Published online Feb 24, 2020. doi: 10.5306/wjco.v11.i2.53
Peer-review started: May 8, 2019
First decision: October 23, 2019
Revised: November 5, 2019
Accepted: November 28, 2019
Article in press: November 28, 2019
Published online: February 24, 2020
Processing time: 297 Days and 18.6 Hours
The tumor objective response rate (ORR) is an important parameter to demonstrate the efficacy of a treatment in oncology. The ORR is valuable for clinical decision making in routine practice and a significant end-point for reporting the results of clinical trials. World Health Organization and Response Evaluation Criteria in Solid Tumors (RECIST) are anatomic response criteria developed mainly for cytotoxic chemotherapy. These criteria are based on the visual assessment of tumor size in morphological images provided by computed tomography (CT) or magnetic resonance imaging. Anatomic response criteria may not be optimal for biologic agents, some disease sites, and some regional therapies. Consequently, modifications of RECIST, Choi criteria and Morphologic response criteria were developed based on the concept of the evaluation of viable tumors. Despite its limitations, RECIST v1.1 is validated in prospective studies, is widely accepted by regulatory agencies and has recently shown good performance for targeted cancer agents. Finally, some alternatives of RECIST were developed as immune-specific response criteria for checkpoint inhibitors. Immune RECIST criteria are based essentially on defining true progressive disease after a confirmatory imaging. Some graphical methods may be useful to show longitudinal change in the tumor burden over time. Tumor tissue is a tridimensional heterogenous mass, and tumor shrinkage is not always symmetrical; thus, metabolic response assessments using positron emission tomography (PET) or PET/CT may reflect the viability of cancer cells or functional changes evolving after anticancer treatments. The metabolic response can show the benefit of a treatment earlier than anatomic shrinkage, possibly preventing delays in drug approval. Computer-assisted automated volumetric assessments, quantitative multimodality imaging in radiology, new tracers in nuclear medicine and finally artificial intelligence have great potential in future evaluations.
Core tips: The tumor objective response rate is an important parameter in oncology. World Health Organization and Response Evaluation Criteria in Solid Tumors (RECIST) are anatomic response criteria developed mainly for cytotoxic chemotherapy. These criteria may not be optimal for biologic agents, some disease sites, and some regional therapies. Some alternatives of RECIST were developed, but RECIST v1.1 is validated in prospective studies, is widely accepted by regulatory agencies and has recently shown good performance for targeted cancer agents. The newest alternatives of RECIST are immune-specific response criteria for checkpoint inhibitors. Metabolic response assessments using positron emission tomography (PET) or PET/computed tomography may reflect the viability of cancer cells or functional changes that occur after anticancer treatments.
- Citation: Aykan NF, Özatlı T. Objective response rate assessment in oncology: Current situation and future expectations. World J Clin Oncol 2020; 11(2): 53-73
- URL: https://www.wjgnet.com/2218-4333/full/v11/i2/53.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i2.53
The tumor objective response rate (ORR) is the assessment of the tumor burden (TB) after a given treatment in patients with solid tumors and has a long history. The ORR is undoubtedly an important parameter to demonstrate the efficacy of a treatment and it serves as a primary or secondary end-point in clinical trials. The first attempts to describe response criteria to anticancer drugs were performed by Karnofsky[1] in 1961, Moertel and Hanley[2] in 1976, Levin et al[3] in 1977 and Macdonald et al[4] in 1990 based on their clinical experiences. Karnofsky and his group classified the therapeutic effects of a drug into three major categories: No clinically useful effect on the course of disease (category 0), clinical benefit with favorable objective changes in all measurable criteria of disease (category I), interruption of or slowing the progression of disease without definite evidence of subjective or objective improvement (category II). Objective improvements include clinical (such as regression of palpable lesions), pathologic, radiologic and biochemical regressions according to the specific type of cancer and individual patient. This classification highlights the importance of response duration; for example, less than one month of duration occurs in category 0. Moertel and Hanley’s assessments were based on tumor size by palpation. The response criteria developed by Levin et al[3] and Macdonald et al[4] were only for malignant brain tumors. Later, World Health Organization (WHO) criteria and Response Evaluation Criteria in Solid Tumors (RECIST) were developed for all solid tumors by working groups, mainly for cytotoxic chemotherapies and based on anatomic imaging[5-8]. These conventional response criteria, especially RECIST, were used for more than two decades in the era of chemotherapy as a surrogate end-point to overall survival (OS) in clinical trials. The efficacy of a chemotherapy was usually correlated with tumor shrinkage over time[9,10]. After the introduction of molecularly targeted treatment with biologic agents into oncology clinics and routine use of an fluorodeoxyglucose-positron emission tomography (FDG-PET) scan and advanced magnetic resonance imaging (MRI) techniques as functional imaging brought new perspectives in response evaluation. Moreover, very recently, immunotherapy by checkpoint inhibitors has led to the development of more specific response criteria because the patterns of response and progression to immunotherapy are different than those of cytotoxic chemotherapy and targeted biologic agents[10].
This review summarizes chronologically standard ORR evaluation criteria [WHO, RECIST v1.0, RECIST v1.1, modified RECIST (mRECIST)], response evaluation in targeted treatments with biologic agents (Choi and morphologic criteria) and then response assessments in immunotherapy (irRC, irRECIST, iRECIST, and imRECIST). We emphasize the importance of early tumor shrinkage (ETS) and depth of response (DpR) by some virtual examples. Later, we give some examples of graphical methods (Waterfall plot, Spider or Spaghetti plot and Swimmer plot) to demonstrate the details of tumor shrinkage, pseudoprogression and a durable response.
Finally, we will discuss the current situation and the limitations of size-based response assessments and alternative metrics of response with the importance of response duration and future expectations.
A systematic literature search was performed using PubMed for “objective response rate”, “WHO criteria”, “RECIST criteria”, “modified RECIST”, “Choi criteria”, “tumor shrinkage”, “early tumor shrinkage”, “depth of response”, “morphologic response”, “immune-related RECIST”, “waterfall plot”, “spider or spaghetti plot”, “swimmer plot”, “PERCIST”, “iPERCIST” and “volumetric response”. Conference abstracts were also included. Furthermore, the references of all selected articles were reviewed to identify any additional information.
WHO criteria were published by The WHO[5] in 1979 and by Miller et al[6] in 1981 to define the objective response, and they were used until 2000. According to the WHO criteria, the tumor size reflects its surface and is determined by bidimensional measurements and multiplication of the longest diameter by the greatest perpendicular diameter for single lesions. The total tumor load of a patient is the sum of the products of the perpendicular diameters for multiple lesions. The objective response to treatment is divided into four categories: Complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) (Table 1).
| WHO | RECIST v1.0 | RECIST v1.1 | |
| Method | Sum of products of two longest diameters in perpendicular dimensions (bidimensional; surface area) | Sum of longest diameters of target lesions (unidimensional) | Sum of longest diameters of non-nodal target lesions and short axis of nodal target lesions (unidimensional) |
| No. of measured lesion | All lesions | Target lesions: maximum 5 per organ, 10 in total | Target lesions: Maximum 2 per organ, 5 in total |
| Response | |||
| CR | Disappearance of all known disease, confirmed at 4 wk2 | Disappearance of all known disease, confirmed at 4 wk | Disappearance of all known disease, confirmed at 4 wk, lymph nodes must be < 10 mm short axis |
| PR | ≥ 50% decrease from baseline, confirmed at 4 wk | ≥ 30% decrease from baseline, confirmed at 4 wk | ≥ 30% decrease from baseline, confirmed at 4 wk |
| SD | Neither PR nor PD criteria met | Neither PR nor PD criteria met | Neither PR nor PD criteria met |
| PD | ≥ 25% increase, no CR, PR, or SD, new lesion (s), ≥ 25% increase in 1 lesion | ≥ 20% increase over smallest sum observed, no CR, PR, or SD, new lesion(s) | ≥ 20% increase over smallest sum observed, no CR, PR, or SD, new lesion(s)3. The sum must also demonstrate an absolute increase of at least 5 mm |
For more than two decades, the WHO response criteria were used as the standard method for tumor evaluation. Due to the development of some problems such as the interobserver variability of the number of lesions, selection of measurable targets, minimum lesion size and definition of PD, WHO criteria became no longer comparable among research organizations[7]. Measuring all lesions was also a time-consuming procedure and carried the risk of measurement errors. Next, to standardize and simplify the methodology, the RECIST version 1.0 was proposed as a new guideline to evaluate the response in 2000[8]. The arrival of new technologies such as computed tomography (CT) and MRI has also led to some new definitions of measurable lesions. RECIST v1.0 criteria brought the term of “target” and “nontarget” lesions. Target lesions are selected based on their size (from those with the longest diameter to shortest ones), are representative lesions of all involved organs, and they should be reproducible by repeated measurements. According to RECIST v1.0, all measurable disease up to a maximum of 5 lesions per organ and 10 lesions in total were representative of all involved organs and should be identified and recorded as target lesions at baseline. All other lesions were nontarget lesions. The RECIST v1.0 guideline was revised in 2009; the maximum number of target lesions per organ was reduced from 5 to 2, and 10 to 5 in total, in RECIST version 1.1[11]. Assessment of the pathological lymph nodes was incorporated as well; nodes with a short axis ≥ 15 mm were considered measurable, pathological and assessable as target lesions. Lymph nodes with a short axis < 10 mm were considered normal. Lymph nodes with a short axis between 10 and 15 mm were included as nonmeasurable nontarget lesions.
Table 1 shows a brief comparison of WHO, RECIST v1.0 and RECIST v1.1 criteria[5,6,8,11,12]. The best overall response is a result of a combination of tumor responses in target and nontarget lesions. If there is any new lesion, the overall best response is always PD. In the absence of any new lesion, CR is the complete disappearance of all target and nontarget lesions. No change in nontarget lesions reduces a CR in target lesions to an overall PR, but no change in nontarget lesions does not reduce a PR in target lesions.
In 2006, the first mRECIST criteria were presented first by Zacharia et al[13] from the United States and then by a Korean group[14-16] in three small-sized studies and in one pooled analysis[17]. As a main modification, they proposed one single largest target lesion per organ instead of 2 in their patients with colorectal cancer (CRC), non-small cell lung cancer and gastric cancer. They reported a high level of concordance with the original RECIST v1.1., but the number of their patients was small (153 patients) for validation.
Another mRECIST was described by Lencioni et al[18] for hepatocellular carcinoma (HCC). In intermediate-stage patients with HCC, according to the Barcelona Clinic Liver Cancer staging classification, the recommended first-line therapy was transarterial chemoembolization (TACE) and, in the advanced-stage patients, the recommended first-line therapy was sorafenib, which is an antiangiogenic tyrosine kinase inhibitor[19,20]. Recent studies have shown a poor correlation between the clinical benefit provided by sorafenib or interventional locoregional therapies such as TACE, transarterial radioembolization or radiofrequency ablation and conventional response criteria[18-22]. mRECIST for HCC was developed based on a new concept of a viable tumor proposed by the European Association for the Study of the Liver (EASL) panel[23]. A viable tumor was defined as showing intratumoral arterial enhancement by contrast agent during dynamic CT or MRI[23]. Therefore, measuring tumor enhancement is a surrogate marker of a viable tumor. Thus, tumor necrosis induced by treatment (TACE or sorafenib) is also considered as a response assessment. According to mRECIST for HCC, CR is the disappearance of any intratumoral arterial enhancement in all target lesions. PR is at least a 30% decrease in the sum of the diameters of viable (contrast enhancement in the arterial phase) target lesions, taking as reference the baseline sum of the diameters of the target lesions. PD is an increase of at least 20% in the sum of the diameters of viable target lesions, and SD is any cases that does not qualify for either PR or PD. Overall response is a result of the combined assessment of target lesions, nontarget lesions and new lesions[18]. The EASL proposed another set of response criteria for HCC[21]. EASL criteria include 2-dimensional methods like WHO, but they consider the enhanced tumor area like mRECIST.
Tumor shrinkage is not always symmetrical; tumors have heterogenous changes due to necrosis, fibrosis or intratumoral hemorrhage, especially after locoregional therapies or biologic agents[24]. The new proposed criterion, quantitative EASL (qEASL), is the 3-dimensional volumetric assessment of enhancing tumor tissue[25]. Table 2 shows a comparison of mRECIST for HCC, EASL and qEASL[18,22-25]. qEASL predicted survival better than RECIST, mRECIST and EASL criteria in patients with HCC[24].
| mRECIST (1D criteria) | EASL (2D criteria) | qEASL (3D criteria) | |
| CR | Disappearance of any intratumoral enhancement in all target lesions | Disappearance of any intratumoral enhancement in all target lesions | Disappearance of any intratumoral enhancement in all target lesions |
| PR | At least 30% decrease in the sum of max. diameters of the enhanced tumor area | At least 50% decrease in the product of max. diameter and its perpendicular of the enhanced tumor area | At least 65% decrease in the enhanced tumor volume |
| SD | Neither PR, nor PD | Neither PR, nor PD | Neither PR, nor PD |
| PD | At least 20% increase in the sum of diameters of the enhanced tumor area | At least 25% increase in the product of max. diameter and its perpendicular of the enhanced tumor area | At least 73% increase in the enhanced tumor volume |
Another alternative of conventional RECIST was developed and validated for malignant pleural mesothelioma (MPM)[26,27]. Due to anatomic reasons such as the curved structure of the chest wall, defining the limits of the longest unidimensional diameter of tumor mass before and after the treatment may be difficult. The mRECIST criteria for MPM consider tumor thickness perpendicular to fixed structures such as the chest wall or vertebral colon in two positions on the same transverse cut of CT scan. The sum of the two measurements in three axial sections (six measurements) defined a pleural unidimensional measure. Nodal, subcutaneous and other bidimensionally measurable lesions are measured unidimensionally per the RECIST criteria and are added to the total TB. PR is at least a 30% reduction in the total tumor measurement, PD is defined as an increase of at least 20% in the total tumor measurement over the nadir, or the appearance of new lesions.
The first targeted antitumor agent that requires re-evaluation of the existing response criteria used to assess treatment response is imatinib mesylate which is a c-KIT receptor tyrosine kinase inhibitor in gastrointestinal stromal tumors (GISTs)[28-30]. Following imatinib treatment, in some cases, the tumor size increases due to intratumoral hemorrhage, necrosis or myxoid degeneration. In many GISTs, the early decrease in the tumor size is minimal. On the other hand, more dramatic changes in responding GISTs is the decrease in the tumor density, which can be measured as the Hounsfield unit on CT. Therefore, Choi criteria were proposed and prospectively validated in patients with imatinib-treated metastatic GIST[28,29]. The response by Choi criteria is a 10% decrease in the tumor size or a 15% decrease in the density on contrast-enhanced CT and was found to be reproducible, more sensitive, and more precise than RECIST, and was correlated significantly with the time to tumor progression and disease-specific survival.
Two major biologic agents whose response rates were largely discussed in new large-scale randomized trials are the anti-vascular endothelial growth factor-targeted monoclonal antibody (MoAb) bevacizumab and an anti-epidermal growth factor receptor (EGFR) MoAb cetuximab. First, in the NO16966 study which is a randomized phase III trial comparing oxaliplatin-based chemotherapy with or without bevacizumab as first-line therapy in metastatic CRC, the response rates were found to be similar in both arms, although the median progression-free survival (PFS) was significantly superior with the addition of bevacizumab to oxaliplatin-based chemotherapy[31]. In a retrospective analysis of the pathological response in patients with CRC liver metastases (LM) following treatment with bevacizumab-containing chemotherapy, the pathological response rate was significantly superior in bevacizumab-treated patients than in those receiving chemotherapy alone (49% vs 27%, P = 0.03), although the RECIST response rates were similar (51%) with and without bevacizumab[32]. Another three previous retrospective studies showed improvement of the pathological response in patients treated with bevacizumab plus chemotherapy[33-35]. The addition of bevacizumab to oxaliplatin-based chemotherapy significantly reduced the degree of tumor viability[33]. Fluoropyrimidine plus oxaliplatin and bevacizumab combination therapy is one of the independent predictors of a pathologic response[34]. Bevacizumab improves the tumor regression grade to chemotherapy significantly[35]. Stremitzer and colleagues[36] prospectively investigated whether the type of MoAb (bevacizumab or cetuximab) with neoadjuvant chemotherapy was associated with the histological response and the pattern of tumor destruction in patients with CRC LM. They found that the addition of bevacizumab to combination chemotherapy showed more necrosis but less fibrosis than cetuximab.
On the other hand, a complete pathologic response (CPR) is a strong predictor of prolonged survival or cure in CRC LM[34,37-39], but total tumor necrosis or CPR does not always indicate a complete clinical response identified by CT[32,37].
As a result, RECIST criteria may not be optimal to evaluate the response to bevacizumab; size-based radiological methods may not differentiate between the tumor and fibrotic tissue due to effective biological therapy[32,33,36]. Recently, new criteria (morphologic response) based on morphologic changes, such as a sharply or poorly defined tumor-liver interface and homogeneous or heterogeneous attenuation observed on contrast-enhanced CT, were defined in patients with CRC LM, undergoing chemotherapy with or without bevacizumab[40,41]. The CT morphology comprises three groups of lesions: Homogeneous lesions with sharp edges (group 1), heterogeneous structures with poorly defined edges (group 3) and intermediate mixed lesions (group 2). Nishioka et al[42] showed that the CT morphology predicts tumor viability and long-term surgical outcomes after chemotherapy in patients with CRC LM. Intratumoral heterogeneity in the primary tumor and its vasculature (angiogenic or non-angiogenic), as well as related lymphatic and hepatic metastasis, may also result in different responses to anticancer therapy[43,44].
Recently, in the FIRE-3 trial comparing cetuximab with bevacizumab, in combination with fluorouracil, folinic acid and irinotecan (FOLFIRI), as a first-line treatment in patients with KRAS exon 2 wild-type metastatic CRC, the primary endpoint was the objective response rate according to RECIST v1.0, and this was found to not be significantly different between the two arms; the median PFS was also similar, but the OS was significantly superior in the FOLFIRI plus cetuximab arm[45]. Several explanations are possible for these surprising results, such as a lack of an independent radiological review of response data and effect or underuse of second-line treatments, and one of them is the inadequacy of RECIST for different targeted therapies[45-47]. Later, an independent, central radiological evaluation showed that the rate of ETS and DpR were significantly greater in patients treated with FOLFIRI plus cetuximab[48]. ETS was defined as a reduction in the tumor diameter of more than 20% at the first tumor assessment (week 6) after the baseline. DpR was defined as the maximal tumor shrinkage observed in a patient. The median time to the maximal tumor response was 3.5 mo in the cetuximab arm and 3.6 months in the bevacizumab arm[49]. DpR is a nadir of tumor shrinkage compared with baseline[50]. According to RECIST, at least a 30% decrease in the sum of the longest diameters indicates PR, and minor tumor progression (1%-19%) and minor tumor shrinkage (0%-29%) cover SD category. ETS, as a categorical measure, includes tumor size reduction in the range of 10%-30% according to a trial as a cut-off percentage, and this was shown to be significantly associated with PFS and OS in several retrospective analyses from previous trials[50-53]. Among these trials, in the BOND study the ETS cut-off was set at 10% and the time of the first response assessment was set as 6 wk[50,53]. Figure 1 shows a simple model of different ETS definitions on a waterfall plot. A systematic review and pooled-analysis of 21 trials showed that patients with ETS were associated with better OS and PFS than early nonresponders in metastatic CRC (mCRC)[54]. On the other hand, early and fast maximum tumor shrinkage (at week 6 or 8) may allow a possible safer resection of CRC LM, before the occurrence of liver damage induced by prolonged chemotherapy[55,56]. The impact of ETS and resection on the outcomes in patients with wild-type (wt) RAS mCRC was investigated in the PRIME trial as a secondary end-point[56]. The PRIME trial compared FOLFOX4 chemotherapy vs FOLFOX4 plus a fully human anti-EGFR MoAb panitumumab as first-line treatment in mCRC and demonstrated that panitumumab+FOLFOX4 significantly improved PFS and OS in patients with wtRAS mCRC[57-59]. Douillard et al[56] indicated that more patients receiving panitumumab+FOLFOX4 had tumor shrinkage and they concluded that ETS appeared to be associated with improved PFS and OS irrespective of the treatment received. ETS and DpR are RECIST-independent parameters; they are not recorded by RECIST-based assessments[45,49].
The early morphologic response was also evaluated in patients with RASwt mCRC in a retrospective analysis[60]. An early optimal morphologic response (EOMR) was defined as a change from group 3 or 2 according to morphologic response criteria at baseline CT to group 1 at the first assessment by CT. In this trial, Masuishi et al[60] found that patients with EOMR had longer PFS than those without EOMR in patients treated with bevacizumab.
