Copyright
©The Author(s) 2025.
World J Clin Oncol. Jun 24, 2025; 16(6): 106798
Published online Jun 24, 2025. doi: 10.5306/wjco.v16.i6.106798
Published online Jun 24, 2025. doi: 10.5306/wjco.v16.i6.106798
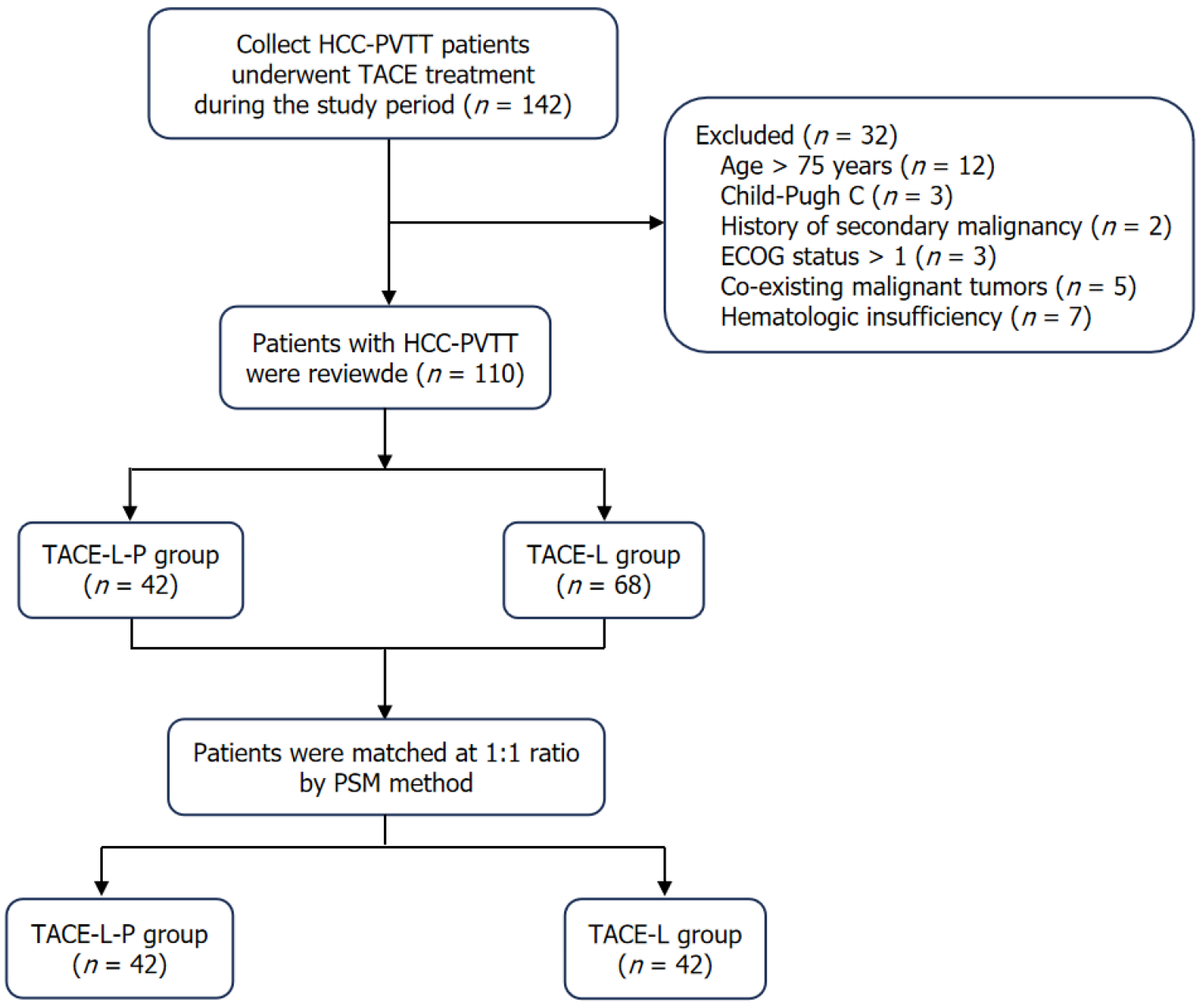
Figure 1 Flow diagram of hepatocellular carcinoma with portal vein tumor thrombus patients treated with transarterial arterial chemoe
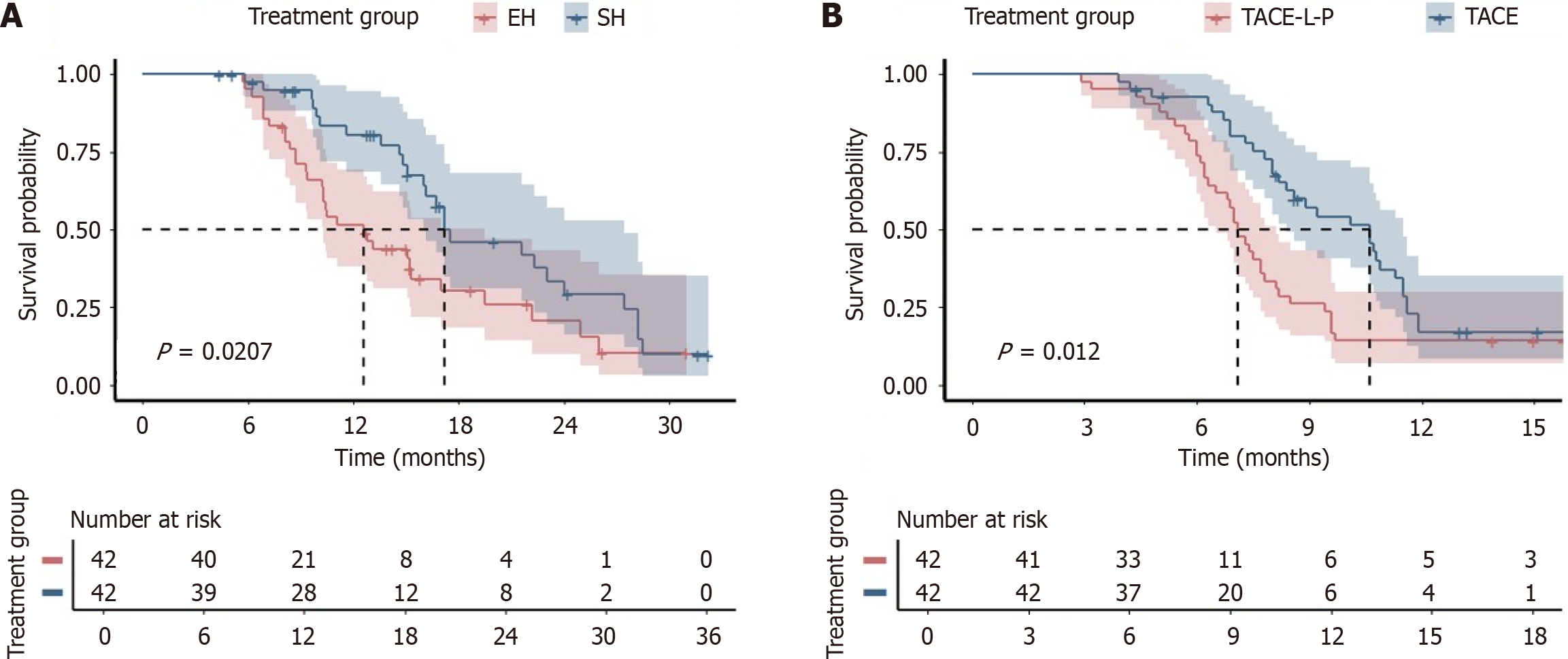
Figure 2 Kaplan-Meier analyses.
A: Overall survival; B: Progression-free survival according to treatment groups after propensity score matching. EH: Emergency hepatectomy; PSM: Propensity score matching; SH: Staged hepatectomy; TACE-L: Transarterial chemoembolization combined with lenvatinib; TACE-L-P: Transarterial arterial chemoembolization combined with lenvatinib plus death protein-1 inhibitors.
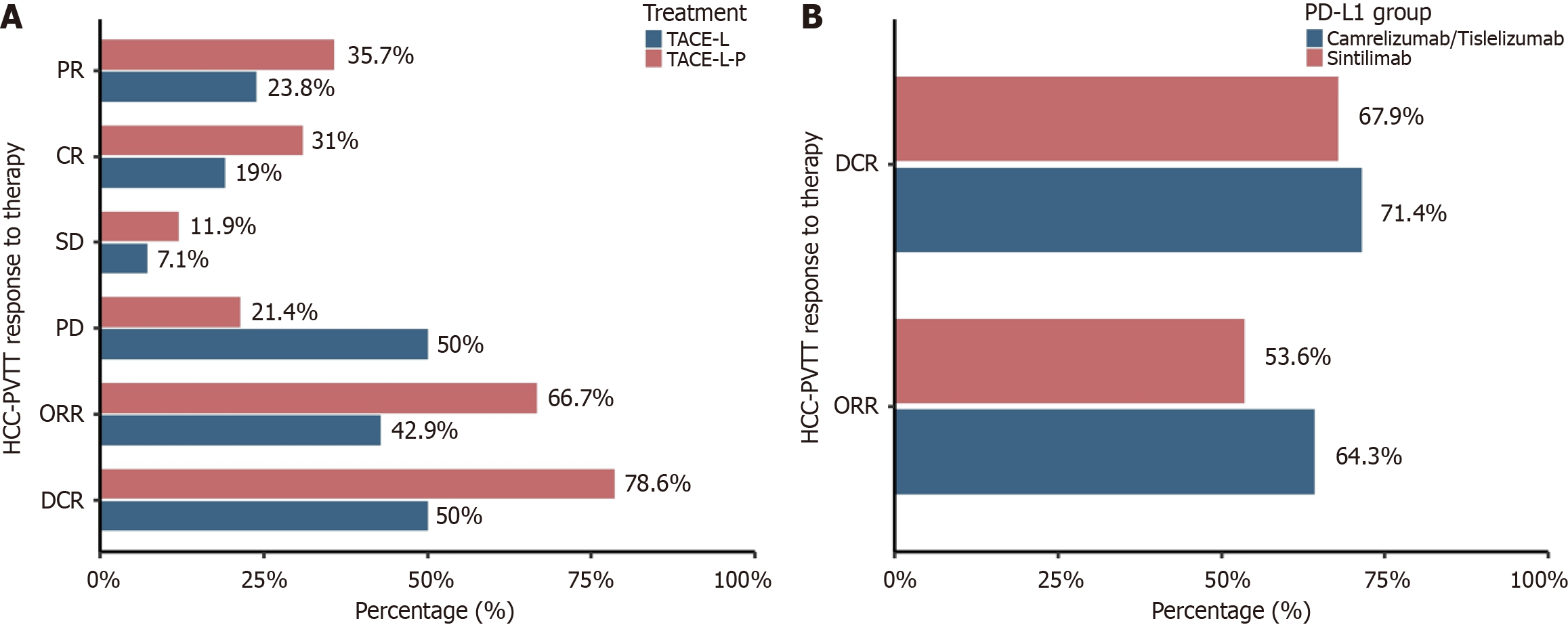
Figure 3 Comparison of objective response rate and disease control rate.
A: Comparison of objective response rate (ORR) and disease control rate (DCR) between transarterial arterial chemoembolization combined with lenvatinib plus death protein-1 inhibitors (TACE-L-P) and transarterial chemoembolization combined with lenvatinib groups based on Modified Response Evaluation Criteria In Solid Tumors criteria after propensity score matching; B: Comparison of ORR and DCR within the TACE-L-P group stratified by programmed death ligand-1 inhibitors (Sintilimab vs Camrelizumab/Tislelizumab). CR: Complete response; DCR: Disease control rate; HCC-PVTT: Hepatocellular carcinoma with portal vein tumor thrombus; ORR: Objective response rate; PD: Death protein; PD-L1: Programmed death ligand-1; PR: Partial response; SD: Stable disease; TACE-L: Transarterial chemoembolization combined with lenvatinib; TACE-L-P: Transarterial arterial chemoembolization combined with lenvatinib plus death protein-1 inhibitors.
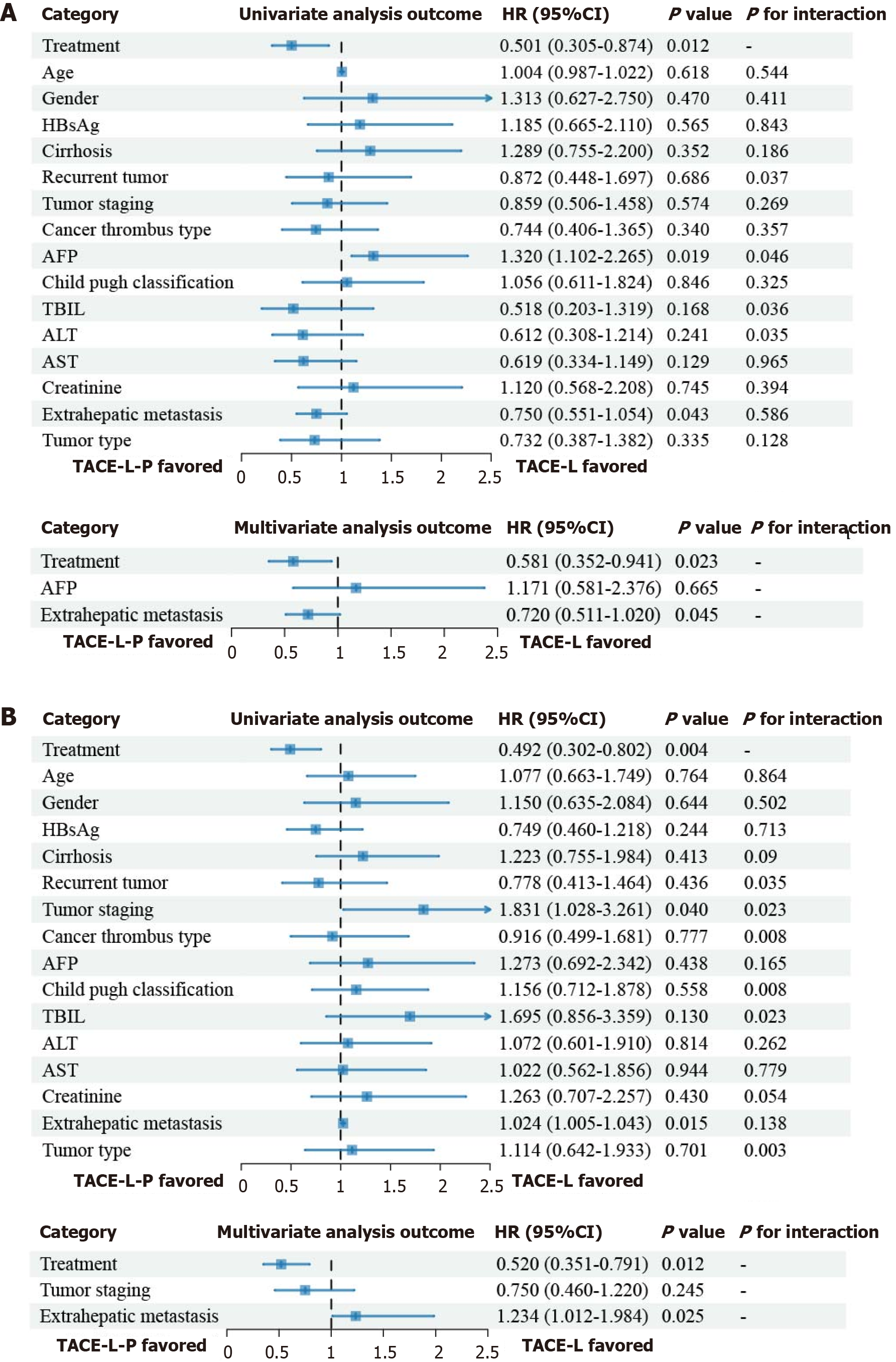
Figure 4 Univariate and multivariate analysis.
A: Overall survival after propensity score matching; B: Univariate and multivariate analysis of progression-free survival after propensity score matching. AFP: Alpha-fetoprotein; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; HbsAg: Hepatitis B surface antigen; HR: Hazard ratio; TACE-L: Transarterial chemoembolization combined with lenvatinib; TACE-L-P: Transarterial arterial chemoembolization combined with lenvatinib plus death protein-1 inhibitors; TBIL: Total bilirubin.
- Citation: Liu S, Liu YH, Ni HB, Li JJ, Wu ZT, Wang LL, Ruan Y, Zhou XH. Transarterial chemoembolization plus lenvatinib with or without protein-1 inhibitor for hepatocellular carcinoma with portal vein tumor thrombus. World J Clin Oncol 2025; 16(6): 106798
- URL: https://www.wjgnet.com/2218-4333/full/v16/i6/106798.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i6.106798