrani L, Mazzei MA, Biagioli M, Altomonte M, Maio M. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58:1297-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 222] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 16. | Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol. 2015;33:3541-3543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 675] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 17. | Cohen JV, Alomari AK, Vortmeyer AO, Jilaveanu LB, Goldberg SB, Mahajan A, Chiang VL, Kluger HM. Melanoma Brain Metastasis Pseudoprogression after Pembrolizumab Treatment. Cancer Immunol Res. 2016;4:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Rocha P, Hardy-Werbin M, Naranjo D, Taus Á, Rodrigo M, Zuccarino F, Roth R, Wood O, Ottensmeier CH, Arriola E. CD103+CD8+ Lymphocytes Characterize the Immune Infiltration in a Case With Pseudoprogression in Squamous NSCLC. J Thorac Oncol. 2018;13:e193-e196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Tabei T, Tsuura Y, Kobayashi K. Pseudoprogression: A case of metastatic renal clear cell carcinoma treated with nivolumab: Letter to the Editor. Pathol Int. 2018;68:627-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Tanizaki J, Hayashi H, Kimura M, Tanaka K, Takeda M, Shimizu S, Ito A, Nakagawa K. Report of two cases of pseudoprogression in patients with non-small cell lung cancer treated with nivolumab-including histological analysis of one case after tumor regression. Lung Cancer. 2016;102:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Borcoman E, Nandikolla A, Long G, Goel S, Le Tourneau C. Patterns of Response and Progression to Immunotherapy. Am Soc Clin Oncol Educ Book. 2018;38:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 22. | Thomas R, Somarouthu B, Alessandrino F, Kurra V, Shinagare AB. Atypical Response Patterns in Patients Treated With Nivolumab. AJR Am J Roentgenol. 2019;1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 23. | Frelaut M, du Rusquec P, de Moura A, Le Tourneau C, Borcoman E. Pseudoprogression and Hyperprogression as New Forms of Response to Immunotherapy. BioDrugs. 2020;34:463-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 24. | Swami U, Smith M, Zhang J. Central Nervous System Pseudoprogression With Nivolumab in a Patient With Squamous Cell Lung Cancer Followed by Prolonged Response. J Thorac Oncol. 2018;13:e183-e184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Yoshimura A, Takumi C, Tsuji T, Hamashima R, Shiotsu S, Yuba T, Urata Y, Hiraoka N. Pulmonary pleomorphic carcinoma with pseudoprogression during nivolumab therapy and the usefulness of tumor markers: A case report. Clin Case Rep. 2018;6:1338-1341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Wong AS, Thian YL, Kapur J, Leong CN, Kee P, Lee CT, Lee MB. Pushing the limits of immune-related response: a case of "extreme pseudoprogression". Cancer Immunol Immunother. 2018;67:1105-1111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Chae YK, Wang S, Nimeiri H, Kalyan A, Giles FJ. Pseudoprogression in microsatellite instability-high colorectal cancer during treatment with combination T cell mediated immunotherapy: a case report and literature review. Oncotarget. 2017;8:57889-57897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Liu G, Chen T, Li R, Zhu L, Liu D, Ding Z. Well-controlled pleural effusion indicated pseudoprogression after immunotherapy in lung cancer: A case report. Thorac Cancer. 2018;9:1190-1193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Sweis RF, Zha Y, Pass L, Heiss B, Chongsuwat T, Luke JJ, Gajewski TF, Szmulewitz R. Pseudoprogression manifesting as recurrent ascites with anti-PD-1 immunotherapy in urothelial bladder cancer. J Immunother Cancer. 2018;6:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Doherty MK, Jao K, Shepherd FA, Hazrati LN, Leighl NB. Central Nervous System Pseudoprogression in a Patient Treated with PD-1 Checkpoint Inhibitor. J Thorac Oncol. 2015;10:e100-e101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Lewis GD, Jonasch E, Shah AY, Fuller GN, Farach AM, Butler EB, Teh BS. Renal cell carcinoma brain metastasis with pseudoprogression and radiation necrosis on nivolumab after previous treatment with stereotactic radiosurgery: An illustrative case report and review of the literature. Pract Radiat Oncol. 2018;8:e262-e265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Saâda-Bouzid E, Defaucheux C, Karabajakian A, Coloma VP, Servois V, Paoletti X, Even C, Fayette J, Guigay J, Loirat D, Peyrade F, Alt M, Gal J, Le Tourneau C. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28:1605-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 452] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 33. | Boland JL, Zhou Q, Martin M, Callahan MK, Konner J, O'Cearbhaill RE, Friedman CF, Tew W, Makker V, Grisham RN, Hensley ML, Zecca N, Iasonos AE, Snyder A, Hyman DM, Sabbatini P, Aghajanian C, Cadoo KA, Zamarin D. Early disease progression and treatment discontinuation in patients with advanced ovarian cancer receiving immune checkpoint blockade. Gynecol Oncol. 2019;152:251-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Faure M, Rochigneux P, Olive D, Taix S, Brenot-Rossi I, Gilabert M. Hyperprogressive Disease in Anorectal Melanoma Treated by PD-1 Inhibitors. Front Immunol. 2018;9:797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Grasselly C, Denis M, Bourguignon A, Talhi N, Mathe D, Tourette A, Serre L, Jordheim LP, Matera EL, Dumontet C. The Antitumor Activity of Combinations of Cytotoxic Chemotherapy and Immune Checkpoint Inhibitors Is Model-Dependent. Front Immunol. 2018;9:2100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 36. | Xiong D, Wang Y, Singavi AK, Mackinnon AC, George B, You M. Immunogenomic Landscape Contributes to Hyperprogressive Disease after Anti-PD-1 Immunotherapy for Cancer. iScience. 2018;9:258-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 37. | Wang F, Li B, Wei Y, Zhao Y, Wang L, Zhang P, Yang J, He W, Chen H, Jiao Z, Li Y. Tumor-derived exosomes induce PD1+ macrophage population in human gastric cancer that promotes disease progression. Oncogenesis. 2018;7:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 38. | Fung KY, Nguyen PM, Putoczki T. The expanding role of innate lymphoid cells and their T-cell counterparts in gastrointestinal cancers. Mol Immunol. 2019;110:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Champiat S, Ferrara R, Massard C, Besse B, Marabelle A, Soria JC, Ferté C. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol. 2018;15:748-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 291] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 40. | Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, Mazieres J, Zalcman G, Brosseau S, Le Moulec S, Leroy L, Duchemann B, Lefebvre C, Veillon R, Westeel V, Koscielny S, Champiat S, Ferté C, Planchard D, Remon J, Boucher M-E, Gazzah A, Adam J, Bria E, Tortora G, Soria J-C, Besse B, Caramella C. Hyperprogressive Disease in Patients With Advanced Non–Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA Oncol. 2018;4:1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 557] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 41. | Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin Cancer Res. 2017;23:4242-4250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 691] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 42. | Lo Russo G, Moro M, Sommariva M, Cancila V, Boeri M, Centonze G, Ferro S, Ganzinelli M, Gasparini P, Huber V, Milione M, Porcu L, Proto C, Pruneri G, Signorelli D, Sangaletti S, Sfondrini L, Storti C, Tassi E, Bardelli A, Marsoni S, Torri V, Tripodo C, Colombo MP, Anichini A, Rivoltini L, Balsari A, Sozzi G, Garassino MC. Antibody-Fc/FcR Interaction on Macrophages as a Mechanism for Hyperprogressive Disease in Non-small Cell Lung Cancer Subsequent to PD-1/PD-L1 Blockade. Clin Cancer Res. 2019;25:989-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 309] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 43. | Gomes de Morais AL, Cardenas JM, de Miguel Luken MJ, Boni V, Moreno I, Ao G, Liu RH, de Hoyos FB, Cubillo A, Calvo E. Comparative assessment of different radiological criteria to identify paradoxical hyperprogression (HPD) to IO drugs. JCO. 2020;38:e15229-e15229. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 44. | Kim JY, Lee KH, Kang J, Borcoman E, Saada-Bouzid E, Kronbichler A, Hong SH, de Rezende LFM, Ogino S, Keum N, Song M, Luchini C, van der Vliet HJ, Shin JI, Gamerith G. Hyperprogressive Disease during Anti-PD-1 (PDCD1) / PD-L1 (CD274) Therapy: A Systematic Review and Meta-Analysis. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 45. | Park JH, Chun SH, Lee Y-G, Chang H, Lee K-W, Kim HR, Shin SH, An HJ, Lee KE, Hwang IG, Ahn M-J, Kim S-B, Keam B. Hyperprogressive disease and its clinical impact in patients with recurrent and/or metastatic head and neck squamous cell carcinoma treated with immune-checkpoint inhibitors: Korean cancer study group HN 18–12. J Cancer Res Clin Oncol. 2020;146:3359-3369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Kim CG, Kim KH, Pyo KH, Xin CF, Hong MH, Ahn BC, Kim Y, Choi SJ, Yoon HI, Lee JG, Lee CY, Park SY, Park SH, Cho BC, Shim HS, Shin EC, Kim HR. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol. 2019;30:1104-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 47. | Kas B, Talbot H, Ferrara R, Richard C, Lamarque JP, Pitre-Champagnat S, Planchard D, Balleyguier C, Besse B, Mezquita L, Lassau N, Caramella C. Clarification of Definitions of Hyperprogressive Disease During Immunotherapy for Non-Small Cell Lung Cancer. JAMA Oncol. 2020;6:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 48. | Aoki M, Shoji H, Nagashima K, Imazeki H, Miyamoto T, Hirano H, Honma Y, Iwasa S, Okita N, Takashima A, Kato K, Higuchi K, Boku N. Hyperprogressive disease during nivolumab or irinotecan treatment in patients with advanced gastric cancer. ESMO Open. 2019;4:e000488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 49. | Sasaki A, Nakamura Y, Mishima S, Kawazoe A, Kuboki Y, Bando H, Kojima T, Doi T, Ohtsu A, Yoshino T, Kuwata T, Akimoto T, Shitara K. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer. 2019;22:793-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 50. | Kim CG, Kim C, Yoon SE, Kim KH, Choi SJ, Kang B, Kim HR, Park SH, Shin EC, Kim YY, Kim DJ, Chung HC, Chon HJ, Choi HJ, Lim HY. Hyperprogressive disease during PD-1 blockade in patients with advanced hepatocellular carcinoma. J Hepatol. 2021;74:350-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 51. | Zheng Z, Wu K, Yao Z, Mu X, Wu H, Zhao W, Cheng L, Liu Z. Hyperprogressive disease in patients with advanced renal cell carcinoma: a new pattern of post-treatment cancer behavior. Immunol Res. 2020;68:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Hao C, Tian J, Liu H, Li F, Niu H, Zhu B. Efficacy and safety of anti-PD-1 and anti-PD-1 combined with anti-CTLA-4 immunotherapy to advanced melanoma: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2017;96:e7325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 53. | Schuiveling M, Tonk EHJ, Verheijden RJ, Suijkerbuijk KPM. Hyperprogressive disease rarely occurs during checkpoint inhibitor treatment for advanced melanoma. Cancer Immunol Immunother. 2021;70:1491-1496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S, Balleyguier C, Planchard D, Gazzah A, Soria JC, Marabelle A, Besse B, Caramella C. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: Comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 250] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 55. | Tozuka T, Kitazono S, Sakamoto H, Yoshida H, Amino Y, Uematsu S, Yoshizawa T, Hasegawa T, Uchibori K, Yanagitani N, Horiike A, Horai T, Seike M, Gemma A, Nishio M. Dissociated responses at initial computed tomography evaluation is a good prognostic factor in non-small cell lung cancer patients treated with anti-programmed cell death-1/ligand 1 inhibitors. BMC Cancer. 2020;20:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 56. | Humbert O, Chardin D. Dissociated Response in Metastatic Cancer: An Atypical Pattern Brought Into the Spotlight With Immunotherapy. Front Oncol. 2020;10:566297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 57. | Zhou H, Sun Y, Xiu W, Han J, Zhong L, Suo J, Wei H, Wang Y, Zhu J. Overall survival benefit of continuing immune checkpoint inhibitors treatment post dissociated response in patients with advanced lung cancer. J Cancer Res Clin Oncol. 2020;146:2979-2988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Humbert O, Cadour N, Paquet M, Schiappa R, Poudenx M, Chardin D, Borchiellini D, Benisvy D, Ouvrier MJ, Zwarthoed C, Schiazza A, Ilie M, Ghalloussi H, Koulibaly PM, Darcourt J, Otto J. 18FDG PET/CT in the early assessment of non-small cell lung cancer response to immunotherapy: frequency and clinical significance of atypical evolutive patterns. Eur J Nucl Med Mol Imaging. 2020;47:1158-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 59. | Reinhorn D, Jacobi O, Icht O, Dudnik E, Rotem O, Zer A, Goldstein DA. Treatment beyond progression with immune checkpoint inhibitors in non-small-cell lung cancer. Immunotherapy. 2020;12:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 60. | Billan S, Kaidar-Person O, Gil Z. Treatment after progression in the era of immunotherapy. Lancet Oncol. 2020;21:e463-e476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 61. | Gandara DR, von Pawel J, Mazieres J, Sullivan R, Helland Å, Han JY, Ponce Aix S, Rittmeyer A, Barlesi F, Kubo T, Park K, Goldschmidt J, Gandhi M, Yun C, Yu W, Matheny C, He P, Sandler A, Ballinger M, Fehrenbacher L. Atezolizumab Treatment Beyond Progression in Advanced NSCLC: Results From the Randomized, Phase III OAK Study. J Thorac Oncol. 2018;13:1906-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |