Published online Jul 24, 2019. doi: 10.5306/wjco.v10.i7.269
Peer-review started: January 4, 2019
First decision: March 15, 2019
Revised: May 25, 2019
Accepted: July 16, 2019
Article in press: July 16, 2019
Published online: July 24, 2019
Processing time: 203 Days and 9.7 Hours
Breast metastasis from extra mammary malignancies is rare. An incidence of 0.2%-1.3% has been reported in the literature, including that from different types of malignant neoplasms.
We present a case of a 29-year-old nonsmoking woman with breast metastasis from lung adenocarcinoma. Computed tomography revealed atelectasis in the right middle lobe of the lung and ipsilateral pleural effusion. Additionally, on physical examination, a small mass was noted in her right breast. The patient underwent bronchoscopy, needle thoracentesis, and breast biopsy. Following cytology, histology and immunohistochemistry, primary lung adenocarcinoma with metastasis to the breast was diagnosed. Only 63 cases, including our patient, have been reported in the literature since 2000, and this is the second in a woman under 30 years of age.
This atypical presentation may cause a significant diagnostic dilemma, but the contribution of immunohistochemistry is crucial to the accuracy of the final diagnosis.
Core tip: We present the second case of lung adenocarcinoma with metastasis to the breast in a patient under 30 years of age. This is a rare entity in oncology and even more so in this age group. There have only been 63 reported cases of breast metastasis from lung adenocarcinoma over the last eighteen years. A clear correlation between the side of primary lung cancer and the side of breast metastasis can be identified. Due to the infrequency of this phenomenon, the diagnosis may cause a significant dilemma. Nevertheless, immunohistochemistry plays a key role in the final diagnosis.
- Citation: Enrico D, Saucedo S, Bravo I. Breast metastasis from primary lung adenocarcinoma in a young woman: A case report and literature review. World J Clin Oncol 2019; 10(7): 269-278
- URL: https://www.wjgnet.com/2218-4333/full/v10/i7/269.htm
- DOI: https://dx.doi.org/10.5306/wjco.v10.i7.269
Primary breast cancer is the most common malignancy in adult females. However, metastatic involvement of the breast is a rare phenomenon, with a reported frequency of approximately 0.2%-1.3%[1]. A variety of neoplasms have been reported to metastasize to the breast, including malignant melanoma, lymphoma, lung, ovary, prostate, kidney, stomach, ileum, thyroid, and cervical cancer[2]. Despite its rarity, metastatic breast disease from lung adenocarcinoma poses a significant diagnostic dilemma.
Lung cancer is the leading cause of cancer death, with one of the highest incidences. However, to date, there have been a few published cases of lung adenocarcinoma metastasizing to the breast. We report the case of a patient with breast metastasis from primary lung adenocarcinoma. To the best of our knowledge, this is the second report of this entity in a woman under 30 years of age.
A 29-year-old nonsmoking nurse presented with a 3-wk history of dry cough to the Eva Perón General Hospital, San Martín (Buenos Aires), Argentina.
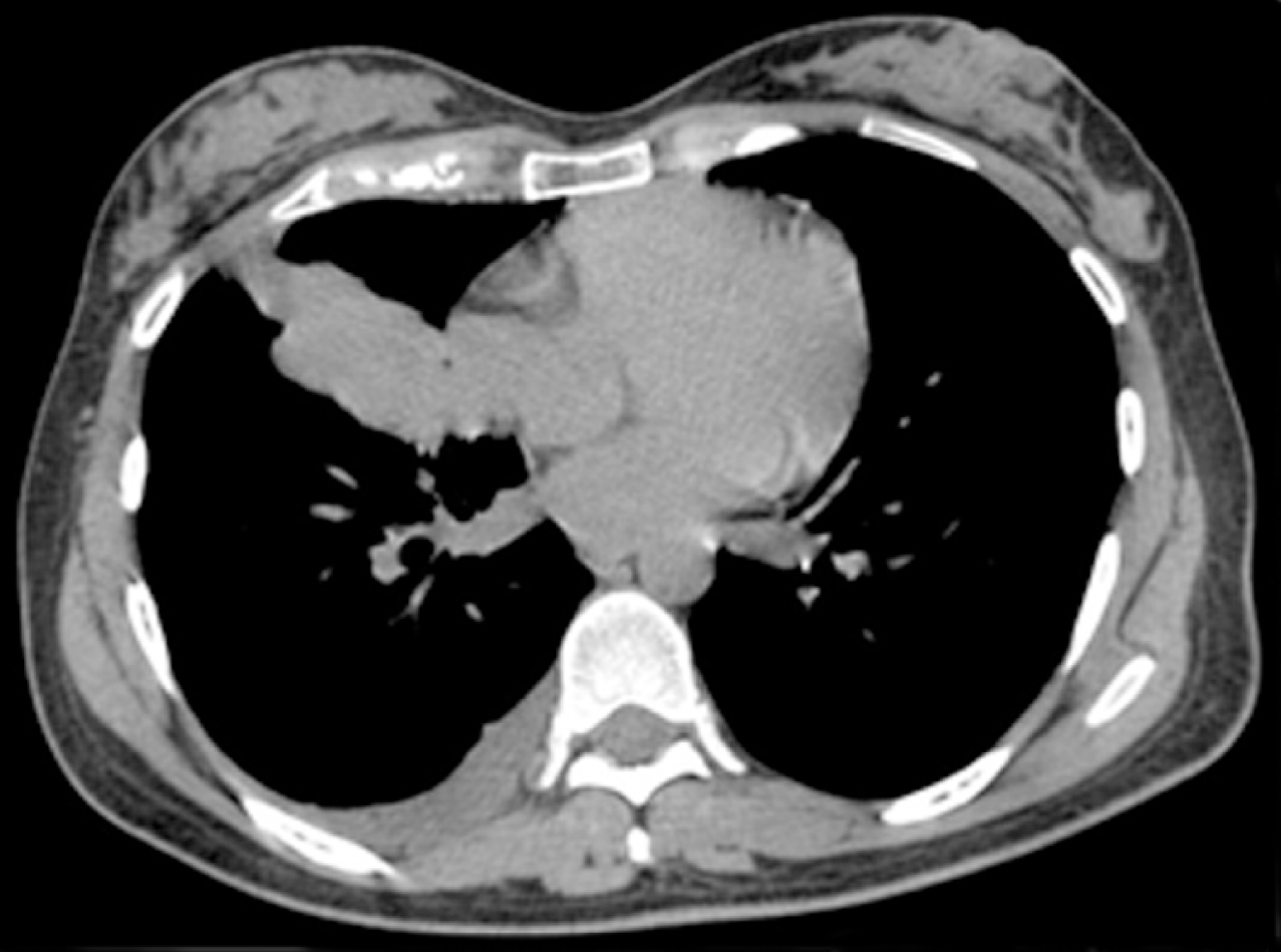
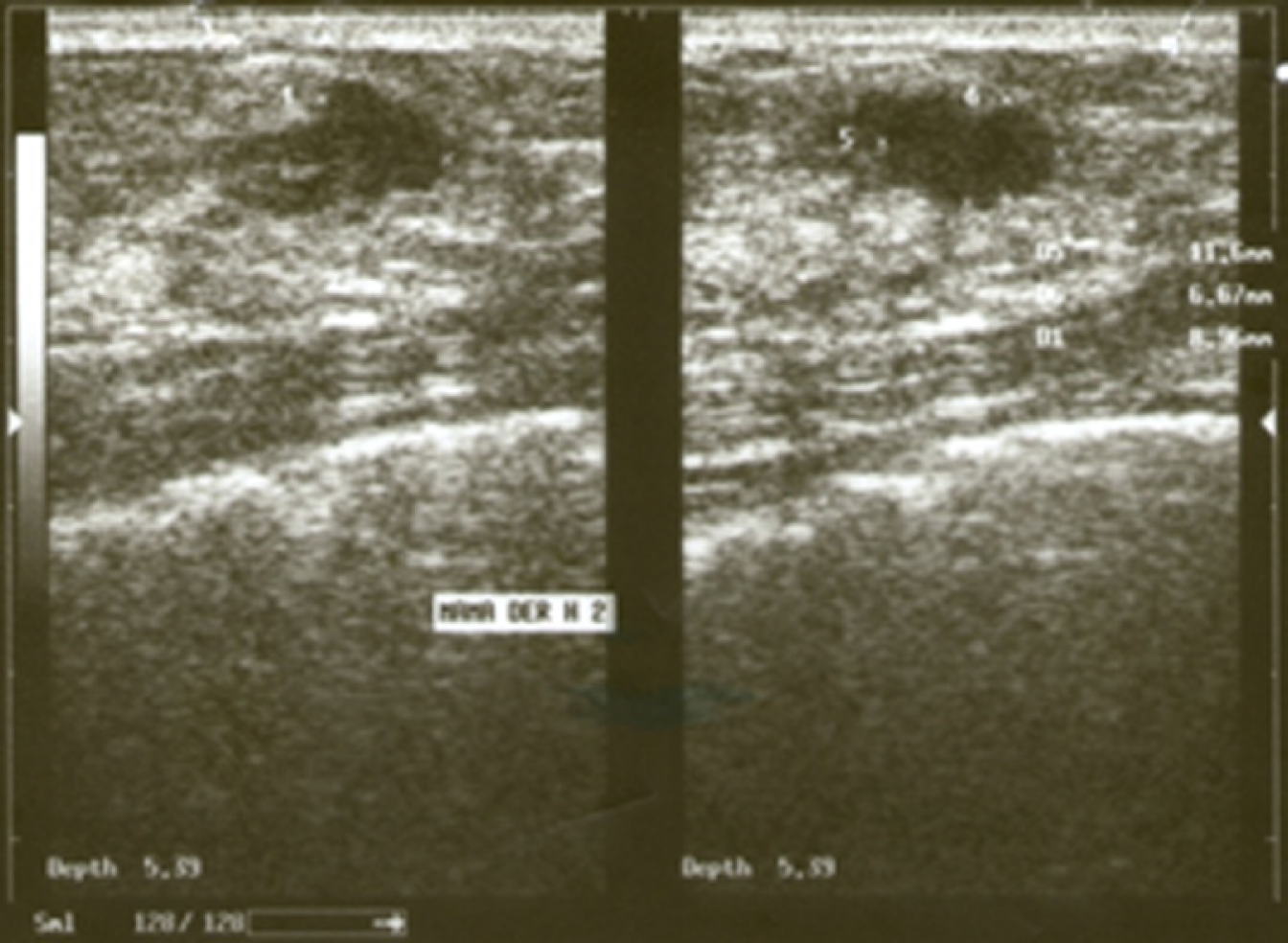
Routine chest X-ray followed by computed tomography (CT) revealed atelectasis in the right middle lobe of the lung, ipsilateral pleural effusion, and enlarged lymph nodes in the mediastinum and right hilum (Figure 1). On physical examination, a small mass was noted in the upper outer field quadrant of her right breast. Axillary and cervical chain lymph nodes were not palpable. Mammography did not reveal any suspicious images. However, ultrasonography (US) satisfactorily showed a hypoechoic solid nodule (11.6 mm x 6.6 mm x 8.9 mm) in the right breast, which was biopsied with a trucut needle (Figure 2).
The patient underwent bronchoscopy, which revealed submucosal infiltration causing a about 50% obstruction of the right middle lobe bronchus. During the bronchial procedure, washing, brushing and biopsies were obtained. Furthermore, needle thoracentesis was performed.
Based on all this information, the main differential diagnoses considered were a primary breast tumor with lung and pleural metastasis or two synchronous primary tumors.
All the cytological specimens (pleural effusion, bronchial washing, and bronchial brushing) were stained using the Papanicolaou technique, and the diagnosis of adenocarcinoma was suggested.
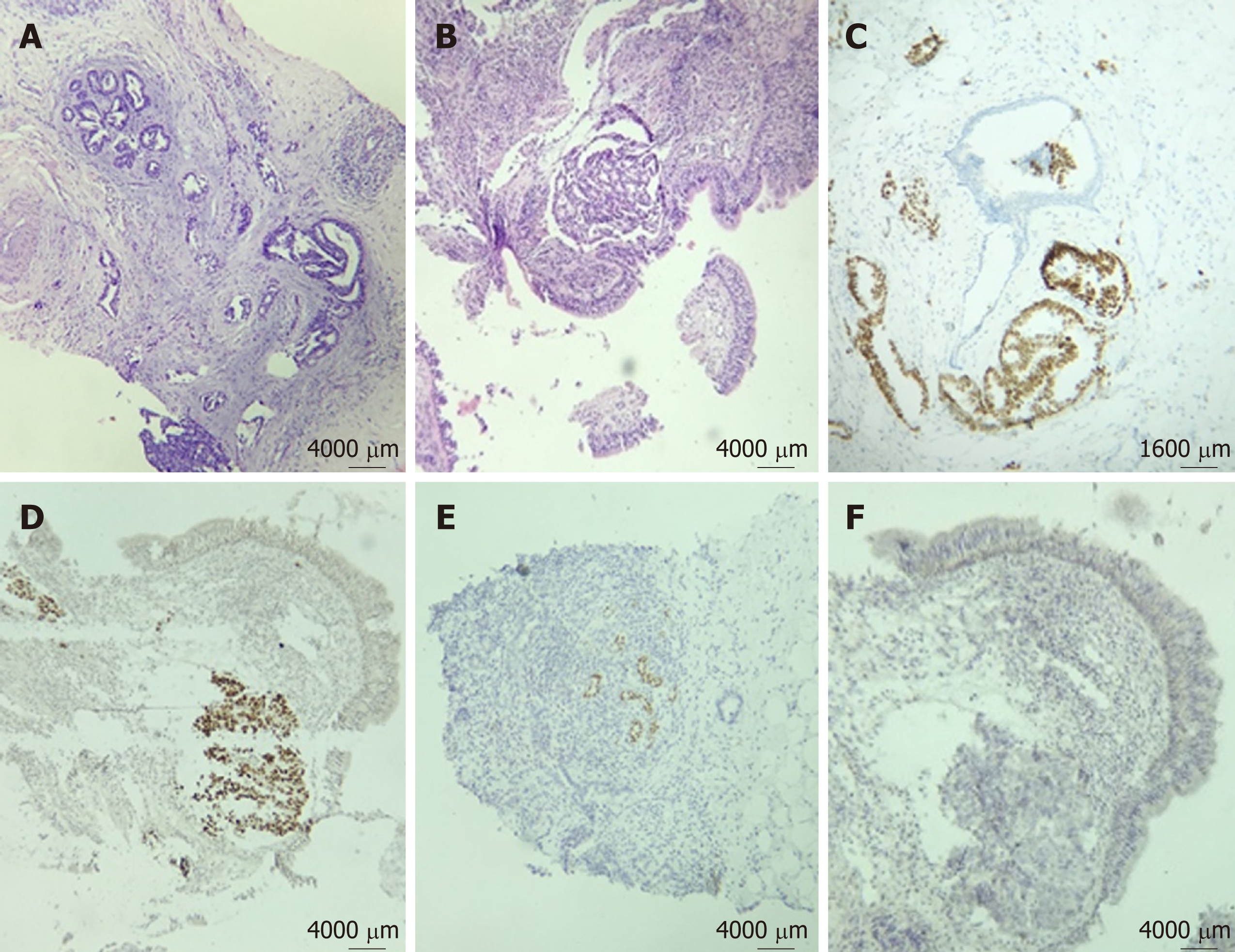
Hematoxylin-eosin (HE) staining and immunohistochemistry (IHC) were performed on formalin-fixed paraffin embedded tissues from bronchoscopy biopsy and core-needle breast biopsy. On both biopsies (bronchial mucosa and breast), HE-stained paraffin sections revealed infiltration by adenocarcinoma (Figure 3). Additionally, no evidence of in situ carcinoma was observed on the breast specimen. IHC (performed on a BenchMark XT autostainer, Ventana Medical Systems Inc, Tucson, AZ) of lung and breast specimens revealed strong immunoreactivity for anti-pancytokeratin AE1AE3, cytokeratin 7 (CK7), thyroid transcription factor-1 (TTF-1), and napsin A. The neoplastic cells lacked expression of cytokeratin 20 (CK20), P63, estrogen receptor (ER), progesterone receptor (PR), HER2/neu, and GATA3 (Figure 3).
Epidermal growth factor receptor mutations in exons 19 to 21 were negative (PCR-based pyrosequencing assay), as was EML4-ALK rearrangement by fluorescence in situ hybridization (FISH).
The histology and immunohistochemical staining pattern were strongly consistent with metastasis to the breast from primary lung adenocarcinoma.
In February 2016, the patient was started on treatment with cisplatin and pemetrexed. After an initial response, she experienced lung progression, and docetaxel was used as a second-line therapy to achieve stable disease.
Due to the deterioration of her clinical conditions, a third-line therapy was not feasible, and she continued with palliative supportive care. Her overall survival was 20 mo.
Since 2000, 63 cases of breast metastasis from a lung adenocarcinoma have been reported in the literature, including our patient (Table 1)[1,3-44]. The median age was 56 years (SD 13.4), and as expected, the majority were female (82.5%), while only 8 (12.7%) patients with breast metastasis were men.
| Ref. | Sex/age | Lung cancer | Breast metastasis | IHC markers of breast biopsy |
| Lee et al[3], 2000 (2 cases) | NA/NA | NA | NA | NA |
| Masmoudi et al[4], 2003 | Female/54 | NA | NA | NA |
| Ramar et al[5], 2003 | Male/56 | Right | Right | CK7-; CK20-; CAM 5.2-; ER-; PR-; CDP- |
| Yeh et al[6], 2004 | Female/44 | NA | Right | NA |
| Komorowski et al[7], 2005 | NA/48 | NA | NA | NA |
| Gómez-Caro et al[8] , 2006 | Male/65 | Left | Left | CK4+; CK7+; TTF-1- |
| Lee[1] , 2007 | Female/64 | NA | NA | NA |
| Ucar et al[9], 2007 | Male/63 | Left | Left | CK7+; TTF-1- |
| Ho et al[10], 2007 | Male/71 | Right | Left | NA |
| Rimner et al[11], 2007 | Female/81 | Left | Left | TTF-1+; ER-; PR-; HER2- |
| Fulciniti et al[12], 2008 | Female/59 | Right | Right | TTF-1+; ER-; PR- |
| Klingen et al[13], 2009 | Female/79 | NA | Left | CK7+; TTF-1+ |
| Male/70 | NA | Right | CK7+; TTF-1+ | |
| Wang et al[14], 2009 | Female/26 | Right | Bilateral | TTF-1+ |
| Babu et al[15], 2009 | Female/51 | NA | Left | CK7+; TTF-1+; ER-; PR- |
| Maounis et al[16], 2010 | Female/73 | Left | Left | TTF-1+; SP-A+; CEA+; CD15+; ER-; GCDFP15-; Mammaglobin-; CK 5/6 -; Calretinin -; CA125-; Thyroglobulin - |
| Yoon et al[17], 2010 | Female/42 | Left | Left | TTF-1+; E-cadherin+; ER-; PR-; p53-; HER2- |
| Nasit et al[18] ,2011 | Female/42 | Right | Bilateral | TTF-1+; CK7+; CEA+; GCDFP15-; ER-, PR-; CK5/6-; Thyroglobulin- |
| Fukumoto et al[19], 2011 | Female/65 | Left | Left | TTF-1+; ER- |
| Li et al[20], 2011 | Female/53 | Left | Left | TTF-1+; ER-; PR- |
| Ko et al[21], 2012 | Female/47 | Right | Right | TTF-1+; ER-; PR-; Mammaglobin- |
| Branica et al[22], 2012 | Female/55 | Left | Left | TTF-1+; CK7+; CK20- |
| Sato et al[23], 2012 | Female/57 | Right | Right | TTF-1+; CK 7+; SP-A+; MUC1+; ER-; PR-; MUC2 -; CK20-; GCDFP15-; HER2- |
| Ji et al[24], 2012 | Female/49 | Right | Left | TTF-1+; ER-; PR-; HER2-; Mammaglobin-; GCDFP15- |
| Female/40 | Left | Right | TTF-1+; ER-; PR-; HER2-; Mammaglobin-; GCDFP15- | |
| Huang et al[25], 2013 | Female/70 | Left | Left | TTF-1+; ER-; PR-; GCDFP15- |
| Female/48 | Right | Right | NA | |
| Female/43 | Right | Right | NA | |
| Female/54 | Left | Left | NA | |
| Female/52 | Left | Left | NA | |
| Female/43 | Left | Left | NA | |
| Sanguinetti et al[26], 2013 | Female/43 | Left | Left | TTF-1+; SP-A+; ER-; GCDFP15-; Mammaglobin- |
| Liam et al[27], 2013 | Female/70 | Right | Right | TTF-1+; ER-; PR-; HER2- |
| Sousaris et al[28], 2013 | Female/55 | Left | Left | TTF-1+; Napsin A+ER-; PR- |
| Jeong et al[29], 2014 | Female/47 | Left | Left | TTF-1+; CK7+; Napsin A+; ER-; PR-; HER2-; GCDFP15-; ALK- |
