Published online Apr 24, 2020. doi: 10.5306/wjco.v11.i4.190
Peer-review started: December 29, 2019
First decision: January 19, 2020
Revised: January 24, 2020
Accepted: March 22, 2020
Article in press: March 22, 2020
Published online: April 24, 2020
Processing time: 115 Days and 6.6 Hours
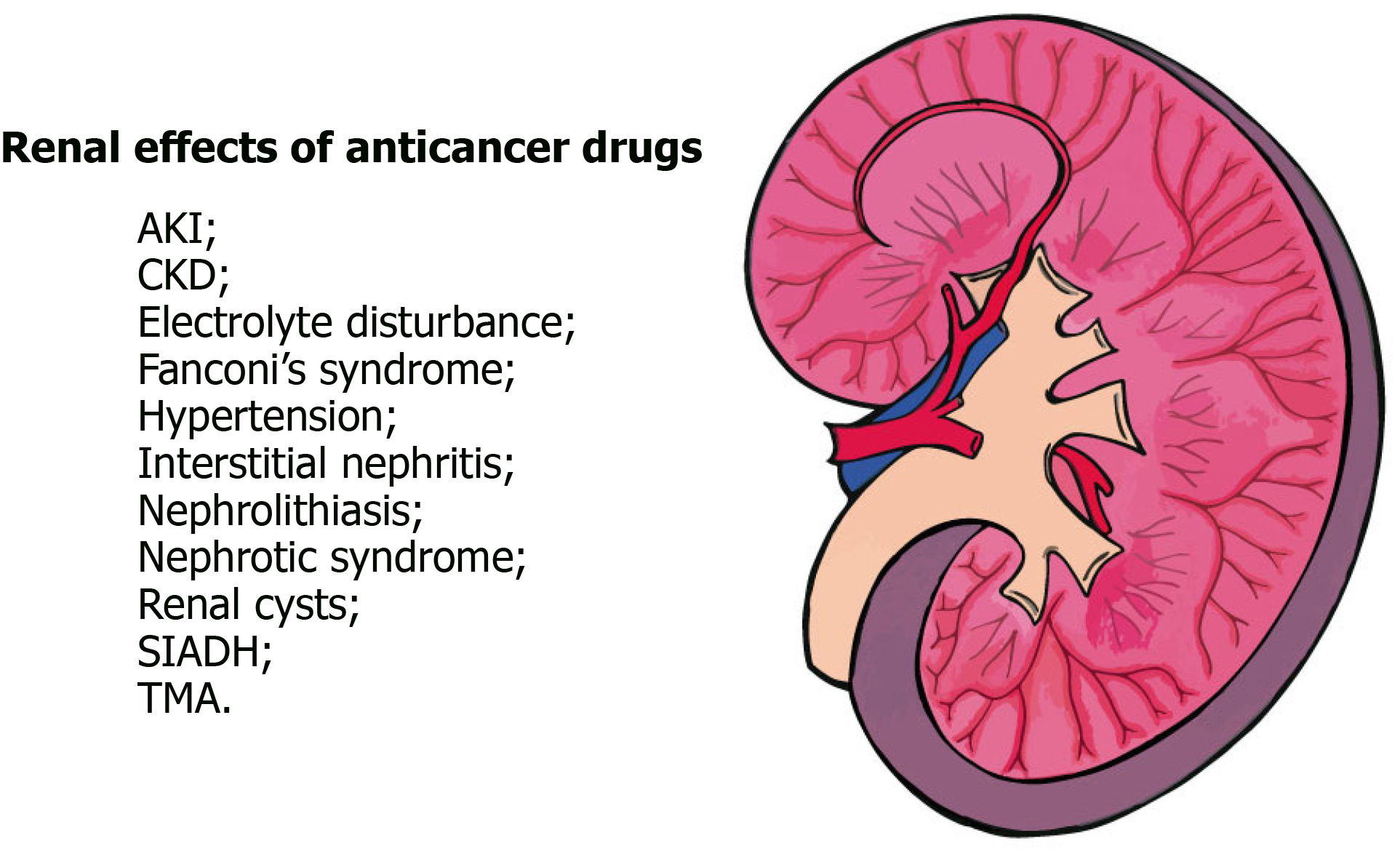
Anticancer drug nephrotoxicity is an important and increasing adverse drug event that limits the efficacy of cancer treatment. The kidney is an important elimination pathway for many antineoplastic drugs and their metabolites, which occurs by glomerular filtration and tubular secretion. Chemotherapeutic agents, both conventional cytotoxic agents and molecularly targeted agents, can affect any segment of the nephron including its microvasculature, leading to many clinical manifestations such as proteinuria, hypertension, electrolyte disturbances, glomerulopathy, acute and chronic interstitial nephritis, acute kidney injury and at times chronic kidney disease. The clinician should be alert to recognize several factors that may maximize renal dysfunction and contribute to the increased incidence of nephrotoxicity associated with these drugs, such as intravascular volume depletion, the associated use of nonchemotherapeutic nephrotoxic drugs (analgesics, antibiotics, proton pump inhibitors, and bone-targeted therapies), radiographic ionic contrast media or radiation therapy, urinary tract obstruction, and intrinsic renal disease. Identification of patients at higher risk for nephrotoxicity may allow the prevention or at least reduction in the development and severity of this adverse effect. Therefore, the aim of this brief review is to provide currently available evidences on oncologic drug-related nephrotoxicity.
Core tip: Nephrotoxicity is an adverse event that is well described with the use of conventional cytotoxic agents and with the advent of new agents directed to specific genes/proteins in recent decades. Its nephrotoxic potential has also been observed, which limits the effectiveness of treatment and increases the morbidity and mortality of these patients. Our objective was to recognize the main chemotherapeutic drugs with nephrotoxic potential and the most common types of kidney injury to prevent or at least reduce their occurrence and severity, allowing better therapeutic indices.
- Citation: Santos MLC, Brito BB, da Silva FAF, Botelho ACDS, Melo FF. Nephrotoxicity in cancer treatment: An overview. World J Clin Oncol 2020; 11(4): 190-204
- URL: https://www.wjgnet.com/2218-4333/full/v11/i4/190.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i4.190
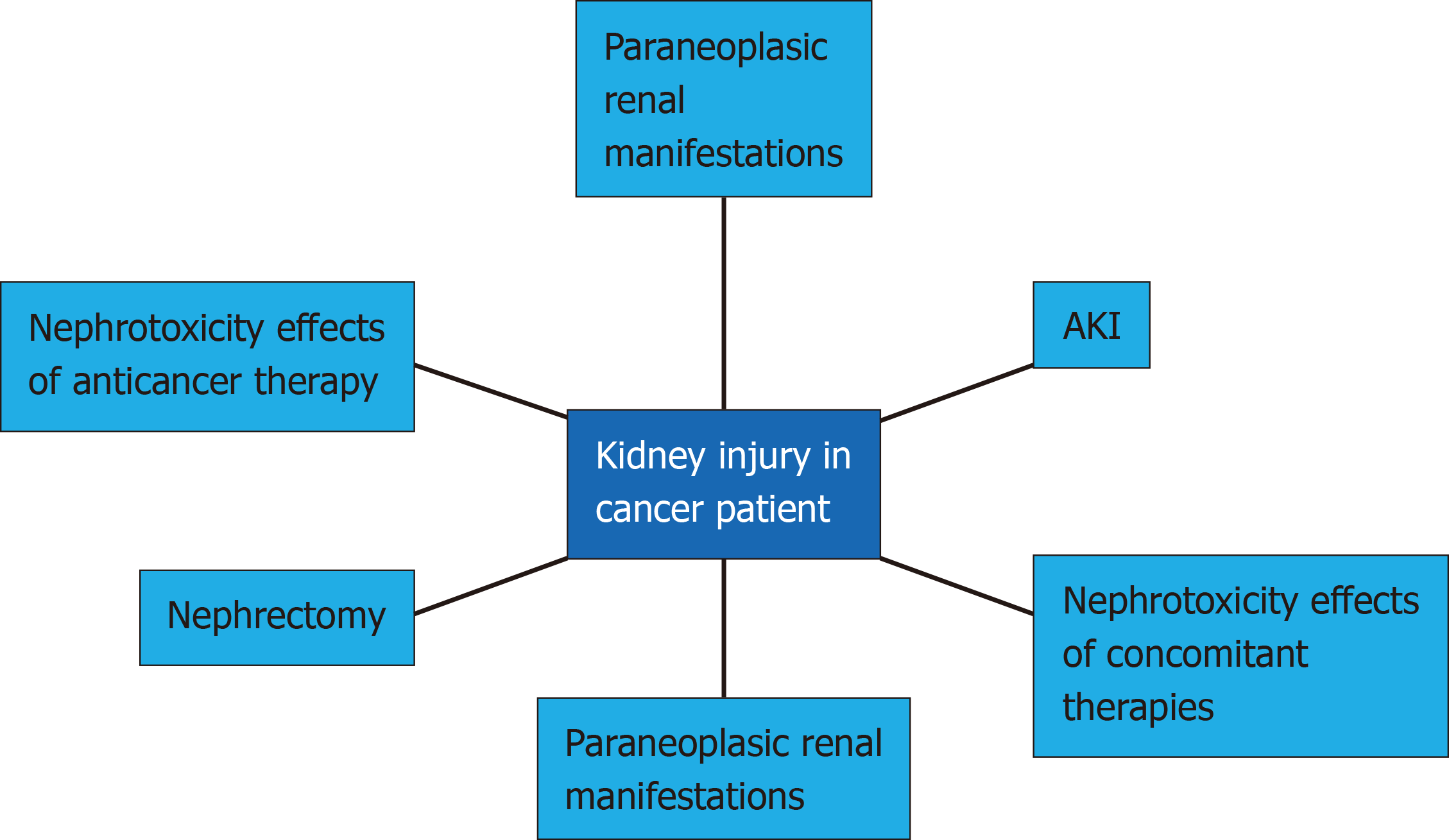
Cancer is an important cause of death worldwide and is associated with significant morbidity related to the underlying disease itself, as well as the adverse effects of chemotherapy. Conventional chemotherapeutic drugs are first-line agents to treat several malignancies but cause kidney toxicity, which occurs when kidney excretion properly due to the action of harmful chemicals[1]. In the last several decades, novel cancer drugs have been developed and used in clinical practice, being more specific against cancer cells and extremely effective against several previously untreatable malignancies, the so-called molecularly targeted agents, but also suffer from nephrotoxicity (Figure 1), which limits the efficacy of the treatment and impact their quality of life and overall survival[2]. A variety of renal complications can occur among cancer patients associated with malignancy (paraneoplastic renal manifestation, need for nephrectomy and urinary tract obstruction) or its treatment (nephrotoxicity effects of chemotherapy: Acute kidney disease (AKI), due to toxic acute tubular necrosis, thrombotic microangiopathy (TMA), and crystal nephropathy; proteinuria/nephrotic syndrome due to TMA and glomerulopathies; tubulopathies due to electrolyte and acid-base disorders; and chronic kidney disease (CKD) due to glomerulopathies or interstitial nephritis) or intrinsic kidney injury related to other pre-existing patient risk factors such as female gender, reduced muscle mass and reduced body water - mainly related to higher age, hypertension, diabetes, congestive heart failure, cirrhosis, hepatic failure, hyperbilirubinemia and hypoalbuminemia. Regarding kidney-related risk factors, are listed nephrosis, previous kidney injury, nephrotic syndrome and hydroelectrolytic disturbance which can be consequence of vomiting, diarrhea and use of diuretics[3]. Kidney injury in cancer patients is shown in Figure 2.
