Published online Dec 24, 2021. doi: 10.5306/wjco.v12.i12.1182
Peer-review started: April 28, 2021
First decision: July 16, 2021
Revised: July 28, 2021
Accepted: November 24, 2021
Article in press: November 24, 2021
Published online: December 24, 2021
Processing time: 240 Days and 1.3 Hours
In recent years, studies have explored different combinations of immunotherapy and chemotherapy. The rationale behind these is the improved survival outcomes of new immunologic therapies used in first-line-treatment of advanced non-small cell lung cancer. Moreover, for the most-studied combinations of anti-programed death-1 (PD-1)/programed death ligand-1 (PD-L1) with the addition of platinum- based chemotherapy, recent research is investigating whether combining different immunologic antitumoral mechanisms of action, such as anti-PD-1/PD-L1 and anti-CTLA-4, or anti-PD-L1 and anti-TIGIT, with or without chemotherapy, can improve efficacy outcomes compared with more classical combinations, or compared with standard chemotherapy alone. Here, we present the data of the main randomized studies that have evaluated these combinations, focusing on the basic rationale behind the different combinations, and the efficacy and tolerability data available to date.
Core Tip: This review presents the results of the main articles reporting on immunotherapy combinations, with or without chemotherapy, focusing principally on efficacy and toxicity data. The convenience of adding shorter chemotherapy regimens vs the standard 4-cycle regimens to immunotherapy doublets is discussed.
- Citation: Sereno M, Higuera O, Cruz Castellanos P, Falagan S, Mielgo-Rubio X, Trujillo-Reyes JC, Couñago F. Immunotherapy combinations and chemotherapy sparing schemes in first line non-small cell lung cancer. World J Clin Oncol 2021; 12(12): 1182-1192
- URL: https://www.wjgnet.com/2218-4333/full/v12/i12/1182.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i12.1182
Lung cancer is the leading cause of cancer-related deaths worldwide. An increased knowledge of lung cancer pathology as well as of tumoral immunity has permitted the discovery of different targeted molecular treatments and immune-therapies involving an important change of the lung cancer paradigm[1].
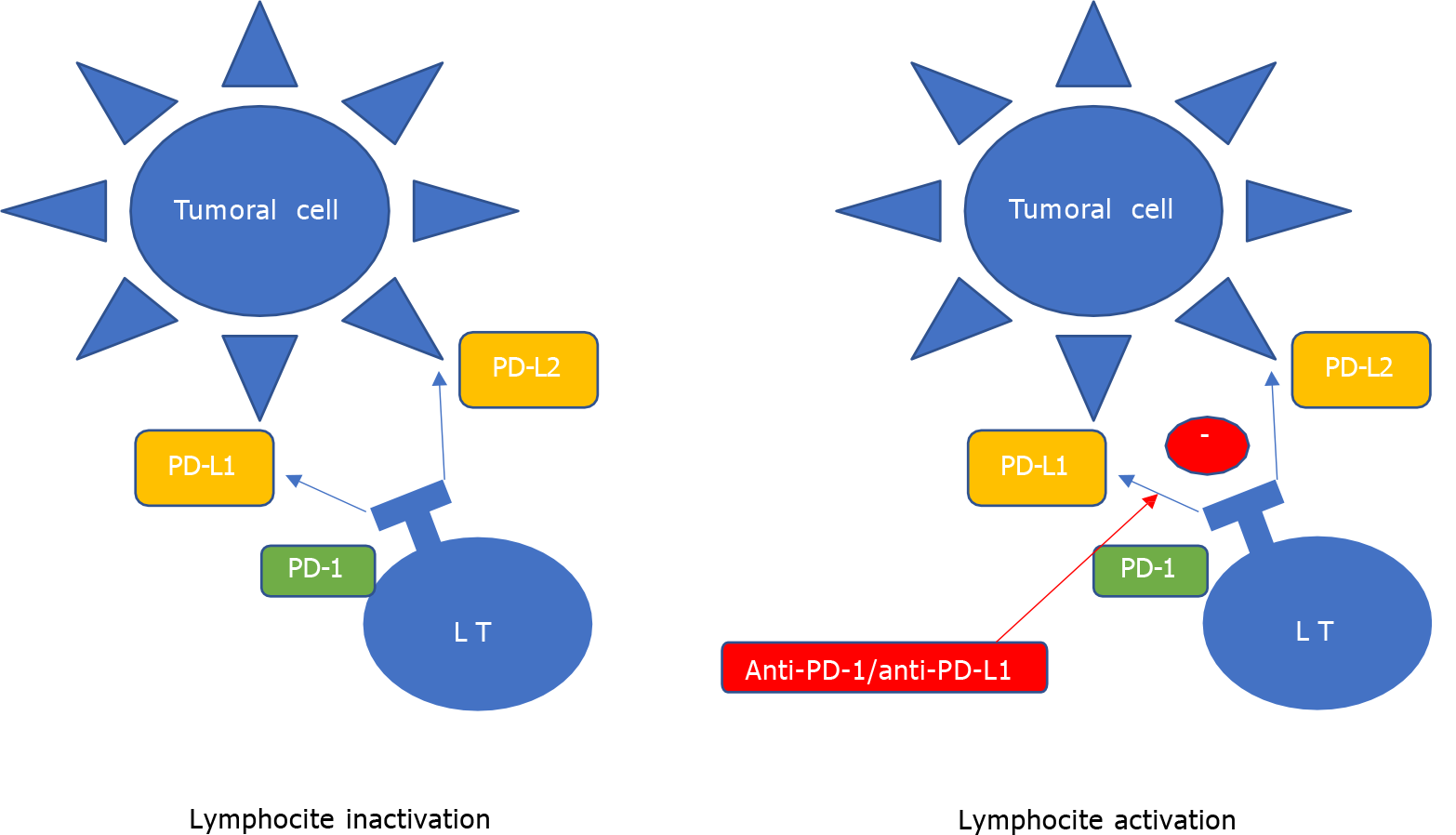
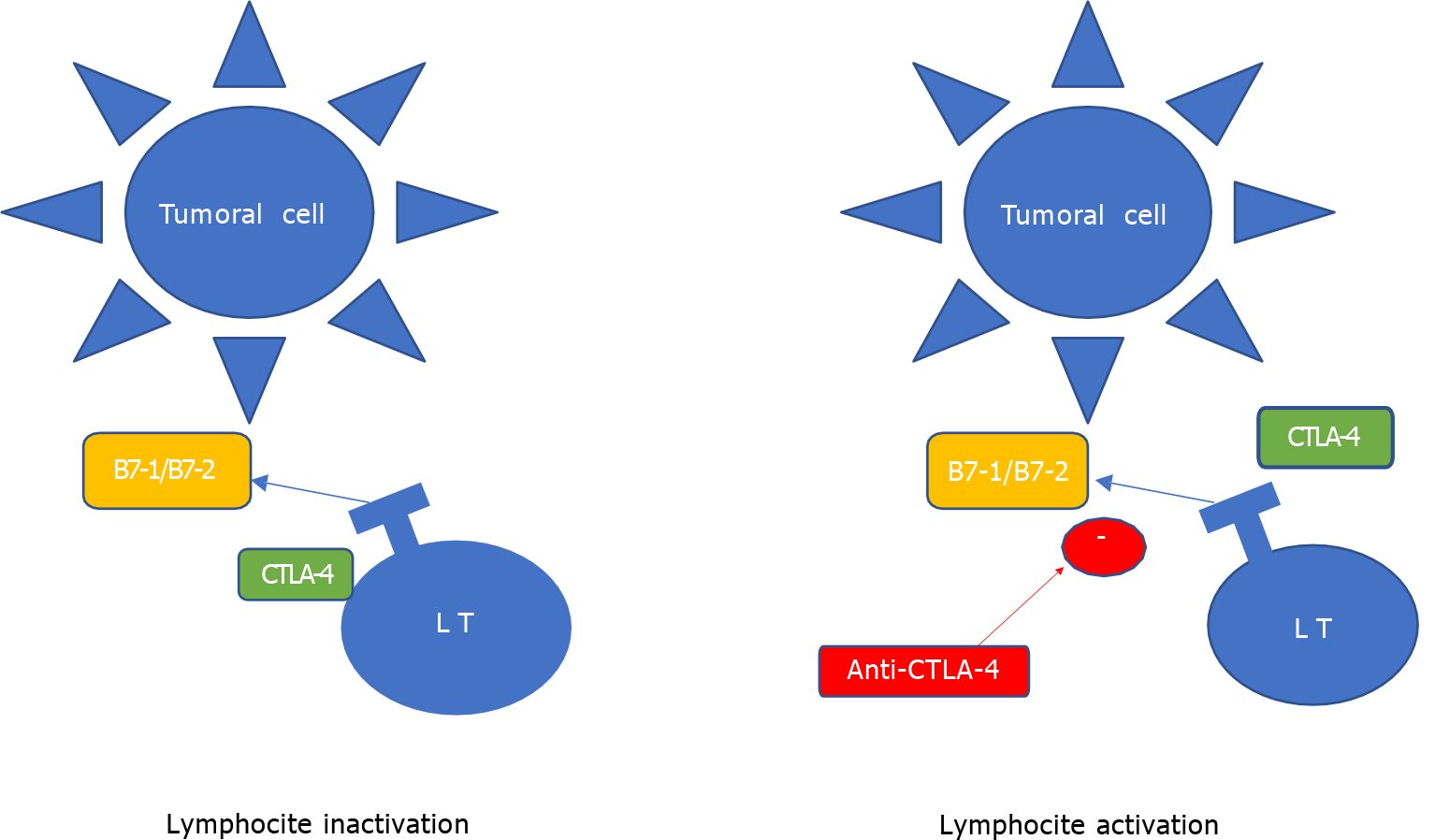
Anti-programed death-1/programed death ligand-1 (PD-1/PD-L1) treatment, recently approved in advanced lung cancer treatment, is an important immune checkpoint of anti-tumor activity in our immune system. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) is another component of these control immune checkpoints and different inhibitors have been developed to look their role in tumor recognition[2] (Figures 1 and 2).
Non-small cell lung cancer (NSCLC) has a high level of heterogeneity secondary to a different molecular immune subtype and a complex immune environment. This complexity justifies heterogenous responses to immunotherapy (IT) and their combinations[2].
The administration of mono-IT (m-IT) with anti-PD-L1/PD-1, or a combination of this type of agent with platinum-based chemotherapy (CT), is the standard therapy for the different subtypes of non-small cell lung cancer without treatable mutations. Several studies focusing on combinations of different checkpoint inhibitors, such as anti-PD-1/PD-L1 and anti-CTLA-4, have shown these to be advantageous compared with CT alone[3,4]. The association of these IT combinations with CT has also been analyzed in two phase III studies [Checkmate 9LA study (CM9LA) and CCTG BR.34], with interesting results in favor of the new combinations[5,6]. The CYTISCAPE study, presented recently in ASCO 2020, proposes a combination of anti-PD-L1 and anti- T-cell immunoreceptor with Ig and ITIM domains (TIGIT), a new checkpoint inhibitor present in activated T-cells. This phase II study reported favorable outcomes for this IT doublet (d-IT) in the population with PD-L1 > 1% vs monotherapy with anti-PD-L1[7].
Here, we briefly summarize the most significant aspects of these new combinations.
The biological rationale for combining IT is based on the complexity of immune surveillance regulation, the effect of other anti-tumor treatments on tumor immunogenicity, and the non- redundancy of immune checkpoint regulation[8].
Most chemotherapeutic agents are regarded as immunosuppressants, exerted this effect by a direct inhibition or killing of effector cells, as well as indirectly by inducing anergy or immune paralysis[9]. However, combinations of CT and ICI have a proven synergistic effect: CT aids in fast tumor regression, and a reduction in tumor burden and immune checkpoint blockade may prolong this effect, thus inducing a long-lasting anti-tumor response[10]. In addition, the non-overlapping toxicity profiles of IT and CT render them good candidates for combination strategies.
In addition to immune regulation, angiogenesis is another hallmark of cancer evolution, promoting tumor vascularity to sustain cancer cell metabolism and invasive capacity, and also favors cancer immune escape. Vascular endothelial growth factor, one of the main regulators of angiogenesis, can also induce T-reg cells and inhibit dendritic cell and T-cell maturation, thus inducing an immunosuppressive status, lymphocyte migration to the tumor site, and negatively regulating immune priming[11,12]. Combining anti-angiogenics with immune checkpoint inhibitors could thus be synergic in reducing tumor vascularization and decreasing the immune suppressive microenvironment. It may also have a synergistic effect by boosting tumor-specific immunity.
Immune checkpoints exert a negative regulatory effect on CD8+ T cells by int
| Checkpoint pathways | Anti-PD-L1 pathway | Anti-CTLA-4 pathway |
| Receptor expression | Activated T-cells | Activated T-cells, B-cells and NK cells |
| Ligands | B 7.1 (CD80); B7.2 (CD86) | PD-L1 (B7-H1), PD-L2 (B7-DC) |
| Mechanism of immune modulation | T-cell activation at initial stage; Competition with co-stimulatory receptor CD-28 for ligand binding; Down regulation of helper T cells CD4 activity; Enhancement Tregs-cells immunosuppressive activity | Suppresses activated T cells in tissues and tumor environment; Express in Tregs-cells may enhance immunosuppressive activity; Limits of B-cells and NK- cells activity; PD-L1 interact with CD-80 to down-modulate T-cells activity |
TIGIT is a novel immune inhibitory receptor. It is a member of the immunoglobulin superfamily and is expressed in a wide variety of human tumors. TIGIT is expressed on the surface of activated T-cell and natural killer (NK)-cell subsets and interacts with high affinity with CD155 [also known as poliovirus receptor (PVR)]. It is also highly correlated with T-cell infiltration and PD-1 expression[14]. Genetic ablation of TIGIT in T cells in mice results in exacerbated T-cell responses in preclinical models of autoimmune diseases and viral infections, demonstrating the role of TIGIT in the inhibition of T-cell responses. Because TIGIT and PD-1 are coordinately expressed by tumor-infiltrating T cells in several human tumors, inhibition of the TIGIT/PVR pathway may complement and potentiate the anti-tumor activity of a PD-L1 pathway inhibitor. In preclinical models, concomitant blockade of both TIGIT and PD-L1/PD-1 pathways demonstrated superior efficacy over the respective single-agent treatments[15].
In conclusion, based on preclinical and clinical studies, there is a strong rationale for combining IT and other agents, and this potentially synergistic effect has been demonstrated with improved outcomes in the clinical setting.
As explained previously, there is a clear justification for combining different the
Since pembrolizumab was first approved in first-line therapy of patients with PDL1 > 50%, several studies, which we describe below, have analyzed the combination of anti PD-1/PDL-1 and anti-CTLA-4, with or without the addition of CT[14] (Table 2).
| Trial | Ph1 | Drugs | Endpoints/outcome | PD-L1/TMB |
| Population | ||||
| Subgroups | ||||
| Checkmate 227 | III | NI vs N vs CT1 | OS PD-L1 > 1% and in non-selected population | PD-L1 ≥ 1%: NI 17 m vs CT 14.9 m, P = 0.007 |
| NI vs CT | PD-L1 < 1%: NI 17 m vs CT 12.2 m, P =0.007 | |||
| 17 m vs 13.9 m | ||||
| MYSTIC | III | DT vs D vs CT1 | OS D vs CT OS and PFS DT vs CT PD-L1 > 25% population | PD-L1 > 25%: D vs CT1 |
| 16 m vs 12 m, P = 0.04 | ||||
| DT vs CT1 11.9 vs 12 m, P = 0.2 | ||||
| TMB > 20 | ||||
| DT vs CT1 21 m vs 10 m | ||||
| Checkmate9LA | III | NI + CT1 X2 vs CT1 X4 | OS in non selected population | NA |
| OS NI-CT 15.6 m vs CT 10.9 m, P = 0.0006 | ||||
| CCTG BR.34 | III | DT+ CT1 x 4 vs Durvalumab + tremelimumab (DT) | OS in non selected population | PD-L1 TPS ≥ 50%, DT-CT vs DT (HR 0.64, 95%CI: 0.40-1.04, P = 0.07). Plasma TMB < 20 mut/Mb was associated with shorter survival in both treatment groups (HR 1.99, 95%CI: 1.3-3.1) |
| 16.6 m DT-CT vs 14 m DT | ||||
| CITYSCAPE | II | TA vs PA | ORR and PFS en PD-L1 > 1% PFS TA 7.7 m vs PA 3.2 m | PD-L1 > 50% |
| ORR TA 37% vs PA 20% | PFS TA NA vs PA 4.1 | |||
| ORR TA 66 % vs PA 24% |
Checkmate 012 (CM012) was the first phase I study to explore the combination of ipilimumab-nivolumab (NI) vs nivolumab (N) in CT-naïve patients with advanced NSCLC. This study assessed three alternative regimens with different doses of I and N, with 6 weekly vs 12 weekly schemes of I, with tolerability as the primary endpoint. This study confirmed the safety of the combination, predicting a high response rate and improved survival (OS)[17].
With the data from CM012, Checkmate 227 (CM227), a phase III study was proposed. This study was comprised of three parts: Part1A that assessed the efficacy of the combination of NI vs N in monotherapy vs standard CT in patients with negative PD-L1 expression, part 1B that compared the combination of NI vs N + CT vs standard CT in PD-L 1 negative patients, and part 2 which evaluated the efficacy of the combination of N + CT, regardless of histology and PD-L1 expression. The study reported a global benefit for the NI doublet in OS, independently of PD-L1 [17.1 ms vs 13.9 ms, hazard ratio (HR) 0.73; 95%CI: 0.64-0.84]. When these data are analyzed in relation to PD-L1, several important observations can be made: in the PD-L1 negative group the benefit in OS in favor of the IT doublet was maintained at 17.2 vs 12.2 mo (HR 0.62, 95%CI: 0.48-0.78). In the PD-L1 positive group (≥ 1%), OS was also better with the combined IT treatment (17.1 ms vs 14.9 ms, HR 0.79; 95%CI: 0.65-0.96). Noteworthily, in this group with 1-49% PD-L1 expression, no differences in OS were found. Also in this study, data of levels of tumor mutational burden (TMB) were reported early on. Hence, in the high TMB group (> 10 mut/Mb), a statistically significant benefit in favor of the combination was reported. Finally, the results of part 2 were communicated, in which the primary final endpoint of OS for N-CT was not reached in patients with non-squamous NSCLC[3].
The CM9LA is a phase III trial with a novel design that adds two cycles of CT to the NI doublet. In the control arm, the CT regimen preferred by the clinician was established, or maintenance with permetrexed in non-squamous histology. With a follow up of 12.7 mo, the experimental arm showed a significant improvement in OS in all subgroups (15.6 ms vs 10.9 ms, HR 0.66; 95%CI: 0.55-0.8)[5].
The combination of Durvalumab (D) and Tremelimumab (T) has also been explored in different phase I-III studies. One of the first phase III trials to analyze this combination in NSCLC was the ARTIC study. This was comprised of two independent sub-studies: one focused on the PD-L1 > 25% population, comparing D vs standard CT in patients pre-treated with platinum, and another in the population with PD-L1 < 25% with three arms: DT vs D vs T in the same patient profile. In both sub-studies, the data from the D and DT arms were more favorable than CT alone[18]. Continuing with this combination of DT in first-line, the MYSTIC phase III study[4] once again focused on the population of PDL-1 > 25%. The patients were randomized to receive D vs DT vs platinum-based CT, in this case in first-line of NSCLC. The efficacy data for the mono-IT vs CT showed a non-significant superiority for the former (16 mo vs 12 mo), although in the d-IT the median OS of 11.9 mo was shorter than that obtained with CT. No benefit in favor of IT was found for PFS either. However, in this study TMB was included as a predictive biomarker of response to IT, with the subgroup TMB > 20 presenting an OS in favor of the IT doublet (21.9 mo vs 10 mo).
Continuing to focus on the DT combination, CGC BR.34, another phase III study emerged, in parallel with CM9LA, to address the need to explore the efficacy of d-IT. In this work, 4 CT cycles (platinum-pemetrexed) were added to the DT doublet in first-line therapy in a non-squamous population. Maintenance treatment was allowed in both arms. After a six-month follow up, no differences were found in OS, although the analysis of PFS revealed statistically significant differences in favor of the combined treatment of IT-CT (7.7 mo vs 3.2 mo)[6].
CITYSCAPE is a phase II trial that evaluates the possible efficacy of combining Tiragolumab (Ti) (an inhibitor of the immune modulatory receptor TIGIT) with Atezolizumab (A) in first-line of NSCLC with PDL > 1%. Preliminary phase I studies (GO 30103) showed the benefit of combining TIGIT inhibition and anti-PD-L1, revealing a greater response rate in pre-treated patients. The first data from this study were presented in ASCO 2020, and the preliminary analysis was positive in favor of the combined approach (TiA vs A-placebo) (5.6 ms vs 3.9 ms, HR 0.58; 95%CI: 0.38-0.89). Moreover, an exploratory analysis in patients with high PD-L1 expression, or with a tumor proportion score over 50%, obtained a reduction of 70% in risk of disease progression or death from the illness with TiA vs A-P (HR 0.30; 95%CI: 0.15-0.61). This study appears to confirm the effectiveness of combining a dual inhibition of immunotherapeutic mechanisms and their potential benefit in clinical practice, although the final results and the study design of the phase III trial are still pending[7].
Several preclinical studies have arrived at an interesting rationale to support CT-IT combinations. These combinations are an important challenge in NSCLC treatment, not only in advanced disease, but also in a neoadjuvant setting, where phase III studies such as CM816 have demonstrated a significant benefit of CT-IT pre-surgery com
Specifically, platinum agents have been shown to partly exert their anti-tumor effect via modulation of the immune system. They attenuate STAT6 signaling by blocking STAT6 phosphorylation, resulting in a downregulation of PD-L2 on DCs and tumor cells. This triggers increased tumor cell recognition by T lymphocytes[23]. In a study of NSCLC cell lines, cisplatin upregulated the MHC class I chain-related molecule A and B expression, and led to enhanced NK cell-mediated antitumor effect[24]. Other mechanisms by which platinum agents stimulate the immune system include increasing human leukocyte 1 gene complex expression encoding MHC-I, which is associated with cytotoxic T-cell function[25].
As mentioned in previous sections, a number of clinical trials, such as the one described above, have focused on the administration of combinations of IT in first-line therapy. Overall, it is found that an unblocking of checkpoints of antitumor response (PD-L1/CTLA-4), using combinations of checkpoint inhibitors, has proved more effective than CT alone or IT in monotherapy. The CM 227 trial, one of the first phase III studies that compared NI vs CT, reported a greater OS in the former treatment, regardless of PD-L1 expression[3].
In spite of the overall positivity of the CM 227 trial, an initial crossing of the curves takes place in the first 3-6 mo of follow up, both for the entire study population and also in patients with PDL-1 > 1%. This means that CT would be superior to IT in a profile of patients with more aggressive disease, who would potentially be more chemosensitive.
Another parallel study to CM227 in the PDL-1 > 25% population, also mentioned previously, is the MYSTIC study[4], which compares DT vs D vs CT. However, the results of this study were more disappointing, reporting no greater superiority for the IT doublet vs CT, although DT was more effective in the subgroup with TMB > 20. The CITYSCAPE study positions a new combination of IT (Ti-A) as another option in first-line therapy, and once again PD-L1 is a good predictor correlated with a greater benefit in favor of the combination.
Therefore, in spite of being a new option in first-line, d-IT was no more beneficial than its comparators in any of the studies analyzed, and which have been described here.
Subsequent studies, which aimed at improving the efficacy data of these schemes, incorporated CT regimens of different durations in an attempt to maximize the benefits of these new combinations.
With the goal of compensating for this initial deficit in OS in patients in the CM227 study, the CM9LA study added two cycles of platinum-based CT to the NI com
Another study that adds CT to the IT doublet is the aforementioned CCTG BR.34 study. This was designed with the same goal, in other words, to try to obtain better outcomes than the MYSTIC study[6]. In this case, efficacy data after a 16-month follow up found no differences in OS (with a non-significant favorable tendency in the PD-L1 > 50% group). However, differences were found in the response rates (28% vs 14%) and in PFS 7.7 vs 3.2 in favor of the combination with CT. A longer follow-up would probably produce more mature conclusions about the benefits of the IT doublet-CT combination vs the comparator with single IT-CT. In this case, in contrast to the MYSTIC study, there are no data to support a benefit in patients with a high TMB, although, once again, the high expression of PD-L1 would seem to predict a greater benefit.
In the debate about combinations of IT and CT, there is some controversy about the number of platinum cycles to use. Hence, the CM9LA study used 2 cycles while in the CCTG BR34 study, 4 cycles were administered. Although there are no data to attribute the difference in results to the number of CT cycles given, it is interesting that in the study with more CT cycles, in which better results would be expected, the data were, in fact, negative. It is, therefore, unclear whether the 2 first cycles are insufficient to compensate for the initial fall in the survival curve due to patients with an early sensitivity to IT.
On the other hand, there is no doubt about the rise in platinum-based toxicity of the longer CT schemes. Therefore, prospective studies may be necessary to determine whether reduced CT schemes, with greater tolerability, in combination with the IT doublet could be at least as effective as standard 4-cycle schemes. Another important aspect to consider about these new combinations concerns the choice of the best biomarker to predict benefits with the IT combination. Compared with PD-L1 expression, in the MYSTIC study higher levels of TMB predicted a greater benefit of the combo-IT. However, these results were not reproduced in other studies that analyzed this marker. For combinations of CT with anti-TIGIT/anti-PD-L1, data have not yet been published.
All these studies have only a short follow up and the results of longer follow up periods are required before we can confirm potential benefits for these new combinations of different and complementary therapeutic strategies.
The toxicity of combinations with IT, whether this corresponds to d-IT or IT-CT, is highly variable, with cytostatic toxicity in addition to immune-mediated adverse effects. The rates of grade 3-4 toxicities for the different studies on the d- IT combination are presented in Table 3.
| Phase II-III studies | G3-4 toxicity (%) | Treatment discontinuation (%) | ||
| Exp arm | Control arm | Exp arm | Control arm | |
| CM 227 | 32.8 NI | 33.8 CT | 9.4 NI | 3.4 CT |
| MYSTIC | 22.9 DT | 36 CT | 12.3 DT | 4.9 CT |
| CM9LA | 47 NI-CT | 38 CT | 16 NI-CT | 9 CT |
| CCTG-BG-34 | 82 DT-CT | 70 DT | NA | NA |
| CITYSCAPE | 19 ATir | 14 PA | 10.3 ATir | 7.5 PlA |
| KEYNOTE-189 | 67 P-CT | 65.8 CT | 13.8 P-CT | 7.9 CT |
Combinations of IT with the NI scheme in the CM227 trial[3], and with DT in the MYSTIC trial[4], show global rates of side effects of 76.7% and 60.1%, respectively; and of 54.2% in arm D. In both studies, higher rates were obtained for the control arm of CT, of 81.9% in CM227 and 83% in MYSTIC. For toxicity rates of G3 or higher, rates of 32.8% were recorded for the NI combination, 22.9% for the DT combination, 14.9% for D in monotherapy, and 33.8% and 36% in the CT arm, thus confirming in both studies that CT was more toxic than IT. Side effects of G3 or higher resulted in interruption of treatment in 9.4%-12.3% in the combination with the IT doublet, in 3.4%-4.9% in the CT arm and in 4.3% in arm D in monotherapy. As expected, the most frequent toxicities of CT were classical toxicities, such as pancytopenia, especially anemia and G3 neutropenia in 10%, and other common effects such as vomiting and asthenia. Immune-mediated adverse events were reported in 13.6% of arm D in monotherapy and in 28.3% with the DT combination. The most frequent immune-mediated toxicities corresponded to cutaneous reactions (30%-35%) and endocrine events (23%-25%). As published previously in other trials and studies, toxicity was also proportional to the level of PD-L1 expression. In CM227, patients with a level of expression of PD-L1 < 1%, presented less treatment-related G 3-4 toxicity (27.0 in PD-1 < 1% vs 32% general population).
As expected, combinations of IT-CT were also associated with an increased toxicity. In the CM 9LA trial[3], the global rate of adverse effects of G3 or higher was 47% in the experimental arm with NI-CT vs 38% in the control arm with CT, and slightly higher in the arms with CT of CM227[3] and MYSTIC[4]. In the CCTG BR.34 study[6], the combination of DT-CT presented side effects of G3 or higher in 82%, and in the control arm with DT alone of 70%, with rates of 22.9% reported in the MYSTIC study[4]. It is noteworthy that this toxicity is also higher, if results are extrapolated, than the toxicities of studies using the CT-IT combination such as Keynote 189, where a G3 toxicity of 67.2% was recorded[26].
The most common treatment-related serious adverse events of any grade were more frequent in the experimental group. These included diarrhea, febrile neutropenia, thrombocytopenia and anemia, with rates of 2%-3%. Treatment-related adverse events of severity G 3-4 that led to interruption of treatment were reported in 16%, and were higher than those reported for IT combinations in CM227[3], MYSTIC[4] and Keynote 189[26].
In studies of the doublet IT-CT, the most frequent treatment-related adverse events of any grade, which are commonly associated with CT, were anemia (23%-38%), neutropenia (10%-17%) and thrombocytopenia (5%-10%) in CM 9LA[5]. Grade 3-4 adverse events in the CCTG BR.34 study corresponded to neutropenia (7%-9%), anemia (6%-14%), diarrhea (1%-4%), elevated lipase levels (1%-6%) and febrile neutropenia (3%-4%). In the CM9LA study[3], the commonest immune-mediated adverse events of level G 3-4 were gastrointestinal (6%), cutaneous (4%) and hepatic (4%). Most of these were successfully resolved, with some patients requiring immune modulating medication, including corticosteroids and TNF antagonists. In the CCTG BR 34 trial[4], the incidence of adverse events related to the immune system was similar in all arms (colitis 11%, pneumonitis 6%, endocrinopathy 21%).
The toxicity of the combination of A and Ti was not negligible, (CITYSCAPE)[7], with global rates of side effects of 80.6% vs 72%, respectively, and G3 toxicity rates of 19.1% vs 14.9%, and rates of interruption of treatment of 10.3% vs 7.5% for the control group. Once again, in the experimental arm the most frequent immune-mediated toxicity effects were skin rashes, perfusion reaction, pancreatitis, thyroid alterations and colitis.
The toxicity of the IT combinations is, therefore, greater than that of studies of single IT, at the expense of more immune-mediated effects, as to be expected. Similarly, toxicity increases when CT is added to these IT doublet schemes because of the toxicity associated with platinum regimens. Indirect comparisons of the more classical schemes of m-IT-CT vs d-IT-CT show greater toxicity in the latter, directly proportional to the number of CT cycles. Hence, in the decision-support algorithm to select the best first-line scheme in our patients, it is essential to take into account the toxicity profiles of each alternative treatment.
The combination of different checkpoint inhibitors, or checkpoint inhibitors combined with other immune modulating strategies, such as TIGIT inhibition, in the context of first-line therapy in advanced NSCLC produces superior outcomes to classical CT regimens. The addition of CT (of 2 to 4 cycles) to this IT doublet is a new strategy to rescue patients who could initially present a detrimental clinical course after first receiving the IT doublet. It is necessary to continue researching to find more accurate predictive biomarkers of response to optimize the selection of patients who should receive these types of therapeutic strategies.
To all the coauthors for the literature review and drafting of the manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen LJ S-Editor: Liu M L-Editor: A P-Editor: Liu M
| 1. | Niu M, Yi M, Li N, Luo S, Wu K. Predictive biomarkers of anti-PD-1/PD-L1 therapy in NSCLC. Exp Hematol Oncol. 2021;10:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 2. | Qu J, Mei Q, Liu L, Cheng T, Wang P, Chen L, Zhou J. The progress and challenge of anti-PD-1/PD-L1 immunotherapy in treating non-small cell lung cancer. Ther Adv Med Oncol. 2021;13:1758835921992968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 3. | Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, de la Mora Jimenez E, Sakai H, Albert I, Vergnenegre A, Peters S, Syrigos K, Barlesi F, Reck M, Borghaei H, Brahmer JR, O'Byrne KJ, Geese WJ, Bhagavatheeswaran P, Rabindran SK, Kasinathan RS, Nathan FE, Ramalingam SS. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2019;381:2020-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1360] [Cited by in RCA: 1978] [Article Influence: 329.7] [Reference Citation Analysis (0)] |
| 4. | Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, van den Heuvel MM, Cobo M, Vicente D, Smolin A, Moiseyenko V, Antonia SJ, Le Moulec S, Robinet G, Natale R, Schneider J, Shepherd FA, Geater SL, Garon EB, Kim ES, Goldberg SB, Nakagawa K, Raja R, Higgs BW, Boothman AM, Zhao L, Scheuring U, Stockman PK, Chand VK, Peters S; MYSTIC Investigators. Durvalumab With or Without Tremelimumab vs Standard Chemotherapy in First-line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6:661-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 487] [Article Influence: 121.8] [Reference Citation Analysis (0)] |
| 5. | Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, Richardet E, Bennouna J, Felip E, Juan-Vidal O, Alexandru A, Sakai H, Lingua A, Salman P, Souquet PJ, De Marchi P, Martin C, Pérol M, Scherpereel A, Lu S, John T, Carbone DP, Meadows-Shropshire S, Agrawal S, Oukessou A, Yan J, Reck M. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 903] [Article Influence: 225.8] [Reference Citation Analysis (0)] |
| 6. | Leighl NB, Laurie SA, Goss GD, Hughes BGM, Stockler MR, Tsao MS, Kulkarni S, Blais N, Joy AA, Mates M, Rana P, Yadav S, Underhill C, Lee CW, Bradbury PA, Hiltz A, Dancey J, Ding K, Vera Badillo FE; Canadian Cancer Trials Group, Australasian Lung Cancer Trials Group. CCTG BR.34: A randomized trial of durvalumab and tremelimumab +/- platinum-based chemotherapy in patients with metastatic (Stage IV) squamous or nonsquamous non-small cell lung cancer (NSCLC). J Clin Oncol. 2020;38:9502. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Rodriguez-abreu D, Johnson ML, Hussein MA, Cobo M, Patel AJ, Secen NM, Lee KH, Massuti B, Hiret S, Yang JC, Barlesi F, Lee DH, Paz-ares LG, Hsieh RW, Miller K, Patil N, Twomey P, Kapp AV, Meng R, Cho BC. Primary analysis of a randomized, double-blind, phase II study of the anti-TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1L) treatment in patients with PD-L1-selected NSCLC (CITYSCAPE). J Clin Oncol. 2020;38:9503. [RCA] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (1)] |
| 8. | Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2373] [Cited by in RCA: 3605] [Article Influence: 450.6] [Reference Citation Analysis (0)] |
| 9. | Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1053] [Cited by in RCA: 1231] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 10. | Champiat S, Ileana E, Giaccone G, Besse B, Mountzios G, Eggermont A, Soria JC. Incorporating immune-checkpoint inhibitors into systemic therapy of NSCLC. J Thorac Oncol. 2014;9:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, Carbone DP. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878-4886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 428] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 12. | Manegold C, Dingemans AC, Gray JE, Nakagawa K, Nicolson M, Peters S, Reck M, Wu YL, Brustugun OT, Crinò L, Felip E, Fennell D, Garrido P, Huber RM, Marabelle A, Moniuszko M, Mornex F, Novello S, Papotti M, Pérol M, Smit EF, Syrigos K, van Meerbeeck JP, van Zandwijk N, Yang JC, Zhou C, Vokes E. The Potential of Combined Immunotherapy and Antiangiogenesis for the Synergistic Treatment of Advanced NSCLC. J Thorac Oncol. 2017;12:194-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 188] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 13. | Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275-4280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1270] [Cited by in RCA: 1492] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 14. | Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, Eaton D, Grogan JL. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 1080] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 15. | Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, Eaton DL, Grogan JL. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26:923-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 884] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 16. | Hanna NH, Schneider BJ, Temin S, Baker S Jr, Brahmer J, Ellis PM, Gaspar LE, Haddad RY, Hesketh PJ, Jain D, Jaiyesimi I, Johnson DH, Leighl NB, Phillips T, Riely GJ, Robinson AG, Rosell R, Schiller JH, Singh N, Spigel DR, Stabler JO, Tashbar J, Masters G. Therapy for Stage IV Non-Small-Cell Lung Cancer Without Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J Clin Oncol. 2020;38:1608-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 17. | Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, Ready NE, Gerber DE, Chow LQ, Juergens RA, Shepherd FA, Laurie SA, Geese WJ, Agrawal S, Young TC, Li X, Antonia SJ. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 775] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 18. | Planchard D, Reinmuth N, Orlov S, Fischer JR, Sugawara S, Mandziuk S, Marquez-Medina D, Novello S, Takeda Y, Soo R, Park K, McCleod M, Geater SL, Powell M, May R, Scheuring U, Stockman P, Kowalski D. ARCTIC: durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer. Ann Oncol. 2020;31:609-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 19. | Spicer J, Wang C, Tanaka F, Saylors GB, Chen K, Liberman M, Vokes EE, Girard N, Lu S, Provencio M, Mitsudomi T, Awad MM, Felip E, Forde PM, Swanson S, Brahmer JR, Kerr K, Dorange C, Cai J, Broderick S. Surgical outcomes from the phase 3 CheckMate 816 trial: Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo alone as neoadjuvant treatment for patients with resectable non-small cell lung cancer (NSCLC). J Clin Oncol. 2021;39:8503-8503. [RCA] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 20. | Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 703] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 21. | Mathew M, Enzler T, Shu CA, Rizvi NA. Combining chemotherapy with PD-1 blockade in NSCLC. Pharmacol Ther. 2018;186:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 22. | Okita R, Yukawa T, Nojima Y, Maeda A, Saisho S, Shimizu K, Nakata M. MHC class I chain-related molecule A and B expression is upregulated by cisplatin and associated with good prognosis in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2016;65:499-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Jackaman C, Majewski D, Fox SA, Nowak AK, Nelson DJ. Chemotherapy broadens the range of tumor antigens seen by cytotoxic CD8(+) T cells in vivo. Cancer Immunol Immunother. 2012;61:2343-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Huang X, Cui S, Shu Y. Cisplatin selectively downregulated the frequency and immunoinhibitory function of myeloid-derived suppressor cells in a murine B16 melanoma model. Immunol Res. 2016;64:160-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Liu WM, Fowler DW, Smith P, Dalgleish AG. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer. 2010;102:115-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 297] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 26. | Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC; KEYNOTE-189 Investigators. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:2078-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3449] [Cited by in RCA: 4811] [Article Influence: 687.3] [Reference Citation Analysis (0)] |