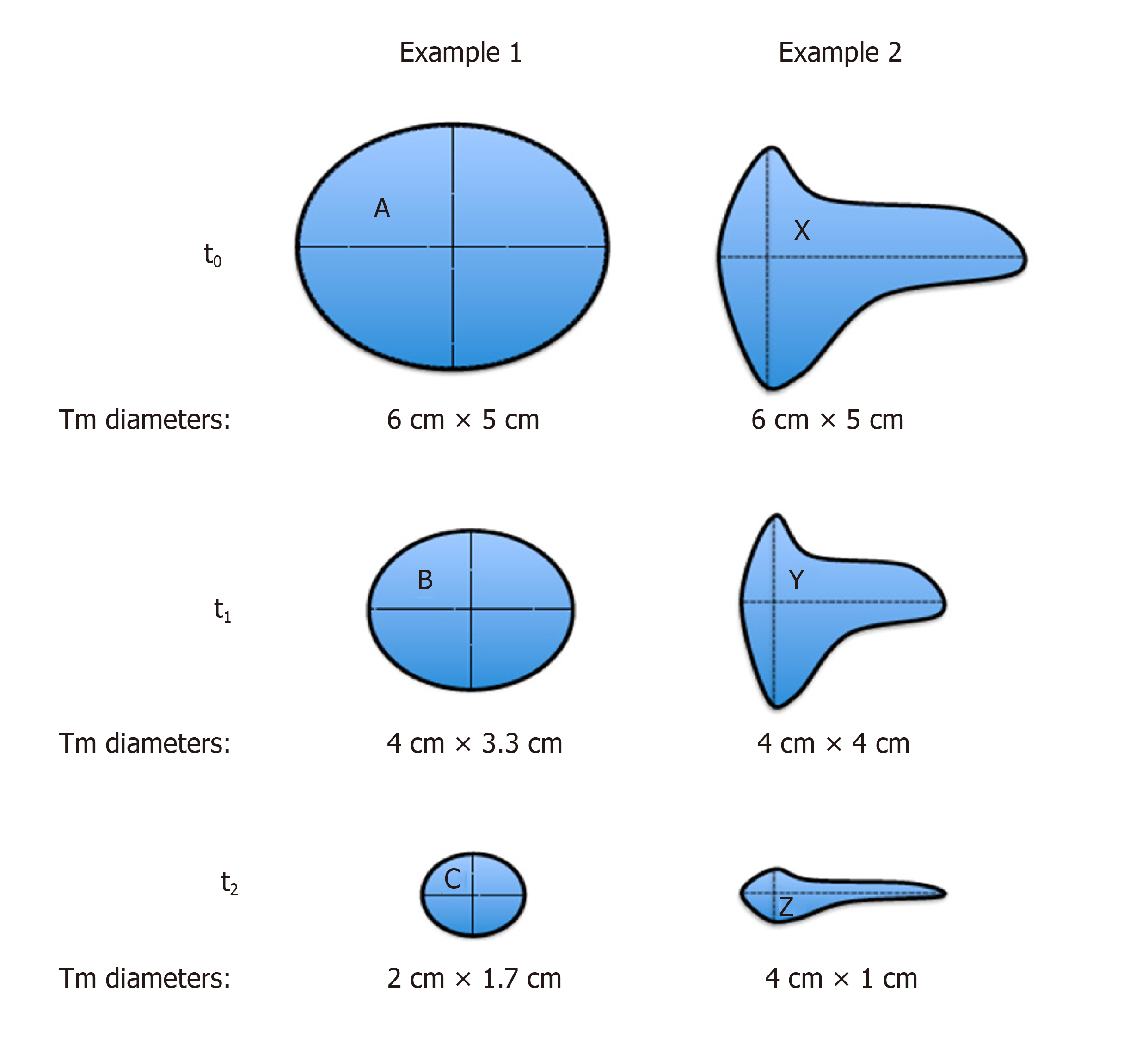
The term of “the depth of response (DpR)” shows that all PRs are not the same. We present here hypothetical 2-D examples to compare and contrast WHO and RECIST criteria (Figure 2). Here, in example 1, we draw a regular spheroid mass (A) with symmetric (proportional) shrinkage following treatment. Example 2 shows an irregular mass (X) with asymmetric shrinkage after treatment. The baseline (at time 0; t0) perpendicular diameters for both tumors are the same; 6 cm × 5 cm.
As shown in Table 3, in example 1, no difference was found in the response evaluation at time point 1 (t1) and time point 2 (t2) between WHO and RECIST. However, in example 2, at t1, the tumor response was PR according to RECIST while the WHO response was SD (Table 3). On the other hand, among PRs, although the longest diameter was the same (4 cm) of tumors B, Y and Z, the smallest tumor according to surface was Z; therefore, WHO criteria could distinguish a DpR, but not RECIST (Table 4). We previously presented this virtual comparison of size-based tumor shrinkage methods and the effect of tumor heterogeneity in 2016[61].
| RECIST | WHO | |
| Example 1 | ||
| t1 (B vs A) | PR | PR |
| t2 (C vs A) | PR | PR |
| Example 2 | ||
| t1 (Y vs X) | PR | SD |
| t2 (Z vs X) | PR | PR |
| Tumor | RECIST (longest diameter) | WHO (surface) | DpR |
| B | 4 cm | PR (13.2 cm2) | No |
| Y | 4 cm | SD (16 cm2) | No |
| Z | 4 cm | PR (4 cm2) | Yes |
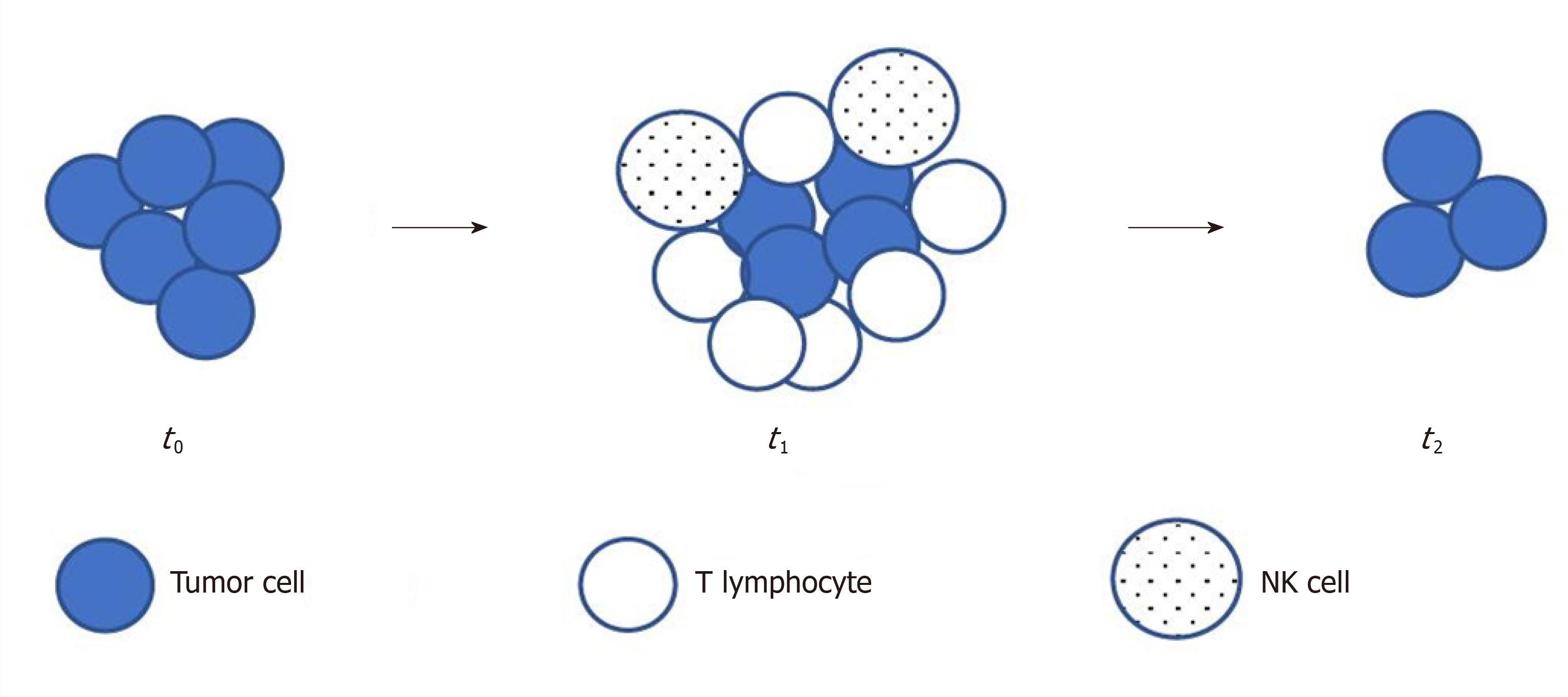
Immune checkpoint inhibitors such as ipilimumab (an anti-CTLA4 MoAb), nivolumab or pembrolizumab (anti-PD 1 antibodies) or atezolizumab (an anti-PD L1 antibody), are active immuno-oncology drugs that have received marketing authorizations since 2011 and have become increasingly available in oncology clinics[10,62,63]. In early studies with these drugs, researchers observed different, atypical and unique response patterns than those with traditional cytotoxic and other molecularly targeted agents[10,63]. This indicated an initial radiological progression by RECIST and subsequent delayed tumor shrinkage; this phenomenon was termed as peudoprogression[62]. During pseudoprogression, biopsy demonstrated inflammatory cell infiltrates, edema or necrosis[62]. The use of RECIST in this situation resulted in premature discontinuation of therapy, although there was a clinical benefit or a later response[10]. Delayed but durable responses were associated with prolonged survival in a subset of patients. These insufficiencies demonstrated the need for more precise criteria in immunotherapy. Figure 3 illustrates pseudoprogression.
Immune-specific response criteria were described chronologically as the irRC (immune-specific related response criteria), irRECIST (immune-related RECIST), iRECIST (immunotherapy RECIST) and imRECIST[63-66]. One of the main differences in these criteria compared with RECIST is the necessity for the confirmation of true PD at least 4 wk after from the first assessment. The baseline measurable minimal target lesion size is 5 mm × 5 mm in irRC and at least 10 mm in irRECIST and iRECIST for non-nodal metastases. irRC represents a bidimensional criteria like the WHO criteria, while the others are unidimensional. The appearance of new lesions during treatment is incorporated into the sum of the measurements; this is another difference from RECIST. Because many immunotherapy trials used conventional RECIST since 2010, making a universal comparison of data from different trials is difficult; thus, a consensus guideline published iRECIST in 2017 to minimize the variability of interpretation[10]. iRECIST is a mRECIST v1.1; the definitions of measurable and nonmeasurable disease, target and nontarget lesions are the same. Table 5 compares immune-specific response criteria.
| irRC (2D), 2009 | irRECIST (1D), 2013 | iRECIST (1D), 2017 | imRECIST (1D), 2018 | |
| Minimum number of target lesions | 10 lesions in total; 5 lesions per organ | 5 lesions in total; 2 lesions per organ | 5 lesions in total; 2 lesions per organ | 5 lesions in total; 2 lesions per organ |
| New lesions | Included in the sum of the measurements | Included in the sum of the measurements | iUPD; becomes iCPD if PD eventually confirmed | Included in the sum of the measurements |
| CR | Disappearance of all lesions | Disappearance of all lesions | Disappearance of all lesions | Disappearance of all lesions |
| PR | ≥ 50% decrease from baseline | ≥ 30% decrease from baseline | ≥ 30% decrease from baseline | ≥ 30% decrease from baseline |
| SD | Neither CR nor PD | Neither CR nor PD | Neither CR nor PD | Neither CR nor PD |
| PD | ≥ 25% increase in the nadir | ≥ 20% increase in the nadir (minimum 5 mm) | ≥ 20% increase in the nadir (minimum 5 mm) | ≥ 20% increase in the nadir (minimum 5 mm) |
| Confirmation of PD | Yes | Yes1 | Yes2 | Yes3 |
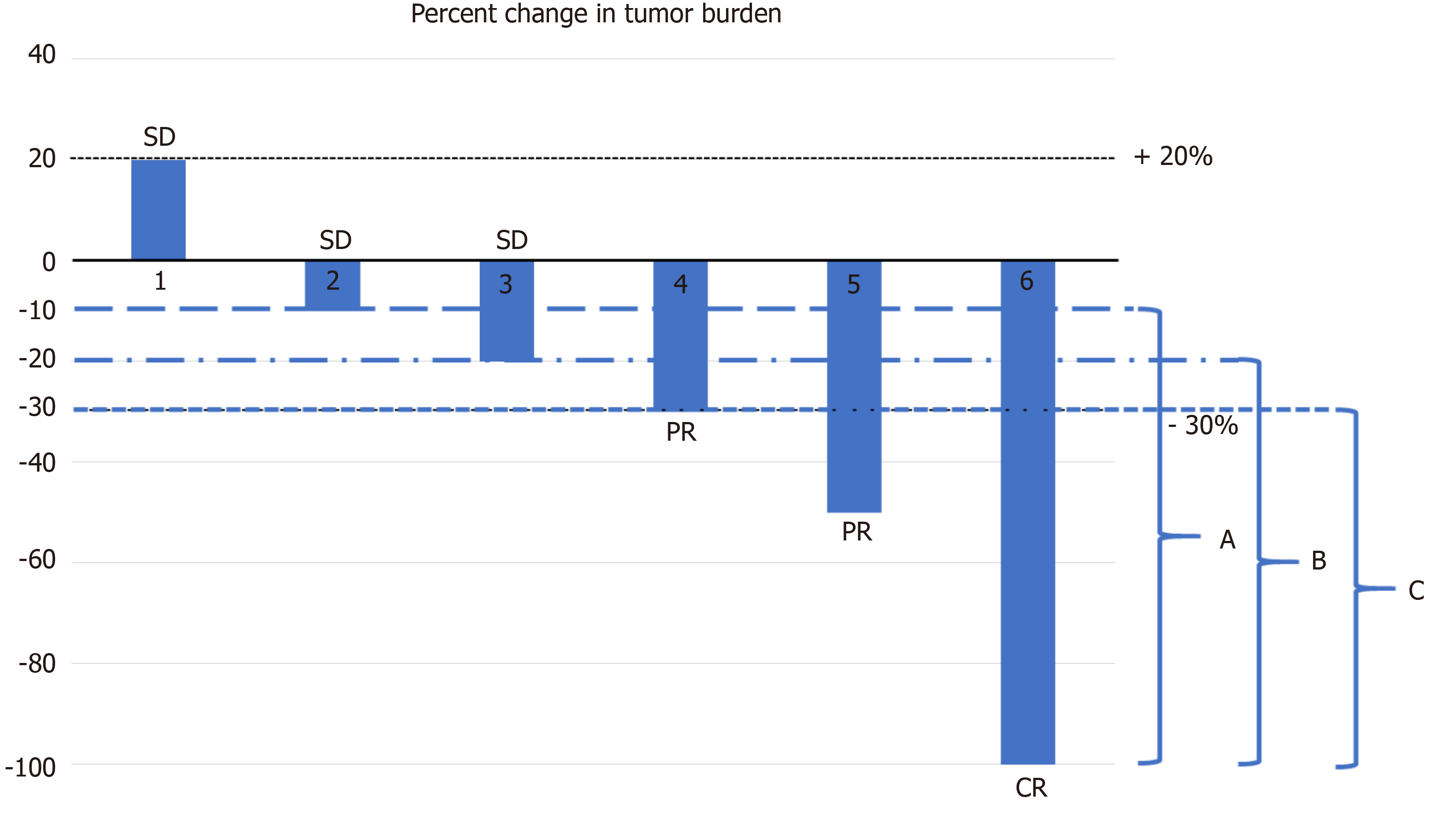
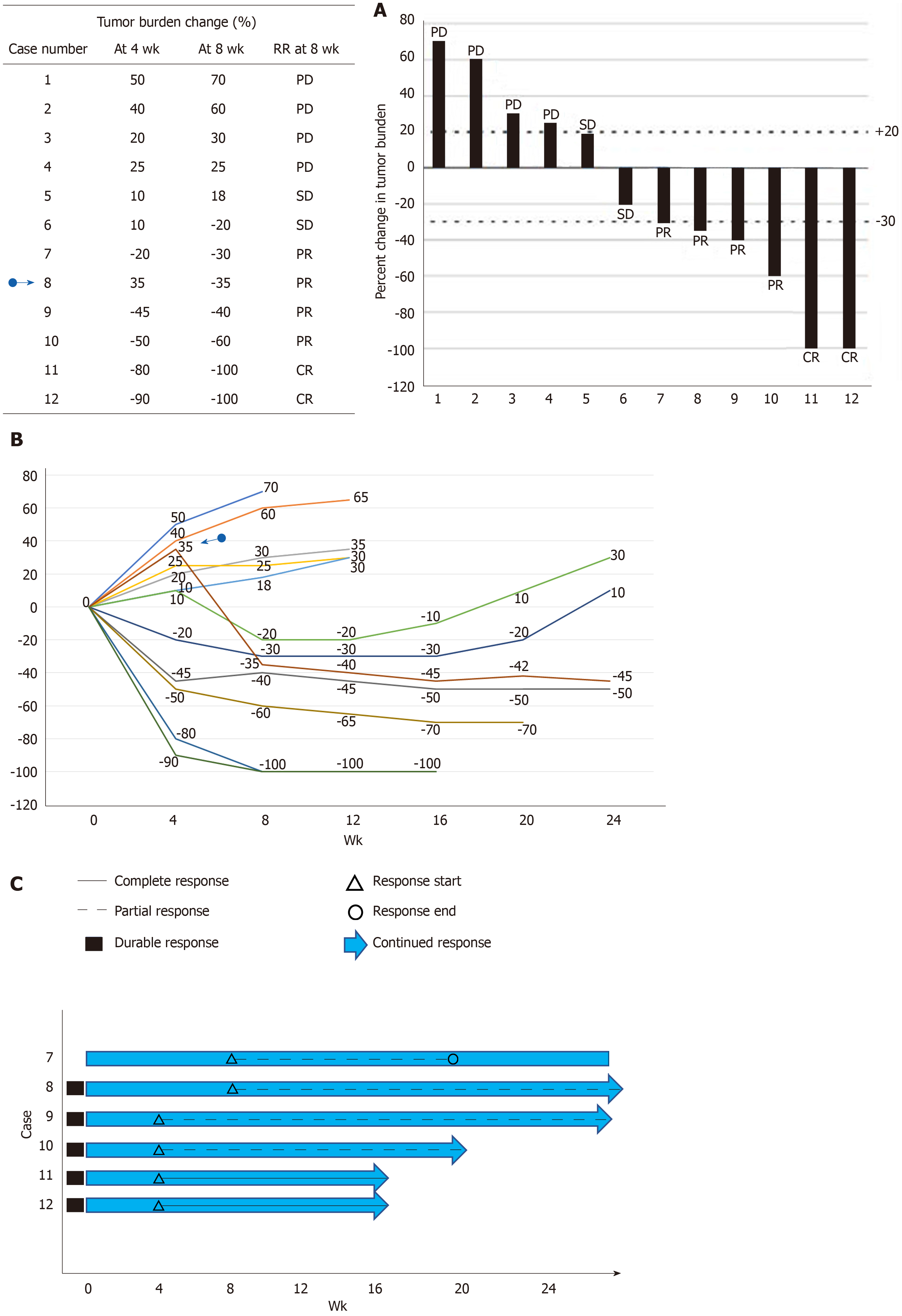
The ORR depends on the categorization of the used response criteria. These categorizations have a certain degree of arbitrary cut-off values such as a 20% increase for PD or a 30% decrease for PR at a certain time point (4 or 8 wk)[8,11,67,68]. It does not demonstrate continuous change in the TB over time. Intermittent measurement of the response by certain intervals to obtain the response duration may result sometimes in the loss of information such as early progression before the time of visit or death. The continuous longitudinal analysis of the TB data requires some graphical methods[67]. The popular graphical methods are the waterfall plot, spider (or spaghetti) plot and swimmer plot[67,69-71] (Figure 4). The waterfall plot is a bar chart that shows the largest percentage change relative to baseline in TB for each patient[67,69]. The horizontal axis (x) is used generally as a baseline measure and the vertical bars may go either above or below the baseline. Each bar represents one patient. The vertical axis (y) is generally used to quantify the response to treatment for example, percentage of growth or reduction. The negative vertical bars demonstrate reduction between 0% and -100%, and positive bars show nonresponders or progression. The bars are usually ordered from the worst (PD) response on the left side to the best (CR) value on the right side of the plot. We can easily see responders, nonresponders and stable patients in an area between the +20% and -30% lines; at a glance, an idea about the effectiveness can be obtained between two different treatments if different color-coded bars are used. The waterfall plot does not demonstrate the timing and durability of the response. Another commonly used graphical method is the spider plot where the percentage change from baseline is plotted against time for each patient. Each line represents one patient. We can observe pseudoprogression and response duration in the spider plot. Both methods have some limitations; they do not provide any statistical results when there is more than one treatment arm, and their use is limited when there are many patients[67,68]. Waterfall plots should be generated by trained radiologists due to the high interobserver variability[72]. A third graphical method is called the swimmer plot that shows an individual subject’s pattern of response[70]. In the swimmer plot, we observe multiple pieces of a patient’s response story, the duration of response and treatment. In Figure 4, we tried to demonstrate these graphical methods using 12 hypothetical cases.
These longitudinal graphical alternative methods of evaluating the response and some new simulation models with survival can be used, especially in early-phase clinical trials to better characterize the treatment effect and inform decision-making[67,73].
WHO criteria, RECIST and RECIST v1.1 are anatomical response criteria. They are based on the visual assessment of the tumor size in morphological images provided by CT or MRI. As mentioned above, the reduction in the viable tumor does not always result in a volume reduction, because the tumor tissue can be replaced by necrotic or fibrotic tissue and morphological changes cannot differentiate among these different tissue types, and volume changes are quite late events such as 2-3 mo[74]. In addition to anatomical and morphological imaging, metabolic imaging has become increasingly important in recent years.
Accelerated glucose metabolism is one of the functional changes observed in cancer cells, and this hallmark of cancer was first recognized by Otto Warburg[75,76]. Radioisotope-based molecular imaging techniques such as PET and single-photon emission CT capture functional pathologic changes[77]. A strong relationship was shown between 18F-FDG uptake and cancer cell number in a substantial number of studies[78]. PET can detect cancers that are smaller than demonstrated on CT. The limit of resolution for detecting cancers by 18F-FDG PET generally ranges between 0.4 and 1.0 cm, corresponding to approximately 108-109 cells[78]. 18F-FDG-PET CT can detect early metabolic changes in tumor cell metabolism before any change in tumor size occurs[79].
In RECIST v1.1, FDG-PET is included in the detection of new lesions in 2009[11]. In the same year, PET response criteria in solid tumors (PERCIST) were published by Wahl et al[78], and a simplified guide to PERCIST 1.0 was published in 2016[80]. Before PERCIST, EORTC PET response criteria were published in 1999[81]. EORTC PET response criteria and PERCIST follow the model of RECIST and define 4 response categories: Complete metabolic response (CMR), partial metabolic response (PMR), stable metabolic disease and progressive metabolic disease[82]. As a quantitative parameter, EORTC PET criteria use the mean standardized uptake value (SUVmean), normalized to the body surface area. PERCIST uses the SUVpeak, normalized to the lean body mass (abbreviated as SUL). The threshold of SUV change to define a response is 25% in EORTC PET criteria. PERCIST uses a change in the FDG uptake by 30% as a threshold for response and progression. These thresholds were calculated based on excluding the fluctuations of measurements of SUVs and have therapeutic effects[82]. Table 6 summarizes EORTC PET criteria and PERCIST 1.0. Recent clinical studies compairing these two metabolic criteria with each other as well as with RECIST and a pooled analysis have indicated that metabolic criteria lead to very similar classifications but there are significant differences between them and RECIST[82,83]. PERCIST is better correlated with patient outcome and may be a better predictor for the effectiveness of new anticancer treatments than RECIST. These findings require confirmation by data from randomized trials.
| EORTC PET, 1999 | PERCIST, 2009 | iPERCIST, 2019 | |
| CMR | Complete Resolution of FDG uptake1 | Complete Resolution of FDG uptake | Complete Resolution of FDG uptake |
| PMR | 15-25% decrease of SUV after one cycle or > 25% decrease after more than one treatment cycle | ≥ 30% decrease in the target tumor SULpeak | ≥ 30% decrease in the target tumor SULpeak |
| SMD | Increase of SUV by < 25% or decrease of SUV by < 15% | Neither CR, PR nor PD | Neither CR, PR nor PD |
| PMD | Increase of SUV by > 25% or Increase of the longest diameter by 20% or new FDG avid lesion(s) | ≥ 30% increase in SULpeak or advent of new FDG avid lesions | ≥ 30% increase in SULpeak or advent of new FDG avid lesions (UPMD); Need to be confirmed by a second PET at 4-8 wk later (CPMD) |
Metabolic response assessments using PET have the potential also after immunotherapy. Goldfarb et al[84] proposed immune PET Response Criteria (iPERCIST), which were adapted from PERCIST and iRECIST[63,80] (Table 6). iPERCIST is a dual-time-point evaluation of “unconfirmed progressive metabolic disease (UPMD)”; baseline PET imaging (SCAN-1) is performed within 1 mo before the start of immunotherapy, follow-up PET (SCAN-2) is peformed at 8 wk after the first dose, and, in the situation of UPMD, a second follow-up FDG-PET (SCAN-3) is performed 4 weeks later to confirm PD.
It appears that 18F-FDG uptake by the tumor is a consequence of an intact metabolic activity and reflects the viability of neoplastic cells[85]. Presently, 18F-FDG-PET CT occurs in the standard management of lymphomas as a reliable biomarker. After two cycles of the ABVD regimen in Hodgkin lymphoma (HL), interim 18F-FDG-PET CT is used to assess the treatment response in patients with early-stage disease. In 2009, the Deauville criteria were defined to interpret this interim and end-of-treatment PET scans[85,86]. Deauville criteria use a 5-point scale (5-PS) based on the visual uptake of 18F-FDG in the involved sites (Table 7). This 5-PS has been validated for use at interim PET (iPET) and end-of-treatment PET in lymphomas[87]. A score of 1 (no uptake) or 2 (uptake ≤ mediastinum) is considered a CMR at iPET and end-of-treatment PET[86-88]. A score of 3 is considered an inadequate response at the time of iPET, and undertreatment of HL should be avoided. A score of 4 or 5 with reduced uptake compared with baseline without new lesions is considered a PMR at iPET. At the end of treatment, a score of 4 or 5 indicates treatment failure even if uptake is reduced. In non-Hodgkin lymphomas (NHLs), Lugano response criteria are based on the metabolic response by PET/CT using 5-PS, and the radiologic response by contrast-enhanced CT[88]. iPET scan can be used after 2 or 4 cycles of chemotherapy in NHL, but has limited prognostic value due to false-positive results[88].
| Score | PET/CT scan result | At iPET | At end-of-treatment |
| 1 | No uptake | CMR | CMR |
| 2 | Uptake ≤ mediastinum | CMR | CMR |
| 3 | Liver ≥ Uptake > mediastinum | Inadequate response | Good prognosis |
| 4 | Uptake moderately higher than liver | PMR | Treatment failure |
| 5 | Uptake markedly higher than liver and/or new lesions | PMR | Treatment failure |
| X | New areas of uptake unlikely to be related to lymphoma |
OS is the gold standard that shows a treatment benefit in oncology. However, sometimes, it takes a long time and waiting for a comparison of the OS difference between treatment arms may delay drug approval by regulatory authorities. The TB data that cover ORR and PFS may be used as surrogate endpoints to OS. As we mentioned in the Introduction section, the assessment of the tumor ORR is important to report the results of clinical trials and for clinical decision making in routine practice[82]. The WHO (bidimensional) and RECIST (unidimensional) criteria are anatomic response criteria that reflect tumor shrinkage, mainly after cytotoxic chemotherapies. The theoretical reason of the unidimensional measurement of tumor size is essentially based on the direct relationship between the number of tumor cells in a spherical tumor and maximum diameter of the tumor mass[89]. Cell killing by chemotherapy is directly related to the dose; a PR is a function of the maximum diameter in the situation of proportionate (symmetrical) reduction of the initial tumor mass. If we consider the tumor tissue heterogeneity, tumor vascularization, necrosis, fibrosis, and intratumoral hemorrhage, tumor shrinkage may not always be symmetrical, especially in the era of biologics[28,29,32,39,40]. Tumor mass decrease is sometimes due to fragmentation rather than shrinkage of the primary tumor, as seen in rectal cancer after chemoradiotherapy[90]. Asymmetric shrinkage is also observed in prostate cancer after external beam radiation therapy[91]. Therefore, anatomic response criteria may not be optimal for biologic agents, some disease sites, some regional therapies. Consequently, modifications of RECIST, Choi criteria and morphologic response criteria were developed based on the concept of the evaluation of a viable tumor (contrast enhancement area and changes in tumor density)[28,41,42]. On the other hand, anatomic assessments alone may take two or three months to detect morphological changes or a shrinkage, whereas metabolic response assessment by PET can show changes as early as 8 d after the start of treatment[74,92,93].
RECIST was introduced based on retrospective clinical data, including more than 4000 patients assessed for the tumor response and then validated in prospective studies[92]. RECIST v1.1 was developed from the assessment of a large database that consists of > 6500 patients and > 18000 target lesions[11]. In a recent survey performed from key partners of the RECIST collaboration, it was shown that 52.3% responders were satisfied with RECIST v1.1[93]. A newest published pooled database analysis from the RECIST Working Group also indicated the good performance of RECIST v1.1 to evaluate the tumor response to targeted cancer agents (TCAs) independent of the subclass of TCA (signal transduction inhibitors or angiogenesis inhibitors) or tumor type[94]. Although validation studies exist and are generally accepted by regulatory agencies, the RECIST v1.1 criteria cannot capture the atypical responses observed with immunotherapy[11,95]. Atypical patterns of response (pseudoprogression) to anti-PD-1 therapy are observed at approximately 6%-10%[96]. Immune RECIST criteria are based essentially on the definition of true PD after confirmatory imaging.
Another clinically important reflection of tumor heterogeneity is contradictory progression of one or a few sites of disease despite an overall TB response to systemic therapy; this concept is called oligoprogression[97,98]. This concept is different than oligometastasis which indicates small number of metastases (generally less than five). In the oligometastatic state of cancer, patients are still candidates for regional potential curative therapy (surgical resection, ablation or RT)[97]. In oligoprogression, there can be diffuse metastatic disease, there is no upper limit of the number of metastases, and a few metastases progress whereas the most of the other tumors are not progressing[98].
With increasing use of immunotherapy, some rapid progression was described, and this immunotherapy-induced acceleration of tumor growth (twofold or greater increase in a time less than 2 mo) was called hyperprogression[10]. Actually, this exceptional situation is a very hot topic of research in immuno-oncology.
In the genomic era, due to smaller homogeneous populations, powering a randomized clinical trial for an end point of OS may no longer be feasible; therefore the ORR and duration of response in single-arm trials may lead to an accelerated approval of new drugs[99]. In a recent analysis of 542 arms for 294 regimens in solid tumors, Oxnard et al[100] found that demonstrating an ORR exceeding 30% with a single agent was highly specific to identify regimens achieving regulatory approval.
For some disease sites, such as the bone and brain, organ-specific response criteria were developed: MD Anderson (MDA) criteria for bone metastases, RANO for brain tumors[101,102]. Bone lesions are not measurable according to RECIST v1.1, only bone lesions with an identifiable soft tissue component may be used as target lesions[11,93]. MDA criteria incorporated CT and MRI to assess the response of bone metastatic lesions besides using plain radiography and skeletal scintigraphy[101]. Sclerosis of previous lytic lesions on CT or regression of measurable lesions on CT or MRI was considered as a PR[101]. In neuro-oncology, the Macdonald criteria, which was widely used until recently, determines the response as a change of a product of the maximal cross-sectional enhancing diameters together with changes in the neurologic status of patients and corticosteroid use[4]. These criteria did not consider the nonenhancing tumor, pseudoprogression and pseudoresponse. To address pseudoprogression, which is a transient increase in contrast enhancement observed in 10%-30% of patients with glioblastoma immediately after chemo-radiotherapy, the RANO criteria recommended, for recurrent disease, clear progression outside the radiation field or clear histologic documentation of progression after the first 12 wk after RT[102]. A pseudoresponse which is a marked decrease in contrast enhancement as early as 1-2 d after antiangiogenic therapies due to a decrease of vascular permeability, does not reflect a true response[103]. The RANO criteria recommended a confirmatory scan at least 4 weeks later to ensure a sustainable response. The RANO criteria defined progression not only as a 25% increase in the contrast-enhancing area but also included any significant enlargement of a nonenhancing T2/FLAIR signal on MRI. Table 8 summarizes current objective response assessment criteria.
| Response assessment criteria | Year | Imaging modalities | Assessment type | Advantages | Disadvantages |
| WHO | 1979 and 1981 | CT | Anatomic, size-based | First objective measurements of images of all lesions | Time-consuming procedure; Interobserver variability |
| RECIST v1.0 | 2000 | CT, MRI | Anatomic, size-based | Easier than WHO; Measurement of "Target" and "Non-target" lesions; Less measurement errors | Only anatomic assessment |
| RECIST v1.1 | 2009 | CT, MRI, PET | Anatomic, size-based | Easier than RECIST v1.0 Lymph nodes incorporated | Only anatomic assessment |
| mRECIST | 2006 | CT, MRI | Anatomic, size-based | Simpler than RECIST v1.1 | Only anatomic assessment, not prospectively validated |
| mRECIST for HCC | 2010 | CT, MRI | Anatomic and functional; Based on contrast enhancement | Measurement of a viable tumor. Appropriate for loco-regional therapies | Only for HCC |
| EASL and qEASL | 2000 and 2012 | CT, MRI | Anatomic and functional; Based on contrast enhancement | qEASL is better than RECIST to predict OS; Measurement of a viable tumor | Only for HCC |
| Choi criteria | 2007 | CT | Anatomic and functional; Based on tumor density | Validated for GIST, more precise than RECIST; Measurement of a viable tumor | Only for GIST |
| Morphologic Response | 2009 | CT | Anatomic and functional; Based on morphologic changes | Appropriate for bevacizumab treatment | For CRC liver met., not prospectively validated |
| irRC | 2009 | CT, MRI | Anatomic, size-based | For the treatment with immune-checkpoint inhibitors, capture of atypical response (pseudoprogression) | The variability of interpretation |
| irRECIST | 2013 | CT, MRI | |||
| iRECIST | 2017 | CT, MRI | |||
| imRECIST | 2018 | CT, MRI | |||
| EORTC PET PERCIST 1.0 iPERCIST | 1999, 2009, 2019 | PET | Metabolic | Detection of early metabolic changes | Limited resolution for tumors less than 0.4 cm. In NSCLC patients, retrospective |
| MDA criteria | 2004 and 2010 | CT, MRI, XR, SS | Anatomic | A comprehensive evaluation of bone metastasis | Only for bone metastasis |
| RANO | 2010 | MRI | Anatomic and functional; Based on contrast enhancement | Capture of pseudoprogression and pseudoresponse | Only for brain tumors |
Tumor tissue is a three-dimensional (3D) mass in the body, and, theoretically volumetric assessment of the objective response may detect precise changes in the TB. In spherical tumors, a decrease in the volume [V = (4/3) πr3] of 65% or greater corresponds to a 50% reduction in the product results [(2r)2] (WHO criteria) or 30% reduction in the diameter (2r) (RECIST) and indicates PR[5,6,89]. PD, which indicates a 20% increase in the diameter according to RECIST, corresponds to a more than 73% increase in volume, but a 25% increase in area according to WHO is corresponds to a 40% increase in volume[89]. Several studies have used 3D or volumetric measurements in different types of tumor and performed different comparisons since 1996[104-119]. Tumor volume measurements using CT initially started for radiotherapy treatment planning in the 1980s and was found to be highly accurate in early trials[120-122]. The comparison of 1D, 2D and 3D assessment of the response in various solid tumors such as lung nodules, metastatic pulmonary nodules, CRC LM, and metastatic lymph nodes was performed in different trials containing a small number of patients. Some of these trials showed good concordance among 1D, 2D and 3D measurements[104,105,111]. Moreover, Hopper et al[104] showed a 23.2% of change in the treatment response category using the 3D volume versus the 2D area methods, and this change was significant. Prasad et al[110] compared these three measurements in patients with LM from breast cancer and found that volumetric measurement produced different results in a considerable proportion of patients. Semiautomated volumetric analysis of lymph node metastases in patients with malignant melanoma was found to be feasible in one study and more reliable than manual measurements in another study evaluating the tumor volume from MRI images in patients with malignant glioma[116,117]. The ORR by volumetric assessment based on brain MRI was used as an approval end point for everolimus in subependymal giant-cell astrocytoma[99,118]. Warren et al[109] found less concordance in the minor response and PD categories between 3D and 1D in childhood brain tumors; however, this concordance was high in detecting PR. In a trial compairing manual 1D measurements versus automated volumetry (AV) in patients with pulmonary metastases, the authors concluded that AV allows better reproducibility and should be preferred[114]. Inversely, some other studies did not support the use of 3D measurements in the place of 1D or 2D assessments because 1D evaluation is comparable to 3D methods and simpler for clinical routine use[106-108,113,115,119]. Galanis et al[115] indicated that response assessment by 1D, 2D and computer-assisted volume methods was similar. A recent trial published in 2017 that compares volumetric versus 1D assessment in mCRC showed that 3D assessment is fairly reproducible and similar to 1D measurement[119]. Briefly, the superiority of volumetric response evaluation was not validated until now in large and prospective trials. On the other hand, tumors do not always have a regular contour; they may be spiculated and may contact various structures, such as the pleura, vessels and chest wall, making the assessment of volume difficult[112].
Imaging quality is the principal issue in radiologic assessments. New imaging techniques, such as diffusion-weighted imaging, dynamic contrast-enhanced MRI and perfusion CT, may add functional information to the morphological evaluation, especially in hepatic and pancreatic tumors[123]. Quantitative multimodality imaging in oncology, such as multiparametric MRI, the PET hybrids (PET-CT, PET-MRI), and new tracers in PET other than FDG (such as radiotracers to image oxygen status, receptor status, and proliferation), may provide a more-comprehensive and more accurate characterization of the tumor phenotype[124]. Importantly, the radiation exposure to patients during diagnosis, staging and response monitoring must be considered. The amount of radiation exposure from an imaging test is shown in Table 9[125]. Hybrid imaging procedures inevitably lead to an increase in patient radiation exposure[126]. MRI has no risk of ionizing radiation.
| Procedure | Approximate effective radiation dose | Comparable to natural background radiation for |
| Chest X-ray | 0.1 mSv | 10 d |
| CT-Head | 2 mSv | 8 mo |
| CT-Thorax | 7 mSv | 2 yr |
| CT–Abdomen and Pelvis | 10 mSv | 3 yr |
| PET/CT | 25 mSv | 8 yr |
Finally, radiologic image pattern analyses (radiomics) by computer-aided detection systems and, very recently artificial intelligence algorithms, particularly deep learning, have demonstrated remarkable progress in image-recognition tasks and are expected to identify wide applications in the evaluation of the treatment response and monitoring[127,128].
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kim HS, Melo F, Patel H S-Editor: Yan JP L-Editor: A E-Editor: Liu MY
| 1. | Karnofsky DA. Meaningful clinical classification of therapeutic responses to anticancer drugs. Clin Pharmacol Ther. 1961;2:709-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 142] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Moertel CG, Hanley JA. The effect of measuring error on the results of therapeutic trials in advanced cancer. Cancer. 1976;38:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Levin VA, Crafts DC, Norman DM, Hoffer PB, Spire JP, Wilson CB. Criteria for evaluating patients undergoing chemotherapy for malignant brain tumors. J Neurosurg. 1977;47:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 136] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Macdonald DR, Cascino TL, Schold SC, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1858] [Cited by in RCA: 1895] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 5. | World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment. World Health Organization Offset Publication No. 48; Geneva (Switzerland), 1979. |
| 6. | Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 7. | Thiesse P, Ollivier L, Di Stefano-Louineau D, Négrier S, Savary J, Pignard K, Lasset C, Escudier B. Response rate accuracy in oncology trials: reasons for interobserver variability. Groupe Français d'Immunothérapie of the Fédération Nationale des Centres de Lutte Contre le Cancer. J Clin Oncol. 1997;15:3507-3514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12751] [Cited by in RCA: 13076] [Article Influence: 523.0] [Reference Citation Analysis (0)] |
| 9. | Burzykowski T, Buyse M, Piccart-Gebhart MJ, Sledge G, Carmichael J, Lück HJ, Mackey JR, Nabholtz JM, Paridaens R, Biganzoli L, Jassem J, Bontenbal M, Bonneterre J, Chan S, Basaran GA, Therasse P. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol. 2008;26:1987-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 273] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 10. | Borcoman E, Nandikolla A, Long G, Goel S, Le Tourneau C. Patterns of Response and Progression to Immunotherapy. Am Soc Clin Oncol Educ Book. 2018;38:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 11. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21598] [Article Influence: 1349.9] [Reference Citation Analysis (1)] |
| 12. | Choi JH, Ahn MJ, Rhim HC, Kim JW, Lee GH, Lee YY, Kim IS. Comparison of WHO and RECIST criteria for response in metastatic colorectal carcinoma. Cancer Res Treat. 2005;37:290-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Zacharia TT, Saini S, Halpern EF, Sumner JE. CT of colon cancer metastases to the liver using modified RECIST criteria: determining the ideal number of target lesions to measure. AJR Am J Roentgenol. 2006;186:1067-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Kim HS, Kim JH, Yang I. Tumor response assessment by measuring the single largest lesion per organ in patients with advanced non-small cell lung cancer. Lung Cancer. 2014;85:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Kim HS, Kim JW, Kim JH, Choi DR, Han AR, Kim MJ, Kim BC, Zang DY. Single-lesion measurement per organ for assessing tumor response in advanced gastric cancer. Oncology. 2015;88:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Kim HS, Kim JH. Ideal number of target lesions per organ to measure in metastatic colorectal cancer. Oncol Lett. 2014;8:1896-1900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Jang HJ, Cho JW, Park B, Choi HC, Kim HS, Kim JH. The assessment of tumor response by measuring the single largest lesion per organ in metastatic tumors: a pooled analysis of previously reported data. J Cancer. 2015;6:169-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3301] [Article Influence: 220.1] [Reference Citation Analysis (36)] |
| 19. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6048] [Article Influence: 864.0] [Reference Citation Analysis (3)] |
| 20. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10261] [Article Influence: 603.6] [Reference Citation Analysis (2)] |
| 21. | Forner A, Ayuso C, Varela M, Rimola J, Hessheimer AJ, de Lope CR, Reig M, Bianchi L, Llovet JM, Bruix J. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 339] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 22. | Kantarci M, Pirimoglu B. Radiological Response to the Locoregional Treatment in Hepatocellular Carcinoma: RECIST, mRECIST, and Others. J Gastrointest Cancer. 2017;48:282-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3242] [Article Influence: 135.1] [Reference Citation Analysis (0)] |
| 24. | Zhao Y, Duran R, Bai W, Sahu S, Wang W, Kabus S, Lin M, Han G, Geschwind JF. Which Criteria Applied in Multi-Phasic CT Can Predict Early Tumor Response in Patients with Hepatocellular Carcinoma Treated Using Conventional TACE: RECIST, mRECIST, EASL or qEASL? Cardiovasc Intervent Radiol. 2018;41:433-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Lin M, Pellerin O, Bhagat N, Rao PP, Loffroy R, Ardon R, Mory B, Reyes DK, Geschwind JF. Quantitative and volumetric European Association for the Study of the Liver and Response Evaluation Criteria in Solid Tumors measurements: feasibility of a semiautomated software method to assess tumor response after transcatheter arterial chemoembolization. J Vasc Interv Radiol. 2012;23:1629-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15:257-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 497] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 27. | Labby ZE, Armato SG, Kindler HL, Dignam JJ, Hasani A, Nowak AK. Optimization of response classification criteria for patients with malignant pleural mesothelioma. J Thorac Oncol. 2012;7:1728-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Benjamin RS. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1094] [Cited by in RCA: 1118] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 29. | Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Charnsangavej C. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 410] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 30. | Choi H. Response evaluation of gastrointestinal stromal tumors. Oncologist. 2008;13 Suppl 2:4-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 31. | Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 2269] [Article Influence: 133.5] [Reference Citation Analysis (0)] |
| 32. | Vera R, Gomez Dorronsoro M, Lopez-Ben S, Viudez A, Queralt B, Hernandez I, Ortiz-Duran MR, Zazpe C, Soriano J, Amat I, Herrera Cabezón J, Diaz E, Codina-Barreras A, Hernandez-Yagüe X, Quera A, Figueras J. Retrospective analysis of pathological response in colorectal cancer liver metastases following treatment with bevacizumab. Clin Transl Oncol. 2014;16:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Ribero D, Wang H, Donadon M, Zorzi D, Thomas MB, Eng C, Chang DZ, Curley SA, Abdalla EK, Ellis LM, Vauthey JN. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer. 2007;110:2761-2767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 280] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 34. | Blazer DG 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, Fogelman D, Eng C, Chang DZ, Wang H, Zorzi D, Ribero D, Ellis LM, Glover KY, Wolff RA, Curley SA, Abdalla EK, Vauthey JN. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344-5351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 475] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 35. | Klinger M, Tamandl D, Eipeldauer S, Hacker S, Herberger B, Kaczirek K, Dorfmeister M, Gruenberger B, Gruenberger T. Bevacizumab improves pathological response of colorectal cancer liver metastases treated with XELOX/FOLFOX. Ann Surg Oncol. 2010;17:2059-2065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Stremitzer S, Stift J, Singh J, Starlinger P, Gruenberger B, Tamandl D, Gruenberger T. Histological response, pattern of tumor destruction and clinical outcome after neoadjuvant chemotherapy including bevacizumab or cetuximab in patients undergoing liver resection for colorectal liver metastases. Eur J Surg Oncol. 2015;41:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Adam R, Wicherts DA, de Haas RJ, Aloia T, Lévi F, Paule B, Guettier C, Kunstlinger F, Delvart V, Azoulay D, Castaing D. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: myth or reality? J Clin Oncol. 2008;26:1635-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 38. | Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, Sartoretti P, Dousset B, Majno PE, Soubrane O, Chaussade S, Mentha G, Terris B. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 394] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 39. | Gruenberger T, Arnold D, Rubbia-Brandt L. Pathologic response to bevacizumab-containing chemotherapy in patients with colorectal liver metastases and its correlation with survival. Surg Oncol. 2012;21:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Chun YS, Vauthey JN, Boonsirikamchai P, Maru DM, Kopetz S, Palavecino M, Curley SA, Abdalla EK, Kaur H, Charnsangavej C, Loyer EM. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302:2338-2344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 400] [Article Influence: 25.0] [Reference Citation Analysis (1)] |
| 41. | Shindoh J, Loyer EM, Kopetz S, Boonsirikamchai P, Maru DM, Chun YS, Zimmitti G, Curley SA, Charnsangavej C, Aloia TA, Vauthey JN. Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol. 2012;30:4566-4572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 42. | Nishioka Y, Shindoh J, Yoshioka R, Gonoi W, Abe H, Okura N, Yoshida S, Oba M, Hashimoto M, Watanabe G, Hasegawa K, Kokudo N. Radiological Morphology of Colorectal Liver Metastases after Preoperative Chemotherapy Predicts Tumor Viability and Postoperative Outcomes. J Gastrointest Surg. 2015;19:1653-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Pan W, Huang S, Zhang J, Zhao B, Wang H, Liu F, Jin K, Mou X. Heterogeneity-related anticancer therapy response differences in metastatic colon carcinoma: new hints to tumor-site-based personalized cancer therapy. Hepatogastroenterology. 2013;60:1927-1934. [PubMed] |
| 44. | Smith NR, Wedge SR, Pommier A, Barry ST. Mechanisms that influence tumour response to VEGF-pathway inhibitors. Biochem Soc Trans. 2014;42:1601-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Müller S, Link H, Niederle N, Rost A, Höffkes HG, Moehler M, Lindig RU, Modest DP, Rossius L, Kirchner T, Jung A, Stintzing S. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1330] [Article Influence: 120.9] [Reference Citation Analysis (0)] |
| 46. | Sclafani F, Cunningham D. Cetuximab or bevacizumab in metastatic colorectal cancer? Lancet Oncol. 2014;15:1040-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Pietrantonio F, Iacovelli R, Di Bartolomeo M, de Braud F. FOLFIRI with cetuximab or bevacizumab: FIRE-3. Lancet Oncol. 2014;15:e581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Stintzing S, Modest DP, Fischer von Weikersthal L, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran S, Heintges T, Lerchenmueller C, Kahl C, Seipelt G, Kullmann F, Scheithauer W, Held S, Giessen C, Moehler M, Jagenburg A, Jung A, Kirchner T, Heinemann V. Independent radiological evaluation of objective response, early tumor shrinkage, and depth of response in FIRE-3 (AIO KRK-0306) in the final RAS evaluable population. Ann Oncol. 2014;25:1-41. |
| 49. | Heinemann V, Stintzing S. FOLFIRI with cetuximab or bevacizumab: FIRE-3-authors' reply. Lancet Oncol. 2014;15:e583-e584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Heinemann V, Stintzing S, Modest DP, Giessen-Jung C, Michl M, Mansmann UR. Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur J Cancer. 2015;51:1927-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 51. | Modest DP, Laubender RP, Stintzing S, Giessen C, Schulz C, Haas M, Mansmann U, Heinemann V. Early tumor shrinkage in patients with metastatic colorectal cancer receiving first-line treatment with cetuximab combined with either CAPIRI or CAPOX: an analysis of the German AIO KRK 0104 trial. Acta Oncol. 2013;52:956-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Piessevaux H, Buyse M, Schlichting M, Van Cutsem E, Bokemeyer C, Heeger S, Tejpar S. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol. 2013;31:3764-3775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 53. | Piessevaux H, Buyse M, De Roock W, Prenen H, Schlichting M, Van Cutsem E, Tejpar S. Radiological tumor size decrease at week 6 is a potent predictor of outcome in chemorefractory metastatic colorectal cancer treated with cetuximab (BOND trial). Ann Oncol. 2009;20:1375-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 54. | Petrelli F, Pietrantonio F, Cremolini C, Di Bartolomeo M, Coinu A, Lonati V, de Braud F, Barni S. Early tumour shrinkage as a prognostic factor and surrogate end-point in colorectal cancer: a systematic review and pooled-analysis. Eur J Cancer. 2015;51:800-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, Rougier P, Nordlinger B. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 519] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 56. | Douillard JY, Siena S, Peeters M, Koukakis R, Terwey JH, Tabernero J. Impact of early tumour shrinkage and resection on outcomes in patients with wild-type RAS metastatic colorectal cancer. Eur J Cancer. 2015;51:1231-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 57. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Oliner KS, Wolf M, Gansert J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-4705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1296] [Cited by in RCA: 1393] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 58. | Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Williams R, Rong A, Wiezorek J, Sidhu R, Patterson SD. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1610] [Cited by in RCA: 1731] [Article Influence: 144.3] [Reference Citation Analysis (0)] |
| 59. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Smakal M, Canon JL, Rother M, Oliner KS, Tian Y, Xu F, Sidhu R. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 422] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 60. | Masuishi T, Taniguchi H, Eto T, Komori A, Mitani S, Hasegawa H, Narita Y, Ishihara M, Tanaka T, Kadowaki S, Ura T, Ando M, Tajika M, Nomura M, Sato Y, Mishima H, Muro K. Morphologic Response and Tumor Shrinkage as Early Predictive Markers in Unresectable Colorectal Liver Metastases. Anticancer Res. 2018;38:6501-6506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Aykan NF, Onat H, Uskent N. The virtual comparison of size-based tumor shrinkage methods and the effect of tumor heterogeneity. Proceedings of the Conference: Cell Symposia Hallmarks of Cancer; 2016 Dec 12; Ghent, Belgium. Abstract: 0065. Available from: https://www.researchgate.net/publication/311706718_The_virtual_comparison_of_size-based_tumor_shrinkage_methods_and_the_effect_of_tumor_heterogeneity. |
| 62. | Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol. 2015;33:3541-3543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 675] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 63. | Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, Hodi FS, Therasse P, Hoekstra OS, Shankar LK, Wolchok JD, Ballinger M, Caramella C, de Vries EGE; RECIST working group. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143-e152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1521] [Cited by in RCA: 1693] [Article Influence: 211.6] [Reference Citation Analysis (0)] |
| 64. | Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412-7420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2311] [Cited by in RCA: 2453] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 65. | Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19:3936-3943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 386] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 66. | Hodi FS, Ballinger M, Lyons B, Soria JC, Nishino M, Tabernero J, Powles T, Smith D, Hoos A, McKenna C, Beyer U, Rhee I, Fine G, Winslow N, Chen DS, Wolchok JD. Immune-Modified Response Evaluation Criteria In Solid Tumors (imRECIST): Refining Guidelines to Assess the Clinical Benefit of Cancer Immunotherapy. J Clin Oncol. 2018;36:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 267] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 67. | Shen Y, Anderson A, Sinha R, Li Y. Joint modeling tumor burden and time to event data in oncology trials. Pharm Stat. 2014;13:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 68. | LoRusso PM, Anderson AB, Boerner SA, Averbuch SD. Making the investigational oncology pipeline more efficient and effective: are we headed in the right direction? Clin Cancer Res. 2010;16:5956-5962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 69. | Gillespie TW. Understanding waterfall plots. J Adv Pract Oncol. 2012;3:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 70. | Tsimberidou AM, Levit LA, Schilsky RL, Averbuch SD, Chen D, Kirkwood JM, McShane LM, Sharon E, Mileham KF, Postow MA. Trial Reporting in Immuno-Oncology (TRIO): An American Society of Clinical Oncology-Society for Immunotherapy of Cancer Statement. J Clin Oncol. 2019;37:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 71. | Johnston S, Puhalla S, Wheatley D, Ring A, Barry P, Holcombe C, Boileau JF, Provencher L, Robidoux A, Rimawi M, McIntosh SA, Shalaby I, Stein RC, Thirlwell M, Dolling D, Morden J, Snowdon C, Perry S, Cornman C, Batten LM, Jeffs LK, Dodson A, Martins V, Modi A, Osborne CK, Pogue-Geile KL, Cheang MCU, Wolmark N, Julian TB, Fisher K, MacKenzie M, Wilcox M, Huang Bartlett C, Koehler M, Dowsett M, Bliss JM, Jacobs SA. Randomized Phase II Study Evaluating Palbociclib in Addition to Letrozole as Neoadjuvant Therapy in Estrogen Receptor-Positive Early Breast Cancer: PALLET Trial. J Clin Oncol. 2019;37:178-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 72. | Shao T, Wang L, Templeton AJ, Jang R, Vera-Badillo FW, McNamara MG, Margolis M, Kim TK, Sinaei M, Shoushtari H, Tannock IF. Use and misuse of waterfall plots. J Natl Cancer Inst. 2014;106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Booth CM, Calvert AH, Giaccone G, Lobbezoo MW, Eisenhauer EA, Seymour LK. Design and conduct of phase II studies of targeted anticancer therapy: recommendations from the task force on methodology for the development of innovative cancer therapies (MDICT). Eur J Cancer. 2008;44:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Stroobants S, Goeminne J, Seegers M, Dimitrijevic S, Dupont P, Nuyts J, Martens M, van den Borne B, Cole P, Sciot R, Dumez H, Silberman S, Mortelmans L, van Oosterom A. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec). Eur J Cancer. 2003;39:2012-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 352] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 75. | Warburg O, Posener K, Negelein E. The metabolism of cancer cells. Biochem Zeitschr. 1924;152:319-344. |
| 76. | Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19834] [Cited by in RCA: 19503] [Article Influence: 780.1] [Reference Citation Analysis (0)] |
| 77. | Vallabhajosula S. (18)F-labeled positron emission tomographic radiopharmaceuticals in oncology: an overview of radiochemistry and mechanisms of tumor localization. Semin Nucl Med. 2007;37:400-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 78. | Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S-150S. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2916] [Cited by in RCA: 2798] [Article Influence: 174.9] [Reference Citation Analysis (0)] |
| 79. | Pietrantonio F, Orlandi A, Inno A, Da Prat V, Spada D, Iaculli A, Di Bartolomeo M, Morosi C, de Braud F. Bevacizumab-based neoadjuvant chemotherapy for colorectal cancer liver metastases: Pitfalls and helpful tricks in a review for clinicians. Crit Rev Oncol Hematol. 2015;95:272-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 80. | O JH, Lodge MA, Wahl RL. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology. 2016;280:576-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 340] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 81. | Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, Pruim J, Price P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1291] [Cited by in RCA: 1315] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 82. | Pinker K, Riedl C, Weber WA. Evaluating tumor response with FDG PET: updates on PERCIST, comparison with EORTC criteria and clues to future developments. Eur J Nucl Med Mol Imaging. 2017;44:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 83. | Kim JH, Kim BJ, Jang HJ, Kim HS. Comparison of the RECIST and EORTC PET criteria in the tumor response assessment: a pooled analysis and review. Cancer Chemother Pharmacol. 2017;80:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 84. | Goldfarb L, Duchemann B, Chouahnia K, Zelek L, Soussan M. Monitoring anti-PD-1-based immunotherapy in non-small cell lung cancer with FDG PET: introduction of iPERCIST. EJNMMI Res. 2019;9:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 85. | Gallamini A, Fiore F, Sorasio R, Meignan M. Interim positron emission tomography scan in Hodgkin lymphoma: definitions, interpretation rules, and clinical validation. Leuk Lymphoma. 2009;50:1761-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 86. | Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C. Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leuk Lymphoma. 2009;50:1257-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 568] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 87. | Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, Schwartz LH, Zucca E, Fisher RI, Trotman J, Hoekstra OS, Hicks RJ, O'Doherty MJ, Hustinx R, Biggi A, Cheson BD. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048-3058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 961] [Cited by in RCA: 1149] [Article Influence: 114.9] [Reference Citation Analysis (0)] |
| 88. | Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; Italian Lymphoma Foundation; European Organisation for Research; Treatment of Cancer/Dutch Hemato-Oncology Group; Grupo Español de Médula Ósea; German High-Grade Lymphoma Study Group; German Hodgkin's Study Group; Japanese Lymphorra Study Group; Lymphoma Study Association; NCIC Clinical Trials Group; Nordic Lymphoma Study Group; Southwest Oncology Group; United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059-3068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2850] [Cited by in RCA: 3863] [Article Influence: 351.2] [Reference Citation Analysis (0)] |
| 89. | James K, Eisenhauer E, Christian M, Terenziani M, Vena D, Muldal A, Therasse P. Measuring response in solid tumors: unidimensional versus bidimensional measurement. J Natl Cancer Inst. 1999;91:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 302] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 90. | Hav M, Libbrecht L, Geboes K, Ferdinande L, Boterberg T, Ceelen W, Pattyn P, Cuvelier C. Prognostic value of tumor shrinkage versus fragmentation following radiochemotherapy and surgery for rectal cancer. Virchows Arch. 2015;466:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 91. | King BL, Butler WM, Merrick GS, Kurko BS, Reed JL, Murray BC, Wallner KE. Electromagnetic transponders indicate prostate size increase followed by decrease during the course of external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2011;79:1350-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 92. | Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer. 2006;42:1031-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 333] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 93. | Liu Y, Litière S, de Vries EG, Sargent D, Shankar L, Bogaerts J, Seymour L. The role of response evaluation criteria in solid tumour in anticancer treatment evaluation: results of a survey in the oncology community. Eur J Cancer. 2014;50:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 94. | Litière S, Isaac G, De Vries EGE, Bogaerts J, Chen A, Dancey J, Ford R, Gwyther S, Hoekstra O, Huang E, Lin N, Liu Y, Mandrekar S, Schwartz LH, Shankar L, Therasse P, Seymour L; RECIST Working Group. RECIST 1.1 for Response Evaluation Apply Not Only to Chemotherapy-Treated Patients But Also to Targeted Cancer Agents: A Pooled Database Analysis. J Clin Oncol. 2019;37:1102-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 95. | Inno A, Lo Russo G, Salgarello M, Corrao G, Casolino R, Galli G, Modena A, Romano L, Pusceddu S, Greco FG, Garassino MC, Gori S. The evolving landscape of criteria for evaluating tumor response in the era of cancer immunotherapy: From Karnofsky to iRECIST. Tumori. 2018;104:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 96. | Queirolo P, Spagnolo F. Atypical responses in patients with advanced melanoma, lung cancer, renal-cell carcinoma and other solid tumors treated with anti-PD-1 drugs: A systematic review. Cancer Treat Rev. 2017;59:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 97. | Rowe SP, Tran PT, Fishman EK, Johnson PT. Oligoprogression: What Radiologists Need to Know About This Emerging Concept in Cancer Therapeutic Decision-making. Acad Radiol. 2017;24:898-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 98. | Cheung P. Stereotactic body radiotherapy for oligoprogressive cancer. Br J Radiol. 2016;89:20160251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 99. | Blumenthal GM, Pazdur R. Response Rate as an Approval End Point in Oncology: Back to the Future. JAMA Oncol. 2016;2:780-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 100. | Oxnard GR, Wilcox KH, Gonen M, Polotsky M, Hirsch BR, Schwartz LH. Response Rate as a Regulatory End Point in Single-Arm Studies of Advanced Solid Tumors. JAMA Oncol. 2016;2:772-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 101. | Hamaoka T, Costelloe CM, Madewell JE, Liu P, Berry DA, Islam R, Theriault RL, Hortobagyi GN, Ueno NT. Tumour response interpretation with new tumour response criteria vs the World Health Organisation criteria in patients with bone-only metastatic breast cancer. Br J Cancer. 2010;102:651-657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 102. | Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ. Response Assessment in Neuro-Oncology Clinical Trials. J Clin Oncol. 2017;35:2439-2449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 319] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 103. | Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2528] [Cited by in RCA: 2930] [Article Influence: 195.3] [Reference Citation Analysis (0)] |
| 104. | Hopper KD, Kasales CJ, Eggli KD, TenHave TR, Belman NM, Potok PS, Van Slyke MA, Olt GJ, Close P, Lipton A, Harvey HA, Hartzel JS. The impact of 2D versus 3D quantitation of tumor bulk determination on current methods of assessing response to treatment. J Comput Assist Tomogr. 1996;20:930-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 105. | Van Hoe L, Van Cutsem E, Vergote I, Baert AL, Bellon E, Dupont P, Marchal G. Size quantification of liver metastases in patients undergoing cancer treatment: reproducibility of one-, two-, and three-dimensional measurements determined with spiral CT. Radiology. 1997;202:671-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 106. | Sohaib SA, Turner B, Hanson JA, Farquharson M, Oliver RT, Reznek RH. CT assessment of tumour response to treatment: comparison of linear, cross-sectional and volumetric measures of tumour size. Br J Radiol. 2000;73:1178-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 107. | Dachman AH, MacEneaney PM, Adedipe A, Carlin M, Schumm LP. Tumor size on computed tomography scans: is one measurement enough? Cancer. 2001;91:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 108. | Werner-Wasik M, Xiao Y, Pequignot E, Curran WJ, Hauck W. Assessment of lung cancer response after nonoperative therapy: tumor diameter, bidimensional product, and volume. A serial CT scan-based study. Int J Radiat Oncol Biol Phys. 2001;51:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 109. | Warren KE, Patronas N, Aikin AA, Albert PS, Balis FM. Comparison of one-, two-, and three-dimensional measurements of childhood brain tumors. J Natl Cancer Inst. 2001;93:1401-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 110. | Prasad SR, Jhaveri KS, Saini S, Hahn PF, Halpern EF, Sumner JE. CT tumor measurement for therapeutic response assessment: comparison of unidimensional, bidimensional, and volumetric techniques initial observations. Radiology. 2002;225:416-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 177] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 111. | Wormanns D, Kohl G, Klotz E, Marheine A, Beyer F, Heindel W, Diederich S. Volumetric measurements of pulmonary nodules at multi-row detector CT: in vivo reproducibility. Eur Radiol. 2004;14:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 229] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 112. | Tran LN, Brown MS, Goldin JG, Yan X, Pais RC, McNitt-Gray MF, Gjertson D, Rogers SR, Aberle DR. Comparison of treatment response classifications between unidimensional, bidimensional, and volumetric measurements of metastatic lung lesions on chest computed tomography. Acad Radiol. 2004;11:1355-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 113. | Shah GD, Kesari S, Xu R, Batchelor TT, O'Neill AM, Hochberg FH, Levy B, Bradshaw J, Wen PY. Comparison of linear and volumetric criteria in assessing tumor response in adult high-grade gliomas. Neuro Oncol. 2006;8:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 114. | Marten K, Auer F, Schmidt S, Kohl G, Rummeny EJ, Engelke C. Inadequacy of manual measurements compared to automated CT volumetry in assessment of treatment response of pulmonary metastases using RECIST criteria. Eur Radiol. 2006;16:781-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 115. | Galanis E, Buckner JC, Maurer MJ, Sykora R, Castillo R, Ballman KV, Erickson BJ. Validation of neuroradiologic response assessment in gliomas: measurement by RECIST, two-dimensional, computer-assisted tumor area, and computer-assisted tumor volume methods. Neuro Oncol. 2006;8:156-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 116. | Fabel M, von Tengg-Kobligk H, Giesel FL, Bornemann L, Dicken V, Kopp-Schneider A, Moser C, Delorme S, Kauczor HU. Semi-automated volumetric analysis of lymph node metastases in patients with malignant melanoma stage III/IV--a feasibility study. Eur Radiol. 2008;18:1114-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 117. | Ertl-Wagner BB, Blume JD, Peck D, Udupa JK, Herman B, Levering A, Schmalfuss IM; Members of the American College of Radiology Imaging Network 6662 Study Group. Reliability of tumor volume estimation from MR images in patients with malignant glioma. Results from the American College of Radiology Imaging Network (ACRIN) 6662 Trial. Eur Radiol. 2009;19:599-609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 118. | Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, Wilson KA, Byars A, Sahmoud T, Franz DN. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 722] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 119. | Lubner MG, Stabo N, Lubner SJ, Del Rio AM, Song C, Pickhardt PJ. Volumetric Versus Unidimensional Measures of Metastatic Colorectal Cancer in Assessing Disease Response. Clin Colorectal Cancer. 2017;16:324-333.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 120. | Quivey JM, Castro JR, Chen GT, Moss A, Marks WM. Computerized tomography in the quantitative assessment of tumour response. Br J Cancer Suppl. 1980;4:30-34. [PubMed] |
| 121. | Breiman RS, Beck JW, Korobkin M, Glenny R, Akwari OE, Heaston DK, Moore AV, Ram PC. Volume determinations using computed tomography. AJR Am J Roentgenol. 1982;138:329-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 224] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 122. | Nawaratne S, Fabiny R, Brien JE, Zalcberg J, Cosolo W, Whan A, Morgan DJ. Accuracy of volume measurement using helical CT. J Comput Assist Tomogr. 1997;21:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 123. | De Robertis R, Tinazzi Martini P, Demozzi E, Puntel G, Ortolani S, Cingarlini S, Ruzzenente A, Guglielmi A, Tortora G, Bassi C, Pederzoli P, D'Onofrio M. Prognostication and response assessment in liver and pancreatic tumors: The new imaging. World J Gastroenterol. 2015;21:6794-6808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 124. | Yankeelov TE, Abramson RG, Quarles CC. Quantitative multimodality imaging in cancer research and therapy. Nat Rev Clin Oncol. 2014;11:670-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 125. | RadiologyInfo. org. How much dose do I get from different imaging procedures? 2018 May 15 [cited 19 March 2019]. Available from: https://www.radiologyinfo.org/en/info.cfm?pg=safety-hiw_07. |
| 126. | Salvatori M, Rizzo A, Rovera G, Indovina L, Schillaci O. Radiation dose in nuclear medicine: the hybrid imaging. Radiol Med. 2019;124:768-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 127. | Cha KH, Hadjiiski L, Chan HP, Weizer AZ, Alva A, Cohan RH, Caoili EM, Paramagul C, Samala RK. Bladder Cancer Treatment Response Assessment in CT using Radiomics with Deep-Learning. Sci Rep. 2017;7:8738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 128. | Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts HJWL. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18:500-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1552] [Cited by in RCA: 1848] [Article Influence: 264.0] [Reference Citation Analysis (2)] |