Many of the drug-related nephrotoxicities do not have a well-defined mechanism of injury or pathophysiology, which makes it difficult to develop strategies to prevent or minimize their occurrence. However, there are some factors that may contribute to the higher incidence of this adverse event including intravascular volume depletion, the associated use of non-chemotherapeutic nephrotoxic drugs (analgesics, antibiotics, proton pump inhibitors and bone-targeted therapies), radiographic ionic contrast media or radiation therapy, urinary tract obstruction, and intrinsic renal disease[4]. These factors should be considered by the oncologist before initiating treatment to minimize the risk of nephrotoxicity. Table 1 summarizes the main classes of chemotherapy and their representatives, the renal lesions associated with its use, and the mechanism that leads to nephrotoxicity. This review provides an update of anticancer drugs that are associated with kidney injury.
| Class | Drugs | Nephrotoxicity | Mechanism of action |
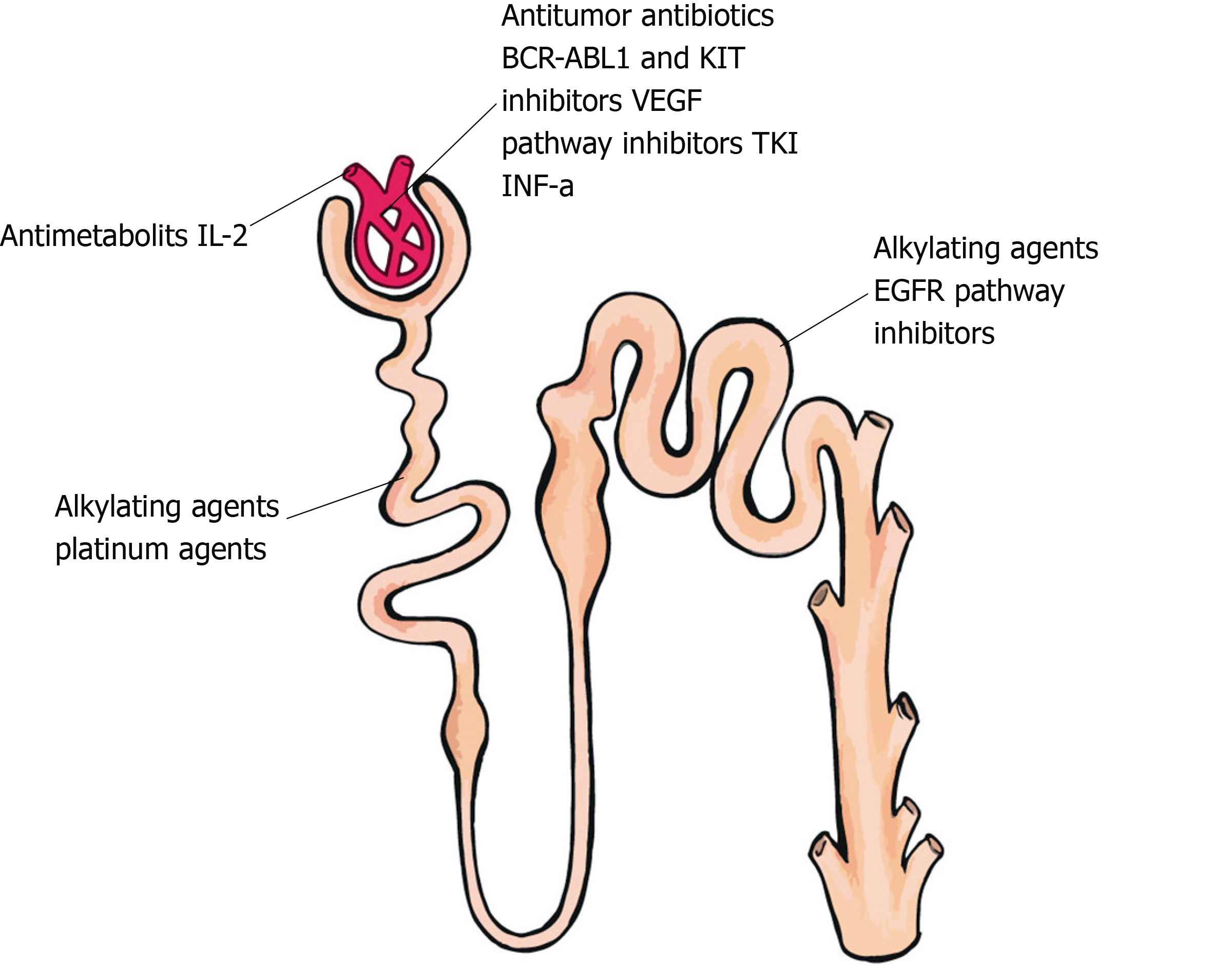
| Alkylating agents | Bendamustine; Cyclophosphamide; Ifosfamide; Melfalano; Nitrosureasnts | AKI; Hemorrhagic cystitis; Inflammatory lesion; SIADH; Hyponatremia; Fanconi's syndrome; Interstitial nephritis; Diabetes | Damage to proximal and distal tubular structures by action of metabolites and increased cellular oxidative stress |
| Antimetabolites | Chlopharabine; Methotrexate; Pemetrexed; Gemcitabine; Pentostatin | AKI; Decreased GFR; Interstitial edema; Tubular acidosis; Diabetes insipidus; Microangiopathic hemolytic anemia; SIADH; Hyponatremia | Decreased GFR due to vasoconstrictor action on afferent renal arteries; Crystal precipitation in tubules and induction of tubular injury |
| Anti-microtubular agents | Paclitaxis; Vincristine; Vinblastine; Vinorelbine | SIADH | Inhibits synthesis of genetic material or causes irreparable DNA damage |
| Antitumor antibiotics | Daunorubicin; Doxorubicin; Mitomycin | Nephrotic syndrome; Focal segmental glomerular sclerosis; TMA; AKI; Hemolytic uremic syndrome | Epithelial lesions (podocytes) |
| Platinum agents | Cisplatin; Carboplatin; Ocaliplatin | AKI; Anemia; Hypomagnesemia; Proximal tubular dysfunction; TMA | Drug accumulation in the proximal renal tubules |
| Cytotoxic agents | Arsenic trioxide; Etoposide; Irinotecan; Topotecan | Tubulointerstitial disease; Rhabdomyolysis; AKI | Increased exposure can be toxic; Higher levels of hematologic toxicity |
| Immunomodulatory drugs | Thalidomide; Lenalidomide; Pomalidomide | Hypercalcemia; Decreased GFR; Nephrolithiasis | Unclear, depending on the drug, may be related to its type of metabolism |
| Proteasome inhibitors | Bortezomib; Carfilzomib; Ixazomib | TMA; Acute interstitial nephritis; AKI | Prerenal causes (e.g., hypovolemia); Tumor lysis-like syndrome |
| EGFR pathway inhibitors | Cetuximab; Panitumumab; Afatinib; Erlotinib; Gefitinib | Electrolyte disturbance; AKI; Diffuse proliferative glomerulonephritis; Nephrotic syndrome | Inhibition of EGFR signaling at the distal convoluted tubule, which regulates transepithelial magnesium transport; AKI mechanism is unclear, however, EGFR plays a role in the maintenance of tubular integrity |
| HER-2 inhibitors | Trastuzumab; Ado-trastuzumab emtansine; Pertuzumab | Proteinuria; AKI; Decreased GFR; Electrolyte disturbance; Hypertension | Unclear |
| BCL-2 inhibitors | Venetoclax; Obituzumab; Ofatumumab | AKI | Tumor lysis syndrome |
| ALK inhibitors | Crizotinib; Alectinib; Brigatinib | Decreased GFR; Development of complex renal cysts; Electrolyte disturbance | Unclear |
| BRAF inhibitors | Vemurafenib; Dabrafenib; Trametinib; Cobimetinib | Decrease GFR; AKI; Glomerulonephritis; Hyponatremia; Hypertension | Unclear |
| MTOR inhibitors | Temsirolimus | Glomerulopathy; AKI; Proteinuria | Unclear |
| BCR-ABL1 and KIT inhibitors | Bosutinib; Dasatinib; Imatinib | Hypophosphatemia; Decreased GFR; AKI; Proteinuria; Nephrotic syndrome; CKD | Rhabdomyolysis; Thrombotic thrombocytopenic purpura; Alterations in glomerular podocytes; Tumor lysis syndrome; Acute tubular injury |
| Anti-angiogenesis drugs (VEGF pathway inhibitors and TKI) | Bevacizumab; Ramucirumab; Aflibercept; Sunitinib; Sorafenib; Pazopanib; Ponatinib; Others | Hypertension; Proteinuria; Nephrotic syndrome; Decreased GFR; TMA; Glomerulopathy; Electrolyte disturbance | Endothelial cell dysfunction and dysregulation of podocyte |
| Inhibitor of Bruton’s tyrosine kinase | Ibrutinib | AKI | Unclear, but tumor lysis syndrome might be contributory |
| Immune checkpoint inhibitors (PD-1, PD-L1, CTLA-4) | Ipilimumab; Pembrolizumab; Nivolumab | Acute tubulointerstitial nephritis; Immune complex glomerulonephritis; TMA; Electrolyte disturbance; AKI (rare) | Unclear, but development of autoantibodies that are pathogenic to the kidney might be contributory |
| Cytokine | INF-a | Proteinuria; Glomerulopathy; TMA; AKI | Minimal change disease or focal segmental glomerulosclerosis |
| Cytokine | IL-2 | AKI | Capillary leak syndrome leading to AKI |
| Peptide receptor radioligand | Lutetium Lu-177 dotatate | Decreased GFR | Kidney irradiation |
Bendamustine is a potent cytotoxic agent that is approved for use in the treatment of chronic lymphocytic leukemia and indolent non-Hodgkin’s lymphoma[5]. In only one study, the relationship of this drug to induction of AKI was observed[6]. Cyclophosphamide has toxicity caused by some of its metabolites, mainly in bone marrow and gonads, and its use has been related to the development of bladder cancer. Hemorrhagic cystitis is the major adverse urological effect, and its severity is related to dose dependence and treatment duration[7]. Cyclophosphamide-induced nephrotoxicity is often not considered in clinical practice, as the change in creatinine levels is of little significance[8]. However, a histological study of cyclophosphamide-treated mice found a significant inflammatory lesion in the animals' renal tissue. This injury has been attributed to increased oxidative stress on renal cells and decreased antioxidant agents[9]. This agent has been associated with the development of syndrome of inappropriate antidiuretic hormone secretion (SIADH), leading to severe hyponatremia in patients receiving high or moderate intravenous doses of this drug[10]. Nephrotoxic effects of ifosfamide are most commonly reported in childhood. In one study, insufficiency renal was observed in more than 80% of patients. In addition, a higher prevalence of Fanconi’s syndrome with hypophosphatemia, hypokalemia, glycosuria, and proteinuria is well documented[11,12]. Melphalan is generally used to treat multiple myeloma and ovarian cancer[13]. The association of high-dose melphalan use with SIADH has been reported[14]. In prolonged therapy with nitrosoureas, a slow, progressive, chronic, and often irreversible interstitial nephritis can be observed. However, the nephrotoxic mechanism of this class is not well understood in the literature[15]. Streptozotocin, which is used in several studies to induce diabetes in animal study models, causes mild proteinuria and elevated serum creatinine levels, followed by significant tubular damage that results in phosphaturia, glycosuria and uricosuria[16,17]. Trabectedin is a marine-derived alkylating agent that is used to treat advanced soft tissue sarcoma and has reports of renal failure, some of which have been attributed to rhabdomyolysis[18,19].