| Mirrielees et al[30], 2014 | Female/58 | Left | Left | TTF-1+; ER+; PR-; HER2- |
| Hachisuka et al[31], 2014 | Male/60 | Left | Right | TTF-1-; Napsin A-; ER-; PR-; HER2-; SP-A-; GCDFP15- |
| Dansin et al[32], 2015 | Female/52 | Left | Left | TTF1+; ER-; PR-; HER2-; GATA3-; GCDFP15-; PAX8- |
| Venkatesulu et al[33], 2015 | Female/30 | Right | Right | TTF1+; ER-; PR-; HER2- |
| Shen et al[34], 2015 | Female/52 | Right | Right | TTF-1+; CK7+; Napsin A+; ER-; PR-; GCDFP15-; Mammaglobin- |
| Gao et al[35], 2016 | Female/45 | Right | Right | TTF-1+; CK7+; Napsin A+; ROS1+; ER-; PR-; GCDFP15-; Mammaglobin-; HER2-; P63-; CK 5/6-; GATA3- |
| Female/43 | Right | Right | TTF-1+; CK7+; Napsin A+; ALK+; ER-; PR-; GCDFP15-; Mammaglobin-; HER2-; P63-; CK 5/6-; GATA3- | |
| Bhanu et al[36], 2016 | Female/30 | Right | Right | TTF-1+; GCDFP15-; Mammaglobin- |
| Erhamamci et al[37], 2016 | Male/74 | Right | Right | NA |
| Ninan et al[38], 2016 | Female/67 | Right | Right | CK7+; TTF-1+; Napsin A+; GCDFP15-; GATA3- |
| Ozturk et al[39], 2017 | Male/63 | Left | Left | TTF-1+; Napsin A+; Mucin +; P63- |
| Cserni[40], 2017 | Female/60 | Right | Left | CK7+; TTF-1+; Napsin A+; ER+; PR-; HER2-; GCDFP15-; Mammaglobin-; GATA3-; P63- |
| Zahedi et al[41], 2017 | Female/45 | Left | Right | CK7+; TTF-1+; Napsin A+; ER-; PR-; HER2-; GCDFP15-; CK20-; Mammaglobin-; Calretinin-; WT1-; CDX2-; Thyroglobulin- |
| Al-Zawi et al[42], 2017 | Female/84 | Left | Left | CK7+; TTF-1+; CK5-; P63-; ER-; PR-; GCDFP15 -; HER2-; ALK- |
| Ali et al[43], 2017 | Female/64 | NA | NA | TTF-1-; ER-; HER2- |
| Female/70 | NA | NA | TTF-1+; Napsin A+; ER+; HER2- | |
| Female/72 | NA | NA | TTF-1+; Napsin A+; ER-; HER2- | |
| Female/59 | NA | NA | TTF-1+; ER-; HER2- | |
| Female/63 | NA | Bilateral | TTF-1+; Napsin A+; ER-; HER2- | |
| Female/45 | NA | NA | TTF-1+; Napsin A-; ER-; HER2- | |
| Female/65 | NA | NA | TTF-1+ | |
| Female/70 | NA | NA | ER- | |
| Female/69 | NA | NA | TTF-1+; Napsin A+; ER-; HER2- | |
| Female/65 | NA | NA | TTF-1-; ER- | |
| Female/64 | NA | NA | TTF-1-; Napsin A-; ER-; HER2- | |
| Ota et al[44], 2018 | Female/69 | Left | Left | CK7+; CK 20-; TTF-1+; Napsin A+; ER-; PR-; HER2-; GCDFP15- |
| Our case | Female/29 | Right | Right | AE1AE3+; CK7+; TTF-1+; Napsin A+; P63-; CK20-; ER-; PR-; GATA3-; HER2- |
Of the 43 patients with data about the side of disease, 35 (81.4%) had evidence of disease in both lung and breast on the same side, while 6 (14%) had contralateral and 3 (7%) had bilateral breast involvement. A statistical correlation was observed between the side of the primary lung cancer and the side of the breast metastasis (P < 0.001).
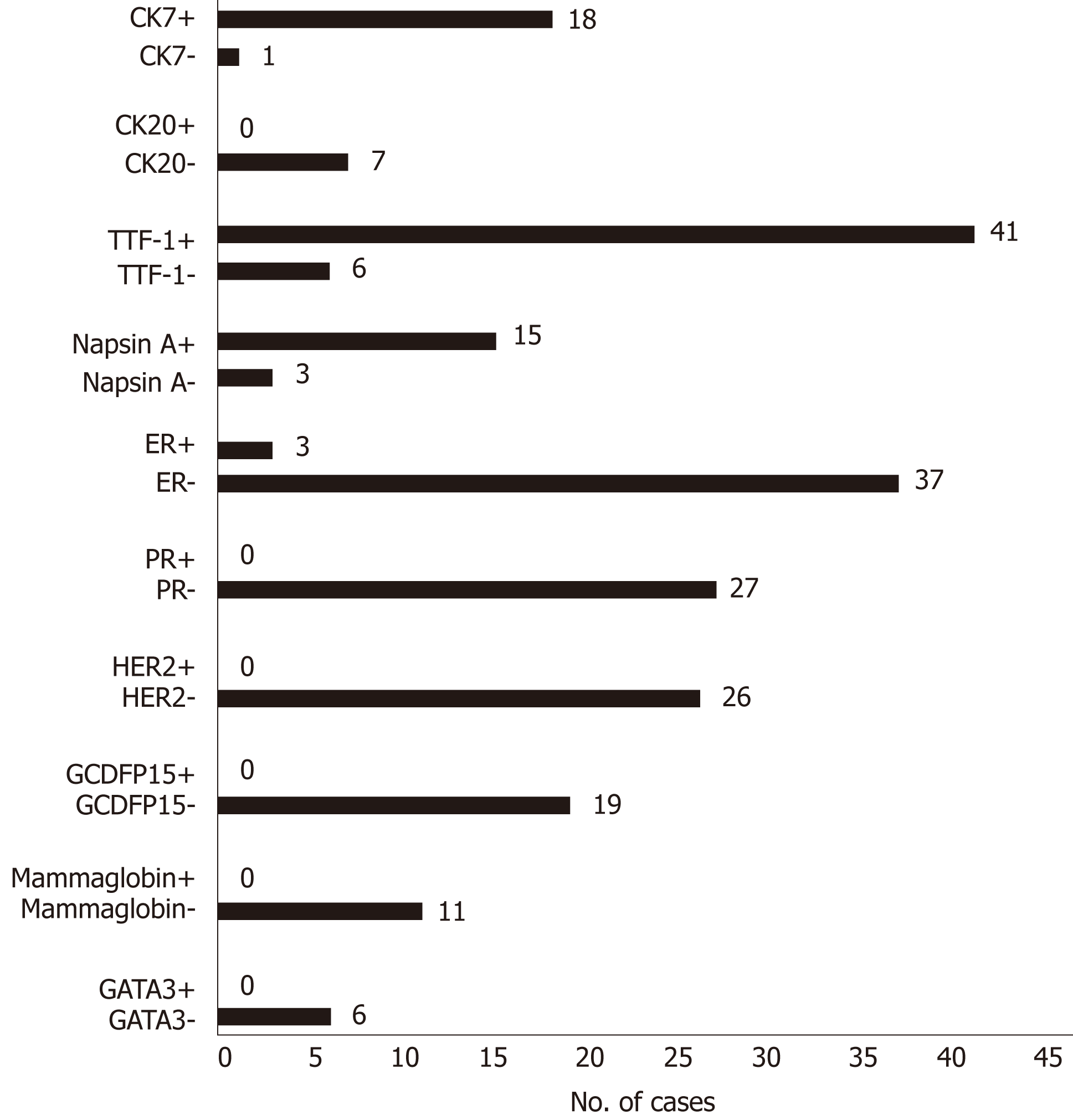
The distribution of immunohistochemical markers in the literature is shown in Figure 4. The most frequent markers analyzed were TTF-1, CK7, CK20, napsin A, ER, PR, HER2, GCDFP-15, mammaglobin, and GATA3. There were six cases with negative TTF-1, three with negative napsin A, and only one with negative CK7.
The most common sites of lung cancer metastasis are the bones, lungs, brain, adrenal glands, liver, and extrathoracic lymph nodes, in descending order[45]. However, autopsy series have revealed that lung cancer may metastasize to nearly any organ. Williams et al[46] published the most extensive series, which included 169 cases of metastases to the breast from extra mammary solid tumors and reported that the most common histological type was malignant melanoma.
Distinguishing a breast metastasis from a primary breast cancer, based on mammography, may be extremely difficult since metastasis can mimic a primary malignancy or even a benign lesion. The absence of micro calcifications is considered a characteristic of metastatic lesions to the breast, with the exception of ovarian cancer[47]. On mammography, usually single lesions are observed, but sometimes multiple well-circumscribed lesions may be present [13]. In our case, there were no mammographic findings, and the breast lesion was discovered by ultrasonography. Although most of the lesions do not show any specific histological features, some authors have described different characteristics of breast metastasis from extra mammary malignancies. These features include a circumscribed tumor with multiple satellite foci, the presence of many lymphatic emboli and the absence of an intraductal component, which is the most relevant characteristic[1].
As outlined above, the distinction between metastasis from lung adenocarcinoma and primary breast adenocarcinoma may cause a diagnostic dilemma. For this, the contribution of immunohistochemistry is crucial. There is no single marker with 100% sensitivity and specificity that can solve this problem, hence an immunohistochemical panel is needed. Both breast and lung adenocarcinomas have overlapping CK7+/CK20- immunoprofiles in most cases. The frequency of ER expression in lung adenocarcinoma has been reported to vary from 7.6% to 27.2%, depending on the antibody used[48]. TTF-1 is positive in 73%–88% of lung adenocarcinoma cases, and there are very few reports of its positivity in breast cancer (less than 3% at least weakly or focally)[49]. Napsin A staining has been reported to be positive in 80%-90% of lung adenocarcinoma cases. This marker is usually negative in breast cancer, even though it has been found to be positive in less than 3% of breast adenocarcinoma cases[50]. Although TTF-1 is a reliable marker for lung adenocarcinoma, napsin A is more sensitive and specific. The combination of both markers provides the maximum benefit. On the other hand, 67%-95% of breast cancer cases express GATA3 (43%–73% of triple-negative cases), and its expression in lung adenocarcinomas is less than 10%[51].
Our patient had metastasis to her right breast, which is the same side affected by the malignant pleural effusion, consistent with the hypothesis by Huang et al[25]. To this end, they considered a stepwise mechanism involving parietal pleural seeding, followed by invasion into chest wall lymphatic vessels draining to ipsilateral axillary lymph nodes and retrograde lymphatic spreading to the breast. This mechanism of breast metastasis could be supported by findings of enlarged homolateral axillary lymph nodes. Moreover, Barber et al[52] demonstrated lymphatic communication between the breast and mediastinal lymphatic channels. These hypotheses could be confirmed by the fact that almost 80% of the cases reported from 2000 to date had ipsilateral lesions. Another potential type of spread could be hematogenous. However, if lung cancer spreads through this route, both breasts should have the same probability of being affected. This is not reflected in the reviewed cases, where only 5.4% of patients had bilateral breast involvement. The last possible explanation could be direct tumor invasion through the chest wall to the breast, but chest CT scans did not reveal this alteration in the reported cases. Therefore, lymphatic spreading might be the most reasonable mechanism of lung cancer dissemination to the breast.
Here, we present a rare case of synchronous isolated metastasis to the breast from lung adenocarcinoma in a young patient. This is the second report, together with that by Wang et al[14], in a woman under 30 years of age. Due to the infrequency of this phenomenon, the diagnosis may cause a significant dilemma. Clinical examination, radiological assessment, and pathological evaluation are essential. Nonetheless, in our opinion, immunohistochemistry makes a difference, playing a key role in the accuracy of the final diagnosis.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: Argentina
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nacak M, Pereira-Vega A S-Editor: Wang JL L-Editor: A E-Editor: Ma YJ
| 1. | Lee AH. The histological diagnosis of metastases to the breast from extramammary malignancies. J Clin Pathol. 2007;60:1333-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Alva S, Shetty-Alva N. An update of tumor metastasis to the breast data. Arch Surg. 1999;134:450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Lee SH, Park JM, Kook SH, Han BK, Moon WK. Metastatic tumors to the breast: mammographic and ultrasonographic findings. J Ultrasound Med. 2000;19:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Masmoudi A, Mathieu MC, Soria JC. Breast metastasis from lung adenocarcinoma: a case report. Anticancer Res. 2003;23:1825-1826. [PubMed] |
| 5. | Ramar K, Pervez H, Potti A, Mehdi S. Breast metastasis from non-small-cell lung carcinoma. Med Oncol. 2003;20:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Yeh CN, Lin CH, Chen MF. Clinical and ultrasonographic characteristics of breast metastases from extramammary malignancies. Am Surg. 2004;70:287-290. [PubMed] |
| 7. | Komorowski AL, Wysocki WM, Mitus J. Metastasis to the breast--a clinical challenge in outpatient. Acta Chir Belg. 2005;105:59-61. [PubMed] |
| 8. | Gómez-Caro A, Piñero A, Roca MJ, Torres J, Ferri B, Galindo PJ, Parrilla P. Surgical treatment of solitary metastasis in the male breast from non-small cell lung cancer. Breast J. 2006;12:366-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Ucar N, Kurt OK, Alpar S, Orsel O, Demirag F, Kurt B. Breast metastasis in a male patient with nonsmall cell lung carcinoma. South Med J. 2007;100:850-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Ho L, Henderson R, Seto J. Breast metastasis from poorly differentiated adenocarcinoma of the lung on PET-CT. Clin Nucl Med. 2007;32:160-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Rimner A, Rosenzweig KE. Palliative radiation for lung cancer metastases to the breast: two case reports. J Thorac Oncol. 2007;2:1133-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Fulciniti F, Losito S, Botti G, Di Mattia D, La Mura A, Pisano C, Pignata S. Metastases to the breast: role of fine needle cytology samples. Our experience with nine cases in 2 years. Ann Oncol. 2008;19:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Klingen TA, Klaasen H, Aas H, Chen Y, Akslen LA. Secondary breast cancer: a 5-year population-based study with review of the literature. APMIS. 2009;117:762-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Wang SC, Tseng JC, Yu CP, Cheng MF, Perng WC, Chen CW. Breast Metastasis from Lung Adenocarcinoma in a 26-year-old Woman: A Case Report. Thorac Med. 2009;24:116–121. [DOI] [Full Text] |
| 15. | Babu KS, Roberts F, Bryden F, McCafferty A, Downer P, Hansell DT, Jones R, Milroy R. Metastases to breast from primary lung cancer. J Thorac Oncol. 2009;4:540-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Maounis N, Chorti M, Legaki S, Ellina E, Emmanouilidou A, Demonakou M, Tsiafaki X. Metastasis to the breast from an adenocarcinoma of the lung with extensive micropapillary component: a case report and review of the literature. Diagn Pathol. 2010;5:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Yoon MY, Song CS, Seo MH, Kim MJ, Oh TY, Jang UH, Kwag HJ, Kim HS, Lim SY, Lim SY, Lee SS. A case of metachronous metastasis to the breast from non-small cell lung carcinoma. Cancer Res Treat. 2010;42:172-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Nasit Jitendra G, Parikh B, Shah M. Bilateral breast metastasis from an adenocarcinoma of lung: a case report. Natl J Med Res. 2011;1:83–86. |
| 19. | Fukumoto K, Usami N, Okasaka T, Kawaguchi K, Okagawa T, Suzuki H, Yokoi K. Late breast metastasis from resected lung cancer diagnosed by epidermal growth factor receptor gene mutation. Lung Cancer. 2011;74:352-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Li CS, Chen T, Tu HY. Metastases to the breast from adenocarcinoma of lung: incidentally detected with routine computed tomography of chest. J Radiol Sci. 2011;36:37–40. |
| 21. | Ko K, Ro JY, Hong EK, Lee S. Micropapillary lung cancer with breast metastasis simulating primary breast cancer due to architectural distortion on images. Korean J Radiol. 2012;13:249-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Branica BV, Meniga IN, Puljić I, Marusić A, Chalfe N, Ivicević A. Breast metastasis from lung adenocarcinoma diagnosed with fine needle aspiration cytology: a case report. Coll Antropol. 2012;36:1461-1465. [PubMed] |
| 23. | Sato K, Takeyama Y, Yoshihara M, Kato T, Hashimoto H, Fukui Y, Gonda H, Suzuki R. CBDCA + Pemetrexed + Bevacizumab and Its Maintenance Chemotherapy in a Case of Solitary Breast Metastasis from a Lung Adenocarcinoma Resistant to Gefitinib. Case Rep Oncol. 2012;5:546-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Ji FF, Gao P, Wang JG, Zhao J, Zhao P. Contralateral breast metastasis from pulmonary adenocarcinoma: two cases report and literature review. J Thorac Dis. 2012;4:384-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 25. | Huang HC, Hang JF, Wu MH, Chou TY, Chiu CH. Lung adenocarcinoma with ipsilateral breast metastasis: a simple coincidence? J Thorac Oncol. 2013;8:974-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Sanguinetti A, Puma F, Lucchini R, Santoprete S, Cirocchi R, Corsi A, Triola R, Avenia N. Breast metastasis from a pulmonary adenocarcinoma: Case report and review of the literature. Oncol Lett. 2013;5:328-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Liam CK, Pang YK, Poh ME, Kow KS, Wong CK, Varughese R. Advanced right lung adenocarcinoma with ipsilateral breast metastasis. Respirol Case Rep. 2013;1:20-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Sousaris N, Mendelsohn G, Barr RG. Lung cancer metastatic to breast: case report and review of the literature. Ultrasound Q. 2013;29:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Jeong YJ, Bong JG, Oh HK, Park SH, Kang SM, Bae SH. Metachronous isolated breast metastasis from pulmonary adenocarcinoma with micropapillary component causing diagnostic challenges. BMC Cancer. 2014;14:736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Mirrielees JA, Kapur JH, Szalkucki LM, Harter JM, Salkowski LR, Strigel RM, Traynor AM, Wilke LG. Metastasis of primary lung carcinoma to the breast: a systematic review of the literature. J Surg Res. 2014;188:419-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Hachisuka A, Takahashi R, Nakagawa S, Takahashi H, Inoue Y, Akashi M, Ichiki M, Momosaki S, Kawahara A, Shirouzu K, Fujii T. Lung adenocarcinoma metastasis to the male breast: a case report. Kurume Med J. 2014;61:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Dansin E, Carnot A, Servent V, Daussay D, Robin YM, Surmei-Pintilie E, Lauridant G, Descarpentries C, Révillion F, Delattre C. EGFR-Mutated Breast Metastasis of Lung Adenocarcinoma: A Case Report. Case Rep Oncol. 2015;8:164-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Venkatesulu BP, Mallick S, Singh A, Julka PK. Non small cell carcinoma of lung with metachronous breast metastasis and cardiac tamponade: Unusual presentation of a common cancer. J Egypt Natl Canc Inst. 2015;27:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Shen YW, Sui YX, Zhang XM, Lv M, Zhang X, Liu PJ, Yang J. Ipsilateral breast metastasis from a pulmonary adenocarcinoma: a case report and a focused review of the literature. Int J Clin Exp Pathol. 2015;8:9647-9654. [PubMed] |
| 35. | Gao Q, Wang B, Zheng Y, Ren G, Zhou J. Breast metastasis from lung cancer: report of two cases of adenocarcinoma with different gene mutation and one case of squamous cell carcinoma. Int J Clin Exp Pathol. 2016;9:443–453. |
| 36. | Bhanu LP, Srinivasa BJ, Hazarika D, Nasiruddin M, Radheshyam N, Mansi K. Breast Metastasis from Adenocarcinoma of Lung: A Case Report. Southeast Asian J Case Rep Rev. 2016;5:2537–2542. |
| 37. | Erhamamci S, Reyhan M, Canpolat T, Nursal GN, Yapar AF. A Case of a Man With Isolated Breast Metastasis From Lung Adenocarcinoma Incidentally Detected by FDG PET/CT. Clin Nucl Med. 2016;41:e146-e148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Ninan J, Naik V, George GM. 'Inflammatory breast cancer' due to metastatic adenocarcinoma of lung. BMJ Case Rep. 2016;2016:bcr2016215857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Ozturk A, Yenibertiz D, Aktas Z, Yılmaz A, Demirag F. A man patient with ipsilateral breast metastasis from pulmonary adenocarcinoma. Cancer Rep Rev. 2017;1:1–2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 40. | Cserni G. Solitary breast metastasis from oestrogen receptor-positive pulmonary adenocarcinoma: report of a case with a potential pitfall. Pol J Pathol. 2017;68:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Zahedi F, Mahdavi H. A Case of Lung Adenocarcinoma with Metastasis to the Breast. Oncol Cancer Case Rep. 2017;3:1–3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 42. | Al-Zawi ASA, Ratajczak A, Idaewor P, Elamass M, Lazarevska A, Tan E, Barron M, Asaad A. Primary lung cancer with metastasis to the ipsilateral breast-a case report. Int J Res Med Sci. 2017;6:334–339. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 43. | Ali RH, Taraboanta C, Mohammad T, Hayes MM, Ionescu DN. Metastatic non-small cell lung carcinoma a mimic of primary breast carcinoma-case series and literature review. Virchows Arch. 2018;472:771-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Ota T, Hasegawa Y, Okimura A, Sakashita K, Sunami T, Yukimoto K, Sawada R, Sakamoto K, Fukuoka M. Breast metastasis from EGFR-mutated lung adenocarcinoma: A case report and review of the literature. Clin Case Rep. 2018;6:1510-1516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Tamura T, Kurishima K, Nakazawa K, Kagohashi K, Ishikawa H, Satoh H, Hizawa N. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol Clin Oncol. 2015;3:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 242] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 46. | Williams SA. Ehlers RA 2nd, Hunt KK, Yi M, Kuerer HM, Singletary SE, Ross MI, Feig BW, Symmans WF, Meric-Bernstam F. Metastases to the breast from nonbreast solid neoplasms: presentation and determinants of survival. Cancer. 2007;110:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 47. | Lee SK, Kim WW, Kim SH, Hur SM, Kim S, Choi JH, Cho EY, Han SY, Hahn BK, Choe JH, Kim JH, Kim JS, Lee JE, Nam SJ, Yang JH. Characteristics of metastasis in the breast from extramammary malignancies. J Surg Oncol. 2010;101:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 48. | Gomez-Fernandez C, Mejias A, Walker G, Nadji M. Immunohistochemical expression of estrogen receptor in adenocarcinomas of the lung: the antibody factor. Appl Immunohistochem Mol Morphol. 2010;18:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Provenzano E, Byrne DJ, Russell PA, Wright GM, Generali D, Fox SB. Differential expression of immunohistochemical markers in primary lung and breast cancers enriched for triple-negative tumours. Histopathology. 2016;68:367-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Turner BM, Cagle PT, Sainz IM, Fukuoka J, Shen SS, Jagirdar J. Napsin A, a new marker for lung adenocarcinoma, is complementary and more sensitive and specific than thyroid transcription factor 1 in the differential diagnosis of primary pulmonary carcinoma: evaluation of 1674 cases by tissue microarray. Arch Pathol Lab Med. 2012;136:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 51. | Miettinen M, McCue PA, Sarlomo-Rikala M, Rys J, Czapiewski P, Wazny K, Langfort R, Waloszczyk P, Biernat W, Lasota J, Wang Z. GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors. Am J Surg Pathol. 2014;38:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 496] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 52. | Barber TW, Hofman MS, Hicks RJ. Breast lymphatic drainage via the pulmonary lymphatic system. Eur J Nucl Med Mol Imaging. 2010;37:2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |