Published online Nov 24, 2021. doi: 10.5306/wjco.v12.i11.983
Peer-review started: March 28, 2021
First decision: June 25, 2021
Revised: July 6, 2021
Accepted: October 12, 2021
Article in press: October 12, 2021
Published online: November 24, 2021
Processing time: 235 Days and 14.2 Hours
Immunotherapy has represented one of the main medical revolutions of recent decades, and is currently a consolidated treatment for different types of tumors at different stages and scenarios, and is present in a multitude of clinical trials. One of the diseases in which it is most developed is non-small cell lung cancer. The combination of radiotherapy and immunotherapy in cancer in general and lung cancer in particular currently represents one of the main focuses of basic and clinical research in oncology, due to the synergy of this interaction, which can improve tumor response, resulting in improved survival and disease control. In this review we present the biochemical and molecular basis of the interaction between radiotherapy and immunotherapy. We also present the current clinical status of this interaction in each of the stages and cases of non-small cell lung cancer, with the main results obtained in the different studies both in terms of tumor response and survival as well as toxicity. Finally, we mention the main studies underway and the challenges of this interaction in the coming years, including how these treatments should be combined to achieve the greatest efficacy with the fewest possible side effects (dose, type of radiotherapy and drugs, sequence of treatments).
Core Tip: Immunotherapy has revolutionised cancer treatment. Its association with radiotherapy has synergistic effects studied at a preclinical and clinical level, especially in metastatic patients. Currently, clinical research in this field is very prolific, and no doubt, as with the PACIFIC trial in non-small cell lung cancer (NSCLC), we will see further changes in the standard of care in the coming years. This review highlights the most important published work in NSCLC in the field of radio-immunotherapy, listing the clinical trials currently existing in each stage of NSCLC.
- Citation: Luna J, Zafra J, Areses Manrique MC, Rodríguez A, Sotoca A, Fírvida JL, Chicas-Sett R, Mielgo X, Reyes JCT, Couñago F. New challenges in the combination of radiotherapy and immunotherapy in non-small cell lung cancer. World J Clin Oncol 2021; 12(11): 983-999
- URL: https://www.wjgnet.com/2218-4333/full/v12/i11/983.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i11.983
Immunotherapy, especially in the form of immune checkpoint inhibitors (ICI), is becoming a revolution in the understanding and treatment of cancer. Their interaction with radiotherapy (RT) generates synergistic effects that have been thoroughly described in preclinical studies. In the clinical setting, the benefits of combining RT and ICI have been evidenced mostly in metastatic patients. However, this concept has evolved since the publication of the PACIFIC trial, which has modified clinical practice in unresectable stage III non-small-cell lung cancer (NSCLC) by demonstrating a significant benefit derived from the addition of sequential durvalumab to standard definitive chemoradiotherapy (CRT). Research on the association of RT and ICI in NSCLC is currently an extraordinarily active field with numerous ongoing clinical trials.
The development of ICI has become a turning point in the standard of care (SoC) of NSCLC through a significant increase in overall survival (OS) in these patients. ICI were initially approved as second-line therapy for patients who had received combination chemotherapy (CT) including platinum following the results of a number of trials: CheckMate 017 and 057 for nivolumab, Keynote-010 for pembrolizumab and the OAK trial for atezolizumab. These studies showed increased OS in comparison to docetaxel, both in squamous and non-squamous NSCLC[1-4]. In particular, Keynote-10[3] was the first to select patients with a programmed death cell protein ligand 1 (PD-L1) expression in tumor cells ≥ 1%, showing that a higher expression of PD-L1 tends to produce better responses.
In the following years, ICI were tested as first-line treatments. Keynote-024 was the first study to evidence that those patients with advanced NSCLC and PD-L1 > 50% receiving pembrolizumab obtained an OS significantly longer than those treated with a platinum doublet. In the updated analysis, with a median follow-up of 25.2 mo (mo), the median OS was 30.0 mo with pembrolizumab and 14.2 mo with chemotherapy [Hazard ratio (HR), 0.63; 95%CI: 0.47 to 0.86][5,6].
Moreover, the addition of pembrolizumab to platinum plus paclitaxel/nab-paclitaxel vs CT alone was assessed in two trials: Keynote-189 (for non-squamous) and Keynote-407 (for squamous NSCLC). A benefit in the pembrolizumab arm was observed in both trials: Progression-free survival (PFS) was 9 mo vs 4.9 mo (HR 0.48) and 8 mo vs 5.1 mo (HR 0.57), respectively. OS was 22 mo vs 10.7 mo (HR 0.56) and 17.1 mo vs 11.6 mo (HR 0.71), respectively[7-10]. These benefits were evidenced in all subgroups and were independent of PD-L1 status.
Atezolizumab has also shown good results in the first-line setting. In the IMpower 150 trial, patients with advanced non-squamous NSCLC who received a combination of carboplatin, paclitaxel, bevacizumab and atezolizumab presented higher PFS (8.3 mo vs 6.8 mo, HR 0.62) and OS (19.8 mo vs 14.9 mo, HR 0.62) when compared to carboplatin plus paclitaxel plus bevacizumab[11]. This benefit was also independent of PD-L1 expression. Interestingly, this has been the only study at this point that included patients with epidermal growth factor receptor and anaplastic lymphoma kinase mutations that had progressed to a tyrosine kinase inhibitor, although OS was not significant in this subgroup. In squamous NSCLC, the IMpower 130 study evidenced that the combination of atezolizumab, carboplatin and nab-paclitaxel improves PFS (7 mo vs 5.5 mo, HR 0.64) and OS (13.9 mo vs 8.6 mo, HR 0.79) compared to CT alone[12].
In unresectable stage III NSCLC, ICI have also changed the SoC. The results of the PACIFIC trial have led to the approval of one year of consolidative durvalumab in patients who have not progressed after definitive CRT and have a PD-L1 ≥ 1%[13,14].
Finally, in small-cell lung cancer, atezolizumab and durvalumab in combination with a doublet of platinum and etoposide have been recently approved after showing a modest improvement in PFS and OS[15,16].
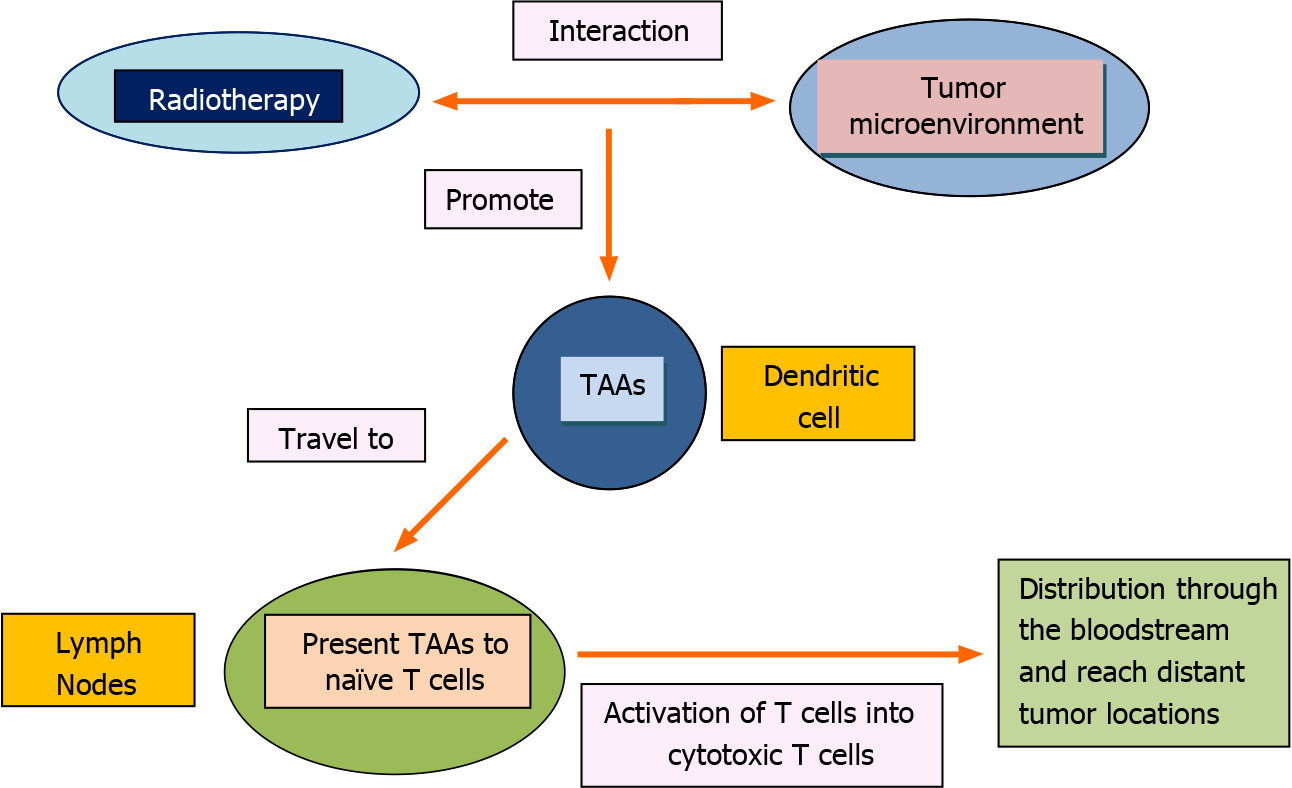
RT plays a role in all stages of NSCLC, both in the radical and palliative settings[17]. Even though RT has traditionally been considered as an exclusively local treatment, limited to the tissues involved in the radiation field, a number of cases reporting the “abscopal effect” (AE) of RT have been published since its first definition by Mole in 1953, who described it as an antitumor effect taking place outside the radiation field[18]. Recent investigations on this matter have shown that the AE might have an immunological explanation. Several preclinical data have evidenced that RT induces immunogenic cell death through the interaction of tumor-associated antigens (TAAs), damage-associated molecular patterns, high mobility group box 1, heat shock proteins, interferon type I (IFN-I), IFN-γ and other immune mediators[19]. This microenvironment favors the incorporation of TAAs by dendritic cells, which transport these to the lymph nodes in order to present them to naïve T cells through the major histocompatibility complex I. This causes the activation of T cells into cytotoxic T cells, which can then be distributed through the bloodstream and reach distant tumor locations[20,21]. This process is summarized in Figure 1.
For many years, this preclinical data has been difficult to translate into the clinical setting, as reports of the AE have been extremely rare[22]. However, since the introduction of ICI, abscopal responses have become much more frequent, with studies reporting up to 65% rates of AE in patients with metastatic NSCLC and melanoma[23]. This is due to the fact that RT and ICI seem to have a synergistic effect: while ICI “take the brakes off” the immune system by blocking inhibitory signals [such as upregulation of PD-1 and cytotoxic T-lymphocyte antigen 4 (CTLA4)-CD28 inhibition of T cell activation], RT serves as an in-situ vaccination that drives immune cells towards the tumor[24,25].
It must be noted, however, that RT can also unleash immunosupressive effects in certain conditions. A prime example is transforming growth factor beta, which participates in regulatory T cell differentiation. Studies have shown that high levels of this substance are associated with diminished antitumor responses, and its blockade is currently being investigated as a way of improving outcomes[26,27]. Although it is considered that RT generally favors a more immunostimulatory state, more data on various treatment variables that might have an influence on this balance (dose, fractionation, treatment sequence) are still required[28,29].
In the last few years, the results of translational and clinical studies have evidenced the synergistic effects obtainable through the combination of immunotherapy with radiotherapy (iRT) in NSCLC[30,31]. Most of these studies are focused on advanced disease. For instance, this combination has been a paradigm shift in the treatment of unresectable stage III NSCLC following the results of the PACIFIC trial, where an improvement in OS and PFS was observed[13,30].
The management of early-stage NSCLC is generating considerable interest in this setting of combined therapies. Although surgery and stereotactic ablative radiotherapy (SABR) have reported good local control rates (70%-92%) in stage I tumors[32], distant failures can represent up to 30%-60% of cases[33,34]. Moreover, many patients at this stage are inoperable due to comorbidities or refuse surgery. In this scenario, ICI may be an alternative to CT given their comparatively better toxicity profile[31]. The main problem, however, is the poor overall response rate (ORR) of ICI in monotherapy in NSCLC (19% for anti-PD-1 and 4.8% for anti-CTLA-4). For this reason, ICI are being combined with other treatments to achieve better results[35]. Forde et al[36] reported a pathological down-staging rate of 40% in patients receiving nivolumab prior to surgical resection (stages I-III). Furthermore, pathological response rates were approximately 10% and no grade ≥ 4 toxicities were observed.
In stage IV NSCLC, iRT in the form of SABR has already achieved an ORR of 36%-41.7% and PFS of 9 mo with a good safety profile[37]. Currently, there are several ongoing clinical trials evaluating iRT in stage I-II NSCLC (Table 1)[38-45]. Although there is still no consensus on the optimal fractionation, ICI agent and treatment sequence, most studies prescribe doses ≥ 6.5 Gy per fraction (fx). The ICI agents include nivolumab, durvalumab, atezolizumab and avelumab. Almost 50% of the registered studies are randomized and evaluate similar primary objectives, such as PFS, local control and toxicity. Among the studies that are already recruiting patients, two of them are randomized phase III studies. The NCT03833159 (PACIFIC-004) trial evaluates sequential durvalumab and SABR in patients with stage I-II NSCLC who are not candidates for surgery. Patients in this study will receive either durvalumab or placebo every four weeks for two years or until treatment discontinuation is necessary[38]. In the NCT04214262, patients will be treated with atezolizumab concurrently with SABR[39]. The exact SABR doses are not specified in either study, but a range of 1-8 fractions will be administered. Great expectations have been placed on these phase III studies, as they could set a new standard in inoperable stage I-II NSCLC.
| Ref. | Phase | n | Stage | SABR dose | ICI agent | ICI sequence | Status |
| NCT03833154[38] | III randomized | 706 | I-II | NM; 3-8 fx | Durvalumab | Sequential | Recruiting |
| NCT04214262[39] | III randomized | 460 | I-II | NM; 3-5 fx | Atezolizumab | Concurrent | Recruiting |
| NCT03110978[40] | II randomized | 140 | I-IIA | 50 Gy/4 fx; 70 Gy/10 fx | Nivolumab | Concurrent | Recruiting |
| NCT03446547[41] | II randomized | 216 | I | NM; 3-4 fx | Durvalumab | Sequential | Recruiting |
| NCT03148327[42] | I-II randomized | 105 | I-IIA | 54 Gy/3 fx; 50 Gy/4 fx; 65 Gy/10 fx | Durvalumab | Concurrent | Recruiting |
| NCT03050554[43] | I-II | 56 | I | 48 Gy/4 fx; 50 Gy/5 fx | Avelumab | Concurrent | Not recruiting |
| NCT03383302[44] | I-II | 31 | I-II | 54 Gy/3 fx; 55 Gy/5 fx | Nivolumab | Sequential | Recruiting |
| NCT02599454[45] | I | 33 | I | 50 Gy/4 fx; 50 Gy/5 fx | Atezolizumab | Induction | Not recruiting |
Stage III NSCLC represents a heterogeneous group of patients with variable prognosis. It includes both resectable tumors in which surgery is the primary curative treatment and CT and RT are administered with neoadjuvant/adjuvant intent, and unresectable tumors in which the SoC is definitive CRT. The suboptimal results of these available treatments have led to the investigation of new therapeutic approaches, such as induction/consolidation CT, RT dose escalation, vaccines or targeted therapies. However, none of these have demonstrated significant improvements over standard treatments. For this reason, recent research is evaluating if the incorporation of ICI could improve results, both as consolidation therapy after CRT or surgery, as well as definitive treatment and neoadjuvant therapy.
Even though there is currently no evidence on its clinical efficacy, the results of ongoing trials combining ICI with RT prior to surgery in resectable tumors could potentially change clinical practice in the years to come (Table 2). At the moment, there are some data on the combination of ICI with CT. For instance, Forde et al[36] reported a 45% of pathological responses in 23 patients with stage I-IIIA NSCLC receiving nivolumab monotherapy, independent of PD-L1 expression. Moreover, the recent NADIM study has observed even higher rates of major pathological response (84.6%) and complete pathological response (71%) with the combination of neoadjuvant nivolumab plus CT[46].
| Ref. | Phase | Design | No.ofpatients | Tumor stage | RT | ICI agent | Sequence | Status |
| CASE4516, NCT02987998[72] | 1 | Neoadjuvant CRT (CDDP-etoposide) + ICI followed by surgery and consolidative ICI | 20 | Resectable IIIA | 45 Gy/25 fx (1.8 Gy/fx) | Pembroli zumab | Concomitant (neoadjuvant) + adjuvant ICI | Active, not recruiting |
| NCT03237377[73] | 2 | Neoadjuvant RT-ICI followed by surgery +/-adjuvant CT | 32 | Resectable IIIA | 45 Gy/25 fx (1.8-2 Gy/fx) | Durvalumab ± tremelimumab | Concomitant(neoadjuvant) | Recruiting |
| NCT04245514, SAKK 16/18[74] | 2 3 RT arms | Neoadjuvant RT-ICI followed by surgery | 90 | Resectable IIIA | Randomized 1:1:1; A: 20 × 2 Gy; B: 5 × 5 Gy; C: 3 × 8 Gy (non -consecutive days) | Durvalumab | Concomitant (neoadjuvant) | Recruiting |
| INCREASE, NL8435[75] | 2 single arm | Neoadjuvant CRT (platinum doublet) + ICI followed by surgery | 29 | Resectable IIB-III (T3-4 N0-1) | 50 Gy/25 fx | Ipilimumab + Nivolumab | Concomitant (neoadjuvant) | Recruiting |
| NCT02904954[76] | 2 randomized | Neoadjuvant ICI +/- SBRT followed by surgery and adjuvant maintenance ICI | 60 | Resectable I-IIIA | SBRT 24 Gy/3 fx | Durvalumab | Concomitantneoadjuvant + adjuvant ICI | Active, not recruiting |
| NCT03871153[77] | 2 single arm | Neoadjuvant CRT (Carbo-taxol) + ICI followed by surgery and adjuvant ICI | 25 | Resectable IIIA N2 | 45-61.2 Gy (25-34 fx a 1.8-2 Gy/fx) | Durvalumab | Concomitant (neoadjuvant) + adjuvant ICI | Recruiting |
| CHIO3, NCT04062708[78] | 2single arm | Concomitant neoadjuvant CT (platinum doublet + ICI followed by surgery + adjuvant RT followed by ICI | 55 | Resectable IIIA-IIIB | 54 Gy | Durvalumab | Concomitant CT-ICI (neoadjuvant) + adjuvant ICI (after adjuvant RT) | Not yet recruiting |
| NCT03102242[79] | 2singlearm | Induction ICI followed by definitive CRT (Carbo-Taxol followed by consolidation CT-ICI | 63 | Unresectable IIIA-IIIB | 60 Gy/30 fx | Atezolizumab | Neoadjuvant + consolidative ICI | Active, notrecruiting |
| NCT02572843[80] | 2 | Neoadjuvant CT (platinum + docetaxel) + ICI followed by surgery +/-RT + ICI | 68 | Resectable IIIA N2 | Convenional RT if R1-R2 and before adjuvant ICI | Durvalumab | Neoadjuvant + adjuvant | Active, not recruiting |
The PACIFIC trial was the first randomized double-blinded phase III study to evaluate maintenance durvalumab for 12 mo after definitive CRT in unresectable stage III NSCLC in patients who had not progressed to CRT. A significant increase in PFS (16.8 mo vs 5.6 mo) and a manageable toxicity profile (G3-4 30.5% vs 26.1%) made this approach the new SoC[13]. Its most recent update in February 2020 reported, with a median follow-up of 33 mo, a 3-year OS of 57% vs 43.5% and a 31% reduction in mortality risk[14]. These results were independent of PD-L1 expression, CT regimen and RT dose. In Europe, durvalumab was approved in September 2018, but only in patients with PD-L1 ≥ 1% based on a post-hoc analysis. This debate on PD-L1 status will be addressed in the PACIFIC 5 trial, as the original trial was not designed with this issue in mind[47]. In contrast, the sequence of administration does seem to be relevant, as an improvement in PFS and OS was reported in those patients that started durvalumab within 14 d after CRT.
On the other hand, the LUN 14-179 study, testing consolidative pembrolizumab 4-8 wk after CRT in 93 patients, showed that ICI can also be effective in delivered with a certain delay after concomitant definitive therapy[48]. With a median follow-up of 18.6 mo, the median time to metastatic disease or death was 22.4 mo, while PFS was 17 mo, with a 2-year OS of 61.9%. In regard to toxicity, only 5.4% of patients developed G3-4 pneumonitis.
Consolidative therapy with nivolumab was also being studied in the RTOG 3505 trial (NCT02768558), a randomized phase III study that was prematurely closed after the results of the PACIFIC trial were published[49]. Among the ongoing clinical trials (Table 3), some are investigating dual ICI and the potential side effects of this combination. An interim analysis of the first 20 patients of the NCT03285321 study has reported higher G ≥ 3 toxicity rates in the nivolumab plus ipilimumab arm, but still manageable according to the authors[50].
| Ref. | Phase | Design | No.of patients | Tumor stage | RT | ICI agent | Sequence | Status |
| BTCRC-LUN16-081, NCT03285321[50] | 2 randomized | Concomitant definitive CRT followed by consolidative ICI (3 CT regimens: CDDP-VP16 vs Carbo-Taxol vs Cisplatin- Pemetrexed) | 108 | Unresectable IIIA-IIIB | 59.4-66.6 Gy | Nivolumab +/-Ipilimumab | Consolidation afterdefinitive treatment | Recruiting |
| NCT03589547[81] | 2 | CRT followed by consolidative ICI and SABR | 25 | III | 60 Gy followed by SBRT 20 Gy/2-3 fx | Durvalumab | Consolidation after definitive treatment (ICI prior to SABR) | Recruiting |
| PACIFIC 6, NCT03693300[82] | 2 | ICI after sequential CRT | 150 | Unresectable III | Conventional RT; 60 Gy/30 fx | Durvalumab | Consolidation after definitive treatment (within 28 d after RT) | Active, not recruiting |
| MK-3475, NCT03379441[83] | 2 | Maintenance ICI after definitive CRT | 126 | Unresectable IIIA-IIIB | Conventional RT | Pembrolizumab | Consolidation afterdefinitive treatment | Not recrutiing |
| DUART,NCT 04249362[84] | 2 single arm | RT followed by ICI | 150 | Unresectable III | Conventional RT 60 Gy Hypofractionated RT 40-54 Gy | Durvalumab | Consolidation after RT (no CT) | Recruiting |
| PACIFIC 5, NCT03706690[47] | 3 randomized, doube-blinded | Consolidative ICI vs placebo after definitive CRT radical (concomitant or sequential) | 360 | Unresectable III | Conventional RT | Durvalumab | Consolidation after definitive treatment | Recruiting |
Given the good results as consolidation therapy, concomitant ICI with definitive CRT is being investigated in order to further improve clinical outcomes while maintaining an adequate toxicity profile. This has been the case for nivolumab and atezolizumab in the ETOP NICOLAS and DETERRED trials, respectively.
The PACIFIC2 phase III study with durvalumab and the KEYNOTE-799 study with pembrolizumab (Table 4) put a focus on toxicity after the previous studies with nivolumab[51] and pembrolizumab[52], which offered promising outcomes but with an increased risk of pneumonitis. In the first one, the ETOP NICOLAS phase II trial[51], nivolumab is added to standard CRT both as concomitant and consolidation therapy. An interim analysis of the initial 21 patients showed a 1-year OS of 79% with no G ≥ 3 pneumonitis, which led to the recruitment of additional patients up to a total number of 80. In this case, the analysis of these 80 patients evidenced 10% G ≥ 3 pneumonitis. 1-year OS in this new cohort has not been published yet.
| Ref. | Phase | Design | No.of patients | Tumor stage | RT | ICI agent | Sequence | Status |
| ARCHON-1, NCT03801902[85] | 1; 2 RT arms | ICI + RT | 24 | Unresectable II-III | Conventional RT (60 Gy/30 fx); Hypofractionated RT (60 Gy/15 fx) | Durvalumab | Concomitant with definitive RT | Recruiting |
| PARTICLE-D, NCT03818776[86] | 1; 2 RT arms (proton beam therapy | ICI + RT | 27 | Unresectable III | RT 60 Gy/20 fx; RT 63 Gy/23 fx | Durvalumab | Concomitant with definitive RT | Recruiting |
| NCT04013542[87] | 1 | ICI + RT | 20 | II-III | Conventional RT | Ipilimumab + Nivolumab | Concomitant with definitive RT and consolidation (nivolumab) | Recruiting |
| NCT03663166[88] | 1; 2 | Concomitant CRT + ICI +/- consolidative ICI | 50 | Unresectable III | 60 Gy/30 fx | Ipilimumab +/-Nivolumab | Concomitant definitive treatment +/- consolidative ICI | Recruiting |
| SPRINT,NCT03523702[54] | 2 | ICI + RT (if PD-L1 ≥ 50%) ; CRT (if PD-L1 < 50%) | 63 | Unresectable III | Conventional RT | Pembrolizumab | Concomitant with definitive RT | Recruiting |
| KEYNOTE-799, NCT03631784[89] | 2 | Concomitant ICI + CRT (platinum doublet) followed by ICI | 216 | Unresectable III | 60 Gy/30 fx | Pembrolizumab | Concomitante and consolidative | Active, not recruiting |
| NCT04092283[90] | 3 randomized | Concomitant CRT + ICI vs CRT followed by ICI (CT: Cisplatin + VP16/ Taxol + Carboplatin/ Cisplatin + Pemetrexed) | 660 | Unresectable III | 60 Gy/30 fx | Durvalumab | Concomitant with definitive treatment vs adjuvant ICI | Recruiting |
| PACIFIC 2, NCT03519971[91] | 3 randomized, double-blinded | ICI vs placebo concomitant to CRT (CDDP-VP16 vs Carbo-Taxol vs Cisplatin or Carbo + Pemetrexed) | 328 | Unresectable III | Conventional RT (60 Gy in 30 fx) | Durvalumab | Concomitant +/- consolidative | Active, not recruiting |
| NCT04026412[92] | 3 randomized | ICI (nivolumab) + CRT followed by ICI (nivolumab + ipilimumab) vs ICI (nivolumab) + CRT followed by ICI (nivolumab) vs CRT followed by durvalumab | 1400 | Unresectable/inoperable III | Conventional RT | Nivolumab; Ipilimumab; Durvalumab | Concomitant + 2 consolidation regimens vs consolidation after CRT | Recruiting |
The phase II trial DETERRED[53] with concomitant atezolizumab has completed recruitment and reported better PFS in the ICI arm (57% vs 50%), with no significant increase in toxicity but with no benefit in OS at this point (79% in both arms).
In addition, several ongoing studies are investigating if CT can be excluded from radical treatment by combining RT and ICI. The SPRINT trial[54] is evaluating the efficacy of induction pembrolizumab in monotherapy and RT in patients with PD-L1 ≥ 50%. Moreover, the DART study[55] is testing the safety and efficacy of concomitant RT and durvalumab followed by consolidative durvalumab in patients who are not candidates for CT. Other strategies include combinations of ICI with different mechanisms, such as anti-CTLA-4 in the NCT03663166 study (Table 4).
As mentioned above, the irruption of ICI has been shifted the treatment paradigm in metastatic NSCLC by increasing survival both as first and second-line therapy[2,3,5,7,11,56,57]. Furthermore, RT in stage IV has evolved from a merely palliative intent to having a key role when associated with ICI. Reports of objective responses in distant locations not included in the radiation field (AE) have multiplied in the era of immunotherapy[31,58].
Preclinical and clinical data suggest that the antitumor efficacy of ICI increases when combined with RT, with a possible impact in survival, which could be the base for designing new combinations that can maximize this synergistic effect[58-65]. Even though most published studies on the combination of RT and ICI in stage IV NSCLC are phase I/II trials with a limited number of patients (Table 5), they have laid the foundation for the possible benefits of this strategy that are being determined in ongoing phase III studies.
| Ref. | Phase | ICI agent | RT dose | Design | Primary endpoints |
| NCT03158883[93] | I | Avelumab | 50 Gy/5 fx | ICI + SABR | ORR |
| NCT03224871[94] | I | Nivolumab; Pembrolizumab; Intratumor IL-2 | 8 Gy/3 fx | ICI + IL-2 + RT | MTD |
| NCT03436056, PRIMING[95] | I | Pembrolizumab | SABR 30 Gy-3 fx SABR 54 Gy/3 fx | ICI + SABR | MTD |
| NCT03812549[96] | I | Sintilimab | SABR 30 Gy/3 fx; LD (low dose)-RT: 2 Gy/1 fx or 4 Gy/2 fx or 10 Gy/5 fx | ICI + SABR; ICI + LD-RT | MTD |
| NCT03223155, COSINR[69] | I | Nivolumab; Ipilimumab | SABR 3-5 fx, 2-4 sites | ICI + SABR | MTD |
| NCT02639026[97] | I | Durvalumab; Tremelimumab | HFRT 24 Gy/3 fx, 17 Gy/1 fx | ICI + HFRT | MTD |
| NCT03275597[98] | I | Durvalumab; Tremelimumab | SABR 30-50 Gy/5 fx | ICI + SABR | MTD |
| NCT03168464[99] | I-II | Nivolumab; Ipilimumab | RT 30 Gy/5 fx | ICI + RT | ORR |
| NCT02239900[100] | I-IIR | Ipilimumab | SABR 50 Gy/4 fx or 60 Gy/10 fx; 1-4 lesions | ICI + SABR | MTD |
| NCT02444741[101] | I-IIR | Pembrolizumab | SABR 4 fx or IMRT, PBRT, 3D-CRT 15 fx | ICI + SABR or IMRT, PBRT, 3D-CRT | MTD, ORR |
| NCT03176173, RRADICAL[102] | II | Nivolumab; Pembrolizumab; Atezolizumab | SABR 1-10 fx | ICI +/- SABR | PFS |
| NCT03965468, CHESS[103] | II | Durvalumab | SABR 1-10 fx | ICI + SABR + CT | PFS |
| NCT03044626, FORCE[104] | II | Nivolumab | RT 20 Gy/5 fx | ICI + RT | ORR |
| NCT02221739[105] | II | Ipilimumab | IMRT or 3D-CRT 30 Gy/5 fx | ICI + RT | ORR |
| NCT02658097[106] | II | Pembrolizumab | RT 8 Gy/1 fx | ICI + RT | ORR |
| NCT03391869, LONESTAR[107] | III | Nivolumab; Ipilimumab | LCT | ICI +/- SABR | OS |
| NCT03867175[108] | III | Pembrolizumab | SABR 3-10 fx | ICI +/- SABR | PFS |
| NCT03774732, NIRVANA-LUNG[109] | III | Pembrolizumab | SABR or 3D-CRT 18 Gy/3 fx | ICI + RT + CT | OS |
Although retrospective data had been the only evidence available for years, phase I studies evaluating the safety of the combination recently started to surface. For instance, Tang et al[66] treated 21 patients with pembrolizumab and RT (SABR or hypofractionated RT) and reported an ORR of 32% while maintaining a good toxicity profile (14% G ≥ 3).
The safety of anti-CTLA-4 agents has also been addressed. Formentiet al[67] designed a phase I/II study with 39 patients treated with ipilimumab plus SABR (28.5 Gy in 3 fx or 30 Gy in 5 fx). ORR was 31%, PFS was 7.1 mo and OS was 13 mo, with only 10.3% G ≥ 3 toxicity[67]. The safety of multisite irradiation was evaluated in the oligometastatic setting by Bauml et al[68], who included 45 patients with oligometastatic NSCLC and delivered local ablative therapy (surgery or SABR) followed by sequential pembrolizumab, showing promising results in terms of PFS (19.1 mo) and OS (41.6 mo), with low toxicity rates.
With the safety of the combination established and the promising survival data reported in these initial studies, randomized evidence on the combination of ICI and RT (mainly in the form of SABR, also known as I-SABR) is finally starting to emerge. At the moment, three randomized trials have published their results. Not only are these reinforcing the idea that a benefit in survival exists, but they are also starting to contemplate some questions regarding the optimal treatment delivery. For instance, the COSINR study by Patel et al[69] is a phase I trial that randomized 35 patients to receive dual ICI (ipilimumab plus nivolumab) and either concurrent or sequential SABR. Global ORR was 68%, while PFS was 6.2 mo in the concomitant arm and 5.9 mo in the sequential arm. Welsh et al[70] recently reported the results of a phase II study that randomized 72 patients to receive RT plus pembrolizumab vs pembrolizumab monotherapy. In the experimental arm, patients received either SABR (50 Gy in 4 fx or 70 Gy in 10 fx) or conventional RT (45 Gy in 15 fx). Globally, there were no significant differences in response or PFS between the combination arm and the pembrolizumab arm, with an ORR of 22% vs 25 and PFS of 9.1 vs 5.1 (P = 1.00). However, in the subanalysis of patients treated in the combination arm, ORR was higher in the SABR group than in the conventional RT group (38% vs 10%), as well as PFS (20.8 mo vs 6.8 mo, P = 0.03)[70]. Finally, the PEMBRO-RT phase II study included 76 patients and randomized them in two arms: sequential pembrolizumab after SABR to a single lesion (24 Gy in 3 fx) vs pembrolizumab monotherapy. ORR was 36% and 18%, respectively. Furthermore, PFS favored the I-SABR arm (6.6 mo vs 1.9 mo), as well as OS (15.6 mo vs 7.6 mo), even though these were not statistically significant[71].
At present, a great number of clinical trials combining RT and ICI in stage IV NSCLC are ongoing (Table 5). These include multiple ICI agents (atezolizumab, avelumab, nivolumab, pembrolizumab, sintilimab, tremelimumab), different combinations, various treatment sequences (induction, sequential or concomitant) and several fractionations and RT techniques such as conventional RT, hypofractionation, SABR, intensity-modulated RT and proton beam RT.
Radio-immunotherapy represents a dynamic area of preclinical and clinical investigation in lung cancer. The synergy between RT and ICI to achieve a greater tumor response has been shown to be a promising option for the treatment of NSCLC. The positive experiences reported with the combination of RT and ICI in early stage, unresectable stage III and stage IV NSCLC have reinforced the interest in the association of these two treatments. In the years to come, the results of ongoing clinical trials will continue to evolve clinical practice in NSCLC.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barros JM, Chen LJ, Gebbia V, Nath J, Pruthi DS S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5686] [Cited by in RCA: 6739] [Article Influence: 673.9] [Reference Citation Analysis (0)] |
| 2. | Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6945] [Cited by in RCA: 7536] [Article Influence: 753.6] [Reference Citation Analysis (0)] |
| 3. | Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4116] [Cited by in RCA: 5052] [Article Influence: 561.3] [Reference Citation Analysis (0)] |
| 4. | Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR; OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2868] [Cited by in RCA: 3704] [Article Influence: 463.0] [Reference Citation Analysis (0)] |
| 5. | Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR; KEYNOTE-024 Investigators. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5948] [Cited by in RCA: 7522] [Article Influence: 835.8] [Reference Citation Analysis (0)] |
| 6. | Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Vandormael K, Riccio A, Yang J, Pietanza MC, Brahmer JR. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol. 2019;37:537-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 1147] [Article Influence: 191.2] [Reference Citation Analysis (0)] |
| 7. | Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC; KEYNOTE-189 Investigators. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:2078-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3449] [Cited by in RCA: 4803] [Article Influence: 686.1] [Reference Citation Analysis (0)] |
| 8. | Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, Hui R, Hochmair MJ, Clingan P, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Garon EB, Novello S, Rubio-Viqueira B, Boyer M, Kurata T, Gray JE, Yang J, Bas T, Pietanza MC, Garassino MC. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol. 2020;38:1505-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 410] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 9. | Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, ÇayŞenler F, Csőszi T, Fülöp A, Rodríguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM; KEYNOTE-407 Investigators. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med. 2018;379:2040-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1941] [Cited by in RCA: 2667] [Article Influence: 381.0] [Reference Citation Analysis (0)] |
| 10. | Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazières J, Hermes B, Cicin I, Medgyasszay B, Rodríguez-Cid J, Okamoto I, Lee S, Ramlau R, Vladimirov V, Cheng Y, Deng X, Zhang Y, Bas T, Piperdi B, Halmos B. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J Thorac Oncol. 2020;15:1657-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 416] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 11. | Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M; IMpower150 Study Group. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. 2018;378:2288-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2076] [Cited by in RCA: 2770] [Article Influence: 395.7] [Reference Citation Analysis (0)] |
| 12. | West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, Zer A, Reinmuth N, Sadiq A, Sandler A, Lin W, Ochi Lohmann T, Archer V, Wang L, Kowanetz M, Cappuzzo F. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 1192] [Article Influence: 198.7] [Reference Citation Analysis (0)] |
| 13. | Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M; PACIFIC Investigators. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2750] [Cited by in RCA: 3240] [Article Influence: 405.0] [Reference Citation Analysis (0)] |
| 14. | Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, Cho BC, Planchard D, Paz-Ares L, Faivre-Finn C, Vansteenkiste JF, Spigel DR, Wadsworth C, Taboada M, Dennis PA, Özgüroğlu M, Antonia SJ. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. J Thorac Oncol. 2020;15:288-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 313] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 15. | Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M, Reck M, Mok T, Lam S, Shames DS, Liu J, Ding B, Lopez-Chavez A, Kabbinavar F, Lin W, Sandler A, Liu SV; IMpower133 Study Group. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. 2018;379:2220-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 2373] [Article Influence: 339.0] [Reference Citation Analysis (0)] |
| 16. | Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH, Voitko O, Poltoratskiy A, Ponce S, Verderame F, Havel L, Bondarenko I, Kazarnowicz A, Losonczy G, Conev NV, Armstrong J, Byrne N, Shire N, Jiang H, Goldman JW; CASPIAN investigators. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 1429] [Article Influence: 238.2] [Reference Citation Analysis (0)] |
| 17. | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Non-Small Cell Lung Cancer Version 2. 2021. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. |
| 18. | Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 810] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 19. | Grass GD, Krishna N, Kim S. The immune mechanisms of abscopal effect in radiation therapy. Curr Probl Cancer. 2016;40:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 20. | Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, Deweese TL, Drake CG. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res. 2015;3:345-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 536] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 21. | Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, Schwendener R, Pruschy M, Knuth A, van den Broek M. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189:558-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 352] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 22. | Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer. 2016;40:25-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 388] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 23. | Chicas Sett R, Zafra Martin J, Castilla Martinez JF, Morales I, Rodriguez D, Benitez G, Lloret M, Lara PC. Immuno-SABR Reboots the Immune Response in Patients with Metastatic NSCLC and Melanoma in Progression to Anti-PD-1 Therapy. Int J Radiat Oncol Biol Phys. 2020;108:S73-S74. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728-734. [PubMed] |
| 25. | Vatner RE, Cooper BT, Vanpouille-Box C, Demaria S, Formenti SC. Combinations of immunotherapy and radiation in cancer therapy. Front Oncol. 2014;4:325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 26. | Formenti SC, Lee P, Adams S, Goldberg JD, Li X, Xie MW, Ratikan JA, Felix C, Hwang L, Faull KF, Sayre JW, Hurvitz S, Glaspy JA, Comin-Anduix B, Demaria S, Schaue D, McBride WH. Focal Irradiation and Systemic TGFβ Blockade in Metastatic Breast Cancer. Clin Cancer Res. 2018;24:2493-2504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 206] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 27. | Rodríguez-Ruiz ME, Rodríguez I, Mayorga L, Labiano T, Barbes B, Etxeberria I, Ponz-Sarvise M, Azpilikueta A, Bolaños E, Sanmamed MF, Berraondo P, Calvo FA, Barcelos-Hoff MH, Perez-Gracia JL, Melero I. TGFβ Blockade Enhances Radiotherapy Abscopal Efficacy Effects in Combination with Anti-PD1 and Anti-CD137 Immunostimulatory Monoclonal Antibodies. Mol Cancer Ther. 2019;18:621-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 28. | Demaria S, Golden EB, Formenti SC. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol. 2015;1:1325-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 554] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 29. | Brooks ED, Chang JY. Time to abandon single-site irradiation for inducing abscopal effects. Nat Rev Clin Oncol. 2019;16:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 231] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 30. | Lin AJ, Roach M, Bradley J, Robinson C. Combining stereotactic body radiation therapy with immunotherapy: current data and future directions. Transl Lung Cancer Res. 2019;8:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Chicas-Sett R, Zafra-Martin J, Morales-Orue I, Castilla-Martinez J, Berenguer-Frances MA, Gonzalez-Rodriguez E, Rodriguez-Abreu D, Couñago F. Immunoradiotherapy as An Effective Therapeutic Strategy in Lung Cancer: From Palliative Care to Curative Intent. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, Karasawa K, Hayakawa K, Niibe Y, Takai Y, Kimura T, Takeda A, Ouchi A, Hareyama M, Kokubo M, Kozuka T, Arimoto T, Hara R, Itami J, Araki T. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys. 2011;81:1352-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 455] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 33. | Chi A, Liao Z, Nguyen NP, Xu J, Stea B, Komaki R. Systemic review of the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: clinical implications. Radiother Oncol. 2010;94:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 272] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 34. | Senthi S, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol. 2012;13:802-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 354] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 35. | Zatloukal P, Heo DS, Park K, Kang J, Butts C, Bradford S Graziano D, Huang B, Healey D. Ramdomized phase II clinical trial comparing tremelimumab (CP-675, 206) with best supportive care (BSC) following first-line platinum-based therapy in patients (PTS) with advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2009;27 Suppl 15:8071. [RCA] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, Zahurak M, Yang SC, Jones DR, Broderick S, Battafarano RJ, Velez MJ, Rekhtman N, Olah Z, Naidoo J, Marrone KA, Verde F, Guo H, Zhang J, Caushi JX, Chan HY, Sidhom JW, Scharpf RB, White J, Gabrielson E, Wang H, Rosner GL, Rusch V, Wolchok JD, Merghoub T, Taube JM, Velculescu VE, Topalian SL, Brahmer JR, Pardoll DM. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med. 2018;378:1976-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 1477] [Article Influence: 211.0] [Reference Citation Analysis (0)] |
| 37. | Theelen WSME, Chen D, Verma V, Hobbs BP, Peulen HMU, Aerts JGJV, Bahce I, Niemeijer ALN, Chang JY, de Groot PM, Nguyen QN, Comeaux NI, Simon GR, Skoulidis F, Lin SH, He K, Patel R, Heymach J, Baas P, Welsh JW. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. 2021;9:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 347] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 38. | AstraZeneca. Durvalumab vs placebo with stereotactic body radiation therapy in early stage unresected non-small cell lung cancer patients (PACIFIC-4). [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/record/NCT03833154 ClinicalTrials.gov Identifier: NCT03833154. |
| 39. | Daly ME. Testing the addition of the drug atezolizumab to the useual radiation treatment for patients with early non-small cell lung cancer. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/record/NCT04214262 ClinicalTrials.gov Identifier: NCT04214262. |
| 40. | Chang JY. Stereotactic body radiation therapy with or without nivolumab in treating patients with stage I-IIA or recurrent non-small cell lung cancer. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/record/NCT03110978ClinicalTrials.gov Identifier: NCT03110978. |
| 41. | Hallqvist A. Ablative stereotactic radiotherapy with durvalumab (MEDI4736) (ASTEROID). [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03446547 ClinicalTrials.gov Identifier: NCT03446547. |
| 42. | Lee P. Astra Zeneca (Immuno stereotactic ablative body radiotherapy) ISABR study: Randomized phase I/II study of stereotactic body radiotherapy. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03148327 ClinicalTrials.gov Identifier: NCT03148327. |
| 43. | Sharabi A. Stereotactic body radiation therapy (SBRT) combined with avelumab (anti-PD-L1) for management of early stage non-small cell lung cancer (NSCLC). [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03050554ClinicalTrials.gov Identifier: NCT03050554. |
| 44. | Ahmed M. SBRT with immunotherapy in early stage non-small cell lung cancer: tolerability and lung effects (STILE). [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03383302 ClinicalTrials.gov Identifier: NCT03383302. |
| 45. | Kelly K. Atezolizumab and stereotactic body radiation therapy in treating patients with non-small cell lung cancer. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02599454 ClinicalTrials.gov Identifier: NCT02599454. |
| 46. | Provencio-Pulla M, Nadal-Alforja E, Cobo M, Insa A, Rivas MC, Majem M, Rodriguez-Abreu D, Lopez-Vivanco G, Domine M, Del Barco Morillo E, Massuti B, Campelo RG, Marti AM, Bernabé R, Franco F, Jove M, Arrabal R, Martin P, Casal J, Calvo V. Neoadjuvant chemo/immunotherapy for the treatment of stages IIIA resectable non-small cell lung cancer (NSCLC): A phase II multicenter exploratory study—NADIM study-SLCG. J Clin Oncol. 2018;36 Suppl 15:8521. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 47. | Wu Y, Wang L, Sendur MAN, Kim Y, Zhu Z, Cheng Y, Li P, Qin Y, Macpherson E, Dennis PA, Lu S. 339TiP - PACIFIC-5: phase 3 study of durvalumab after either concurrent or sequential chemoradiotherapy (CRT) in patients with stage III NSCLC. Ann Oncol. 2019;30:339. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 48. | Durm GA, Jabbour SK, Althouse SK, Liu Z, Sadiq AA, Zon RT, Jalal SI, Kloecker GH, Williamson MJ, Reckamp KL, Langdon RM, Kio EA, Gentzler RD, Adesunloye BA, Harb WA, Walling RV, Titzer ML, Hanna NH. A phase 2 trial of consolidation pembrolizumab following concurrent chemoradiation for patients with unresectable stage III non-small cell lung cancer: Hoosier Cancer Research Network LUN 14-179. Cancer. 2020;126:4353-4361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 49. | Gerber DE, Urbanic JJ, Langer C, Hu C, Chang IF, Lu B, Movsas B, Jeraj R, Curran WJ, Bradley JD. Treatment Design and Rationale for a Randomized Trial of Cisplatin and Etoposide Plus Thoracic Radiotherapy Followed by Nivolumab or Placebo for Locally Advanced Non-Small-Cell Lung Cancer (RTOG 3505). Clin Lung Cancer. 2017;18:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 50. | Yan M, Durm GA, Mamdani H, GantiAK, Hrinczenko B, Jabbour SK, Feldman LE, Kloecker GH, Leal T, Almokadem S, Naidoo J, Fujioka N, Hanna NH. Interim safety analysis of consolidation nivolumab and ipilimumab vs nivolumab alone following concurrent chemoradiation for unresectable stage IIIA/IIIB NSCLC: Big Ten Cancer Research Consortium LUN 16-081. J Clin Oncol. 2019;37 Suppl 15:8535-8535. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Peters S, Felip E, Dafni U, Belka C, Guckenberger M, Irigoyen A, Nadal E, Becker A, Vees H, Pless M, Martinez-Marti A, Tufman A, Lambrecht M, Andratschke N, Piguet AC, Kassapian M, Roschitzki-Voser H, Rabaglio-Poretti M, Stahel RA, Vansteenkiste J, De Ruysscher D. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer-The ETOP NICOLAS trial. Lung Cancer. 2019;133:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 52. | Jabbour SK, Berman AT, Decker RH, Lin Y, Feigenberg SJ, Gettinger SN, Aggarwal C, Langer CJ, Simone CB 2nd, Bradley JD, Aisner J, Malhotra J. Phase 1 Trial of Pembrolizumab Administered Concurrently With Chemoradiotherapy for Locally Advanced Non-Small Cell Lung Cancer: A Nonrandomized Controlled Trial. JAMA Oncol. 2020;6:848-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 53. | Lin SH, Lin Y, Yao L, Kalhor N, Carter BW, Altan M, Blumenschein G, Byers LA, Fossella F, Gibbons DL, Kurie JM, Lu C, Simon G, Skoulidis F, Chang JY, Jeter MD, Liao Z, Gomez DR, O'Reilly M, Papadimitrakopoulou V, Thall P, Heymach JV, Tsao AS. Phase II Trial of Concurrent Atezolizumab With Chemoradiation for Unresectable NSCLC. J Thorac Oncol. 2020;15:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 54. | Ohri N, Cheng H, Jolly S, Gadgeel SM, Cooper BT, Shum E, Halmos B. The selective personalized radioimmunotherapy for locally advanced NSCLC trial (SPRINT). J Clin Oncol. 2019;37 Suppl 15:TPS8571. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Patel SP, Othus M, Chae YK, Giles FJ, Hansel DE, Singh PP, Fontaine A, Shah MH, Kasi A, Baghdadi TA, Matrana M, Gatalica Z, Korn WM, Hayward J, McLeod C, Chen HX, Sharon E, Mayerson E, Ryan CW, Plets M, Blanke CD, Kurzrock R. A Phase II Basket Trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin Cancer Res. 2020;26:2290-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 236] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 56. | Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, Peters S; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29 Suppl 4:iv192-iv237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1392] [Cited by in RCA: 1607] [Article Influence: 229.6] [Reference Citation Analysis (0)] |
| 57. | Mazieres J, Rittmeyer A, Gadgeel S, Hida T, Gandara DR, Cortinovis DL, Barlesi F, Yu W, Matheny C, Ballinger M, Park K. Atezolizumab Versus Docetaxel in Pretreated Patients With NSCLC: Final Results From the Randomized Phase 2 POPLAR and Phase 3 OAK Clinical Trials. J Thorac Oncol. 2021;16:140-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 58. | Ko EC, Raben D, Formenti SC. The Integration of Radiotherapy with Immunotherapy for the Treatment of Non-Small Cell Lung Cancer. Clin Cancer Res. 2018;24:5792-5806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 199] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 59. | Ng J, Dai T. Radiation therapy and the abscopal effect: a concept comes of age. Ann Transl Med. 2016;4:118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 60. | Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 867] [Cited by in RCA: 1050] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 61. | Foster CC, Sher DJ, Rusthoven CG, Verma V, Spiotto MT, Weichselbaum RR, Koshy M. Overall survival according to immunotherapy and radiation treatment for metastatic non-small-cell lung cancer: a National Cancer Database analysis. Radiat Oncol. 2019;14:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 62. | Yang H, Jin T, Li M, Xue J, Lu B. Synergistic effect of immunotherapy and radiotherapy in non-small cell lung cancer: current clinical trials and prospective challenges. Precision Clin Med. 2019;2:57-70. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 63. | Mondini M, Levy A, Meziani L, Milliat F, Deutsch E. Radiotherapy-immunotherapy combinations - perspectives and challenges. Mol Oncol. 2020;14:1529-1537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 64. | Low JL, Walsh RJ, Ang Y, Chan G, Soo RA. The evolving immuno-oncology landscape in advanced lung cancer: first-line treatment of non-small cell lung cancer. Ther Adv Med Oncol. 2019;11:1758835919870360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 65. | Spaas M, Lievens Y. Is the Combination of Immunotherapy and Radiotherapy in Non-small Cell Lung Cancer a Feasible and Effective Approach? Front Med (Lausanne). 2019;6:244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 66. | Tang C, de Groot P, Hess K, Shabaan S, Gomez DR, Chang JY, Lin SH, Paraskevopoulos T, Raju U, Simon GR, Papadimitrakopoulou V, Liao Z, Fossella FV, Glisson BS, Komaki RU, Hahn SM, Heymach J, Welsh JW. Phase I study of pembrolizumab and stereotactic or hypofractionated radiation for metastatic non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;99:160. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 67. | Formenti SC, Rudqvist NP, Golden E, Cooper B, Wennerberg E, Lhuillier C, Vanpouille-Box C, Friedman K, Ferrari de Andrade L, Wucherpfennig KW, Heguy A, Imai N, Gnjatic S, Emerson RO, Zhou XK, Zhang T, Chachoua A, Demaria S. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24:1845-1851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 702] [Article Influence: 100.3] [Reference Citation Analysis (0)] |
| 68. | Bauml JM, Mick R, Ciunci C, Aggarwal C, Davis C, Evans T, Deshpande C, Miller L, Patel P, Alley E, Knepley C, Mutale F, Cohen RB, Langer CJ. Pembrolizumab After Completion of Locally Ablative Therapy for Oligometastatic Non-Small Cell Lung Cancer: A Phase 2 Trial. JAMA Oncol. 2019;5:1283-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 232] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 69. | Patel JD, Bestvina CM, Karrison T, Jelinek MJ, Juloori A, Pointer K, Hoffman PC, Pitroda PS, Vokes EE, Chmura SJ. Randomized phase I trial to evaluate concurrent or sequential ipilimumab, nivolumab and stereotactic body radiotherapy in patients with stage IV non-small cell lung cancer (COSINR study). J Clin Oncol. 2020;38 Suppl 15:9616. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 70. | Welsh J, Menon H, Chen D, Verma V, Tang C, Altan M, Hess K, de Groot P, Nguyen QN, Varghese R, Comeaux NI, Simon G, Skoulidis F, Chang JY, Papdimitrakopoulou V, Lin SH, Heymach JV. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: a randomized phase I/II trial. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 175] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 71. | Theelen WSME, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts JGJV, Dumoulin DW, Bahce I, Niemeijer AN, de Langen AJ, Monkhorst K, Baas P. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5:1276-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 697] [Article Influence: 116.2] [Reference Citation Analysis (0)] |
| 72. | Pennell N. Neoadjuvant Chemoradiation Plus Pembrolizumab Followed By Consolidation Pembrolizumab in NSCLC. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02987998 ClinicalTrials.gov Identifier: NCT02987998. |
| 73. | Forde P. Neoadjuvant Immunoradiation for Resectable Non-Small Cell Lung Cancer. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03237377 ClinicalTrials.gov Identifier: NCT03237377. |
| 74. | Rothschild S. Multimodality Treatment in Stage III Non-small Cell Lung Cancer (NSCLC). [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04245514 ClinicalTrials.gov Identifier: NCT04245514. |
| 75. | Dickhoff C, Senan S, Schneiders FL, Veltman J, Hashemi S, Daniels JMA, Fransen M, Heineman DJ, Radonic T, van de Ven PM, Bartelink IH, Meijboom LJ, Garcia-Vallejo JJ, Oprea-Lager DE, de Gruijl TD, Bahce I. Ipilimumab plus nivolumab and chemoradiotherapy followed by surgery in patients with resectable and borderline resectable T3-4N0-1 non-small cell lung cancer: the INCREASE trial. BMC Cancer. 2020;20:764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 76. | Altorki NK. Durvalumab (MEDI4736) With or Without SBRT in Clinical Stage I, II and IIIA Non-small Cell Lung Cancer. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02904954 ClinicalTrials.gov Identifier: NCT02904954. |
| 77. | Durm G. Chemoradiation Plus Durvalumab Followed by Surgery Followed by Adjuvant Durvalumab in Patients With Surgically Resectable Stage III (N2) Non-Small Cell Lung Cancer. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03871153 ClinicalTrials.gov Identifier: NCT03871153. |
| 78. | Bertagnolli M. CHIO3 Trial: CHemotherapy Combined With Immune Checkpoint Inhibitor for Operable Stage IIIA/B Non-Small Cell Lung Cancer. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04062708 ClinicalTrials.gov Identifier: NCT04062708. |
| 79. | Ross H. Atezolizumab Immunotherapy in Patients With Advanced NSCLC. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03102242 ClinicalTrials.gov Identifier: NCT03102242. |
| 80. | Rothschild S. Anti-PD-L1 in Stage IIIA(N2) Non-small Cell Lung Cancer (NSCLC). [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02572843 ClinicalTrials.gov Identifier: NCT02572843. |
| 81. | Khan H. Durvalumab and Consolidation SBRT Following Chemoradiation for Locally Advanced Stage III Non-Small Cell Lung (358). [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03589547 ClinicalTrials.gov Identifier: NCT03589547. |
| 82. | AstraZeneca. A Study to Determine Safety of Durvalumab After Sequential Chemo Radiation in Patients With Unresectable Stage III Non-Small Cell Lung Cancer. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03693300 ClinicalTrials.gov Identifier: NCT03693300. |
| 83. | Novello S. Pembrolizumab (MK-3475) as Maintainance in Treated Patients With Unresectable Stage III NSCLC (MP-LALC). [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03379441 ClinicalTrials.gov Identifier: NCT03379441. |
| 84. | Filippi AR. Study of Durvalumab Following Radiation Therapy in Patients With Stage 3 Unresectable NSCLC Ineligible for Chemotherapy (DUART). [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04249362 ClinicalTrials.gov Identifier: NCT04249362. |
| 85. | Lin SH. Accelerated Hypofractionated or Conventionally Fractionated Radiotherapy and Durvalumab in Treating Patients With Stage II-III Non-small Cell Lung Cancer. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03801902 ClinicalTrials.gov Identifier: NCT03801902. |
| 86. | Biswas T. Proton Based Cardiac Sparing Accelerated Fractionated RadioTherapy in Unresectable NSCLC. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03818776 ClinicalTrials.gov Identifier: NCT03818776. |
| 87. | Tsao AS. Ipilimumab and Nivolumab in Combination With Radiation Therapy in Treating Patients With Stage 2-3 Non-small Lung Cancer. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04013542 ClinicalTrials.gov Identifier: NCT04013542. |
| 88. | Perez B. Radiation and Chemotherapy With Ipilimumab Followed by Nivolumab for Patients With Stage III Unresectable Non-Small Cell Lung Cancer (NSCLC). [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03663166 ClinicalTrials.gov Identifier: NCT03663166. |
| 89. | Merck Sharp and Dohme Corp. A Trial of Pembrolizumab in Combination With Chemotherapy and Radiotherapy in Stage III NSCLC (KEYNOTE-799, MK-3475-799). (KEYNOTE-799). [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03631784 ClinicalTrials.gov Identifier: NCT03631784. |
| 90. | Pennell NA. Testing the Addition of an Antibody to Standard Chemoradiation Followed by the Antibody for One Year to Standard Chemoradiation Followed by One Year of the Antibody in Patients With Unresectable Stage III Non-Small Cell Lung Cancer. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04092283 ClinicalTrials.gov Identifier: NCT04092283. |
| 91. | Bradley J. Study of Durvalumab Given With Chemoradiation Therapy in Patients With Unresectable Non-small Cell Lung Cancer. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03519971 ClinicalTrials.gov Identifier: NCT03519971. |
| 92. | Bristol-Myers Squibb. A Study of Nivolumab and Ipilimumab in Untreated Patients With Stage 3 Non-small Cell Lung Cancer (NSCLC) That is Unable or Not Planned to be Removed by Surgery (CheckMate73L). [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04026412 ClinicalTrials.gov Identifier: NCT04026412. |
| 93. | Daly M. UCDCC#270: Avelumab and Stereotactic Ablative Radiotherapy in Non-responding and Progressing NSCLC Patients. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03158883 ClinicalTrials.gov Identifier: NCT03158883. |
| 94. | Monjazeb A. UCDCC#269: A Pilot Study of Interlesional IL-2 and RT in Patients With NSCLC. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03224871 ClinicalTrials.gov Identifier: NCT03224871. |
| 95. | Royal Marsden NHS Foundation Trust. PembRolIzuMab and Stereotactic Body Radiotherapy In Metastatic Non-small-cell lunG Cancer Patients (PRIMING). [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03436056 ClinicalTrials.gov Identifier: NCT03436056. |
| 96. | Lu Y. Safety and Tolerability Evaluation of Sintilimab in Combination With Radiation in Stage IV NSCLC Patients. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03812549 ClinicalTrials.gov Identifier: NCT03812549. |
| 97. | O'Hara MH. Trial Of Hypofractionated Radiotherapy In Combination With MEDI4736 And Tremelimumab For Patients With Metastatic Melanoma And Lung, Breast And Pancreatic Cancers. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02639026 ClinicalTrials.gov Identifier: NCT02639026. |
| 98. | Bassetti M. Phase Ib Study of Stereotactic Body Radiotherapy (SBRT) in Oligometastatic Non-small Lung Cancer (NSCLC) With Dual Immune Checkpoint Inhibition. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03275597 ClinicalTrials.gov Identifier: NCT03275597. |
| 99. | Formenti S. Radiation and Immune Checkpoints Blockade in Metastatic NSCLC (BMS # CA209-632). [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03168464 ClinicalTrials.gov Identifier: NCT03168464. |
| 100. | Welsh J. Ipilimumab and Stereotactic Body Radiation Therapy (SBRT) in Advanced Solid Tumors. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02239900 ClinicalTrials.gov Identifier: NCT02239900. |
| 101. | Welsh J. Pembrolizumab and Stereotactic Body Radiation Therapy or Non-Stereotactic Wide-Field Radiation Therapy in Treating Patients With Non-small Cell Lung Cancer. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02444741 ClinicalTrials.gov Identifier: NCT02444741. |
| 102. | Gensheimer M. Radical-Dose Image Guided Radiation Therapy in Treating Patients With Metastatic Non-small Cell Lung Cancer Undergoing Immunotherapy. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03176173 ClinicalTrials.gov Identifier: NCT03176173. |
| 103. | Guckenberger M, Schmitt-Opitz I. Immunotherapy, Chemotherapy, Radiotherapy and Surgery for Synchronous Oligo-metastatic NSCLC (CHESS). [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03965468 ClinicalTrials.gov Identifier: NCT03965468. |
| 104. | Bozorgmehr F. Fostering Efficacy of Anti - PD-1 - Treatment: Nivolumab Plus Radiotherapy in Advanced NSCLC (FORCE). [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03044626 ClinicalTrials.gov Identifier: NCT03044626. |
| 105. | Chachoua A. Study of Combined Ionizing Radiation and Ipilimumab in Metastatic Non-small Cell Lung Cancer (NSCLC). [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02221739 ClinicalTrials.gov Identifier: NCT02221739. |
| 106. | Pennell N. Pembrolizumab Alone or Sequentially Following Single Fraction Non-ablative Radiation to One of the Target Lesions, in Previously Treated Patients With Stage IV NSCLC. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02658097 ClinicalTrials.gov Identifier: NCT02658097. |
| 107. | Heymach J. Nivolumab and Ipilimumab With or Without Local Consolidation Therapy in Treating Patients With Stage IV Non-Small Cell Lung Cancer. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03391869 ClinicalTrials.gov Identifier: NCT03391869. |
| 108. | Farris M. Immunotherapy With or Without SBRT in Patients With Stage IV Non-small Cell Lung Cancer. [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03867175 ClinicalTrials.gov Identifier: NCT03867175. |
| 109. | Doyen J, Levy A, Besse B. PD-1 Inhibitors and Chemotherapy With Concurrent Irradiation at Varied Tumour Sites in Advanced Non-small Cell Lung Cancer (NIRVANA-LUNG). [accessed 2021 March 28]. In: ClinicalTrial.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03774732 ClinicalTrials.gov Identifier: NCT03774732. |