Clofarabine is approved for the treatment of acute lymphoblastic leukemia in children, as well as acute myeloid leukemia and acute lymphoblastic leukemia in adults. Two reports of severe kidney injury have been documented following administration of this drug as proteinuria and anuria requiring dialysis[20,21]. Renal insufficiency has been reported in some patients that undergone allogeneic hematopoietic cell transplantation[22,23]. Methotrexate is one of the most widely used antineoplastic drugs. Treatment with high doses may lead to acute kidney injury due to crystal precipitation in the tubules and induction of tubular injury[24]. This drug may transiently decrease the glomerular filtration rate (GFR) due to an afferent arteriolar constriction[25]. Pemetrexed is a methotrexate derivative used to treat advanced non-small cell lung cancer, and its nephrotoxic effects include acute tubular necrosis, interstitial edema, tubular acidosis, and diabetes insipidus[26-28]. Gemcitabine is used in many advanced neoplasms, being associated with a kidney injury such as hypertension and TMA in a case series. Moreover, hemolytic uremic syndrome may be a potential adverse effect[29]. Pentostatin is effective in treating hairy cell leukemia, and its use has been associated with mild renal dysfunction in some patients[30].
Paclitaxel is a microtubule inhibitor that is used in various antineoplastic treatments. Its nephrotoxicity was observed in a histological study with mice, in which dose-dependent cellular apoptosis and parenchymal necrosis were reported[31]. Other antimicrotubule agents, vincristine, vinblastine, and vinorelbine are related to a small number of SIADH cases[32,33].
Anthracyclines such as daunorubicin and doxorubicin cause nephrotic syndrome with significant renal lesions and focal segmental glomerular sclerosis. Pegylated liposomal doxorubicin has been linked to renal TMA and AKI[34,35]. Mitomycin nephrotoxicity is well documented due to direct damage to the renal parenchyma[36]. There are numerous cases reported in the literature of hemolytic uremic syndrome related to the cumulative dose of this drug. This syndrome leads, in most cases, to a slowly progressive renal failure and hypertension[37,38].
Cisplatin is one of the most widely used agents in cancer treatment and is known as one of the most nephrotoxic drugs. This nephrotoxic effect is dose-dependent and can be reversed with discontinuation of the drug[39]. The most common findings of renal damage in individuals using cisplatin are AKI, hypomagnesemia, proximal tubular dysfunction, and TMA[40]. Hydration and dose adjustment in patients with preexisting renal impairment is important to prevent cisplatin-induced nephrotoxicity[40,41]. Standardization of what is recommended is still necessary, mainly because current guidelines follow empirical treatments rather than clinical trials results[42]. However, this reasoning still depends much more on clinical manifestations and professional practice than isolated laboratory findings[41].
Another important platinum agent is carboplatin, which has a similar effect to cisplatin but with a significant reduction in expected adverse effects. The main one is hypomagnesemia, which is still less important than expected with cisplatin[42,43]. One caution that should be exercised is for patients who have already taken cisplatin and switched to carboplatin, because although it is not related to AKI, the patient is still at risk for this complication due to contact with cisplatin[44].
Among the new platinum agents, the third generation is mainly represented by oxaliplatin, a recent agent that has few adverse effects over previous ones and has relevant safety in patients with preexisting renal impairment, and does not require dose adjustment for these patients[45,46].
Arsenic trioxide is one of the main cytotoxic agents used in cancer treatments. At low doses commonly used, nephrotoxic effects are not common, which are usually represented by tubulointerstitial disease of the kidney and rhabdomyolysis[47]. However, because its metabolism is mainly renal, it is important to be careful and closely monitor its use in patients with preexisting renal impairment[48].
Etoposide is another cytotoxic agent and its use is related to renal insufficiency[49]. Some guidelines attempt to standardize etoposide’s dose adjustment in patients with renal dysfunction, and although data are different between societies, there is consensus on dose reduction in these patients, as well as discontinuation of their use for lower creatinine clearance[42,50].
Finally, there are also in this group irinotecan and topotecan. The former has hepatic metabolism and poor renal excretion[51]. Although lower renal complications are therefore expected, there are reports of toxicity in patients with preexisting renal impairment even at low doses[52]. There is also no standardization regarding its use in these patients. Topotecan is predominantly cleared by the kidneys, and increased toxicity may be seen in patients with moderate renal insufficiency[50,53]. There is still no worldwide consensus on dose adjustment for its use, although some studies are already moving towards standardization of drug dose reduction in patients with renal dysfunction.
This group of antineoplastic drugs is especially important for the treatment of multiple myeloma. Among them, thalidomide stands out first. Studies have shown that there is no relationship between AKI and the use of thalidomide, but the progression of the underlying disease itself. However, some case reports have shown hyperkalemia, requiring no dose adjustment, but close observation of potassium levels[54,55]. Lenalidomide, a thalidomide analog, mainly undergoes renal metabolism, which puts patients with prior renal injury at risk when exposed to this type of treatment and therefore requires dose adjustment[56,57]. Among the findings, AKI was reported including severe renal dysfunction and dialysis necessity[58-60].
Pomalidomide, although its metabolism is mainly hepatic, was related to AKI and nephrolithiasis. However, there are no standardizations for dose adjustment, except for patients on hemodialysis[61,62].
Proteasome inhibitors are primarily used for the treatment of multiple myeloma, and the drug Bortezomib has reports of TMA and acute interstitial nephritis with granuloma formation[63]. However, there are no standardizations regarding drug dose adjustment for patients with renal dysfunction. Carfilzomib, another drug of this class, has reports of TMA and AKI in several studies, such as Siegel et al[64] and Hájek et al[65]. The main mechanisms of action are described in Table 1[66,67]. Despite these data, there is still no standardization for dose adjustments for patients with renal injury. Another representative is ixazomib, which has reports of TMA development, since its excretion is primarily renal[68]. Thus, guidelines already indicate dose adjustment for patients with renal dysfunction.
Activation of epidermal growth factor receptor (EGFR) results in phosphorylation of the receptor tyrosine kinase, triggering signaling pathways that modulate cell differentiation, proliferation, and survival[69]. Such a receptor is a crucial target for the treatment of some cancers such as non-small cell lung cancer, which can be performed by administrating EGFR-tyrosine kinase inhibitors (EGFR-TKIs) or anti-EGFR monoclonal antibodies[70,71].
Rare cases of nephrotic syndrome accompanied by minimal-change disease and membranous nephropathy have been related to the use of gefitinib, an EGFR-TKI[72-74]. Moreover, this drug family might also cause hypomagnesemia, hypophosphatemia, and hypokalemia[75]. On the other hand, increased renal magnesium loss is associated with the administration of anti-EGFR monoclonal antibodies (cetuximab and panitumumab). That electrolyte wasting leads to hypomagnesemia in up to 37% of patients, which resolves with therapy discontinuation[76,77]. In addition, cetuximab causes hypokalemia in about 8% of treated individuals[78]. Furthermore, this drug is linked to other very rare complications such as acute kidney injury, nephrotic syndrome, and proliferative glomerulonephritis[75,79].
Some molecularly targeted therapies aim to inhibit human epidermal growth factor receptor 2 (HER-2), an overexpressed receptor in some breast and gastric /gastroesophageal junction cancers[80-82]. Trastuzumab is the precursor and the most successful example among antibodies aiming HER-2 antagonism[83]. However, this drug, as well as pertuzumab (a humanized monoclonal antibody), have been implicated in the occurrence of renal impairment, exemplified by acute kidney injury, proteinuria, elevated serum creatinine, and nephritis. In addition, hypertension, hypokalemia, hyponatremia, and hypomagnesemia have also been reported[75]. The administration of lapatinib, a TKI, has been associated with the development of acute kidney injury and hypokalemia. In a few patients, hyponatremia, hypomagnesemia, and hypertension have also been observed[75,84].
The cell intrinsic apoptotic pathway has B-cell leukemia/lymphoma-2 (BCL-2) proteins as crucial regulators. When such a pathway is disturbed, the improper perpetuation of malignant cells can occur[85]. Given the context, the emergence of venetoclax, a BCL-2 inhibitor, represents a promising solution for the treatment of refractory chronic lymphocytic leukemia. Despite the benefits, such a drug has been associated with significant incidence of tumor lysis syndrome, leading to huge electrolyte disturbances and AKI. To prevent these effects, the gradual escalation of drug dose administration to the patient is recommended[86,87].
Anaplastic lymphoma kinase 1 (ALK-1) belongs to the insulin receptor tyrosine kinase family, which plays an important role in cell growth regulation[88]. Mutations involving the gene that encodes this kinase are related to malignancies such as anaplastic large cell lymphoma, Hodgkin lymphoma, non-small cell lung cancer, neuroblastoma, and rhabdomyosarcoma[89-92]. Crizotinib is the first-developed ALK inhibitor and has been widely used for the treatment of advanced non-small cell lung cancer with positivity for the ALK fusion gene. Reductions in GFR have been observed in patients who underwent this therapy. However, the premature emergence of this involvement, its negligible cumulative effect, and the quick reversibility after drug discontinuation indicate that the occurrence of this phenomenon is not due to direct nephrotoxicity from crizotinib[93]. Moreover, this drug is also related to the emergence of complex renal cysts, hyponatremia, and hypokalemia in a limited number of patients; however, all of these are reversible with therapy interruption[94,95].
BRAF is the encoding gene of a human protein called B-Raf, and if muted, can promote cell proliferation and carcinogenesis[96]. Molecularly targeted therapies aimed toward its inhibition have been used in individuals with malignant melanoma containing the BRAF V600E mutation[97]. Vemurafenib is one of the approved drugs for such patients, being related to a reduction in creatinine clearance (reversible with treatment discontinuation) as well as rare cases of AKI, which are more commonly observed in men[98,99]. At a lower frequency, the use of another BRAF inhibitor named dabrafenib can also lead to AKI[99].
A serine/threonine kinase called mammalian target of rapamycin (mTOR) participates in the signaling pathways of growth factors and cytokines related to oncogenic activity, and its inhibition leads to cell cycle arrest[100]. The administration of mTOR inhibitors, such as temsirolimus, is associated with the development of proteinuria, and in some cases, has kidney dysfunction as a consequence[101].
The t(9;22)(q34;q11) chromosomal translocation that originates from the Philadelphia chromosome results in a BCR-ABL1 gene rearrangement observed in patients with chronic myeloid leukemia (CML)[102,103]. ABL can be targeted by bosutinib, which is used in the treatment of refractory CML and can lead to hypophosphatemia, as well as to a reversible decrease in GFR. With the goal of preventing advanced kidney disturbances due to adverse events from bosutinib, monitoring of renal function is recommended at baseline as well as while the patient undergoes this treatment. Moreover, dose reduction should be conducted when renal impairment due to the therapy occurs[104]. Besides BCR-ABL1 inhibition, a TKI named desatinib also acts to restrain the platelet-derived growth factor receptor and tyrosine kinase receptor KIT (CD117). This drug is rarely associated with AKI and proteinuria[105,106]. Another BCR-ABL1 and KIT inhibitor, imatinib, can be applied for the treatment of gastrointestinal stromal tumors beyond CML. If used for a long period, such an agent can lead to AKI and CKD, and kidney injury seems to be dose-dependent, with higher doses associated with a higher risk of renal impairment[107,108]. Moreover, imatinib administration is related to the occurrence of hypophosphatemia[109].
Vascular endothelial growth factor (VEGF) is an essential growth factor that plays a key role in angiogenesis during embryogenesis, wound healing, and tumor growth. It was first investigated as a potential anticancer agent over the past few decades[110].
There are two types of VEGF pathway inhibitors: VEGF ligand inhibitors, which are antagonists of the VEGF receptor and are represented by ramucirumab, bevacizumab, and aflibercept; and small molecule TKIs (ponatinib, sunitinib, regorafenib, sorafenib, cabozantinib, pazopanib, axitinib, vandetanib, cabozantinib, lenvatinib), which prevent the activation of the VEGF receptor intracellular domain[111].
In normal conditions, VEGF is produced by the podocytes and binds to its receptors found in glomerular and peritubular endothelium, as well as in mesangial cells. This process maintains the structure of the glomerular basement membrane and the proper glomerular functioning[112]. Therefore, all drugs that block the VEGF pathway may induce renal abnormalities. Their renal toxicity is mainly renovascular in nature including hypertension and proteinuria, occasionally causing nephrotic syndrome, decreased GFR, and TMA which remains rare[113]. However, the exact mechanism underlying proteinuria and the factors associated with the occurrence and severity of proteinuria are unknown. It is suggested that preexisting renal disease (including higher baseline urine protein levels and hypertension) and renal cell carcinoma, may be predisposing factors to proteinuria[114,115].
Interruption of anti-VEGF drugs use improves kidney dysfunction, but persistent proteinuria is not unusual. Although angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may lower intraglomerular pressure and diminish protein excretion, no recommendation for use of these agents can be made as there are no controlled studies on the subject[116].
Although there is a lack of information regarding kidney biopsies in patients that undergo VEGF-targeted agents treatment, studies have demonstrated the presence of collapsing glomerulopathy, TMA, and isolated reports of immune complex glomerulonephritis and cryoglobulinemic[117]. The most common causative agent is bevacizumab. Less common histologic findings have been reported with bevacizumab such as nephritic syndrome and AKI[118].
Regarding TKIs, proteinuria and hypertension can be seen with their use. In addition, AKI and diabetes insipidus have been reported in clinical trials of vandetanib, although causality has not been proven. Decreased GFR during therapy has been reported with axitinib, sunitinib, and sorafenib, although renal failure is rare. Patients treated with lenvatinib may progress to renal failure or impairment, while regorafenib has been associated with several electrolyte abnormalities, including hypophosphatemia, hypocalcemia, hyponatremia, and hypokalemia[119,120]. Sorafenib and sunitinib have been associated with acute and chronic interstitial nephritis in case reports[121,122]. Sorafenib is also known to cause hypophosphatemia and hypocalcemia[123].
Ibrutinib is an irreversible inhibitor of Burton's tyrosine kinase. This drug has activity in B cell malignancies and is approved for the patients with mantle cell lymphoma or chronic lymphocytic leukemia. It may be related to AKI and the mechanism of this injury is unclear, but tumor lysis syndrome might be contributory[124].
Moxetumomab pasudotox is used to ameliorate the prognosis of patients with relapsing or refractory hairy cell leukemia. Such a drug can be associated with AKI and proteinuria[125].
Inhibitors of poly-adenosine diphosphate ribose polymerase are approved for treatment of BRCA-mutated breast cancer and for platinum-sensitive relapsed epithelial ovarian cancer. Increased creatinine have been reported in some patients treated with olaparib, but, in most cases, it is mild[126].
The monoclonal antibodies known as checkpoint inhibitors (CPIs) target specific inhibitory receptors present in T cells, as well as in tumor cells and in other immune cells. The primary targets for checkpoint inhibition include programmed cell death 1 receptor (PD-1) and programmed cell death 1 ligand (PD-L1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), that play an important role in negatively regulating T cell activation/function, thus tumor cells carrying PDL-1 or CTLA-4 are protected from immune reactions. The objective of checkpoint inhibitors is to restore or generate the activation of the immune system, directed to tumor cells[127].
Drugs targeting PD-1 (nivolumab and pembrolizumab) and PD-L1 (atezolizumab, avelumab, and durvalumab) have recently demonstrated their potential efficacy in different tumor types (e.g., urothelial carcinoma, non-small cell lung cancer, melanoma, head and neck cancer, Merkel cell carcinoma, renal cell carcinoma, Hodgkin lymphoma, and microsatellite instability-high or mismatch repair deficient [dMMR] solid tumors) and drugs targeting CTLA-4 (ipilimumab) is approved for use in patients with advanced melanoma[128].
The immune response generated by CPIs is complicated by a number of immune-related adverse events related to many different organs, including the kidneys[129]. AKI is a rare complication of checkpoint inhibitor immunotherapy, being mainly associated with ipilimumab/nivolumab combination therapy (4.9%)[130]. The most commonly reported underlying pathology is acute tubulointerstitial nephritis, but immune complex glomerulonephritis and TMA have also been observed[131]. There is also an association between CPIs treatment and electrolyte abnormalities, with hypocalcemia being the most significant. Discontinuation of checkpoint inhibitor immunotherapy and treatment with corticosteroids are indicated for patients with severe renal injury[132].
Interferon-alpha activates interleukin-2 (IL-2) release, which leads to cancer cell death[133]. Recombinant interferon-alpha can cause proteinuria, which can be in the nephrotic range and AKI, the histology is consistent with minimal change disease or focal segmental glomerulosclerosis[134,135]. Rarely, TMA is seen and in this situation prompt drug discontinuation is critical[136].
IL-2 is used for the management of metastatic renal cell carcinoma and metastatic melanoma. IL-2 therapy causes cytokine-driven capillary leak syndrome, leading to intravascular volume depletion, edema, and a reversible fall in GFR. In this context, AKI is a consequence of prerenal azotemia from capillary leak. It is important to highlight that ischemic acute tubular injury may also occur due to severe hypotension[137]. Cytokine-mediated inflammatory kidney injury may also occur[138].
Chemotherapy-induced nephron specific segment injury is shown in Figure 3. The peptide receptor radioligand Lutetium Lu 177-dotatate is a radiolabeled somatostatin analog with potential antineoplastic activities. Lutetium Lu 177-dotatate binds to somatostatin receptors expressed by various neuroendocrine tumor cells. Once the radioligand binds to that receptor, this complex in internalized, resulting in beta radiation delivery to cells that express somatostatin receptors. Kidney irradiation may result in glomerular damage with renal impairment. An amino acid solution infusion is needed to protect the kidneys from the radiation effects of the therapeutic radionuclide. This protocol results in low rates of nephrotoxicity during therapy. No risk factors for renal toxicity have been identified[139].
Onco-nephrology has emerged as a new specialized therapeutic perspective for cancer patients. Despite the improvement of patients survival resulting from the use of conventional and molecularly targeted agents, several therapeutic complications due to nephrotoxic effects of these drugs have been reported. Given the background, it is important to know the possible adverse events related to these therapies, allowing early diagnosis of these effects, avoiding further issues due to this treatment. It is important to highlight that the approach in patients affected by nephrotoxicity due to anti-cancer drugs include, in addition to the forms previously mentioned specifically for each drug, close monitoring, proper hydration and dose reduction, with suspension of the agent use if necessary. It is also important to conduct more controlled studies in the sense of creating guidelines for dose adjustment in patients with renal impairment, since most of the dose adjustment standards for these drugs are being created recently through studies with small numbers of subjects and in initial stages of clinical trials. The development of these studies is also important to look at other treatment-associated nephrotoxic effects, as well as to reduce short and long-term complications related to such therapies.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Brazil
Peer-review report´s scientific quality classification
Grade A (Excellent):
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor):
P-Reviewer: Manenti A, Tanabe S, Sridharan G S-Editor: Zhang L L-Editor: Filipodia E-Editor: Liu MY
| 1. | Rosner MH, Perazella MA. Acute Kidney Injury in Patients with Cancer. N Engl J Med. 2017;376:1770-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (3)] |
| 2. | Perazella MA. Onco-nephrology: renal toxicities of chemotherapeutic agents. Clin J Am Soc Nephrol. 2012;7:1713-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 3. | Perazella MA, Izzedine H. New drug toxicities in the onco-nephrology world. Kidney Int. 2015;87:909-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Jhaveri KD, Fishbane S. Nephrology Crossword: Onco-nephrology--chemotherapy agents and nephrotoxicity. Kidney Int. 2013;84:421-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Owen JS, Melhem M, Passarell JA, D'Andrea D, Darwish M, Kahl B. Bendamustine pharmacokinetic profile and exposure-response relationships in patients with indolent non-Hodgkin's lymphoma. Cancer Chemother Pharmacol. 2010;66:1039-1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Prediletto I, Farag SA, Bacher U, Jeker B, Mansouri Taleghani B, Brégy R, Zander T, Betticher D, Egger T, Novak U, Pabst T. High incidence of reversible renal toxicity of dose-intensified bendamustine-based high-dose chemotherapy in lymphoma and myeloma patients. Bone Marrow Transplant. 2019;54:1923-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (2)] |
| 7. | Ponticelli C, Escoli R, Moroni G. Does cyclophosphamide still play a role in glomerular diseases? Autoimmun Rev. 2018;17:1022-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 8. | Lim SR, Hyun SH, Lee SG, Kim JY, Kim SH, Park SJ, Moon KS, Sul D, Kim DH, Choi HK. Potential urinary biomarkers of nephrotoxicity in cyclophosphamide-treated rats investigated by NMR-based metabolic profiling. J Biochem Mol Toxicol. 2017;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Bhat N, Kalthur SG, Padmashali S, Monappa V. Toxic Effects of Different Doses of Cyclophosphamide on Liver and Kidney Tissue in Swiss Albino Mice: A Histopathological Study. Ethiop J Health Sci. 2018;28:711-716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Liamis G, Filippatos TD, Elisaf MS. Electrolyte disorders associated with the use of anticancer drugs. Eur J Pharmacol. 2016;777:78-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Ensergueix G, Karras A. [Ifosphamide nephrotoxicity]. Nephrol Ther. 2018;14 Suppl 1:S125-S131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Farry JK, Flombaum CD, Latcha S. Long term renal toxicity of ifosfamide in adult patients--5 year data. Eur J Cancer. 2012;48:1326-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Falco P, Bringhen S, Avonto I, Gay F, Morabito F, Boccadoro M, Palumbo A. Melphalan and its role in the management of patients with multiple myeloma. Expert Rev Anticancer Ther. 2007;7:945-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Greenbaum-Lefkoe B, Rosenstock JG, Belasco JB, Rohrbaugh TM, Meadows AT. Syndrome of inappropriate antidiuretic hormone secretion. A complication of high-dose intravenous melphalan. Cancer. 1985;55:44-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Narins RG, Carley M, Bloom EJ, Harrison DS. The nephrotoxicity of chemotherapeutic agents. Semin Nephrol. 1990;10:556-564. [PubMed] |
| 16. | Graham ML, Janecek JL, Kittredge JA, Hering BJ, Schuurman HJ. The streptozotocin-induced diabetic nude mouse model: differences between animals from different sources. Comp Med. 2011;61:356-360. [PubMed] |
| 17. | Malyszko J, Kozlowska K, Kozlowski L, Malyszko J. Nephrotoxicity of anticancer treatment. Nephrol Dial Transplant. 2017;32:924-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Pick AM, Nystrom KK. Fatal hepatic and renal toxicity as a complication of trabectedin therapy for radiation-induced sarcoma. J Oncol Pharm Pract. 2010;16:269-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Skorupa A, Beldner M, Kraft A, Montero AJ. Fatal rhabdomyolysis as a complication of ET-743 (Yondelis) chemotherapy for sarcoma. Cancer Biol Ther. 2007;6:1015-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Kintzel PE, Visser JA, Campbell AD. Clofarabine-associated acute kidney injury and proteinuria. Pharmacotherapy. 2011;31:923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Jhaveri KD, Chidella S, Allen SL, Fishbane S. Clofarabine-induced kidney toxicity. J Oncol Pharm Pract. 2014;20:305-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | van Besien K, Stock W, Rich E, Odenike O, Godley LA, O'Donnell PH, Kline J, Nguyen V, Del Cerro P, Larson RA, Artz AS. Phase I-II study of clofarabine-melphalan-alemtuzumab conditioning for allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:913-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Petri CR, O'Donnell PH, Cao H, Artz AS, Stock W, Wickrema A, Hard M, van Besien K. Clofarabine-associated acute kidney injury in patients undergoing hematopoietic stem cell transplant. Leuk Lymphoma. 2014;55:2866-2873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 461] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 25. | Howell SB, Carmody J. Changes in glomerular filtration rate associated with high-dose methotrexate therapy in adults. Cancer Treat Rep. 1977;61:1389-1391. [PubMed] |
| 26. | Zattera T, Londrino F, Trezzi M, Palumbo R, Granata A, Tatangelo P, Corbani V, Falqui V, Chiappini N, Mathiasen L, Cavallini M, Rolla D. Pemetrexed-induced acute kidney failure following irreversible renal damage: two case reports and literature review. J Nephropathol. 2017;6:43-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 27. | Vootukuru V, Liew YP, Nally JV. Pemetrexed-induced acute renal failure, nephrogenic diabetes insipidus, and renal tubular acidosis in a patient with non-small cell lung cancer. Med Oncol. 2006;23:419-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Stavroulopoulos A, Nakopoulou L, Xydakis AM, Aresti V, Nikolakopoulou A, Klouvas G. Interstitial nephritis and nephrogenic diabetes insipidus in a patient treated with pemetrexed. Ren Fail. 2010;32:1000-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Glezerman I, Kris MG, Miller V, Seshan S, Flombaum CD. Gemcitabine nephrotoxicity and hemolytic uremic syndrome: report of 29 cases from a single institution. Clin Nephrol. 2009;71:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Iwasaki T, Ohara H, Matsumoto S, Matsudaira H. Test of magnetic sensitivity in three different biological systems. J Radiat Res. 1978;19:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Rabah SO. Acute Taxol nephrotoxicity: Histological and ultrastructural studies of mice kidney parenchyma. Saudi J Biol Sci. 2010;17:105-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Garrett CA, Simpson TA. Syndrome of inappropriate antidiuretic hormone associated with vinorelbine therapy. Ann Pharmacother. 1998;32:1306-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Cutting HO. Inappropriate secretion of antidiuretic hormone secondary to vincristine therapy. Am J Med. 1971;51:269-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 60] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Carron PL, Padilla M, Maurizi Balzan J. Nephrotic syndrome and acute renal failure during pegylated liposomal doxorubicin treatment. Hemodial Int. 2014;18:846-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Shavit L, Lifschitz MD, Gabizon A, Kwa M, Muggia F, Slotki I. Pegylated liposomal doxorubicin and renal thrombotic microangiopathy: an under-recognized complication of prolonged treatment for ovarian cancer. Kidney Int. 2014;85:213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Groff JA, Kozak M, Boehmer JP, Demko TM, Diamond JR. Endotheliopathy: a continuum of hemolytic uremic syndrome due to mitomycin therapy. Am J Kidney Dis. 1997;29:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Cordonnier D, Vert-Pré FC, Bayle F, Alix JL, Couderc P. [Nephrotoxicity of mitomycin C (apropos of 25 case reports). Results of a multicenter survey organized by the Society of Nephrology]. Nephrologie. 1985;6:19-26. [PubMed] |
| 38. | Giroux L, Bettez P, Giroux L. Mitomycin-C nephrotoxicity: a clinico-pathologic study of 17 cases. Am J Kidney Dis. 1985;6:28-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Bitran JD, Desser RK, Billings AA, Kozloff MF, Shapiro CM. Acute nephrotoxicity following cis-dichlorodiammine-platinum. Cancer. 1982;49:1784-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Crona DJ, Faso A, Nishijima TF, McGraw KA, Galsky MD, Milowsky MI. A Systematic Review of Strategies to Prevent Cisplatin-Induced Nephrotoxicity. Oncologist. 2017;22:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 273] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 41. | Raj GV, Iasonos A, Herr H, Donat SM. Formulas calculating creatinine clearance are inadequate for determining eligibility for Cisplatin-based chemotherapy in bladder cancer. J Clin Oncol. 2006;24:3095-3100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Kintzel PE, Dorr RT. Anticancer drug renal toxicity and elimination: dosing guidelines for altered renal function. Cancer Treat Rev. 1995;21:33-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 250] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | Joel SP, Shah R, Clark PI, Slevin ML. Predicting etoposide toxicity: relationship to organ function and protein binding. J Clin Oncol. 1996;14:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 44. | Ries F, Klastersky J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am J Kidney Dis. 1986;8:368-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 235] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 45. | McDonald BR, Kirmani S, Vasquez M, Mehta RL. Acute renal failure associated with the use of intraperitoneal carboplatin: a report of two cases and review of the literature. Am J Med. 1991;90:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Labaye J, Sarret D, Duvic C, Hérody M, Didelot F, Nédélec G, Noël LH. Renal toxicity of oxaliplatin. Nephrol Dial Transplant. 2005;20:1275-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Takimoto CH, Remick SC, Sharma S, Mani S, Ramanathan RK, Doroshow J, Hamilton A, Mulkerin D, Graham M, Lockwood GF, Ivy P, Egorin M, Schuler B, Greenslade D, Goetz A, Knight R, Thomas R, Monahan BP, Dahut W, Grem JL; National Cancer Institute Organ Dysfunction Working Group Study. Dose-escalating and pharmacological study of oxaliplatin in adult cancer patients with impaired renal function: a National Cancer Institute Organ Dysfunction Working Group Study. J Clin Oncol. 2003;21:2664-2672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | Robles-Osorio ML, Sabath-Silva E, Sabath E. Arsenic-mediated nephrotoxicity. Ren Fail. 2015;37:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 49. | Sweeney CJ, Takimoto C, Wood L, Porter JM, Tracewell WG, Darwish M, D'Andrea DM, Remick SC. A pharmacokinetic and safety study of intravenous arsenic trioxide in adult cancer patients with renal impairment. Cancer Chemother Pharmacol. 2010;66:345-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Bennett WM, Aronoff GR, Morrison G, Golper TA, Pulliam J, Wolfson M, Singer I. Drug prescribing in renal failure: dosing guidelines for adults. Am J Kidney Dis. 1983;3:155-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 114] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Slatter JG, Schaaf LJ, Sams JP, Feenstra KL, Johnson MG, Bombardt PA, Cathcart KS, Verburg MT, Pearson LK, Compton LD, Miller LL, Baker DS, Pesheck CV, Lord RS. Pharmacokinetics, metabolism, and excretion of irinotecan (CPT-11) following I.V. infusion of [(14)C]CPT-11 in cancer patients. Drug Metab Dispos. 2000;28:423-433. [PubMed] |
| 52. | Ashizawa T, Iwahori T, Yokoyama T, Kihara Y, Konnno O, Jyojima Y, Akashi I, Nakamura Y, Hama K, Iwamoto H, Segawa M, Takeuchi H, Hirano T, Nagao T. Irinotecan hydrochloride (CPT-11) in dialysis patients with gastrointestinal cancer. Acta Med Okayama. 2010;64:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 53. | O'Reilly S, Rowinsky EK, Slichenmyer W, Donehower RC, Forastiere AA, Ettinger DS, Chen TL, Sartorius S, Grochow LB. Phase I and pharmacologic study of topotecan in patients with impaired renal function. J Clin Oncol. 1996;14:3062-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Harris E, Behrens J, Samson D, Rahemtulla A, Russell NH, Byrne JL. Use of thalidomide in patients with myeloma and renal failure may be associated with unexplained hyperkalaemia. Br J Haematol. 2003;122:160-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Fakhouri F, Guerraoui H, Presne C, Peltier J, Delarue R, Muret P, Knebelmann B. Thalidomide in patients with multiple myeloma and renal failure. Br J Haematol. 2004;125:96-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Niesvizky R, Naib T, Christos PJ, Jayabalan D, Furst JR, Jalbrzikowski J, Zafar F, Mark T, Lent R, Pearse RN, Ely S, Leonard JP, Mazumdar M, Chen-Kiang S, Coleman M. Lenalidomide-induced myelosuppression is associated with renal dysfunction: adverse events evaluation of treatment-naïve patients undergoing front-line lenalidomide and dexamethasone therapy. Br J Haematol. 2007;138:640-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Dimopoulos M, Alegre A, Stadtmauer EA, Goldschmidt H, Zonder JA, de Castro CM, Masliak Z, Reece D, Olesnyckyj M, Yu Z, Weber DM. The efficacy and safety of lenalidomide plus dexamethasone in relapsed and/or refractory multiple myeloma patients with impaired renal function. Cancer. 2010;116:3807-3814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 58. | Lipson EJ, Huff CA, Holanda DG, McDevitt MA, Fine DM. Lenalidomide-induced acute interstitial nephritis. Oncologist. 2010;15:961-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Batts ED, Sanchorawala V, Hegerfeldt Y, Lazarus HM. Azotemia associated with use of lenalidomide in plasma cell dyscrasias. Leuk Lymphoma. 2008;49:1108-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 60. | Specter R, Sanchorawala V, Seldin DC, Shelton A, Fennessey S, Finn KT, Zeldis JB, Dember LM. Kidney dysfunction during lenalidomide treatment for AL amyloidosis. Nephrol Dial Transplant. 2011;26:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 61. | Richardson PG, Siegel DS, Vij R, Hofmeister CC, Baz R, Jagannath S, Chen C, Lonial S, Jakubowiak A, Bahlis N, Song K, Belch A, Raje N, Shustik C, Lentzsch S, Lacy M, Mikhael J, Matous J, Vesole D, Chen M, Zaki MH, Jacques C, Yu Z, Anderson KC. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. 2014;123:1826-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 303] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 62. | Baird P, Leung S, Hoang H, Babalola O, Devoe CE, Wanchoo R, Jhaveri KD. A case of acute kidney injury from crystal nephropathy secondary to pomalidomide and levofloxacin use. J Oncol Pharm Pract. 2016;22:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Cheungpasitporn W, Leung N, Rajkumar SV, Cornell LD, Sethi S, Angioi A, Fervenza FC. Bortezomib-induced acute interstitial nephritis. Nephrol Dial Transplant. 2015;30:1225-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 64. | Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, Trudel S, Kukreti V, Bahlis N, Alsina M, Chanan-Khan A, Buadi F, Reu FJ, Somlo G, Zonder J, Song K, Stewart AK, Stadtmauer E, Kunkel L, Wear S, Wong AF, Orlowski RZ, Jagannath S. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817-2825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 518] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 65. | Hájek R, Masszi T, Petrucci MT, Palumbo A, Rosiñol L, Nagler A, Yong KL, Oriol A, Minarik J, Pour L, Dimopoulos MA, Maisnar V, Rossi D, Kasparu H, Van Droogenbroeck J, Yehuda DB, Hardan I, Jenner M, Calbecka M, Dávid M, de la Rubia J, Drach J, Gasztonyi Z, Górnik S, Leleu X, Munder M, Offidani M, Zojer N, Rajangam K, Chang YL, San-Miguel JF, Ludwig H. A randomized phase III study of carfilzomib vs low-dose corticosteroids with optional cyclophosphamide in relapsed and refractory multiple myeloma (FOCUS). Leukemia. 2017;31:107-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 66. | Jhaveri KD, Chidella S, Varghese J, Mailloux L, Devoe C. Carfilzomib-related acute kidney injury. Clin Adv Hematol Oncol. 2013;11:604-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 67. | Liberman V, D'Agati VD, Masani NN, Drakakis J, Mattana J. Acute Tubular Necrosis in a Patient With Myeloma Treated With Carfilzomib. Kidney Int Rep. 2016;1:89-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 68. | Yui JC, Dispenzieri A, Leung N. Ixazomib-induced thrombotic microangiopathy. Am J Hematol. 2017;92:E53-E55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 69. | Singh D, Attri BK, Gill RK, Bariwal J. Review on EGFR Inhibitors: Critical Updates. Mini Rev Med Chem. 2016;16:1134-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 70. | Gelatti ACZ, Drilon A, Santini FC. Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC). Lung Cancer. 2019;137:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 71. | Khan K, Valeri N, Dearman C, Rao S, Watkins D, Starling N, Chau I, Cunningham D. Targeting EGFR pathway in metastatic colorectal cancer- tumour heterogeniety and convergent evolution. Crit Rev Oncol Hematol. 2019;143:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 72. | Kumasaka R, Nakamura N, Shirato K, Osawa H, Takanashi S, Hasegawa Y, Yamabe H, Nakamura M, Tamura M, Okumura K. Side effects of therapy: case 1. Nephrotic syndrome associated with gefitinib therapy. J Clin Oncol. 2004;22:2504-2505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Maruyama K, Chinda J, Kuroshima T, Kabara M, Nakagawa N, Fujino T, Yamamoto Y, Ohsaki Y, Ogawa Y, Hasebe N. Minimal change nephrotic syndrome associated with gefitinib and a successful switch to erlotinib. Intern Med. 2015;54:823-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Kaneko T, Shimizu A, Aoki M, Tsuruoka S. A case of gefitinib-associated membranous nephropathy in treatment for pulmonary adenocarcinoma. CEN Case Rep. 2015;4:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 75. | Jhaveri KD, Sakhiya V, Wanchoo R, Ross D, Fishbane S. Renal effects of novel anticancer targeted therapies: a review of the Food and Drug Administration Adverse Event Reporting System. Kidney Int. 2016;90:706-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 76. | Schrag D, Chung KY, Flombaum C, Saltz L. Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer Inst. 2005;97:1221-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 77. | Tejpar S, Piessevaux H, Claes K, Piront P, Hoenderop JG, Verslype C, Van Cutsem E. Magnesium wasting associated with epidermal-growth-factor receptor-targeting antibodies in colorectal cancer: a prospective study. Lancet Oncol. 2007;8:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 78. | Cao Y, Liu L, Liao C, Tan A, Gao F. Meta-analysis of incidence and risk of hypokalemia with cetuximab-based therapy for advanced cancer. Cancer Chemother Pharmacol. 2010;66:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Harari PM. Epidermal growth factor receptor inhibition strategies in oncology. Endocr Relat Cancer. 2004;11:689-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 265] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 80. | Nagaraja V, Shaw N, Morey AL, Cox MR, Eslick GD. HER2 expression in oesophageal carcinoma and Barrett's oesophagus associated adenocarcinoma: An Australian study. Eur J Surg Oncol. 2016;42:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 81. | Gowryshankar A, Nagaraja V, Eslick GD. HER2 status in Barrett's esophagus & esophageal cancer: a meta analysis. J Gastrointest Oncol. 2014;5:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 82. | Burstein HJ. The distinctive nature of HER2-positive breast cancers. N Engl J Med. 2005;353:1652-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 376] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 83. | Albanell J, Baselga J. Trastuzumab, a humanized anti-HER2 monoclonal antibody, for the treatment of breast cancer. Drugs Today (Barc). 1999;35:931-946. [PubMed] |
| 84. | de Souza JA, Davis DW, Zhang Y, Khattri A, Seiwert TY, Aktolga S, Wong SJ, Kozloff MF, Nattam S, Lingen MW, Kunnavakkam R, Stenson KM, Blair EA, Bozeman J, Dancey JE, Vokes EE, Cohen EE. A phase II study of lapatinib in recurrent/metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2012;18:2336-2343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 85. | Ryan CE, Davids MS. BCL-2 Inhibitors, Present and Future. Cancer J. 2019;25:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 86. | Wanchoo R, Bernabe Ramirez C, Barrientos J, Jhaveri KD. Renal involvement in chronic lymphocytic leukemia. Clin Kidney J. 2018;11:670-680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 87. | Cheson BD, Heitner Enschede S, Cerri E, Desai M, Potluri J, Lamanna N, Tam C. Tumor Lysis Syndrome in Chronic Lymphocytic Leukemia with Novel Targeted Agents. Oncologist. 2017;22:1283-1291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 88. | Ladanyi M, Cavalchire G, Morris SW, Downing J, Filippa DA. Reverse transcriptase polymerase chain reaction for the Ki-1 anaplastic large cell lymphoma-associated t(2;5) translocation in Hodgkin's disease. Am J Pathol. 1994;145:1296-1300. [PubMed] |
| 89. | Orscheschek K, Merz H, Hell J, Binder T, Bartels H, Feller AC. Large-cell anaplastic lymphoma-specific translocation (t[2;5] [p23;q35]) in Hodgkin's disease: indication of a common pathogenesis? Lancet. 1995;345:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 90. | Pillay K, Govender D, Chetty R. ALK protein expression in rhabdomyosarcomas. Histopathology. 2002;41:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 91. | Chen Y, Takita J, Choi YL, Kato M, Ohira M, Sanada M, Wang L, Soda M, Kikuchi A, Igarashi T, Nakagawara A, Hayashi Y, Mano H, Ogawa S. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455:971-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 686] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 92. | Shinmura K, Kageyama S, Tao H, Bunai T, Suzuki M, Kamo T, Takamochi K, Suzuki K, Tanahashi M, Niwa H, Ogawa H, Sugimura H. EML4-ALK fusion transcripts, but no NPM-, TPM3-, CLTC-, ATIC-, or TFG-ALK fusion transcripts, in non-small cell lung carcinomas. Lung Cancer. 2008;61:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 93. | Camidge DR, Brosnan EM, DeSilva C, Koo PJ, Chonchol M. Crizotinib effects on creatinine and non-creatinine-based measures of glomerular filtration rate. J Thorac Oncol. 2014;9:1634-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 94. | Lin YT, Wang YF, Yang JC, Yu CJ, Wu SG, Shih JY, Yang PC. Development of renal cysts after crizotinib treatment in advanced ALK-positive non-small-cell lung cancer. J Thorac Oncol. 2014;9:1720-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 95. | Izzedine H, El-Fekih RK, Perazella MA. The renal effects of ALK inhibitors. Invest New Drugs. 2016;34:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 96. | Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH, Kaempgen E, Martín-Algarra S, Karaszewska B, Mauch C, Chiarion-Sileni V, Martin AM, Swann S, Haney P, Mirakhur B, Guckert ME, Goodman V, Chapman PB. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2237] [Cited by in RCA: 2276] [Article Influence: 175.1] [Reference Citation Analysis (0)] |
| 97. | Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O'Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA; BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5824] [Cited by in RCA: 5945] [Article Influence: 424.6] [Reference Citation Analysis (0)] |
| 98. | Hurabielle C, Pillebout E, Stehlé T, Pagès C, Roux J, Schneider P, Chevret S, Chaffaut C, Boutten A, Mourah S, Basset-Seguin N, Vidal-Petiot E, Lebbé C, Flamant M. Mechanisms Underpinning Increased Plasma Creatinine Levels in Patients Receiving Vemurafenib for Advanced Melanoma. PLoS One. 2016;11:e0149873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 99. | Jhaveri KD, Sakhiya V, Fishbane S. Nephrotoxicity of the BRAF Inhibitors Vemurafenib and Dabrafenib. JAMA Oncol. 2015;1:1133-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 100. | Temsirolimus 2012, In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–2013 Dec 10. [PubMed] |
| 101. | Izzedine H, Escudier B, Rouvier P, Gueutin V, Varga A, Bahleda R, Soria JC. Acute tubular necrosis associated with mTOR inhibitor therapy: a real entity biopsy-proven. Ann Oncol. 2013;24:2421-2425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 102. | Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3005] [Cited by in RCA: 2869] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 103. | Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends Genet. 1993;9:138-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1217] [Cited by in RCA: 1129] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 104. | Cortes JE, Gambacorti-Passerini C, Kim DW, Kantarjian HM, Lipton JH, Lahoti A, Talpaz M, Matczak E, Barry E, Leip E, Brümmendorf TH, Khoury HJ. Effects of Bosutinib Treatment on Renal Function in Patients With Philadelphia Chromosome-Positive Leukemias. Clin Lymphoma Myeloma Leuk. 2017;17:684-695.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 105. | Ozkurt S, Temiz G, Acikalin MF, Soydan M. Acute renal failure under dasatinib therapy. Ren Fail. 2010;32:147-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 106. | Calizo RC, Bhattacharya S, van Hasselt JGC, Wei C, Wong JS, Wiener RJ, Ge X, Wong NJ, Lee JJ, Cuttitta CM, Jayaraman G, Au VH, Janssen W, Liu T, Li H, Salem F, Jaimes EA, Murphy B, Campbell KN, Azeloglu EU. Disruption of podocyte cytoskeletal biomechanics by dasatinib leads to nephrotoxicity. Nat Commun. 2019;10:2061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 107. | Marcolino MS, Boersma E, Clementino NC, Macedo AV, Marx-Neto AD, Silva MH, van Gelder T, Akkerhuis KM, Ribeiro AL. Imatinib treatment duration is related to decreased estimated glomerular filtration rate in chronic myeloid leukemia patients. Ann Oncol. 2011;22:2073-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 108. | Pou M, Saval N, Vera M, Saurina A, Solé M, Cervantes F, Botey A. Acute renal failure secondary to imatinib mesylate treatment in chronic myeloid leukemia. Leuk Lymphoma. 2003;44:1239-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 109. | Berman E, Nicolaides M, Maki RG, Fleisher M, Chanel S, Scheu K, Wilson BA, Heller G, Sauter NP. Altered bone and mineral metabolism in patients receiving imatinib mesylate. N Engl J Med. 2006;354:2006-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 110. | Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1110] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 111. | Launay-Vacher V, Aapro M, De Castro G, Cohen E, Deray G, Dooley M, Humphreys B, Lichtman S, Rey J, Scotté F, Wildiers H, Sprangers B. Renal effects of molecular targeted therapies in oncology: a review by the Cancer and the Kidney International Network (C-KIN). Ann Oncol. 2015;26:1677-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 112. | Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129-1136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1264] [Cited by in RCA: 1130] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 113. | Launay-Vacher V, Deray G. Hypertension and proteinuria: a class-effect of antiangiogenic therapies. Anticancer Drugs. 2009;20:81-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 114. | Tomita Y, Uemura H, Fujimoto H, Kanayama HO, Shinohara N, Nakazawa H, Imai K, Umeyama Y, Ozono S, Naito S, Akaza H; Japan Axitinib Phase II Study Group. Key predictive factors of axitinib (AG-013736)-induced proteinuria and efficacy: a phase II study in Japanese patients with cytokine-refractory metastatic renal cell Carcinoma. Eur J Cancer. 2011;47:2592-2602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 115. | Shord SS, Bressler LR, Tierney LA, Cuellar S, George A. Understanding and managing the possible adverse effects associated with bevacizumab. Am J Health Syst Pharm. 2009;66:999-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 116. | Patel TV, Morgan JA, Demetri GD, George S, Maki RG, Quigley M, Humphreys BD. A preeclampsia-like syndrome characterized by reversible hypertension and proteinuria induced by the multitargeted kinase inhibitors sunitinib and sorafenib. J Natl Cancer Inst. 2008;100:282-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 117. | Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D, McKendrick J, Polikoff J, Tellier A, Castan R, Allegra C. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 1024] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 118. | Shord SS, Bressler LR, Tierney LA, Cuellar S, George A. Understanding and managing the possible adverse effects associated with bevacizumab. Am J Health Syst Pharm. 2009;66:999-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 119. | Gurevich F, Perazella MA. Renal effects of anti-angiogenesis therapy: update for the internist. Am J Med. 2009;122:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 120. | Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D; CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2194] [Cited by in RCA: 2114] [Article Influence: 176.2] [Reference Citation Analysis (0)] |
| 121. | Winn SK, Ellis S, Savage P, Sampson S, Marsh JE. Biopsy-proven acute interstitial nephritis associated with the tyrosine kinase inhibitor sunitinib: a class effect? Nephrol Dial Transplant. 2009;24:673-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 122. | Izzedine H, Brocheriou I, Rixe O, Deray G. Interstitial nephritis in a patient taking sorafenib. Nephrol Dial Transplant. 2007;22:2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 123. | Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM; TARGET Study Group. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3788] [Cited by in RCA: 3764] [Article Influence: 209.1] [Reference Citation Analysis (0)] |
| 124. | Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, Pristupa A, Janssens A, Mayer J, Bartlett NL, Dilhuydy MS, Pylypenko H, Loscertales J, Avigdor A, Rule S, Villa D, Samoilova O, Panagiotidis P, Goy A, Mato A, Pavlovsky MA, Karlsson C, Mahler M, Salman M, Sun S, Phelps C, Balasubramanian S, Howes A, Hallek M; HELIOS investigators. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17:200-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 347] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 125. | Kreitman RJ, Dearden C, Zinzani PL, Delgado J, Karlin L, Robak T, Gladstone DE, le Coutre P, Dietrich S, Gotic M, Larratt L, Offner F, Schiller G, Swords R, Bacon L, Bocchia M, Bouabdallah K, Breems DA, Cortelezzi A, Dinner S, Doubek M, Gjertsen BT, Gobbi M, Hellmann A, Lepretre S, Maloisel F, Ravandi F, Rousselot P, Rummel M, Siddiqi T, Tadmor T, Troussard X, Yi CA, Saglio G, Roboz GJ, Balic K, Standifer N, He P, Marshall S, Wilson W, Pastan I, Yao NS, Giles F. Moxetumomab pasudotox in relapsed/refractory hairy cell leukemia. Leukemia. 2018;32:1768-1777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 190] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 126. | Dean E, Middleton MR, Pwint T, Swaisland H, Carmichael J, Goodege-Kunwar P, Ranson M. Phase I study to assess the safety and tolerability of olaparib in combination with bevacizumab in patients with advanced solid tumours. Br J Cancer. 2012;106:468-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 127. | Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer--preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 224] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 128. | Food and Drug Administration. Pembrolizumab (KEYTRUDA), checkpoint inhibitor. [published 2016 October 25 Oct; cited 2019 December 16]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/pembrolizumab-keytruda-checkpoint-inhibitor. |
| 129. | Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F, Cauquil C, Chanson P, Collins M, Durrbach A, Ederhy S, Feuillet S, François H, Lazarovici J, Le Pavec J, De Martin E, Mateus C, Michot JM, Samuel D, Soria JC, Robert C, Eggermont A, Marabelle A. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27:559-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 649] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 130. | Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, Le DT, Lipson EJ, Glezerman IG, Wolchok J, Cornell LD, Feldman P, Stokes MB, Zapata SA, Hodi FS, Ott PA, Yamashita M, Leaf DE. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016;90:638-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 443] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 131. | Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A, Gaber L, Lahoti A, Workeneh B, Chen S, Lin J, Abdel-Wahab N, Tayar J, Lu H, Suarez-Almazor M, Tannir N, Yee C, Diab A, Abudayyeh A. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J Immunother Cancer. 2019;7:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 132. | Shirali AC, Perazella MA, Gettinger S. Association of Acute Interstitial Nephritis With Programmed Cell Death 1 Inhibitor Therapy in Lung Cancer Patients. Am J Kidney Dis. 2016;68:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (1)] |
| 133. | Lesinski GB, Anghelina M, Zimmerer J, Bakalakos T, Badgwell B, Parihar R, Hu Y, Becknell B, Abood G, Chaudhury AR, Magro C, Durbin J, Carson WE. The antitumor effects of IFN-alpha are abrogated in a STAT1-deficient mouse. J Clin Invest. 2003;112:170-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 134. | Markowitz GS, Nasr SH, Stokes MB, D'Agati VD. Treatment with IFN-{alpha}, -{beta}, or -{gamma} is associated with collapsing focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010;5:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 135. | Ozturk M, Basoglu F, Yilmaz M, Ozagari AA, Baybas S. Interferon β associated nephropathy in a Multiple Sclerosis patient: A case and review. Mult Scler Relat Disord. 2016;9:50-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 136. | Zuber J, Martinez F, Droz D, Oksenhendler E, Legendre C; Groupe D'stude Des Nephrologues D'ile-de-France (GENIF). Alpha-interferon-associated thrombotic microangiopathy: a clinicopathologic study of 8 patients and review of the literature. Medicine (Baltimore). 2002;81:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 137. | Webb DE, Austin HA, Belldegrun A, Vaughan E, Linehan WM, Rosenberg SA. Metabolic and renal effects of interleukin-2 immunotherapy for metastatic cancer. Clin Nephrol. 1988;30:141-145. [PubMed] |
| 138. | Marabondo S, Kaufman HL. High-dose interleukin-2 (IL-2) for the treatment of melanoma: safety considerations and future directions. Expert Opin Drug Saf. 2017;16:1347-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 139. | Bergsma H, Konijnenberg MW, van der Zwan WA, Kam BL, Teunissen JJ, Kooij PP, Mauff KA, Krenning EP, Kwekkeboom DJ. Nephrotoxicity after PRRT with (177)Lu-DOTA-octreotate. Eur J Nucl Med Mol Imaging. 2016;43:1802-1811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |