Published online Apr 24, 2021. doi: 10.5306/wjco.v12.i4.249
Peer-review started: September 21, 2020
First decision: November 16, 2020
Revised: November 25, 2020
Accepted: March 7, 2021
Article in press: March 7, 2021
Published online: April 24, 2021
Processing time: 211 Days and 6.9 Hours
18F-fluorodeoxyglucose-positron emission tomography (PET)/computed tomography is useful in diagnosing lymph node and distant metastases of esophageal cancer. However, its value for predicting survival is controversial.
To evaluate the value of PET complete metabolic response (CMR) as a prognostic predictor for esophageal cancer.
Between June 2013 and December 2017, 58 patients with squamous cell esophageal cancer who underwent neoadjuvant chemotherapy (NAC) in Oita University were enrolled in this retrospective cohort study. Tumors were clinically staged using fluorodeoxyglucose-PET/computed tomography before and after NAC. After NAC, maximal standardized uptake value ≤ 2.5 was defined as PET-CMR, and maximal standardized uptake value > 2.5 was defined as non-PET-CMR. We compared short-term outcomes between the PET-CMR group and non-PET-CMR group and evaluated prognostic factors by univariate and multivariate analyses.
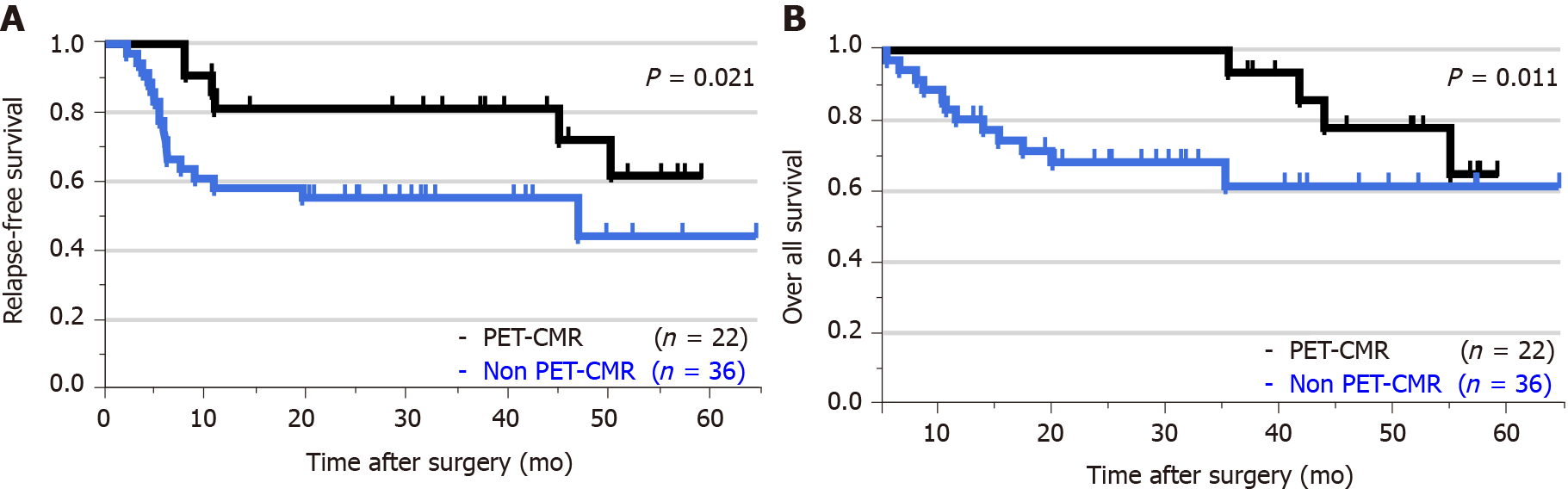
The PET-CMR group included 22 patients, and the non-PET-CMR group included 36 patients. There were no significant differences in intraoperative and postoperative complications between the two groups. Five-year relapse-free survival and overall survival in the PET-CMR group were significantly more favorable than those in the non-PET-CMR group (38.6 mo vs 20.8 mo, P = 0.021; 42.8 mo vs 25.1 mo, P = 0.011, respectively). PET-CMR was a significant prognostic factor in terms of relapse-free survival by univariate analysis (hazard ratio: 2.523; 95% confidence interval: 1.034–7.063; P < 0.041). Particularly, PET-computed tomography negative N was an independent prognostic factor of relapse-free survival and overall survival by multivariate analysis.
PET-CMR after NAC is considered a favorable prognostic factor for esophageal cancer. Evaluation by PET-computed tomography could be useful in clinical decision making for esophageal cancer.
Core Tip: The study aimed to evaluate the value of positron emission tomography (PET)/computed tomography complete metabolic response (CMR) as a prognostic predictor for esophageal cancer. Fifty-eight patients with esophageal cancer who underwent neoadjuvant chemotherapy were enrolled. The PET-CMR group included 22 patients, and the non-PET-CMR group included 36 patients. Five-year relapse-free survival and overall survival in the PET-CMR group were significantly more favorable than those in the non-PET-CMR group. PET-CMR was a significant prognostic factor in terms of relapse-free survival by univariate analysis. PET-CMR after neoadjuvant chemotherapy is considered a favorable prognostic factor for relapse-free survival in patients with esophageal cancer.
- Citation: Suzuki K, Etoh T, Shibata T, Nishiki K, Fumoto S, Ueda Y, Shiroshita H, Shiraishi N, Inomata M. Positron emission tomography complete metabolic response as a favorable prognostic predictor in esophageal cancer following neoadjuvant chemotherapy with docetaxel/cisplatin/5-fluorouracil. World J Clin Oncol 2021; 12(4): 249-261
- URL: https://www.wjgnet.com/2218-4333/full/v12/i4/249.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i4.249
Esophageal cancer is the eighth most common type of cancer worldwide, with a 5-yr overall survival (OS) of 35% after primary esophagectomy[1]. Neoadjuvant chemotherapy/radiotherapy (CRT) has been introduced to improve survival of patients with advanced esophageal cancer[2,3]. CRT followed by esophagectomy is considered a standard strategy for locally advanced esophageal cancer in Western countries[3,4]. In contrast, neoadjuvant chemotherapy (NAC) with cisplatin plus 5-fluorouracil (CF) followed by esophagectomy is the standard strategy according to the JCOG 9907 trial in Japan[5].
With the expectation of further anticancer effect, triplet NAC with docetaxel, cisplatin plus 5-fluorouracil (DCF) was introduced for locally advanced esophageal cancer, and anticancer effects and survival benefits of DCF were reported in previous studies[6,7]. Furthermore, to clarify the feasibility and effectiveness of DCF and DCF/radiotherapy compared to CF as neoadjuvant therapy for locally advanced esophageal cancer, a randomized controlled study is underway in Japan (JCOG1109)[8].
Proper evaluation of preoperative chemotherapy response and lymph node (LN) metastasis before surgery is important for predicting prognosis in patients who have received neoadjuvant therapy[9,10]. Computed tomography (CT) is widely used to determine staging in esophageal cancer. However, the accuracy of CT in detecting regional LN metastasis in esophageal cancer is still unsatisfactory[11]. Recent studies have shown that 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) can determine the degree of metabolic activity in tumor cells and can improve tumor staging for patients with esophageal cancer[12,13]. Furthermore, several studies have shown that FDG-PET/CT is useful for diagnosing metastatic LNs and distant metastasis and for detecting the recurrence of esophageal cancer after surgery[12-14]. However, the value of FDG-PET/CT to predict survival in patients with esophageal cancer is controversial. In this study, we aimed to evaluate the value of PET complete metabolic response (CMR) in esophageal cancer following NAC as a prognostic predictor.
We reviewed data from 70 consecutive patients with squamous cell esophageal cancer who were preoperatively evaluated with FDG-PET/CT before and after NAC with DCF between June 2013 and December 2017 in Oita University. Those who received definitive radio/chemotherapy after NAC (n = 11) and underwent esophagectomy in another hospital (n = 1) were excluded. Thus, 58 patients received esophagectomy after NAC and were enrolled in this retrospective study. NAC was recommended for patients with resectable clinical stage T2-T4 esophageal cancer and for those with tumors and clinical LN metastases or resectable supraclavicular LN metastasis (clinical M1 3) according to the TNM Classification of Malignant Tumors, 7th edition[15]. Indications for NAC with DCF in our institution are age < 80-years-old and performance status 0 or 1. Patients with a history of adverse events from docetaxel were excluded from receiving NAC with DCF. The clinicopathologic profiles of the tumors were based on the TNM Classification of Malignant Tumors, 7th edition[15]. This study was approved by the institutional Ethical Review Board of Oita University Faculty of Medicine (Approval No. 1602).
The NAC carried out with DCF consisted of docetaxel 70 mg/m2 administered intravenously for 1 h on day 1; 5-fluorouracil 750 mg/m2 administered as a 24-h continuous intravenous infusion on days 1-5; and cisplatin 70 mg/m2 administered intravenously for 2 h on day 1. This regimen was conducted every 3 wk. The tumor was evaluated by FDG-PET/CT and endoscopy 1 to 2 wk after completing NAC. Patients underwent esophagectomy 4 to 6 wk after completing NAC.
The clinical disappearance of the primary tumor was evaluated by endoscopy and FDG-PET/CT. Endoscopic tumor disappearance was evaluated according to the Classification of Esophageal Cancer published by the Japan Esophageal Society[16], which describes disappearance as no visible tumor lesions with no mucosa and with an irregular surface, active esophagitis, ulceration and protruding changes with submucosal tumor and the absence of cancer cells in biopsy specimens. The pathological complete disappearance of the primary tumor was defined as grade 3 (pathological complete response).
Tumors were clinically staged using systematic FDG-PET/CT imaging before and after NAC. The patients were assessed by FDG-PET/CT 1 to 2 wk after completing NAC. The cut-off for primary tumor or LN metastasis was a maximal standardized uptake value (SUVmax) of 2.5 as described previously[17-19]. After the NAC, a SUVmax ≤ 2.5 was defined as PET-CMR, and a SUVmax > 2.5 was defined as non-PET-CMR in this study. We compared clinicopathological factors and short- and long-term outcomes between patients with PET-CMR and non-PET-CMR before and after NAC.
Subtotal esophagectomy with two- or three-field LN dissection was performed for thoracic esophageal cancer. When esophageal cancer was in the upper and middle third of the thoracic esophagus and LN metastasis was present in the superior mediastinum, cervical LN dissection was added. When esophageal cancer was in the lower thoracic esophagus and LN metastasis in the superior mediastinum was not suspected, cervical LN dissection was not necessary. If LN metastasis in the superior mediastinum was revealed pathologically after the esophagectomy, cervical LN dissection was performed post esophagectomy. A gastric tube was subsequently lifted via the posterior mediastinal route, and high thoracic esophagogastrostomy was performed with a circular stapler. Cervical esophagectomy or pharyngeal-laryngeal-cervical esophagectomy with cervical LN dissection was performed for cervical esophageal cancer.
Categorical variables were analyzed using Chi-square tests, and continuous variables were analyzed using unpaired t-tests. Survival outcomes were evaluated for 70 consecutive patients surgically treated before June 2013 and who were followed up for at least 3 yrs. Survival was analyzed using Kaplan-Meier curves and compared using log-rank tests. Relapse-free survival (RFS) was defined as the interval between the date of surgery and the first event (recurrence or death from any cause) or the most recent follow-up evaluation. OS was defined as the interval between the date of surgery and death from any cause or the most recent follow-up evaluation. The effects of various clinicopathologic parameters on survival were assessed using univariate analysis and multivariate Cox proportional hazards analysis. Covariates with a P value < 0.05 in the univariate analysis were entered into the multivariate analyses. All data were statistically analyzed using SPSS software version 24 (IBM Corporation, Armonk, NY, United States).
Twenty-two (38%) of the 58 patients were diagnosed as having CMR with FDG-PET/CT (PET-CMR group), and 36 patients (62%) were diagnosed as not having CMR (non-PET-CMR group). Patient characteristics are summarized in Table 1. There were no significant differences in age, sex, primary tumor location and LN and distant metastasis before NAC between the two groups. Downstaging was observed in 15 patients (68.1%) in the PET-CMR group and in 17 patients (56.7%) in the non-PET-CMR group. There were no patients with clinical complete response in either group. The SUVmax of the primary tumor before NAC was 16.6 ± 6.5 in the PET-CMR group and 17.5 ± 6.5 in the non-PET-CMR group. FDG accumulation in LNs before NAC was observed in 14 patients (63.6%) in the PET-CMR group and in 21 patients (58.3%) in the non-PET-CMR group. There were no significant differences in FDG accumulation in the primary tumor or in LNs between the two groups.
| PET-CMR, n = 22 | Non-PET-CMR, n = 36 | P value | |
| Age, mean | 65.9 (48.0-76.0) | 65.0 (47.0-80.0) | 0.82 |
| Male/Female | 20/2 | 33/3 | 0.41 |
| Comorbidity | 15 | 28 | |
| Location | |||
| Ce | 1 | 4 | 0.58 |
| Ut | 4 | 3 | |
| Mt | 10 | 17 | |
| Lt | 7 | 12 | |
| Before NAC | |||
| cStage | |||
| cT | |||
| T1 | 2 | 0 | 0.044 |
| T2 | 6 | 3 | |
| T3 | 13 | 32 | |
| T4 | 1 | 1 | |
| cN | |||
| N0 | 5 | 9 | 0.27 |
| N1 | 12 | 14 | |
| N2 | 4 | 13 | |
| N3 | 1 | 0 | |
| cM | |||
| M0 | 17 | 28 | 0.96 |
| M1 | 5 | 8 | |
| cStage | |||
| I | 3 | 4 | 0.94 |
| II | 4 | 5 | |
| III | 10 | 19 | |
| IVA | 5 | 8 | |
| IVB | 0 | 0 | |
| Primary, SUVmax mean | 16.4 ± 6.5 | 15.7 ± 6.5 | 0.98 |
| LN+ | 14 (63.6%) | 21 (58.3%) | 0.69 |
| After NAC | |||
| CT-Stage | |||
| T | |||
| T1 | 9 | 2 | 0.0058 |
| T2 | 12 | 26 | |
| T3 | 1 | 6 | |
| T4 | 0 | 2 | |
| N | |||
| N0 | 21 | 21 | 0.0081 |
| N1 | 1 | 8 | |
| N2 | 0 | 7 | |
| N3 | 0 | 0 | |
| M | |||
| M0 | 22 | 36 | - |
| M1 | 0 | 0 | |
| Stage | |||
| 0 | 5 | 0 | 0.0017 |
| I | 4 | 1 | |
| II | 12 | 23 | |
| III | 1 | 10 | |
| IVA | 0 | 2 | |
| IVB | 0 | 0 | |
| Down stage | 15 | 17 | 0.119 |
Surgical outcomes are shown in Table 2. There were no significant differences in operative procedures and fields of LN dissection between the two groups. There were 7 cases (19.4%) of noncurative resection (R1) in the non-PET-CMR group, whereas all esophagectomies were curatively performed in the PET-CMR group (P = 0.027). Blood loss was greater in the non-PET-CMR group compared to the PET-CMR group (836 ± 540 mL vs 559 ± 219 mL, P = 0.026). Bronchial injury requiring repair of the bronchus occurred in one patient during surgery in each group. There were no significant differences in postoperative complications (Clavien-Dindo classification ≥ 3) between the two groups.
| PET-CMR, n = 22 | Non-PET-CMR, n = 36 | P value | |
| Esophagectomy procedures | |||
| Subtotal | 20 | 32 | 0.31 |
| Cervical | 1 | 0 | |
| Pharyngeal laryngeal | 1 | 4 | |
| LN dissection | |||
| Cervical | 1 | 2 | 0.89 |
| 2 fields | 5 | 10 | |
| 3 fields | 16 | 24 | |
| Harvested LN1 | 72 ± 7 | 73 ± 5 | 0.91 |
| Blood loss in mL1 | 559 ± 219 | 836 ± 540 | 0.026 |
| OP time in min1 | 527 ± 105 | 580 ± 121 | 0.092 |
| Curability | |||
| R0 | 22 | 29 | 0.027 |
| R1 | 0 | 7 | |
| Intraoperative complication | |||
| Bronchus injury | 1 | 1 | 0.72 |
| Postoperative complication | |||
| All | 14 | 22 | 0.85 |
| Anastomotic leakage2 | 1 | 1 | 0.72 |
| Anastomotic stenosis2 | 2 | 3 | 0.92 |
| Recurrent nerve palsy2 | 7 | 12 | 0.91 |
| Respiratory2 | 3 | 8 | 0.42 |
| Others2 | 2 | 3 | 0.92 |
| Surgical mortality | 0 | 0 | - |
Pathological findings are shown in Table 3. Pathological grade 3 was achieved in 9 of 22 patients (41%) in the PET-CMR group and in 3 of 36 patients (8%) in the non-PET-CMR group. The non-PET-CMR group showed more aggressive behavior in terms of pT, pStage, lymphovascular invasion and pathological grade. However, there was no significant difference in pN between the two groups.
| PET-CMR, n = 22 | Non-PET-CMR, n = 36 | P value | |
| T | |||
| T0 | 9 | 5 | < 0.0001 |
| T1 | 11 | 4 | |
| T2 | 1 | 9 | |
| T3 | 1 | 12 | |
| T4 | 0 | 6 | |
| N | |||
| N0 | 13 | 14 | 0.052 |
| N1 | 7 | 7 | |
| N2 | 2 | 8 | |
| N3 | 0 | 7 | |
| M | |||
| M0 | 20 | 32 | 0.81 |
| M1 | 2 | 4 | |
| Stage | |||
| 0 | 9 | 3 | 0.033 |
| I | 3 | 8 | |
| II | 8 | 8 | |
| III | 0 | 10 | |
| IVA | 2 | 4 | |
| IVB | 0 | 3 | |
| Ly+ | 4 | 22 | 0.001 |
| v+ | 1 | 15 | 0.002 |
| Pathological grade | |||
| 0 | 0 (0%) | 3 (8%) | 0.029 |
| 1a | 4 (18%) | 14 (39%) | |
| 1b | 3 (14%) | 6 (17%) | |
| 2 | 6 (27%) | 10 (28%) | |
| 3 | 9 (41%) | 3 (8%) |
The accuracy of PET-CMR is shown in Table 4. The accuracy of PET-CMR for the T factor was 69.0%. Similarly, sensitivity was 68.2%, and specificity was 71.4%. The accuracy, sensitivity and specificity of PET-CMR for the N factor were 65.5%, 41.9%, and 92.6%, respectively. When the T and N factors were combined, the accuracy was 72.4%, sensitivity was 71.7%, and specificity was 75.0%.
| TP | FP | TN | FN | Accuracy | Sensitivity | Specificity | |
| T factor | 30 | 4 | 10 | 14 | 69.0% | 68.2% | 71.4% |
| N factor | 13 | 2 | 25 | 18 | 65.5% | 41.9% | 92.6% |
| T and N | 33 | 3 | 13 | 9 | 72.4% | 71.7% | 75.0% |
Five-year RFS and 5-yr OS are shown in Figure 1. The mean follow-up period was 44.3 mo. Five-year RFS of the PET-CMR group was significantly more favorable than that of the non-PET-CMR group (38.6 ± 17.7 mo vs 20.8 ± 17.8 mo, P = 0.021). Similarly, 5-yr OS of the PET-CMR group was also significantly more favorable than that of the non-PET-CMR (42.8 ± 14.8 mo vs 25.1 ± 16.0 mo, P = 0.011). Five patients (22.7%) had postoperative recurrence in the PET-CMR group vs 17 patients (47.2%) in the non-PET-CMR group (Table 5). There were no significant differences in the distribution of the sites of recurrence between the two groups (P = 0.66).
| PET-CMR, n = 5 | Non-PET-CMR, n = 17 | P value | |
| Local | 0 | 4 | 0.66 |
| Distant | 5 | 16 | |
| Extraregional LN | 3 | 6 | |
| Liver | 0 | 5 | |
| Bone | 1 | 4 | |
| Lung | 1 | 3 | |
| Pleura | 0 | 2 |
Various clinicopathologic factors and PET findings of the primary tumor and LNs were evaluated as prognostic factors using Cox regression models (Table 6 and 7). The univariate analysis of RFS showed that tumor difference, pathologic T, N, stage, complete response, CT-PET negative T and N and PET-CMR were statistically significant. The multivariate analysis subsequently selected CT-PET negative N (hazard ratio: 22.570; 95% confidence interval: 4.69-177.80; P < 0.01) as the independent covariate for RFS. CT-PET-T and CT-PET-CMR were excluded from the multivariate analysis due to multicollinearity with CT-PET-N. The univariate analysis of OS showed pathologic T, N, M, stage, complete response and CT-PET negative T and N to be statistically significant. Then, the multivariate analysis subsequently selected CT-PET negative N (hazard ratio: 8.268; 95% confidence interval: 1.74-63.60; P < 0.01) as the only independent covariate for OS.
| Relapse-free survival | ||||||
| Univariate | Multivariate | |||||
| RR | 95%CI | P value | RR | 95%CI | P value | |
| Age | 0.982 | 0.935-1.033 | 0.468 | |||
| Male/Female | 2.211 | 0.638-5.920 | 0.1885 | |||
| Location, MtLtAe/CeUt | 1.230 | 0.406-3.090 | 0.689 | |||
| Tumor grade, other/por | 2.612 | 1.050-6.026 | 0.040 | 1.412 | 0.498-3.785 | 0.503 |
| pT, 0▪1▪2/3▪4 | 4.554 | 1.983-10.770 | 0.0004 | 1.195 | 0.433-3.432 | 0.734 |
| pN, 0/1▪2▪3 | 5.287 | 2.062-16.320 | 0.0003 | 3.172 | 1.074-10.710 | 0.036 |
| pM, 0/1 | 2.543 | 0.726-6.977 | 0.131 | |||
| pStage, 0▪1▪2/3▪4 | 8.655 | 3.652-22.060 | < 0.0001 | 2.449 | 0.356-16.800 | 0.357 |
| pCR (primary tumor), CR (+)/nonCR (-) | 5.199 | 1.501-32.770 | 0.0063 | 1.878 | 0.337-15.060 | 0.487 |
| CT-PET-T, (-)/(+) | 2.561 | 1.059-7.120 | 0.0036 | |||
| CT-PET-N, (-)/(+) | 10.810 | 4.251-28.160 | < 0.0001 | 22.570 | 4.694-177.800 | < 0.0001 |
| CT-PET-CMR, (-)/(+) | 2.523 | 1.034-7.063 | 0.041 | |||
| Overall survival | ||||||
| Univariate | Multivariate | |||||
| RR | 95%CI | P value | RR | 95%CI | P value | |
| Age | 1.036 | 0.972-1.112 | 0.285 | |||
| Male/Female | 1.302 | 0.204-4.683 | 0.736 | |||
| Location, MtLtAe/CeUt | 1.057 | 0.242-3.291 | 0.931 | |||
| Tumor grade, other/por | 1.603 | 0.499-4.466 | 0.403 | |||
| 0▪1▪2/3▪4 | 8.167 | 2.912-25.340 | < 0.0001 | 1.644 | 0.430-8.039 | 0.486 |
| pN, 0/1▪2▪3 | 5.653 | 1.803-24.800 | 0.0021 | 0.924 | 0.045-7.556 | 0.946 |
| pM, 0/1 | 5.043 | 1.354-15.690 | 0.019 | 1.518 | 0.302-6.353 | 0.588 |
| pStage, 0▪1▪2/3▪4 | 15.52 | 5.062-58.400 | < 0.0001 | 5.760 | 0.415-160.800 | 0.197 |
| pCR (primary tumor), CR (+)/nonCR (-) | 3.202 | 0.887-20.470 | 0.0793 | |||
| CT-PET-T, (-)/(+) | 3.227 | 1.106-11.680 | 0.0313 | 1.450 | 0.307-6.866 | 0.630 |
| CT-PET-N, (-)/(+) | 25.270 | 6.361-168.100 | < 0.0001 | 8.268 | 1.748-63.630 | 0.006 |
| CT-PET-CMR, (-)/(+) | 2.927 | 0.996-10.660 | 0.0510 | |||
NAC is a standard strategy for locally advanced esophageal cancer, and triplet NAC with DCF instead of CF has been applied in clinical practice due to its effectiveness. FDG/PET-CT is useful for assessing tumor progression before surgery, but the usefulness of FDG/PET-CT as a prognostic predictor has not been clarified. In this study, we confirmed that the patients with PET-CMR had a more favorable prognosis than those with non-PET-CMR after NAC with DCF. Furthermore, the presence of PET-positive LNs after NAC could be an independent prognostic factor.
CT is a widely used diagnostic modality to determine staging in esophageal cancer. The sensitivity and specificity of CT for the detection of LN metastasis in esophageal cancer were reported to be 38.57% and 93.93%, respectively[20]. In contrast, the accuracy of CT in detecting regional LN metastasis in esophageal cancer was reported to be unsatisfactory[11]. Similarly, the sensitivity, specificity and accuracy of CT for the detection of LN metastasis in esophageal cancer in our study were 34.4%, 84.6%, 56.9%, respectively. 18F-FDG-PET/CT has moderate/low sensitivity and high/moderate specificity for the detection of regional nodal metastasis in patients with esophageal squamous cell carcinoma. Furthermore, previous reports have shown that FDG-PET/CT is useful for diagnosing metastatic LN and distant metastasis and for detecting recurrent esophageal cancer after surgery. An FDG-PET/CT SUVmax> 2.6 and a CT attenuation pattern were reported to accurately detect regional LN metastasis with an accuracy of 84.5%, sensitivity of 70.7% and specificity of 86.7%[21,22]. Novel PET/CT using 4′-(methyl-11C) thiothymidine was reported, and the accuracy, sensitivity and specificity of 4′-(methyl-11C) thiothymidine-PET/CT were 92.3%, 90.0% and 93.8%, respectively[23], whereas those of PET-CMR in our study were 72.4%, 71.7% and 75.0%, respectively. These results are equivalent to previous reports, but assessment of the N factor with PET-CMR can result in slightly different interpretations. The sensitivity and specificity of PET-CMR for the N factor are 41.9% and 92.6%, respectively, indicating that even if FDG accumulation in LNs is positive, the result may not necessarily be accurate. However, if FDG accumulation in LNs is negative, negative involvement of cancer can be determined with high probability. Therefore, PET/CT could be a useful tool for the detection of LN metastasis in esophageal cancer compared with CT.
In the present study, PET-CMR was an independent prognostic predictor in the univariate analysis of RFS. One of the reasons is that curative resection was performed in all cases of PET-CMR group, whereas noncurative resection was performed in only 19% of cases in the non-PET-CMR group. Intraoperative blood loss may also have affected RFS. However, PET-CMR was not an independent prognostic predictor in the multivariate analysis of OS. We speculate that this indicates that OS might depend on the therapeutic regimen used to treat recurrence.
PET-negative LNs were confirmed as one prognostic predictor. In general, LN metastasis occurring after NAC is the most important prognostic factor in patients with resectable esophageal cancer[24]. However, the value of FDG-PET/CT in predicting pathologic LN metastasis and survival among patients with esophageal cancer is controversial. FDG uptake in primary tumors has been helpful in evaluating responses to neoadjuvant therapy and survival[25,26]. It was reported that the metabolic nodal response of esophageal cancer after NAC could be an independent predictor[27]. That report analyzed pathological nodal status and metabolic nodal status as a single factor, so metabolic nodal status alone may not be an independent prognostic factor. In the present study, PET-negative LNs after NAC were an independent prognostic factor in the univariate and multivariate analyses. We previously reported that some molecular biomarkers might be predictive factors for esophageal cancer in patients treated with NAC[28]. If these predictive molecular biomarkers can be clarified further, it is possible that patients with PET-negative LNs after NAC could avoid esophagectomy and undergo strict surveillance.
There are some limitations in this study. First, this is a retrospective study with a small sample size. Because NAC with DCF is a relatively novel regimen and was only introduced in our department in 2013, the follow-up periods are currently limited. We are presently collecting prospective data. Then, larger sample size studies are needed to confirm our results in the future. Second, although neoadjuvant CRT is considered a standard option for esophageal cancer in western countries, a randomized control trial (JCOG1109) is now underway in Japan; therefore, we do not yet have enough data on neoadjuvant CRT. Third, the patients who received definitive CRT after NAC were excluded from this study. Further investigations that include patients treated with neoadjuvant CRT and definitive CRT are necessary.
PET-CMR after NAC is a favorable prognostic factor for esophageal cancer in terms of RFS. Evaluation by PET-CT after NAC could be useful in clinical decision making for esophageal cancer. Furthermore, in limited patients with PET-negative LNs after NAC, a watch-and-see strategy may be one possible option to avoid unnecessary esophagectomy in the future.
Recent studies have shown that 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) can determine the degree of metabolic activity in tumor cells and can improve tumor staging for patients with esophageal cancer. Furthermore, several studies have shown that FDG-PET/computed tomography (CT) (FDG-PET/CT) is useful for diagnosing metastatic lymph nodes and distant metastasis and for detecting the recurrence of esophageal cancer after surgery. However, the value of FDG-PET/CT to predict survival in patients with esophageal cancer is controversial.
Accurate assessment of cancer remnants after neoadjuvant chemotherapy (NAC) in esophageal cancer may lead to a watch-and-see treatment strategy to avoid surgery.
We aimed to evaluate the value of PET complete metabolic response in esophageal cancer following NAC as a prognostic predictor.
We reviewed data from 70 consecutive patients with squamous cell esophageal cancer who were preoperatively evaluated with FDG-PET/CT before and after NAC with docetaxel, cisplatin and 5-fluorouracil between June 2013 and December 2017 at Oita University. Those who received definitive radiotherapy/chemotherapy after NAC (n = 11) and underwent esophagectomy in another hospital (n = 1) were excluded. Thus, 58 patients received esophagectomy after NAC and were enrolled in this retrospective cohort study.
Five-year relapse-free survival and overall survival (OS) in the PET complete metabolic response group were significantly more favorable than those in the non-PET complete metabolic response group (38.6 mo vs 20.8 mo, P = 0.021, 42.8 mo vs 25.1 mo, P = 0.011, respectively). The univariate analysis of OS showed pathologic T, N, M, stage, complete response and CT-PET negative T and N to be statistically significant. Then, the multivariate analysis subsequently selected CT-PET negative N (hazard ratio: 8.268; 95% confidence interval 1.74-63.60; P < 0.01) as the only independent covariate for OS.
The multivariate analysis subsequently selected CT-PET negative N (hazard ratio: 8.268; 95% confidence interval: 1.74-63.60; P < 0.01) as the only independent covariate for OS.
In limited patients with PET-negative lymph nodes after NAC, a watch-and-see strategy may be one possible option to avoid unnecessary esophagectomy in the future.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shu X S-Editor: Zhang L L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Omloo JM, Lagarde SM, Hulscher JB, Reitsma JB, Fockens P, van Dekken H, Ten Kate FJ, Obertop H, Tilanus HW, van Lanschot JJ. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg. 2007;246:992-1000; discussion 1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 514] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 2. | Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, Ackland S, Gotley DC, Joseph D, Millar J, North J, Walpole ET, Denham JW; Trans-Tasman Radiation Oncology Group; Australasian Gastro-Intestinal Trials Group. Surgery alone vs chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 703] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 3. | Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, Gebski V; Australasian Gastro-Intestinal Trials Group. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1263] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 4. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A; CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4057] [Article Influence: 312.1] [Reference Citation Analysis (0)] |
| 5. | Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, Ikeda K, Kanda T, Tsujinaka T, Nakamura K, Fukuda H. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil vs preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 1046] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 6. | Watanabe M, Baba Y, Yoshida N, Ishimoto T, Nagai Y, Iwatsuki M, Iwagami S, Baba H. Outcomes of preoperative chemotherapy with docetaxel, cisplatin, and 5-fluorouracil followed by esophagectomy in patients with resectable node-positive esophageal cancer. Ann Surg Oncol. 2014;21:2838-2844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Izumi D, Yoshida N, Watanabe M, Shiraishi S, Ishimoto T, Kosumi K, Tokunaga R, Taki K, Higashi T, Harada K, Miyata T, Ida S, Imamura Y, Iwagami S, Baba Y, Sakamoto Y, Miyamoto Y, Yamashita Y, Baba H. Tumor/normal esophagus ratio in (18)F-fluorodeoxyglucose positron emission tomography/computed tomography for response and prognosis stratification after neoadjuvant chemotherapy for esophageal squamous cell carcinoma. J Gastroenterol. 2016;51:788-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Nakamura K, Kato K, Igaki H, Ito Y, Mizusawa J, Ando N, Udagawa H, Tsubosa Y, Daiko H, Hironaka S, Fukuda H, Kitagawa Y; Japan Esophageal Oncology Group/Japan Clinical Oncology Group. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) vs docetaxel, cisplatin plus 5-FU (DCF) vs radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol. 2013;43:752-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 9. | Miyata H, Yamasaki M, Takiguchi S, Nakajima K, Fujiwara Y, Mori M, Doki Y. Pre- and post-therapy nodal status equally affects survival of patients with oesophageal squamous cell carcinoma receiving preoperative chemoradiation. Oncol Rep. 2010;23:1331-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Hamai Y, Hihara J, Emi M, Furukawa T, Murakami Y, Nishibuchi I, Ibuki Y, Yamakita I, Kurokawa T, Nagata Y, Okada M. Evaluation of Prognostic Factors for Esophageal Squamous Cell Carcinoma Treated with Neoadjuvant Chemoradiotherapy Followed by Surgery. World J Surg. 2018;42:1496-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Jiang C, Chen Y, Zhu Y, Xu Y. Systematic review and meta-analysis of the accuracy of 18F-FDG PET/CT for detection of regional lymph node metastasis in esophageal squamous cell carcinoma. J Thorac Dis. 2018;10:6066-6076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Hamai Y, Hihara J, Emi M, Ibuki Y, Murakami Y, Nishibuchi I, Nagata Y, Aoki Y, Furukawa T, Okada M. Clinical Significance of 18F-Fluorodeoxyglucose-Positron Emission Tomography-Positive Lymph Nodes to Outcomes of Trimodal Therapy for Esophageal Squamous Cell Carcinoma. Ann Surg Oncol. 2019;26:1869-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Schmidt T, Lordick F, Herrmann K, Ott K. Value of functional imaging by PET in esophageal cancer. J Natl Compr Canc Netw. 2015;13:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Goense L, van Rossum PS, Reitsma JB, Lam MG, Meijer GJ, van Vulpen M, Ruurda JP, van Hillegersberg R. Diagnostic Performance of ¹⁸F-FDG PET and PET/CT for the Detection of Recurrent Esophageal Cancer After Treatment with Curative Intent: A Systematic Review and Meta-Analysis. J Nucl Med. 2015;56:995-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Leslie S, Mary G, Christian W. TNM Classification of Malignant Tumours, 7th Edition. International Union Against Cancer, 2010 [cited 4 March 2021]. Available from: https://wenku.baidu.com/view/1329e228915f804d2b16c1e9.html. |
| 16. | Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus. 2017;14:1-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 704] [Article Influence: 88.0] [Reference Citation Analysis (1)] |
| 17. | Yasuda T, Yano M, Miyata H, Yamasaki M, Takiguchi S, Fujiwara Y, Doki Y. Prognostic Significance of (18)F-fluorodeoxyglucose Positron Emission Tomography (FDG-PET)-Positive Lymph Nodes Following Neoadjuvant Chemotherapy and Surgery for Resectable Thoracic Esophageal Squamous Cell Carcinoma. Ann Surg Oncol. 2015;22:2599-2607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Yasuda T, Higuchi I, Yano M, Miyata H, Yamasaki M, Takiguchi S, Fujiwara Y, Hatazawa J, Doki Y. The impact of ¹⁸F-fluorodeoxyglucose positron emission tomography positive lymph nodes on postoperative recurrence and survival in resectable thoracic esophageal squamous cell carcinoma. Ann Surg Oncol. 2012;19:652-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Gillies RS, Middleton MR, Han C, Marshall RE, Maynard ND, Bradley KM, Gleeson FV. Role of positron emission tomography-computed tomography in predicting survival after neoadjuvant chemotherapy and surgery for oesophageal adenocarcinoma. Br J Surg. 2012;99:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | National Comprehensive Cancer Network. Esophageal and esophagogastric junction cancers [cited 4 March 2021]. In: Clinical practice guidelines in oncology (NCCN guideline). Version 2. Available from: http://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. . |
| 21. | Lee JY, Kim YH, Park YJ, Park SB, Chung HW, Zo JI, Shim YM, Lee KS, Choi JY. Improved detection of metastatic lymph nodes in oesophageal squamous cell carcinoma by combined interpretation of fluorine-18-fluorodeoxyglucose positron-emission tomography/computed tomography. Cancer Imaging. 2019;19:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Eyck BM, Onstenk BD, Noordman BJ, Nieboer D, Spaander MCW, Valkema R, Lagarde SM, Wijnhoven BPL, van Lanschot JJB. Accuracy of Detecting Residual Disease After Neoadjuvant Chemoradiotherapy for Esophageal Cancer: A Systematic Review and Meta-analysis. Ann Surg. 2020;271:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 23. | Hotta M, Minamimoto R, Yamada K, Nohara K, Soma D, Nakajima K, Toyohara J, Takase K. Efficacy of 4'-[methyl-11C] thiothymidine PET/CT before and after neoadjuvant therapy for predicting therapeutic responses in patients with esophageal cancer: a pilot study. EJNMMI Res. 2019;9:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Davarzani N, Hutchins GGA, West NP, Hewitt LC, Nankivell M, Cunningham D, Allum WH, Smyth E, Valeri N, Langley RE, Grabsch HI. Prognostic value of pathological lymph node status and primary tumour regression grading following neoadjuvant chemotherapy - results from the MRC OE02 oesophageal cancer trial. Histopathology. 2018;72:1180-1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Hamai Y, Hihara J, Emi M, Furukawa T, Yamakita I, Kurokawa T, Okada M. Ability of Fluorine-18 Fluorodeoxyglucose Positron Emission Tomography to Predict Outcomes of Neoadjuvant Chemoradiotherapy Followed by Surgical Treatment for Esophageal Squamous Cell Carcinoma. Ann Thorac Surg. 2016;102:1132-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Cong L, Wang S, Gao T, Hu L. The predictive value of 18F-FDG PET for pathological response of primary tumor in patients with esophageal cancer during or after neoadjuvant chemoradiotherapy: a meta-analysis. Jpn J Clin Oncol. 2016;46:1118-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Findlay JM, Dickson E, Fiorani C, Bradley KM, Mukherjee S, Gillies RS, Maynard ND, Middleton MR. Temporal validation of metabolic nodal response of esophageal cancer to neoadjuvant chemotherapy as an independent predictor of unresectable disease, survival, and recurrence. Eur Radiol. 2019;29:6717-6727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Fujishima H, Fumoto S, Shibata T, Nishiki K, Tsukamoto Y, Etoh T, Moriyama M, Shiraishi N, Inomata M. A 17-molecule set as a predictor of complete response to neoadjuvant chemotherapy with docetaxel, cisplatin, and 5-fluorouracil in esophageal cancer. PLoS One. 2017;12:e0188098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |