Published online Oct 24, 2021. doi: 10.5306/wjco.v12.i10.947
Peer-review started: April 30, 2021
First decision: June 16, 2021
Revised: June 21, 2021
Accepted: August 20, 2021
Article in press: August 20, 2021
Published online: October 24, 2021
Processing time: 174 Days and 21.3 Hours
Inflammation is crucial to tumor progression. A traumatic event at a specific site in the brain activates the signaling molecules, which triggers inflammation as the initial response within the tumor and its surroundings. The educated immune cells and secreted proteins then initiate the inflammatory cascade leading to persistent chronic inflammation. Therefore, estimation of the circulating inflammatory indicators kynurenine (KYN), interleukin-6 (IL-6), tissue-inhibitor of matrix-metalloproteinase-1 and human telomerase reverse transcriptase (hTERT) along with neutrophil-lymphocyte ratio (NLR) has prognostic value.
To assess the utility of chosen inflammatory marker panel in estimating systemic inflammation.
The chosen markers were quantitatively evaluated in 90 naive, molecularly sub-typed plasma samples of glioma. A correlation between the markers and con
Median values of circulating KYN, IL-6, hTERT, tissue-inhibitor of matrix-metalloproteinase-1 and NLR in isocitrate-dehydrogenase-mutant/wildtype and within the astrocytic sub-groups were estimated, which differed from controls, reaching statistical significance (P < 0.0001). All markers negatively correlated with mortality (P < 0.0001). Applying combination-statistics, the panel of KYN, IL-6, hTERT and NLR achieved higher sensitivity and specificity (> 90%) than stand-alone markers, to define survival. The inflammatory panel could discriminate between WHO grades, and isocitrate-dehydrogenase-mutant/wildtype and define differential survival between astrocytic isocitrate-dehydrogenase-mutant/ wildtype. Therefore, its assessment for precise disease prognosis is indicated. Association of KYN with NLR, IL-6 and hTERT was significant. Cox-regression described KYN, IL-6, NLR, and hTERT as good prognostic markers, independent of confounders. Multivariate linear-regression analysis confirmed the association of KYN and hTERT with inflammation marker IL-6.There was a concomitant significant decrease in their levels in a 3-mo follow-up.
The first evidence-based study of circulating-KYN in molecularly defined glio
Core Tip: The current study is the first-ever analysis of the circulatory levels of ky
- Citation: Gandhi P, Shrivastava R, Garg N, Sorte SK. Novel molecular panel for evaluating systemic inflammation and survival in therapy naïve glioma patients . World J Clin Oncol 2021; 12(10): 947-959
- URL: https://www.wjgnet.com/2218-4333/full/v12/i10/947.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i10.947
The role of inflammation and its partakers in glioma, especially the molecular markers secreted at the tumor initiation stage, is still not completely worked out. Since the brain is unique in comparison to other organ systems, being confined within the blood-brain barrier, it presents a local innate immune response to an inflammatory stimulus, like tumor antigens[1], injury, or oncogene over-expression, by the pro
One of the significant inflammatory metabolites in the brain is kynurenine (KYN) of the KYN pathway; it is produced and secreted by endothelial cells and pericytes of the blood-brain barrier, which have been stimulated by inflammation. In the absence of effective immune regulation, chronic inflammation is generated by aggressively proliferating cells. Excess KYN production is triggered by inflammation in the tumor environment (TE), which leads to local immune tolerance, and the inflammatory signals generated by the tumor results in KYN being transduced across the intact blood-brain barrier to be detected in systemic circulation[5,6]. Over-activation of KYN was first described in glioblastoma (GB) cell lines and tumor tissues as early as 2012 by Opitz and colleagues[7]. Excessive production of this metabolite was linked to increased tumor immunity and decreased survival as recorded by Adams et al[8] in cultured cells and 18 GB patient samples[8].
The tumor-infiltrating immune cells - neutrophils and lymphocytes, are also markers of systemic inflammation[9]. Their involvement as local inflammation indicators in glial tumors has been documented by Zadora et al[10]. Neutrophil recruitment initiates cytokine secretion, which strengthens the initial in situ neuro-inflammatory response by activating more neutrophils and macrophages. Brain tumors, including glioma, express high levels of different cytokines, specifically interleukin-6 (IL-6), involved in multiple pathways of tumor pathology[11]. During gliomagenesis, IL-6 executes a more critical role than other interleukins since it predominately triggers the pro-inflammatory cascade. In the last few years, a couple of blood-based investigations analyzing the expression of IL-6 in different grades of glioma suggested that IL-6 expression may indicate disease prognosis[12,13].
Involved in this scenario of brain inflammation is another set of proteins, the tissue-inhibitor of matrix-metalloproteinases (TIMPs), known to perform multiple functions, including regulating inflammation in the TE. In this context, there is a lone study by Lin et al[14] reporting plasma levels of TIMP-1 to be associated with an inflammatory response in glioma, proposing a role of this molecule in prognosis[14].
In this landscape of inflammation partakers in the TE, the association between increased chronic inflammation and telomerase activity is often overlooked. The human telomerase reverse transcriptase (hTERT) is an enzyme active in the immune system cells and regulates inflammation; however, its activity beyond a critical limit initiates uncontrolled proliferation. Several studies suggest that TERT activates the nuclear factor kappa-B target genes such as IL-6, which is crucial to inflammation and cancer progression[15-17]. Research groups, including ours, have demonstrated that hTERT executes many vital functions independent of its telomere maintenance, namely angiogenesis, inflammation, and stemness in glioma[18,19]. These findings suggested that the feedback loop of the hTERT signaling pathway may reinforce the inflammatory signaling via cytokine secretion, leading to the development of chronic inflammation in the TE.
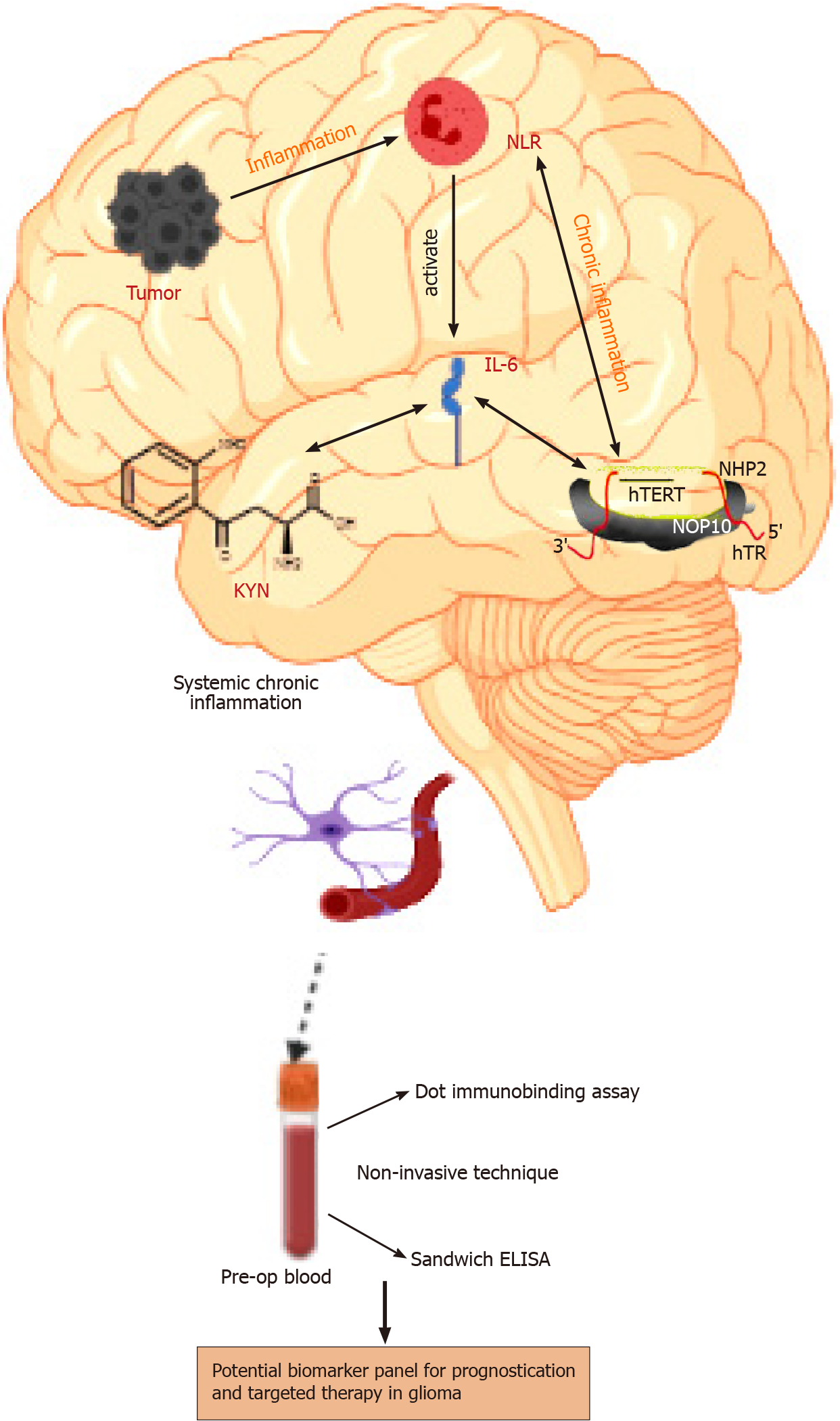
Mechanistically, a traumatic event at a specific site in the brain leads to the secretion of inflammatory molecules such as IL-6 and KYN, which trigger the inflammatory cascade leading to over-activation of telomerase. The abnormal hTERT physiology is responsible for immune system dysfunction, followed by persistent chronic inflammation leading to a malignant phenotype. Further, this unresolved chronic inflammation also begins contributing to disease progression. The current exploratory study is the first ever analysis of the circulatory levels of KYN, IL-6, neutrophil-lymphocyte ratio (NLR), hTERT, and TIMP-1 in a sizeable cohort of glioma samples categorized according to their histological grade and isocitrate-dehydrogenase (IDH) expression. The ability of this panel to differentiate survival in glioma subgroups has been assessed.
Patients with symptoms of recurrent seizures and headache radiologically diagnosed and histopathologically confirmed as glioma grade II, III, or IV.
Patients less than 18 years of age or patients histopathologically confirmed as glioma grade I.
Ninety treatment-naive samples, presenting with clinical symptoms like recurrent seizures, headache, increased intracranial pressure, and radiological diagnosis of glial tumor, were collected from the in-patient ward of the Neurosurgery department. Informed consent in writing was taken from all the participants of the study cohort (IRB/21/Res/11). Forty-five healthy subjects without any recent clinical history of inflammation or autoimmune disease, were taken as controls for blood samples. Whole blood was collected just before surgery; plasma and serum were separated and preserved at -80°C until further testing for target proteins was undertaken. A follow-up sample of all patients was done 3 mo post-surgery. According to the established lab protocol, the systemic levels of the marker KYN were correlated with its in situ tumor expression by immunofluorescence-immunohistochemistry for validation (Supplementary File Method A).
Qualitative screening of circulating markers was done using 20μL of serum after removing high abundance proteins. Subsequently, the samples were loaded onto nitrocellulose membrane and incubated overnight at 4°C. Then the membrane was probed with KYN, IL-6, TIMP-1, and hTERT antibody (1:2000 dilution, Monoclonal, Santa Cruz Biotechnology, United States) for 2 h followed by host-specific respective alkaline-phosphatase-conjugated antibody tagging (Santa Cruz Biotechnology, United States; 1:2500 dilutions) for 1 h. The label was detected using an alkaline-phosphatase substrate (BCIP, Sigma Aldrich, United States), which produced a visible signal corresponding to the concentration of the target protein.
Quantification of the biomarkers in plasma samples was done by enzyme-linked immunosorbent assay (ELISA) for KYN (Creative Diagnostics, United States), TIMP-1 (R&D systems, United States), and hTERT (Elabscience, United States) according to details provided in the instruction manuals. The plasma concentrations were noted in ng/mL for KYN and TIMP-1, and ng/L for hTERT. IL-6 test results were computed from the patients’ clinical reports and compared with the baseline values earlier established for this marker in the lab[13]. The standard reference range of each marker was defined and set as per kit insert. Complete blood counts of all enrolled subjects were recorded from the pre-surgery blood profile; the neutrophil and lymphocyte counts were extrapolated into the mathematical formula and calculated[18] to establish the NLR in patients and controls.
Non-parametric Kruskal-Wallis test was used to assess the difference between samples of different histological grades for plasma values of all biomarkers. Mann Whitney test was applied on the groups stratified according to IDH status and for marker panel KYN, TIMP-1, NLR, IL-6, and hTERT, to differentiate their significance. Analysis of area under the curve for receiver operating characteristic was performed to define the cut-off values and sensitivity of the markers between the grades and within subgroups that attained ≥ 80% specificity. To establish the diagnostic accuracy of the markers, CombiROC software (https://combiroc.eu) was used, and the predictive probability of the inflammatory panel for prognostication was subjected to receiver operating characteristic analysis. To discern the influence of covariates on plasma maker levels, univariate analysis was carried out to identify the confounding factors, namely, age, site, extent of resection, therapy, KYN, TIMP-1, NLR, IL-6 and, hTERT. Parameters attaining a level of significance in this analysis were entered into the multivariate functionally to create the final model. Furthermore, the Cox proportional regression model was used to compute hazard ratios with 95% confidence interval (CI) to establish the independent status of prognostic markers.
Overall survival (OS), defined as the time from randomization to death from any cause, was considered as a direct measure of clinical benefit to the patient. Patients alive or lost to follow-up were treated as censored. Curves defining total survival period with reference to the identified biomarkers were drawn on Kaplan-Meier estimates and differences compared between IDH-mutant/wildtype (IDH-m/w) subgroups for statistical significance using the log-rank test. Spearman’s rho co
Plasma samples from healthy controls (n = 45) and glioma patients (n = 90, IDH-m: n = 60 inclusive of astrocytic and oligo-component and IDH-w, astrocytic n = 30) formed the study cohort. Details of patients, relevant demographics, and clinical data are presented in Table 1.
| No. | Factors | Controls | IDH-mutant | IDH-wildtype |
| 1 | Sample size, (n) | (n = 45) | 60 | 30 |
| 2 | Sex, M/F | M = 30; F = 15 | M = 51; F = 9 | M = 25; F = 5 |
| 3 | Age (yr) | 25 (21-50) | 35.0 (13-75) | 51.5 (25-76) |
| 4 | Site | - | Frontal = 37/Non-frontal = 23 | Frontal = 12/Non-frontal = 18 |
| 5 | EOR | - | STR = 38; GTR = 14 | STR = 30; GTR = 0 |
| 6 | OS | - | 28.5 (1-158) | 6.5 (1-38) |
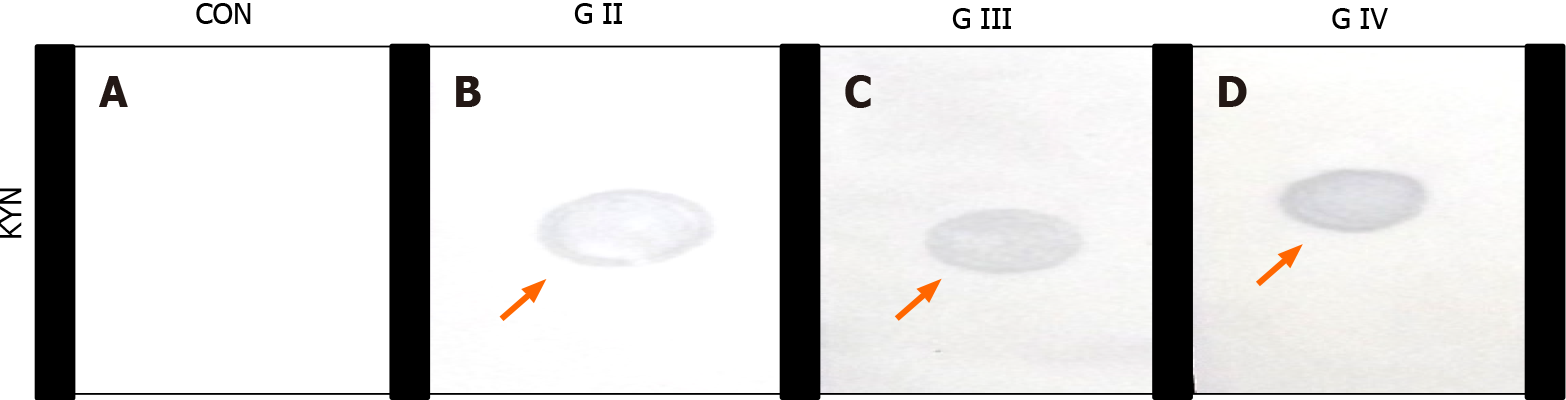
The presence/absence of KYN, IL-6, TIMP-1, and hTERT in circulation was screened on nitrocellulose membrane by dot immune-binding assay. A colored signal (blue) indicated immune-complex formation and presence of the inflammatory molecule KYN (Figure 1) .The signal intensity of each marker corresponded to the pathological grading of the tumor.
Median values of plasma KYN in grade-II, III, and IV were 69.86ng/mL, 112.15ng/mL, 237.318 ng/mL, respectively. Levels of circulating KYN, IL-6, TIMP-1, and hTERT emerged as substantially higher (P < 0.0001) with increasing histological grade when the Kruskal-Wallis test was applied. The statistical difference between IDH-m/w groups (Table 2) using Mann Whitney test was highly significant for KYN (P < 0.0001), NLR (P = 0.0002), TIMP-1 (P = 0.0405) and hTERT (P < 0.0001).
| No. | Factors | Controls (n = 45) | IDH-mutant (n = 60) | IDH-wildtype (n = 30) | P value |
| 1 | KYN (ng/mL) | 5.009 (0.052-34.43) | 77.155 (1.56-387.44) | 237.318 (53.43-894.95) | < 0.0001c |
| 2 | IL-6 (pg/mL) | 37.009 (6.715-62.49) | 68.62 (21.85-209.73) | 197.672 (59.217-803.711) | < 0.0001c |
| 3 | NLR | 1.59 (0.87-2.8) | 3.67 (1.23-11.5) | 5.585 (3.1-18) | 0.0002c |
| 4 | TIMP-1 (ng/mL) | 38.03 (10.26-73.99) | 82.54 (25.09-131.30) | 107.01 (37.175-185.09) | 0.0405a |
| 5 | hTERT (ng/mL) | 1.029 (0.075-1.58) | 1.364 (0.1-6.886) | 2.185 (0.565-8.84) | < 0.0001c |
The association of plasma levels (preoperative) of all markers yielded a positive correlation with the grade; however, the best value was observed for IL-6 (r = 0.64, P < 0.0001, Table 3), while the most significant inverse correlation of KYN (r = 0.6154, P < 0.0001) was attained with OS (Table 3). When the samples were molecularly stratified, there was an inverse correlation of all biomarkers with survival outcome, being worse for IDH-w ascompared to IDH-m. The Spearman coefficient for inflammatory markers KYN, TIMP-1, NLR, IL-6, and hTERT was significant and positive for tumor grade, but there was an inverse association with OS, suggesting a poor prognosis with increasing systemic levels of these markers in therapy naïve glioma patients.
| No. | Circulatory markers | Spearman r | 95%CI | P value |
| Correlation with OS | ||||
| 1 | KYN (ng/mL) | -0.6154 | -0.7324 to -0.463 | < 0.0001c |
| 2 | IL-6 (pg/mL) | -0.5531 | -0.6854 to -0.3855 | < 0.0001c |
| 3 | NLR | -0.5696 | -0.698 to -0.4058 | < 0.0001c |
| 4 | TIMP-1 (ng/mL) | -0.4831 | -0.6312 to -0.3011 | < 0.0001c |
| 5 | hTERT (ng/mL) | -0.4386 | -0.5960 to -0.2489 | < 0.0001c |
| Correlation with grade | ||||
| 6 | KYN (ng/mL) | 0.5409 | 0.3705 to 0.6761 | < 0.0001c |
| 7 | IL-6 (pg/mL) | 0.6400 | 0.4944 to 0.7507 | < 0.0001c |
| 8 | NLR | 0.4982 | 0.3190 to 0.6430 | < 0.0001c |
| 9 | TIMP-1 (ng/mL) | 0.2614 | 0.05118 to 0.4495 | 0.0128a |
| 10 | hTERT (ng/mL) | 0.3000 | 0.06441 to 0.4834 | 0.0102a |
| Correlation with KYN | ||||
| 11 | IL-6 (pg/mL) | 0.4919 | 0.3115 to 0.638 | < 0.0001c |
| 12 | NLR | 0.4084 | 0.214 to 0.5717 | < 0.0001c |
| 13 | TIMP-1 (ng/mL) | 0.4718 | 0.2877 to 0.6223 | < 0.0001c |
| 14 | hTERT (ng/mL) | 0.3504 | 0.1485 to 0.5243 | 0.0007c |
The tissue expression of KYN was concomitant with its plasma levels and increased with increasing histological grade (Supplementary Figure 1).
The relation of OS with KYN, TMIP-1, IL-6, NLR, hTERT, age, site, the extent of resection and therapy was calculated using univariate and multivariate Coxregression models to identify the plausible prognostic factors (Table 4). Univariate analysis delineated shorter patient survival to be associated with KYN, IL-6, NLR, TIMP-1, hTERT (P = 0.0001), and age (P = 0.0004). When multivariate Cox-regression model was applied, higher levels of KYN (P = 0.0003), IL-6 (P = 0.0004), NLR (P = 0.0001) and hTERT (P = 0.0026) were found to independently define prognosis. Based on these results, it was assumed that TIMP-1 could not be considered a sensitive marker for inflammation. So, the final maker panel of four markers, KYN, IL-6, NLR, and hTERT, was taken further for validation.
| No. | Variable | Univariate | Multivariate | ||
| HR (95%CI) | P value | HR (95%CI) | P value | ||
| 1 | KYN (ng/mL) | 1.0037 (1.0026-1.0048) | 0.0001c | 1.0024 (1.0011-1.0037) | 0.0003c |
| 2 | IL-6 (pg/mL) | 1.0032 (1.0021-1.0042) | 0.0001c | 1.0022 (1.0010-1.0035) | 0.0004c |
| 3 | NLR | 1.2437 (1.1621-1.3312) | 0.0001c | 1.2085 (1.1138-1.3114) | 0.0001c |
| 4 | TIMP-1 (ng/mL) | 1.0150 (1.0075-1.0225) | 0.0001c | 1.0046 (0.9975-1.0118) | 0.2042 |
| 5 | hTERT (ng/mL) | 1.2681 (1.1498-1.3987) | 0.0001c | 1.2303 (1.0749-1.4080) | 0.0026b |
| 5 | Age(yr) | 1.0245 (1.0108-1.0383) | 0.0004c | 1.0090 (0.9938-1.0233) | 0.2165 |
| 6 | EOR | 1.3379 (1.8805-3.0328) | 0.1726 | NA | NA |
| 7 | Site | 0.9527 (0.6221-1.4589) | 0.8236 | NA | NA |
| 8 | Therapy | 0.8507 (0.2201-1.3578) | 0.906 | NA | NA |
The cut-off thresholds based on area under the curve for the four circulatory bio
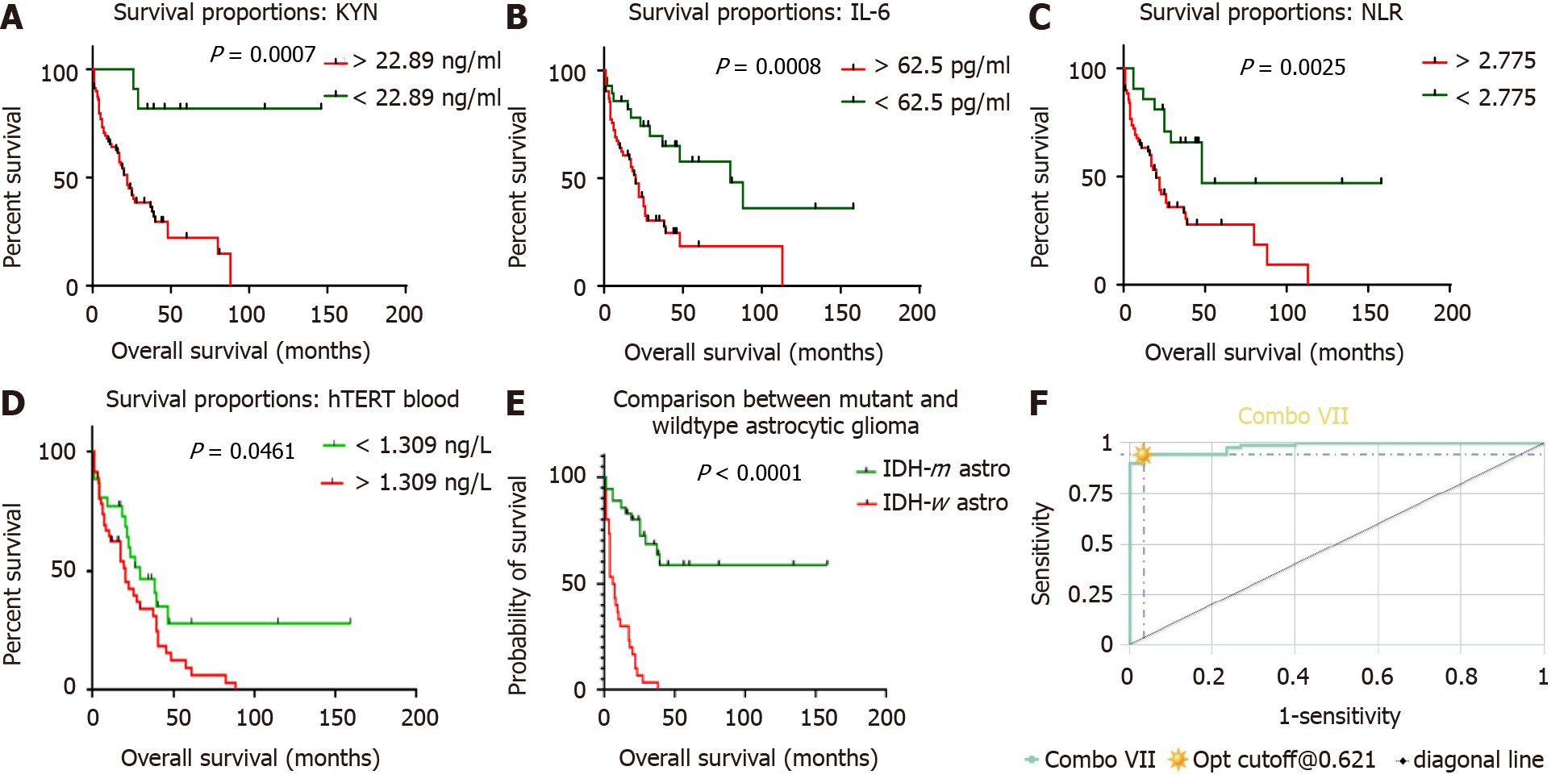
Based on the AUROC analysis, optimal cut-off points were determined for the best balance of sensitivity and specificity and the highest value of likelihood ratio to predict survival through log-rank analysis, and thereafter Kaplan Meier curves were con
Circulating concentrations of KYN, NLR, IL-6 and hTERT were set at 22.89 ng/mL, 2.775, 62.5 pg/mL and 1.309 ng/L, respectively. Patients with values exceeding these thresholds were observed to have a shorter survival period. The OS was defined for KYN (18 vs undefined months, HR 3.176 with 95%CI: 1.626-6.206, P = 0.0007), NLR (20 mo vs 48 mo, HR 0.4167 with 95%CI: 0.204-0.8512, P = 0.0025), IL-6 (20 mo vs 80 mo, HR 0.25 with 95%CI: 0.1318-0.4741, P = 0.0008), and hTERT (18 mo vs 48 mo, HR 0.21 with 95%CI: 0.1178-0.4741, P = 0.0006).
On follow-up, paired t-test between pre-and 3-mo post-surgery levels of KYN (P = 0.0148), IL-6 (P = 0.0107), NLR (P = 0.0038), and hTERT (P = 0.0016) showed a significant difference, and lower values post-intervention indicate that inflammation has plausibly reduced on debulking of the tumor (Table 5).
| No | Markers | Preoperative concentration | 3-mo postoperative concentration | P value | rs value |
| 1 | KYN (ng/mL) | 126.1 (19.410-387.440) | 75.405 (16.340-227.400) | 0.0148a | 0.4509 |
| 2 | IL-6 (pg/mL) | 75.65 (23.96-209.73) | 60.03 (21.85-140.77) | 0.0107a | 0.1850 |
| 3 | NLR | 4.30 (1.43-15.00) | 2.93 (0.80-6.90) | 0.0038b | 0.7260 |
| 4 | hTERT (ng/L) | 2.27 (1.16-6.86) | 1.33 (0.78-4.78) | 0.0016b | 0.6574 |
The predicting accuracy of these chosen candidate markers to ascertain systemic inflammation status in glioma patients was increased substantially in combination as a panel rather than standalone markers. Applying CombiROC enhanced the sensitivity of the biomarker panel. The sensitivity of KYN, IL-6, NLR, and hTERT was 86.11%, 72.22%, 77.78%, and 80% individually, which increased to 94.4% in combination with 96.7% specificity and an area under the curve of 0.983 as seen in combination VII (Figure 2F).
Based on the above results and multivariate linear regression, it can be inferred that there exists anassociation between the tumor secreted inflammatory molecules (Figure 3); therefore, these markers can be evaluated to assess systemic inflammation and prognosis.
The link between a tumor and inflammation is a well-established fact and is one of the major attributes of malignancy[19]. Inflammation in the TE mediates all aspects of glial oncogenesis, including in situ progressive development of vasculature and tissue remodeling[20]. Thus, by and large, the inflammatory molecules orchestrate the extrinsic and intrinsic stimuli, thereby initiating and contributing to tumor progression[21].
Although KYN is an important inflammatory metabolite in the glial-onco-transformation process, there are limited studies on the role of this molecule in glioma, with three research groups recording the ratio of KYN and tryptophan in a very small number of patients. In reference is the study on the plasma of 18 GB patients by Adams et al[8], who presented data to show that because of activation of the KYN pathway, the KYN/TRP-ratio was significantly higher in GB as compared to healthy volunteers. The other group of investigators presented a different opinion on KYN as a marker, stating that there was no significant difference in the levels before and after surgical intervention, as seen in 10 GB samples[22]. However, the research by Lenzen et al[23] documented significantly decreased serological values of KYN after a vaccine treatment with heat shock protein-peptide complex-96, but they did not provide any baseline data of KYN for comparison.
Therefore, the present therapy-naive dataset is unique as it establishes KYN levels in circulation for glioma, with values screened qualitatively at baseline and recorded quantitatively at two time points, pre-and post-surgery. This marker was able to differentiate between IDH-m/w groups with high significance (Table 2), along with histo
When a chronic inflammatory response is generated within the tumor, the KYN pathway proteins trigger the over-expression of pro-inflammatory cytokine IL-6[8]. We noted a raised expression of the inflammatory marker IL-6 in our enrolled glioma patients. Similarly, limited systemic studies available on GB reveal that aberrant production of this circulating cytokine is directly associated with tumor growth and poor survival, demonstrating IL-6 as an independent prognostic factor for survival[13,24,25]. These studies suggest that the cytokine plays an important role in the in situ inflammatory response within the tumor.
Despite the established association between telomerase activity and inflammation, the related molecular pathway in glioma has not been elucidated. Elevated telomerase activity for the sustained proliferation of tumor cells begins a smoldering response of acute followed by chronic inflammation[26,27]. The work of Shervington and Patel[28] in glioma subtypes suggests a noteworthy variation of TERT protein expression levels in GB when compared with control. Two case reports presented from our lab also correlate hTERT expression to proliferation, stemness, and survival in glioma sub-types[13,29]. In a first-of-its-kind investigation, the correlation between tissue and blood concentrations of hTERT marker was also established by us in glioma[30]. Findings of the current analysis are also in line, and there was a significant difference of this marker among grades, IDH-m/w, and a positive association with KYN and IL-6.
In the present cohort analysis, the NLR score emerged as a good indicator of prognosis and survival in the molecularly typed sub-groups. Concurrence of this result can be found in studies conducted on smaller groups of GB patients[31,32] which show that there is a significant association of NLR with OS[33-36]. Our previous work on diffuse gliomas, along with the current study on IDH characterized gliomas, provides substantial laboratory-based evidence that NLR is a marker of inflammation and can be used as a prognosticator in the clinical setting[18].
There is an increasing acceptance of the proactive role of glial tumor cells in brain inflammation, leading to suppression of innate immune response via secretion of matrix metalloproteases[37,38]. In a study conducted earlier in the decade, Sree
The systemic levels of target molecules discussed herein can be considered to represent the cascade of events leading to chronic systemic inflammation in glioma, as depicted in Figure 3. The present work is a one of its kind experiment-based qua
Chronic persistent inflammation is a hallmark of glioma and a major contributor to the disease progression. Currently there are no serological or molecular markers that are routinely evaluated before deciding treatment and in the follow-up period for monitoring survival and therapeutic efficacy.
A non-invasive inflammatory marker panel is essential to define survival and plan for a better clinical outcome.
The objective of this investigation was to assess the utility of the non-invasive biomarker panel to estimate systemic inflammation and whether it can define and differentiate survival in glioma subgroups.
Dot-immune assay for screening of the expression of molecular makers followed by estimation of circulatory levels by enzyme-linked immunosorbent assay are easy, cost-effective and sensitive methods even in resource limited settings. The expression of marker kynurenine (KYN) has been validated by immunofluorescence-immunohistochemistry in situ.
The molecular marker panel of KYN, interleukin-6, human telomerase reverse transcriptase and neutrophil-lymphocyte ratio were negatively correlated with mortality (P < 0.0001) and achieved higher sensitivity and specificity (> 90%) than stand-alone markers, to define survival. It could discriminate between WHO-grades, isocitrate-dehydrogenase-mutant/wildtype and define differential survival between astrocytic isocitrate-dehydrogenase-mutant/wildtype. Association of KYN with neu
This is a first of its kind evidence-based study of a non-invasive panel that can estimate chronic systemic inflammation, wherein the identified over-expressed molecular markers can be targeted to design a personalized therapy.
The study model when replicated in a bigger sized multicentric cohort can pave way for the use of the inflammatory molecular screen for routine patient care.
The authors acknowledge the review of histopathological slides by Dr. Jharna Mishra, Head Pathology, Bansal Hospital, Bhopal (M.P).
Manuscript source: Invited manuscript
Corresponding Author’s Membership in Professional Societies: Indian Society of Neurooncology, No. 385.
Specialty type: Oncology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ding L S-Editor: Fan JR L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med. 2012;209:1057-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 285] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 2. | Ha ET, Antonios JP, Soto H, Prins RM, Yang I, Kasahara N, Liau LM, Kruse CA. Chronic inflammation drives glioma growth: cellular and molecular factors responsible for an immunosuppressive microenvironment. Neuroimmunol Neuroinflamm. 2014;1:66-76. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2011;59:1169-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 405] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 4. | Serra MB, Barroso WA, da Silva NN, Silva SDN, Borges ACR, Abreu IC, Borges MODR. From Inflammation to Current and Alternative Therapies Involved in Wound Healing. Int J Inflam. 2017;2017:3406215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 5. | Owe-Young R, Webster NL, Mukhtar M, Pomerantz RJ, Smythe G, Walker D, Armati PJ, Crowe SM, Brew BJ. Kynurenine pathway metabolism in human blood-brain-barrier cells: implications for immune tolerance and neurotoxicity. J Neurochem. 2008;105:1346-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Lovelace MD, Varney B, Sundaram G, Franco NF, Ng ML, Pai S, Lim CK, Guillemin GJ, Brew BJ. Current Evidence for a Role of the Kynurenine Pathway of Tryptophan Metabolism in Multiple Sclerosis. Front Immunol. 2016;7:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 7. | Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1207] [Cited by in RCA: 1458] [Article Influence: 104.1] [Reference Citation Analysis (0)] |
| 8. | Adams S, Teo C, McDonald KL, Zinger A, Bustamante S, Lim CK, Sundaram G, Braidy N, Brew BJ, Guillemin GJ. Involvement of the kynurenine pathway in human glioma pathophysiology. PLoS One. 2014;9:e112945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Li X, Han Z, Cheng Z, Yu J, Liu S, Yu X, Liang P. Preoperative neutrophil-to-lymphocyte ratio is a predictor of recurrence following thermal ablation for recurrent hepatocellular carcinoma: a retrospective analysis. PLoS One. 2014;9:e110546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Zadora P, Dabrowski W, Czarko K, Smolen A, Kotlinska-Hasiec E, Wiorkowski K, Sikora A, Jarosz B, Kura K, Rola R, Trojanowski T. Preoperative neutrophil-lymphocyte count ratio helps predict the grade of glial tumor - a pilot study. Neurol Neurochir Pol. 2015;49:41-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Mostofa AG, Punganuru SR, Madala HR, Al-Obaide M, Srivenugopal KS. The Process and Regulatory Components of Inflammation in Brain Oncogenesis. Biomolecules. 2017;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Shan Y, He X, Song W, Han D, Niu J, Wang J. Role of IL-6 in the invasiveness and prognosis of glioma. Int J Clin Exp Med. 2015;8:9114-9120. [PubMed] |
| 13. | Gandhi P, Khare R, Garg N, Sorte S. Immunophenotypic signature of primary glioblastoma multiforme: A case of extended progression free survival. World J Clin Cases. 2017;5:247-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Lin Y, Wang JF, Gao GZ, Zhang GZ, Wang FL, Wang YJ. Plasma levels of tissue inhibitor of matrix metalloproteinase-1 correlate with diagnosis and prognosis of glioma patients. Chin Med J (Engl). 2013;126:4295-4300. [PubMed] |
| 15. | Ghosh A, Saginc G, Leow SC, Khattar E, Shin EM, Yan TD, Wong M, Zhang Z, Li G, Sung WK, Zhou J, Chng WJ, Li S, Liu E, Tergaonkar V. Telomerase directly regulates NF-κB-dependent transcription. Nat Cell Biol. 2012;14:1270-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 296] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 16. | Chen R, Zhang K, Chen H, Zhao X, Wang J, Li L, Cong Y, Ju Z, Xu D, Williams BR, Jia J, Liu JP. Telomerase Deficiency Causes Alveolar Stem Cell Senescence-associated Low-grade Inflammation in Lungs. J Biol Chem. 2015;290:30813-30829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Liu H, Yang Y, Ge Y, Liu J, Zhao Y. TERC promotes cellular inflammatory response independent of telomerase. Nucleic Acids Res. 2019;47:8084-8095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Gandhi P, Khare R, VasudevGulwani H, Kaur S. Circulatory YKL-40 & NLR: Underestimated Prognostic Indicators in Diffuse Glioma. Int J Mol Cell Med. 2018;7:111-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 19. | Wang J, Li D, Cang H, Guo B. Crosstalk between cancer and immune cells: Role of tumor-associated macrophages in the tumor microenvironment. Cancer Med. 2019;8:4709-4721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 236] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 20. | Lin Q, Jin S, Han M, Zheng W, Liu J, Wei X. Inflammation in the Tumor Microenvironment. J Immunol Res. 2018;2018:1965847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, Gong Z, Zhang S, Zhou J, Cao K, Li X, Xiong W, Li G, Zeng Z, Guo C. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8:761-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1071] [Cited by in RCA: 953] [Article Influence: 119.1] [Reference Citation Analysis (0)] |
| 22. | Zhai L, Dey M, Lauing KL, Gritsina G, Kaur R, Lukas RV, Nicholas MK, Rademaker AW, Dostal CR, McCusker RH, Raizer JJ, Parsa AT, Bloch O, Wainwright DA. The kynurenine to tryptophan ratio as a prognostic tool for glioblastoma patients enrolling in immunotherapy. J Clin Neurosci. 2015;22:1964-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Lenzen A, Zhai L, Lauing KL, Gritsina G, Ladomersky E, Genet M, James CD, Bloch O, Wainwright DA. The Kynurenine/Tryptophan Ratio and Glioblastoma Patients Treated with Hsppc-96 Vaccine. Immunotherapy (Los Angel). 2016;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Li Z, Huang Q, Chen H, Lin Z, Zhao M, Jiang Z. Interferon Regulatory Factor 7 Promoted Glioblastoma Progression and Stemness by Modulating IL-6 Expression in Microglia. J Cancer. 2017;8:207-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Holst CB, Christensen IJ, Skjøth-Rasmussen J, Hamerlik P, Poulsen HS, Johansen JS. Systemic Immune Modulation in Gliomas: Prognostic Value of Plasma IL-6, YKL-40, and Genetic Variation in YKL-40. Front Oncol. 2020;10:478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Kordinas V, Ioannidis A, Chatzipanagiotou S. The Telomere/Telomerase System in Chronic Inflammatory Diseases. Cause or Effect? Genes (Basel). 2016;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 27. | Wu L, Fidan K, Um JY, Ahn KS. Telomerase: Key regulator of inflammation and cancer. Pharmacol Res. 2020;155:104726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Shervington A, Patel R. Differential hTERT mRNA processing between young and older glioma patients. FEBS Lett. 2008;582:1707-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Gandhi P, Khare R, Niraj K, Garg N, Sorte SK, Gulwani H. Unique case of oligoastrocytoma with recurrence and grade progression: Exhibiting differential expression of high mobility group-A1 and human telomerase reverse transcriptase. World J Clin Cases. 2016;4:296-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Gandhi P, Khare R, Garg N. Evaluating the potential of circulating hTERT levels in glioma: can plasma levels serve as an independent prognostic marker? J Neurooncol. 2017;135:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Dharmajaya R, Sari DK. Role and value of inflammatory markers in brain tumors: A case controlled study. Ann Med Surg (Lond). 2021;63:102107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Alexiou GA, Vartholomatos E, Zagorianakou P, Voulgaris S. Prognostic significance of neutrophilto-lymphocyte ratio in glioblastoma. Neuroimmunol Neuroinflamm. 2014;1:131-134. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Bambury RM, Teo MY, Power DG, Yusuf A, Murray S, Battley JE, Drake C, O'Dea P, Bermingham N, Keohane C, Grossman SA, Moylan EJ, O'Reilly S. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J Neurooncol. 2013;114:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 34. | Han S, Liu Y, Li Q, Li Z, Hou H, Wu A. Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer. 2015;15:617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 174] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 35. | McNamara MG, Lwin Z, Jiang H, Templeton AJ, Zadeh G, Bernstein M, Chung C, Millar BA, Laperriere N, Mason WP. Factors impacting survival following second surgery in patients with glioblastoma in the temozolomide treatment era, incorporating neutrophil/lymphocyte ratio and time to first progression. J Neurooncol. 2014;117:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 36. | Sharma G, Jain SK, Sinha VD. Peripheral Inflammatory Blood Markers in Diagnosis of Glioma and IDH Status. J Neurosci Rural Pract. 2021;12:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Pullen NA, Pickford AR, Perry MM, Jaworski DM, Loveson KF, Arthur DJ, Holliday JR, Van Meter TE, Peckham R, Younas W, Briggs SEJ, MacDonald S, Butterfield T, Constantinou M, Fillmore HL. Current insights into matrix metalloproteinases and glioma progression: transcending the degradation boundary. Metalloproteinases Med. 2018;5:13-30. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Muller L, Muller-Haegele S, Mitsuhashi M, Gooding W, Okada H, Whiteside TL. Exosomes isolated from plasma of glioma patients enrolled in a vaccination trial reflect antitumor immune activity and might predict survival. Oncoimmunology. 2015;4:e1008347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 39. | Sreekanthreddy P, Srinivasan H, Kumar DM, Nijaguna MB, Sridevi S, Vrinda M, Arivazhagan A, Balasubramaniam A, Hegde AS, Chandramouli BA, Santosh V, Rao MR, Kondaiah P, Somasundaram K. Identification of potential serum biomarkers of glioblastoma: serum osteopontin levels correlate with poor prognosis. Cancer Epidemiol Biomarkers Prev. 2010;19:1409-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 40. | Crocker M, Ashley S, Giddings I, Petrik V, Hardcastle A, Aherne W, Pearson A, Bell BA, Zacharoulis S, Papadopoulos MC. Serum angiogenic profile of patients with glioblastoma identifies distinct tumor subtypes and shows that TIMP-1 is a prognostic factor. Neuro Oncol. 2011;13:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Zeng WJ, Yang YL, Wen ZP, Chen P, Chen XP, Gong ZC. Identification of gene expression and DNA methylation of SERPINA5 and TIMP1 as novel prognostic markers in lower-grade gliomas. PeerJ. 2020;8:e9262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |