Published online Nov 24, 2021. doi: 10.5306/wjco.v12.i11.1064
Peer-review started: February 23, 2021
First decision: May 7, 2021
Revised: May 18, 2021
Accepted: September 30, 2021
Article in press: September 30, 2021
Published online: November 24, 2021
Processing time: 268 Days and 21.7 Hours
An increasing number of studies report the beneficial effects of regional hyperthermia in association with chemotherapy (CHT) and radiotherapy for the treatment of pancreatic cancer; in particular, the use of modulated electro-hyperthermia (mEHT) results in increased survival and tumor response.
To compare outcomes of CHT alone or in association with mEHT for the treatment of stage III and IV pancreatic cancer.
This was an observational retrospective study; data were collected for patients with stage III-IV pancreatic cancer that were treated with CHT alone or in combination with mEHT from 2003 to 2019. A total of 158 patients were included in the study out 270 patients screened in four Italian hospitals; 58 (37%) of these received CHT + mEHT and 100 (63%) CHT. CHT was mainly gemcitabine-based regimens in both groups.
Overall (19.5 mo vs 11.02 mo, P < 0.001) and progression-free (12 mo vs 3 mo, P < 0.001) survival were better for the CHT + mEHT group compared to the CHT group. The association of mEHT resulted also in an improvement of tumor response with disease control rate 95% vs 58% (P < 0.001) at 3 mo. Toxicity was comparable in the two study groups, and mEHT related adverse events were limited in 8 patients presenting G1-2 skin burns.
The addition of mEHT to systemic CHT improved overall and progression-free survival and local tumor control with comparable toxicity.
Core Tip: Modulated electro-hyperthermia is a relatively new regional hyperthermia method. It targets tumor cell membranes and extracellular matrix to increase their temperature. New studies have appeared in tumor palliation reporting incremental benefits of chemotherapy and radiotherapy and few additional side effects. In patients with stage III and IV pancreatic cancer, modulated electro-hyperthermia in association with chemotherapy results in significant improvements of overall and progression-free survival and tumor response with comparable toxicity.
- Citation: Fiorentini G, Sarti D, Ranieri G, Gadaleta CD, Fiorentini C, Milandri C, Mambrini A, Guadagni S. Modulated electro-hyperthermia in stage III and IV pancreatic cancer: Results of an observational study on 158 patients. World J Clin Oncol 2021; 12(11): 1064-1071
- URL: https://www.wjgnet.com/2218-4333/full/v12/i11/1064.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i11.1064
Pancreatic cancer has one of the worst prognoses in oncology, with a 5-year overall survival (OS) of 9%, and is the seventh most common cause of cancer deaths in the world in 2018[1]. Adenocarcinoma is the main morphology of this tumor (90%), and its incidence and mortality are increased during the last 2 decades[1]. Surgery followed by adjuvant therapy is the only curative treatment of pancreatic tumors; however, only 10%-20% of them are resectable at diagnosis, whereas 30%–40% are locally advanced and 50%-60% are metastatic.
Non-metastatic, locally advanced pancreatic cancer (LAPC) can be treated with neoadjuvant chemotherapy or chemoradiotherapy to allow surgical resection. Gemcitabine in association with nab-paclitaxel and FOLFIRINOX (leucovorin, fluorouracil, irinotecan, oxaliplatin) for fit patients are two current standard first-line options in LAPC, but they have high toxicity and often low efficacy[2-4]. Pancreatic cancer is quite resistant to radiotherapy (RT) and chemotherapy (CHT) because of its hypoxic microenvironment that diminishes sensitivity to these therapies. In order to increase tumor response, the use of regional hyperthermia is often associated to CHT and RT[5]. Regional hyperthermia (RHT) efficacy in cancer remission is well known, indeed, it enhances drug delivery and diffusion inside the tumor cells, improves blood flow, reduces hypoxia, and inhibits DNA repair, resulting in apoptosis[6].
RHT is achieved by increasing the cancer cells’ temperature to 39.5-43 °C with an external device. A new method of RHT has been recently developed: The modulated electro-hyperthermia (mEHT) that is performed with a 13.56 MHz capacitive coupled device. It targets malignant cell membranes and extracellular matrix, allowing to overcome the issue of reaching deep tumors and achieving homogenous heating[7,8]. The mEHT has comparable benefits to other RHT methods and improves survival and local tumor control for several tumors, including pancreatic cancer[9-11].
This was a large retrospective observational multicentric study aimed to compare outcomes of chemotherapy alone or in association with mEHT for the treatment of locally advance pancreatic cancer, in terms of survival and tumor response. Data were collected retrospectively, and patients were selected according to the following inclusion criteria: > 18 years, stage III and IV pancreatic cancer, treatment with CHT alone or in combination with mEHT, and informed consent signed. Patients were excluded from the study if they had a pacemaker, bilirubin, or transaminase level > 3 times the normal value upper range level or bleeding.
From January 2003 to December 2019, 270 patients with stage III and IV pancreatic cancer were screened in four Italian hospitals; 158 of these patients met the inclusion criteria and were included in the study, 58 (37%) of these received CHT + mEHT and 100 (63%) CHT alone. CHT was mainly gemcitabine-based regimens in both groups.
Modulated electro-hyperthermia was performed using the EHY-2000plus device (CE0123, Oncotherm, Torisdorf, Germany), applying a radiofrequency current of 13.56 MHz as carrier frequency that was modulated by time-fractal fluctuation. The energy was transferred by capacitive coupling, with precise impedance matching[12].
The hyperthermia protocol included three mEHT treatments/week for 2 mo, starting at a 60 W power for 40 min. Following treatments were performed by increasing the power up to 150 W and the time up to 90 min in 2 wk. mEHT was administered after CHT or within 48 h, in order to couple the high drug blood concentration with the modulated electro hyperthermia and optimize their synergy.
The majority (95%) of gemcitabine-based treatments were administered on the same day of electro-hyperthermia treatment. In a minority of patients (5%), it was administered the following day or within the following 72 h because of precarious clinical conditions and geographic accessibility. Even if gemcitabine had a half-life of 42-94 min and was eliminated within 5-11 h after infusion, the pharmacokinetic elimination half-life for dFdU varies between 2 and 24 h, and it is still present systemically in concentrations greater than 1 μmol/L up to 1 wk after infusion[13].
The primary outcome was to monitor OS and progression free survival (PFS). OS was considered from diagnosis to death or last follow up date; PFS was considered from treatment start to date of progression.
Secondary outcome was local tumor control and toxicity. Response Evaluation Criteria in Solid Tumors version 1.4 was used for tumor assessment from magnetic resonance imaging or computed tomography scans. Complete response (CR) was considered when all target lesions disappeared. Partial response (PR) was considered when the sum of diameters of all target lesions was reduced of at least 30%. Progressive disease (PD) was considered when the sum of diameters of all target lesions was increased of at least a 20%, or one or more new lesions appeared. Stable disease (SD) was considered when the sum of diameters of all target lesions reduced < 30%, increased < 20%, or did not change.
Toxicity was assessed with Common Terminology Criteria for Adverse Events version 5.0.
Age and survival were reported as median and ranges; frequencies were reported as percentages. Kaplan-Meier non parametric estimates was used for OS and PFS analysis, reporting survival probability on the y axis and time in months on the x axis. Chi square test, Student’s t test, and log-rank test were used for statistical significance, and P ≤ 0.05 was used to indicate statistically significant differences.
The study included 158 consecutive patients, 58 (37%) of these received CHT + mEHT and 100 (63%) CHT alone. The two sub groups had similar characteristics concerning gender distribution, presence and site of metastases, and previous surgery (Table 1). Some differences were found between CHT + mEHT and CHT groups in median age (64 vs 69 years, P = 0.013), previous RT (2% vs 12%, P = 0.023), number of previous CHT lines (2 Lines: 19% vs 35%, P = 0.037), and type of chemotherapy. Gemox was the most used chemotherapy in CHT + mEHT group (P = 0.004), whereas FOLFOX and FOLFIRINOX were used only in CHT group (P < 0.05).
| CHT + mEHT, n (%) | CHT, n (%) | P value | |
| Median age (range) | 64 (38-82) | 69 (34-92) | 0.013 |
| M | 39 (67) | 57 (57) | 0.204 |
| F | 19 (33) | 43 (43) | |
| Non-Metastatic | 16 (28) | 37 (37) | 0.223 |
| Metastatic | 42 (72) | 63 (63) | |
| Site of Metastases | |||
| Liver | 40 (61) | 51 (70) | 0.164 |
| Peritoneum | 16 (24) | 6 (8) | |
| Lymph nodes | 9 (14) | 6 (8) | |
| Lung | 1 (2) | 8 (11) | |
| Previous surgery | 14 (24) | 24 (24) | 0.981 |
| Previous RT | 1 (2) | 12 (12) | 0.023 |
| Previous CHT | 52 (90) | 52 (52) | < 0.001 |
| Number of previous CHT lines | |||
| 1 | 31 (60) | 25 (48) | 0.334 |
| 2 | 10 (19) | 18 (35) | 0.037 |
| > 3 | 11 (21) | 9 (17) | 0.619 |
| Type of CHT | |||
| Gemox | 28 (54) | 12 (23) | 0.004 |
| Gemcitabine | 12 (23) | 7 (13) | 0.205 |
| Gemcitabine abraxane | 16 (31) | 15 (29) | 0.830 |
| FOLFIRINOX | 0 (0) | 5 (10) | 0.022 |
| FOLFOX | 0 (0) | 4 (8) | 0.041 |
| Other | 2 (4) | 9 (17) |
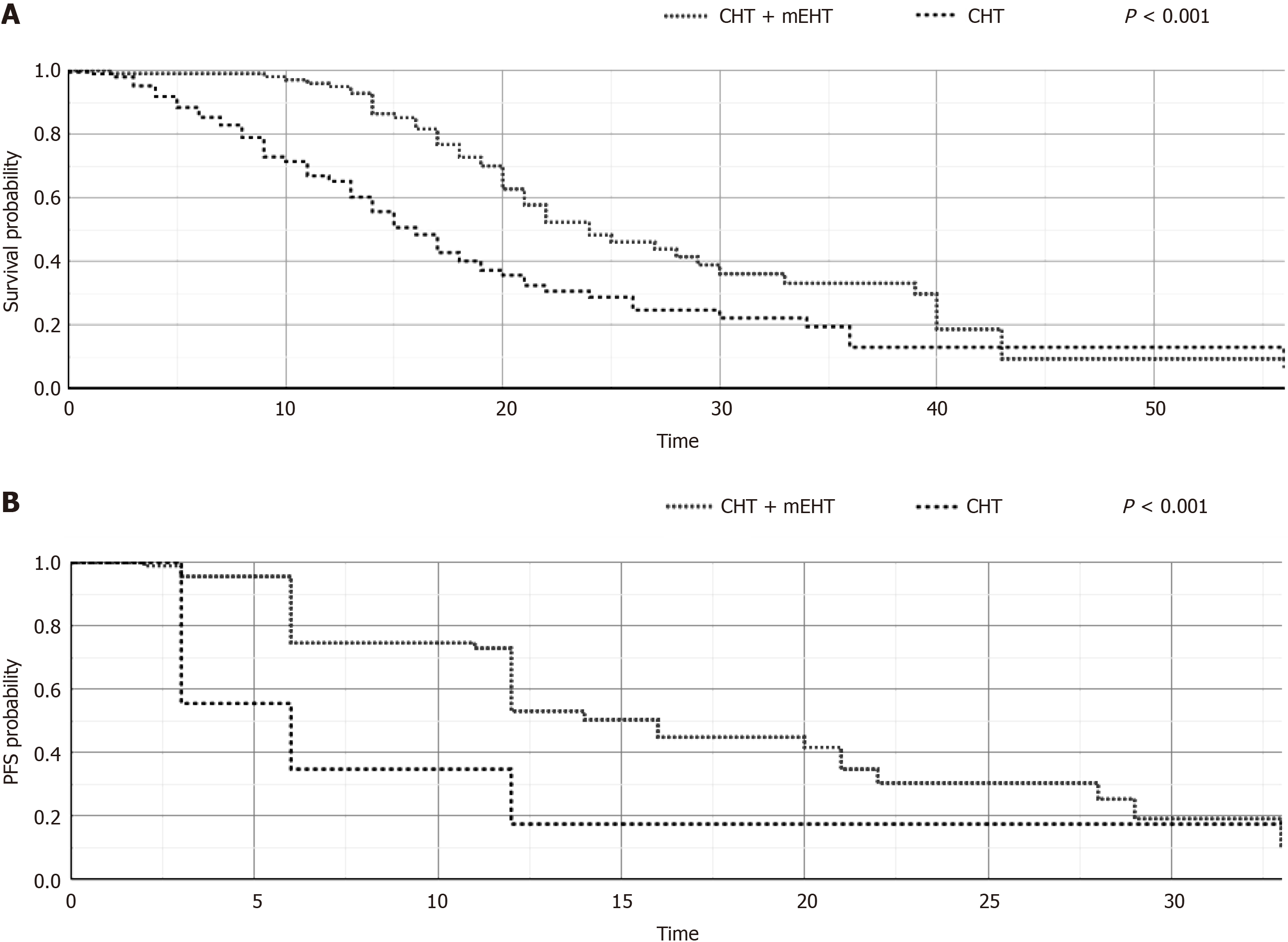
Median OS was greater for CHT + mEHT group than CHT group (19.5 mo vs 11.02 mo, P < 0.001); also PFS was improved (12 mo vs 3 mo, P < 0.001) for the CHT + mEHT group (Figure 1A and B).
The association of mEHT resulted also in an improvement of tumor response at the 3 mo time point, with a disease control rate (DCR) 95% vs 58% (P < 0.001) in CHT + mEHT group and CHT group, respectively (Table 2). CHT + mEHT group, in particular, had a greater PR (52% vs 14%, P < 0.001) and lower PD (5% vs 58%, P < 0.001) than CHT group.
| CHT + mEHT, n (%) | CHT, n (%) | P value | |
| DCR | 53 (95) | 40 (58) | < 0.001 |
| PR | 29 (52) | 13 (14) | < 0.001 |
| SD | 24 (43) | 27 (28) | 0.064 |
| PD | 3 (5) | 56 (58) | < 0.001 |
Each patient received an average of 13 (range = 4-28) sessions of mEHT. Out of a total of 754 mEHT delivered sessions, the safety assessment of mEHT showed a limited number of adverse events 23/754 (4%). mEHT toxicity consisted of skin pain in 15 (3%) patients and burns in 8 (1%).
All these side effects were G1-G2 intensity and resolved with local medications and discontinuation of treatment for 1 wk. All patients were evaluated before and after mEHT with electrocardiogram and cardiac ultrasound. No one had cardiac toxicity.
Hyperthermia has been used as cancer therapy for decades, especially for its benefits in enhancing chemotherapy and radiotherapy efficacy[6,14-22]. mEHT has been more recently introduced among hyperthermia methods, targeting malignant cell membranes and the extracellular matrix and overcoming the issue of homogenous tissue heating[7,8]. The efficacy of mEHT was shown for several types of tumors, including pancreatic cancer, increasing tumor response and survival[5,9,15-21].
The current study showed that mEHT improved OS (19.5 mo vs 11.02 mo, P < 0.001) and PFS (12 mo vs 3 mo, P < 0.001), resulting also in an increased tumor response with DCR 95% vs 58% (P < 0.001) than CHT alone. Toxicity was comparable in the two study groups and hyperthermia-related adverse events were mainly G1-2.
The beneficial effect of hyperthermia on survival of locally advanced pancreatic cancer was reported if combined with chemotherapy by Tschoep-Lechner, reporting in 23 patients an OS 12.9 mo (95%CI: 9.9-15.9) and a DCR in 16 patients with available CT scans of 50%[20].
Similar results are observed when associated to chemo-radiotherapy (CRT). Three studies, in particular, showed OS of 8.8-15 mo vs 4.9-11 mo (P < 0.05) in the association group than CRT alone group and PFS = 18.6 mo vs 9.6 mo (P = 0.01)[17-19]. The association of CHT to RHT also result in encouraging survival: Median OS of 12.9-17.7 mo, 1-year OS = 41% and 2-year OS = 15%[15-17]. Similar survivals are reported by other four studies on mEHT for the treatment of locally advanced pancreatic carcinoma, OS of 8.9-19 mo and PFS of 3.9-12.9 mo[9-11]. The results of the present study were in agreement with the above data, showing OS of 19.5 mo and PFS of 12 mo for CHT + mEHT group. In OS analysis, the survival curve was crossed; this may be due to the fact that stage III-IV pancreatic cancer has always had a poor outcome and, inevitably, when comparing the curves of two treatments, they cross because all patients had died.
Improvements were reported also in tumor response for locally advanced pancreatic carcinoma a consequence of the association of CHT to mEHT with a DCR of 95% that were in agreement with previous data: DCR of 71%-96%[8-11] and resulted higher than that reported by CHT associated with local hyperthermia, showing a DCR of 50%-61%[17-19]. The level of hyperthermia related toxicity was 5% as reported by other studies, showing G2 pain and a skin rash and 5% grade III–IV toxicity[15,18]. These data suggest that RHT increases CRT and CHT benefit both in terms of median OS and in DCR in pancreatic cancer with a low toxicity.
The main limitations of this study were due to the observational nature of this study that resulted in some imbalances distribution between the two subgroups as concerning number of patients and median age, since CHT group had a greater number of subjects (100 vs 58) that were of median older age (69 years vs 64 years) than CHT + mEHT group. Age should not be considered an issue, since the improvement of survival and tumor response was observed also in older patients (> 65 years) with stage III and IV pancreatic cancer[9]. Further prospective, randomized trials on a larger number of patients are required to develop these initial data.
The addition of mEHT to systemic CHT improved OS and PFS and local tumor control with comparable toxicity.
Hyperthermia has been used as cancer therapy for decades, especially for its benefits in enhancing chemotherapy and radiotherapy efficacy. Modulated electro-hyperthermia (mEHT) is a relatively new method of hyperthermia that targets malignant cell membranes and the extracellular matrix, overcoming the issue of homogenous tissue heating.
The efficacy of mEHT is known for several types of tumors, including pancreatic cancer. Pancreatic cancer has a poor prognosis, and the combination of mEHT with chemo- and/or radiotherapy might be important to increase tumor response and improve survival.
The aim of this study was to compare outcomes of chemotherapy (CHT) alone or in association with mEHT for the treatment of locally advanced pancreatic cancer.
Data were collected retrospectively from a cohort of 158 consecutive patients with stage III-IV pancreatic cancer that were treated with CHT alone (63%) or in combination with mEHT (37%) from 2003 to 2019. These data included patients’ characteristics, type of chemotherapy, previous surgery or radiotherapy, tumor response, survival, progression free survival, and adverse events.
The evaluation of survival showed that CHT + mEHT group had a longer overall (19.5 mo vs 11.02 mo, P < 0.001) and progression free (12 mo vs 3 mo, P < 0.001) survival. The association of mEHT improved also tumor response with disease control rate 95% vs 58% (P < 0.001). Toxicity was comparable in the two study groups and hyperthermia-related adverse events were mainly G1-2.
The results obtained in this study provided new evidence that mEHT improved survival and tumor response, delaying the progression insurgence. The introduction of mEHT, moreover, did not influence chemotherapy tolerability, and hyperthermia-related adverse events were limited.
This observational study provides further evidence that mEHT association to chemotherapy can enhance its benefit in pancreatic cancer patients. Further studies are required to confirm these results in a large cohort study and to evaluate treatment safety and efficacy.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bredt LC, Gao W, Yang Y S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | International Agency for Research on Cancer; World Health Organization. Global Cancer Observatory 2018. Available from: http://gco.iarc.fr/. |
| 2. | Feliu J, Jorge Fernández M, Macarulla T, Massuti B, Albero A, González González JF, Quintero-Aldana G, Delgado-Mingorance JI, Fernández Montes A, García Piernavieja C, Valladares-Ayerbes M, López Muñoz AM, Mondéjar Solís R, Vicente P, Casado Gonzalez E, González Cebrián I, López-Vivanco G. Phase II clinical trial of nab-paclitaxel plus gemcitabine in elderly patients with previously untreated locally advanced or metastatic pancreatic adenocarcinoma: the BIBABRAX study. Cancer Chemother Pharmacol. 2021;87:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Ye LF, Ren C, Bai L, Liang JY, Hu MT, Yang H, Wang ZQ, Wang FH, Xu RH, Li YH, Wang DS. Efficacy and safety of modified FOLFIRINOX as salvage therapy for patients with refractory advanced biliary tract cancer: a retrospective study. Invest New Drugs. 2021;39:836-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Liu X, Yang X, Zhou G, Chen Y, Li C, Wang X. Gemcitabine-Based Regional Intra-Arterial Infusion Chemotherapy in Patients With Advanced Pancreatic Adenocarcinoma. Medicine (Baltimore). 2016;95:e3098. [PubMed] [DOI] [Full Text] |
| 5. | van der Horst A, Versteijne E, Besselink MGH, Daams JG, Bulle EB, Bijlsma MF, Wilmink JW, van Delden OM, van Hooft JE, Franken NAP, van Laarhoven HWM, Crezee J, van Tienhoven G. The clinical benefit of hyperthermia in pancreatic cancer: a systematic review. Int J Hyperthermia. 2018;34:969-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Datta NR, Ordóñez SG, Gaipl US, Paulides MM, Crezee H, Gellermann J, Marder D, Puric E, Bodis S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev. 2015;41:742-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 325] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 7. | Szasz AM, Minnaar CA, Szentmártoni G, Szigeti GP, Dank M. Review of the Clinical Evidences of Modulated Electro-Hyperthermia (mEHT) Method: An Update for the Practicing Oncologist. Front Oncol. 2019;9:1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Alshaibi HF, Al-Shehri B, Hassan B, Al-Zahrani R, Assiss T. Modulated Electrohyperthermia: A New Hope for Cancer Patients. Biomed Res Int. 2020;2020:8814878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Fiorentini G, Sarti D, Casadei V, Milandri C, Dentico P, Mambrini A, Nani R, Fiorentini C, Guadagni S. Modulated Electro-Hyperthermia as Palliative Treatment for Pancreatic Cancer: A Retrospective Observational Study on 106 Patients. Integr Cancer Ther. 2019;18:1534735419878505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Sarti D, Milandri C, Fiorentini C, Mambrini A, Fiorentini G. Modulated electro-hyperthermia for the treatment of elderly pancreatic cancer patients. Argum Geriatr Oncol. 2020;5:1-7. |
| 11. | Volovat C, Volovat SR, Scripcaru V, Miron L. Second-line chemotherapy with gemcitabine and oxaliplatin in combination with loco-regional hyperthermia (EHY-2000) in patients with refractory metastatic pancreatic cancer—preliminary results of a prospective trial. Rom Rep Phys. 2014;66:166-174. |
| 12. | Szasz O. Bioelectromagnetic Paradigm of Cancer Treatment—Modulated Electro-Hyperthermia (mEHT). Open J Biophys. 2019;9:98-109. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Peters GJ, Clavel M, Noordhuis P, Geyssen GJ, Laan AC, Guastalla J, Edzes HT, Vermorken JB. Clinical phase I and pharmacology study of gemcitabine (2', 2'-difluorodeoxycytidine) administered in a two-weekly schedule. J Chemother. 2007;19:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Lee SY, Szigeti GP, Szasz AM. Oncological hyperthermia: The correct dosing in clinical applications. Int J Oncol. 2019;54:627-643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Maebayashi T, Ishibashi N, Aizawa T, Sakaguchi M, Sato T, Kawamori J, Tanaka Y. Treatment outcomes of concurrent hyperthermia and chemoradiotherapy for pancreatic cancer: Insights into the significance of hyperthermia treatment. Oncol Lett. 2017;13:4959-4964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Ohguri T, Imada H, Yahara K, Narisada H, Morioka T, Nakano K, Korogi Y. Concurrent chemoradiotherapy with gemcitabine plus regional hyperthermia for locally advanced pancreatic carcinoma: initial experience. Radiat Med. 2008;26:587-596. [PubMed] |
| 17. | Maluta S, Schaffer M, Pioli F, Dall'oglio S, Pasetto S, Schaffer PM, Weber B, Giri MG. Regional hyperthermia combined with chemoradiotherapy in primary or recurrent locally advanced pancreatic cancer : an open-label comparative cohort trial. Strahlenther Onkol. 2011;187:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Ono E, Yano M, Ohshiro T, Shishida M, Sumitani D, Okamoto Y, Och M. Effectiveness of hyperthermia in clinical stage IV pancreatic cancer. Oncothermia J. 2019;27:88-93. |
| 19. | Ishikawa T, Kokura S, Sakamoto N, Ando T, Imamoto E, Hattori T, Oyamada H, Yoshinami N, Sakamoto M, Kitagawa K, Okumura Y, Yoshida N, Kamada K, Katada K, Uchiyama K, Handa O, Takagi T, Yasuda H, Sakagami J, Konishi H, Yagi N, Naito Y, Yoshikawa T. Phase II trial of combined regional hyperthermia and gemcitabine for locally advanced or metastatic pancreatic cancer. Int J Hyperthermia. 2012;28:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Tschoep-Lechner KE, Milani V, Berger F, Dieterle N, Abdel-Rahman S, Salat C, Issels RD. Gemcitabine and cisplatin combined with regional hyperthermia as second-line treatment in patients with gemcitabine-refractory advanced pancreatic cancer. Int J Hyperthermia. 2013;29:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 21. | Peeken JC, Vaupel P, Combs SE. Integrating Hyperthermia into Modern Radiation Oncology: What Evidence Is Necessary? Front Oncol. 2017;7:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 22. | Sauer R, Creeze H, Hulshof M, Issels R, Ott O; Interdisciplinary Working Group for Clinical Hyperthermia (Atzelsberg Circle) of the German Cancer Society and the German Society of Radiooncology. Concerning the final report "Hyperthermia: a systematic review" of the Ludwig Boltzmann Institute for Health Technology Assessment, Vienna, March 2010. Strahlenther Onkol. 2012;188:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |