Published online Jul 24, 2020. doi: 10.5306/wjco.v11.i7.504
Peer-review started: February 14, 2020
First decision: May 28, 2020
Revised: June 2, 2020
Accepted: June 10, 2020
Article in press: June 10, 2020
Published online: July 24, 2020
Processing time: 156 Days and 20.1 Hours
Bevacizumab is an antiangiogenic agent, and that synergizes with chemotherapeutic drugs. When used in combination therapies, Bevacizumab is associated with adverse events such as hemorrhage, gastrointestinal perforation, delayed wound healing, and pneumothorax. However, the molecular mechanisms underlying these adverse events are not fully understood.
A 45-year-old female with multiple lung metastases that were derived from primary breast cancer, was placed on Bevacizumab + paclitaxel therapy, since this combination has a potent antitumor effect. She reported dyspnea before cycle 3, day 1 and we therefore ran a chest X-ray, which detected a right pneumothorax. The coronal plane computed tomography revealed that one solid mass rapidly necrosed and was replaced by a cavity that passed through the bronchus in the right lower lobe. The cavity eventually ruptured the pleura and made the bronchopleural fistula that led to this pneumothorax. Thoracic cavity drainage using an intercostal catheter was performed. On the 7th day of drainage, the patient was discharged from our hospital on recovery. Recurrence of pneumothorax was not reported, and continuation of chemotherapy was made possible by changing the regimen.
Patients with lung metastases surrounding the bronchi and on the pleura should be monitored for pneumothorax by Bevacizumab-containing chemotherapies.
Core tip: Combination therapy with Bevacizumab and chemotherapy is used to expect its high antitumor effect. However, Bevacizumab-containing combination therapies can induce severe adverse events. Mechanisms and risk factors that underlie the onset of pneumothorax are not clear. Here, we report a case of pneumothorax associated with a combination therapy of Bevacizumab with chemotherapy, and discuss the mechanisms and risk factors. Patients with multiple lung metastases surrounding the bronchi and on the pleura, should be monitored for pneumothorax, an adverse event that can be induced by the rapid necrosis due to Bevacizumab-containing combination chemotherapies.
- Citation: Ozaki Y, Yoshimura A, Sawaki M, Hattori M, Gondo N, Kotani H, Adachi Y, Kataoka A, Sugino K, Horisawa N, Endo Y, Nozawa K, Sakamoto S, Iwata H. Mechanisms and anatomical risk factors of pneumothorax after Bevacizumab use: A case report. World J Clin Oncol 2020; 11(7): 504-509
- URL: https://www.wjgnet.com/2218-4333/full/v11/i7/504.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i7.504
Bevacizumab (BV) is a monoclonal humanized antibody targeting vascular endothelial growth factor (VEGF). BV exerts its high antitumor effect by suppressing tumor angiogenesis and improving the delivery of chemotherapeutic drugs through normalization of the vascular plexus in tumors[1-4]. BV also synergizes with chemotherapeutic agents to promote tumor regression. However, BV-containing combination therapies can induce severe adverse events such as gastrointestinal perforation[5-7], hemorrhage[8,9], delayed wound healing[10], and pneumothorax[11-14]. Although there have been few reports of pneumothorax, the risk factors and molecular mechanisms that underlie the onset of pneumothorax are not clear[11,14]. Here, we report a case of pneumothorax associated with a combination therapy of BV with chemotherapy, and discuss the molecular mechanisms and risk factors that were associated with this serious adverse event.
A 45-year-old female who was diagnosed with lung metastases of breast cancer and received combination therapy with Bevacizumab and chemotherapy developed dyspnea.
The case is a 45-year-old female. She visited our hospital because of an abnormality in her mammography and was diagnosed with left breast cancer (cStageT3N1M0). Pathological analysis of a core needle biopsy sample indicated a triple negative subtype (estrogen receptor-negative, progesterone receptor-negative and human epidermal growth factor receptor 2-negative). We planned 4 cycles of 5-fluorouracil + epirubicin + cyclophosphamide (FEC) followed by weekly paclitaxel (PTX) given 12 times as neoadjuvant chemotherapy. However neoadjuvant chemotherapy was stopped because of progression during weekly PTX; a left mastectomy and axillary lymph node dissection was then performed. The patient received postoperative chemotherapy (2 cycles of FEC followed by 8 cycles of capecitabine), but bilateral lung metastases were detected after the 5th cycle of capecitabine following a chest computed tomography (CT) that was performed due to her complaint of a persistent cough.
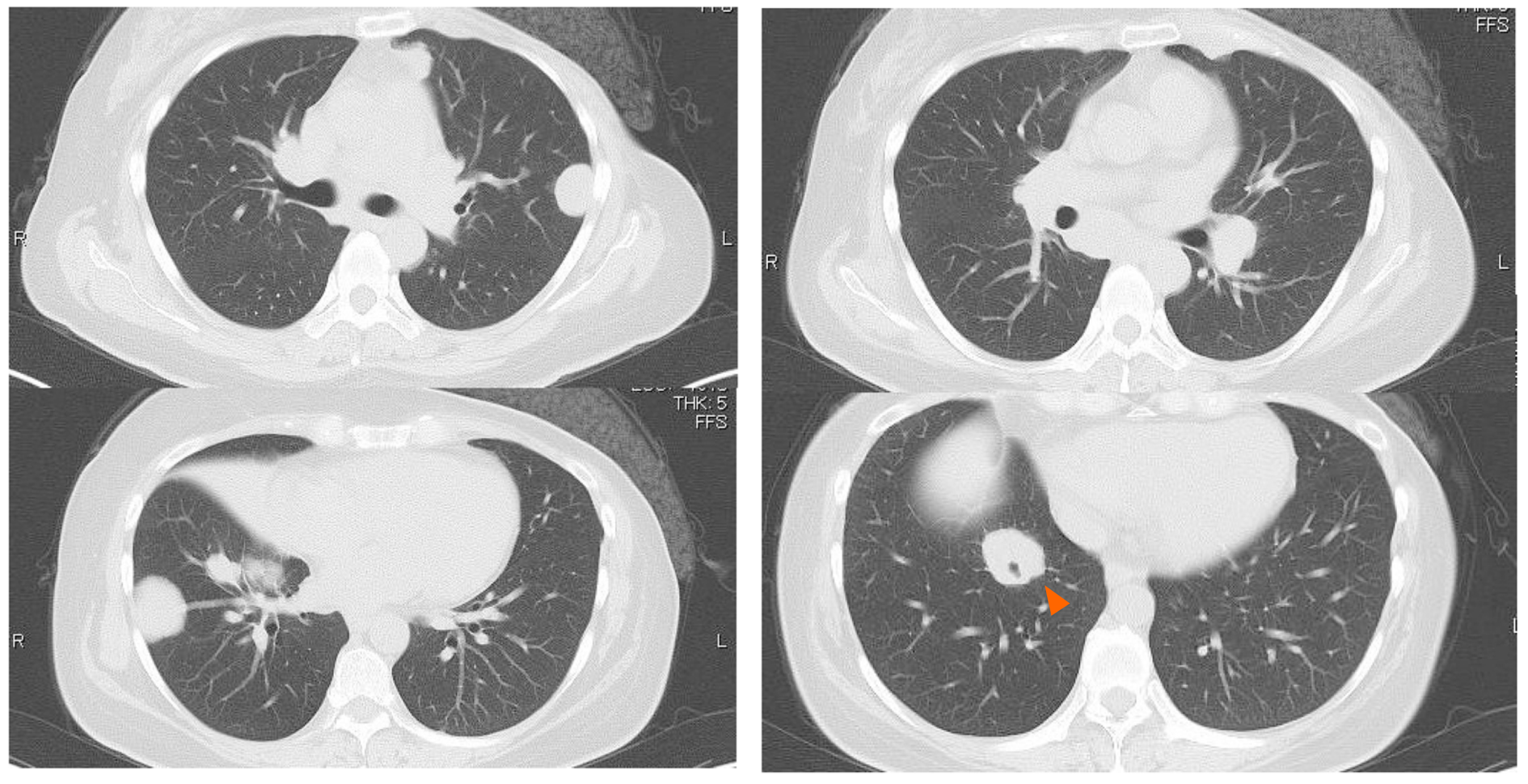
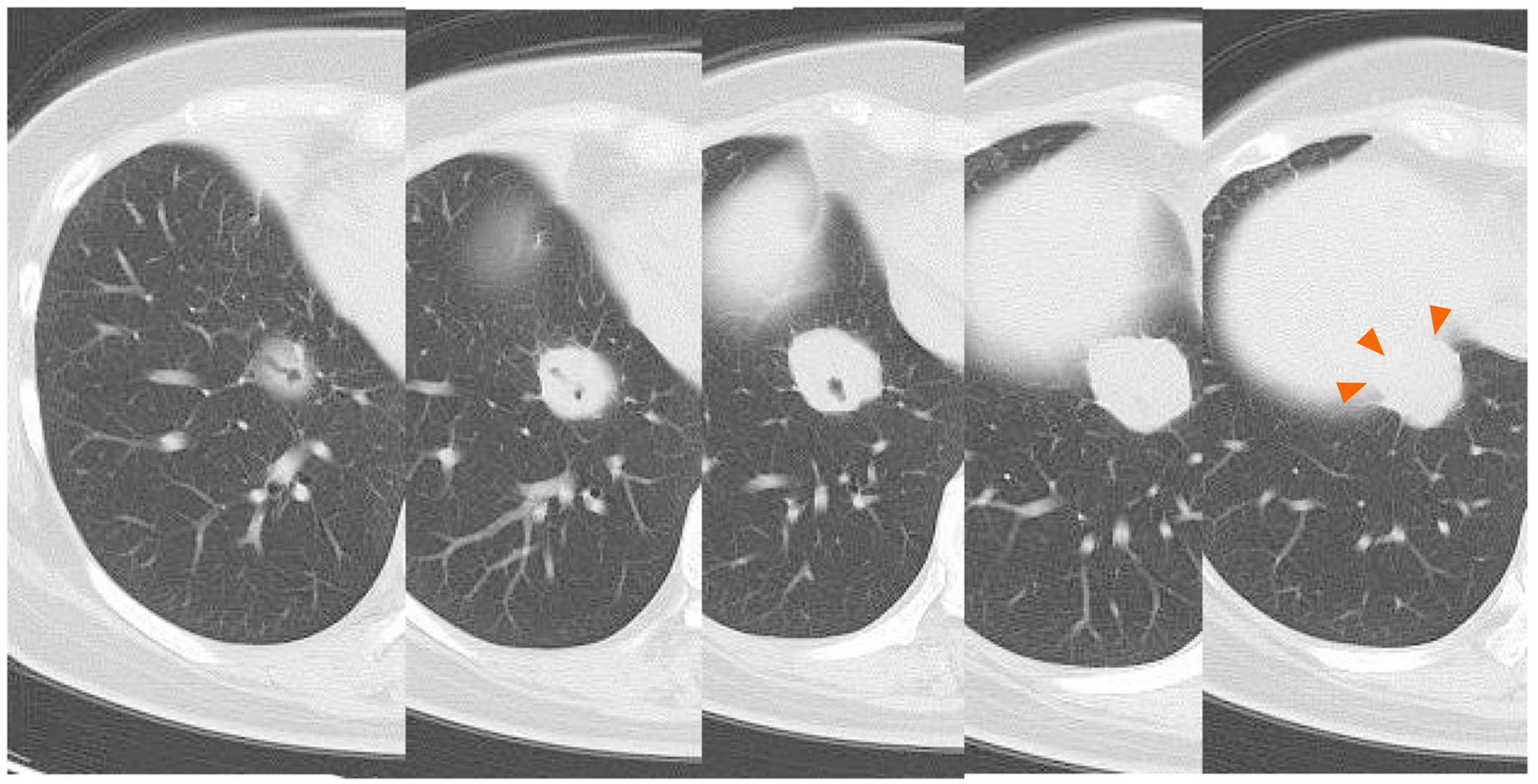
Some masses were on the pleura and one of the masses developed surrounding a bronchus at the right lower lobe (Figure 1 and 2). We made a diagnosis of multiple lung metastases of breast cancer. BV + PTX therapy was started due to its expected high antitumor effect. One cycle was 28 days, and BV was administered at 10 mg/kg on days 1 and 8 and PTX was administered at 80 mg/m2 on days 1, 8 and 15. The patient noticed dyspnea before cycle 3, day 1.
She was tachypnea. The oxygen saturation at atmospheric pressure was 95%.
There were no noticeable abnormalities.
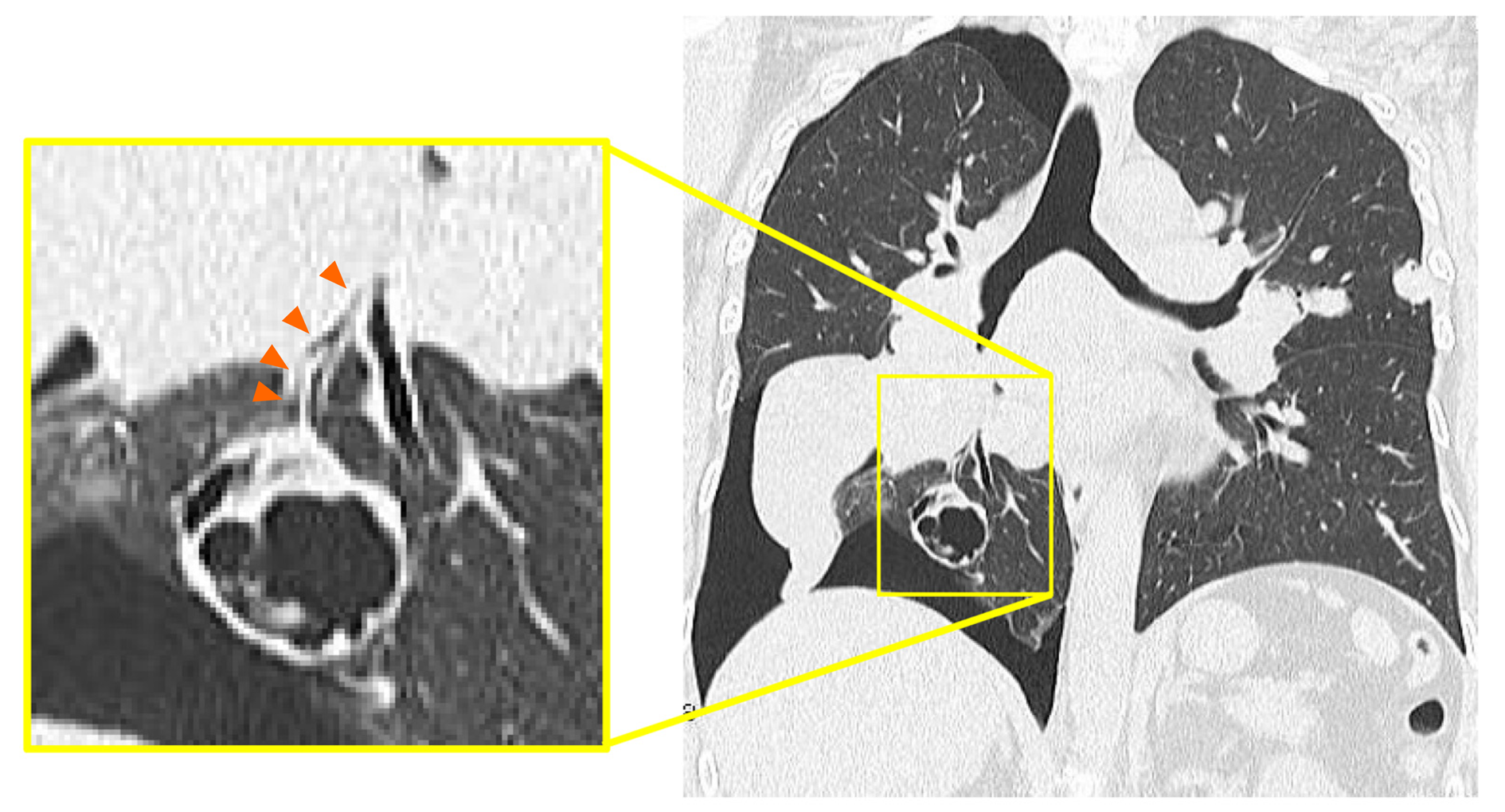
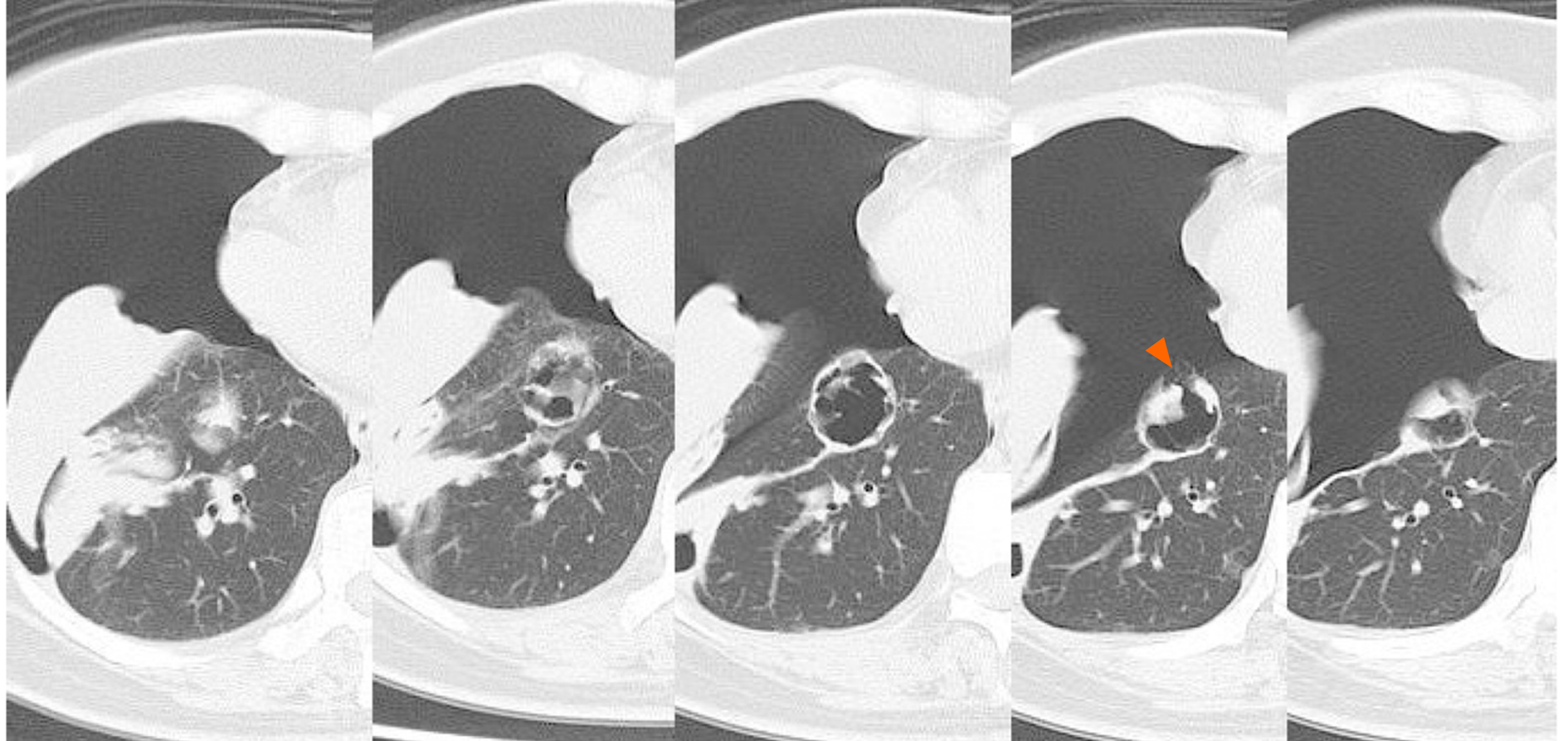
A right pneumothorax was diagnosed following a chest X-ray. The coronal plane CT revealed one solid mass replaced by a cavity that passed through the bronchus in the right lower lobe (Figure 3). The cavity eventually ruptured the pleura and made the bronchopleural fistula that led to this pneumothorax (Figure 4).
We diagnosed pneumothorax due to bronchopleural fistula that is associated with rapid necrosis of the lung metastasis.
The patient was admitted to our hospital for management of the pneumothorax. Thoracic cavity drainage using an intercostal catheter was performed. Supplemental oxygen was not required. On the 2nd day, the leakage disappeared. On the 4th, the right lung was fully expanded on the X-ray and the drain was clamped. On the 6th, the drain was withdrawn. On the 7th, the patient was discharged from our hospital on recovery.
No recurrence of pneumothorax was observed following release from hospital. The continuance of chemotherapy was made possible by changing the regimen (irinotecan, gemcitabine + carboplatin). The patient died due to breast cancer 23 mo after her first treatment, which was 9 mo from the occurrence of the pneumothorax.
BV is associated with the occurrence of several adverse events, such as gastrointestinal perforation[5-7], hemorrhage[8,9], delayed wound healing[10] and pneumothorax[11-14]. Although there have been few reports of pneumothorax, the risk factors and molecular mechanisms that underlie the onset of pneumothorax are not clear[11,14].
A more effective delivery of chemotherapeutic agents during BV treatment may explain the onset of gastrointestinal perforation in colon cancer[5]. This is because the drugs induced rapid necrosis in the tumor, which infiltrates the serosa. Fatal hemoptysis in patients with lung squamous carcinoma has been reported in a phase II trial[9]. This suggests that centrally located lung tumors close to major blood vessels might be linked to severe pulmonary hemorrhage, since they may lead to collapsed vessels. And, as for breast cancer, a case was reported in which axillary arterial bleeding occurred after BV chemotherapy induced axillary metastatic lymph node necrosis[8].
BV promotes tumor regression by normalizing the vascular plexus in tumors, which is associated with improved delivery of chemotherapeutic drugs. This causes necrosis of tumors that lie close to important organs, and leads to severe adverse events[1-4]. Another adverse effect associated with BV is delayed wound healing, as it inhibits the physiological endothelial repair processes mediated by VEGF[10].
The response of tumors to BV chemotherapy can to some extent explain why the solid mass in this case developed a cavity. The tumor surrounded the bronchi, and therefore induction of rapid necrosis and discharge of breakdown products via the bronchi would lead to cavity formation. This may be unique to this particular case, where only one solid mass surrounded the bronchi and became cavitary.
Another anatomical feature of this case is that the mass was on the pleura, and was therefore also susceptible to necrosis in this location. The cavity most likely ruptured the pleura, creating the bronchopleural fistula that led to this pneumothorax. The delayed wound healing caused by BV may also be associated with fistula formation.
Patients with multiple lung metastases surrounding the bronchi and on the pleura, should be monitored for pneumothorax, an adverse event that can be induced by the rapid necrosis due to Bevacizumab-containing combination chemotherapies.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aykan NF S-Editor: Gong ZM L-Editor: A E-Editor: Zhang YL
| 1. | Gray J, Murren J, Sharma A, Kelley S, Detterbeck F, Bepler G. Perforated viscus in a patient with non-small cell lung cancer receiving bevacizumab. J Thorac Oncol. 2007;2:571-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2468] [Cited by in RCA: 2569] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 3. | Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2025] [Cited by in RCA: 2184] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 4. | Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4319] [Cited by in RCA: 3955] [Article Influence: 197.8] [Reference Citation Analysis (0)] |
| 5. | Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol. 2009;10:559-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 307] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 6. | Van Cutsem E, Rivera F, Berry S, Kretzschmar A, Michael M, DiBartolomeo M, Mazier MA, Canon JL, Georgoulias V, Peeters M, Bridgewater J, Cunningham D; First BEAT investigators. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20:1842-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 411] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 7. | Shord SS, Bressler LR, Tierney LA, Cuellar S, George A. Understanding and managing the possible adverse effects associated with bevacizumab. Am J Health Syst Pharm. 2009;66:999-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 8. | Abe N, Ohtake T, Abe S, Aoto K, Okano M, Tachibana K, Yoshida S, Yasuda M, Kimijima I, Takenoshita S. [A Case of Axillary Arterial Bleeding after Axillary Metastatic Lymph Node Necrosis during Treatment with Paclitaxel and Bevacizumabfor Breast Cancer]. Gan To Kagaku Ryoho. 2016;43:2062-2064. [PubMed] |
| 9. | Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF, Gaudreault J, Damico LA, Holmgren E, Kabbinavar F. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1564] [Cited by in RCA: 1464] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 10. | Sharma K, Marcus JR. Bevacizumab and wound-healing complications: mechanisms of action, clinical evidence, and management recommendations for the plastic surgeon. Ann Plast Surg. 2013;71:434-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Iida T, Yabana T, Nakagaki S, Adachi T, Kondo Y. A Rupture of a Lung Metastatic Lesion of Colon Cancer, Leading to Pneumothorax Caused by Bevacizumab. Intern Med. 2016;55:3125-3129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Upadya A, Amoateng-Adjepong Y, Haddad RG. Recurrent bilateral spontaneous pneumothorax complicating chemotherapy for metastatic sarcoma. South Med J. 2003;96:821-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Interiano RB, McCarville MB, Wu J, Davidoff AM, Sandoval J, Navid F. Pneumothorax as a complication of combination antiangiogenic therapy in children and young adults with refractory/recurrent solid tumors. J Pediatr Surg. 2015;50:1484-1489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Yang SH, Lin JK, Chen WS, Lin TC, Yang SH, Jiang JK, Chang SC, Lan YT, Chao TC, Yen CC, Tzeng CH, Teng HW. Pneumothorax after bevacizumab-containing chemotherapy: a case report. Jpn J Clin Oncol. 2011;41:269-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |